Abstract
Advanced therapy medicinal products (ATMPs) hold promise as treatments for previously untreatable and high-burden diseases. Expectations are high and active company pipelines are observed, yet only 10 market authorizations were approved in Europe. Our aim was to identify challenges experienced in European ATMP clinical development by companies. A survey-based cohort study was conducted among commercial ATMP developers. Respondents shared challenges experienced during various development phases, as well as developer and product characteristics. Descriptions of challenges were grouped in domains (clinical, financial, human resource management, regulatory, scientific, technical, other) and further categorized using thematic content analysis. A descriptive analysis was performed. We invited 271 commercial ATMP developers, of which 68 responded providing 243 challenges. Of products in development, 72% were in early clinical development and 40% were gene therapies. Most developers were small- or medium-sized enterprises (65%). The most often mentioned challenges were related to country-specific requirements (16%), manufacturing (15%), and clinical trial design (8%). The European ATMP field is still in its early stages, and developers experience challenges on many levels. Challenges are multifactorial and a mix of ATMP-specific and generic development aspects, such as new and orphan indications, novel technologies, and inexperience, adding complexity to development efforts.
Keywords: ATMP, gene therapy, cell-based therapy, challenges, development, Europe, regulation, manufacturing
Introduction
Advancements in biomedical sciences are leading to new treatment options for disease with high unmet medical need and create possibilities to improve the quality of life in aging populations. Medicines derived through these advancements include genetic therapy medicinal products (GTMPs), cell-based therapy medicinal products (CTMPs), tissue-engineered products (TEPs), and products integrally combined with medical devices, in Europe known as advanced therapy medicinal products (ATMPs).1
Recent reports show high development activity in the ATMP field that does not seem to match with the limited number of ATMPs currently available on the European market.2 Even though over 500 clinical trials were performed with ATMPs between 2009 and 2017, this led to only 19 market authorization applications to the European Medicines Agency (EMA).2 Ten ATMPs received centralized marketing authorization (MA). Of these, three companies later withdrew the license and one discontinued product marketing, all for commercial reasons. Thorough understanding of stakeholder challenges experienced during development is needed to properly value the potential of ATMPs.
In the literature, no comprehensive overview of the challenges encountered by European-wide commercial ATMP developers is available. Given ATMP development occurs to a greater extent in the public domain compared with more traditional pharmaceutical development, the available literature of ATMPs and their development challenges mostly describes individual issues in academic and hospital settings.3 Difficulties described are complex manufacturing processes,4, 5, 6, 7 implementation of Good Manufacturing Practices (GMP) specifically for cell and gene products,8, 9, 10 complex trial designs,4, 11 and heterogeneous national procedures at member state level.12 The few reports on development by companies describe manufacturing difficulties, uncertain reimbursement perspectives, and the use of hospital exemption (HE).13, 14, 15 Plagnol et al.16 are the first to comprehensively describe industry commercialization barriers collected via interviews specifically for regenerative medicines in the United Kingdom. Their study suggests that commercial ATMP developers encounter both ATMP-specific challenges and more general barriers similar to other emerging industries. Additionally, ATMPs also include a diverse set of technologies developed by a heterogeneous group of developers.3, 17 Challenges may be linked to certain product categories or developers. For example, biotech small- and medium-sized enterprises (SMEs) are known to have more difficulties in acquiring funds and addressing regulatory requirements compared with larger companies.18 Also, manufacturers of biologicals encounter challenges specific for protein manufacturing and formulation.19, 20, 21
The aim of this study was to assess the challenges experienced by companies developing ATMPs in Europe. Experiences were collected via a survey distributed among identified ATMP developers active in Europe. The study contributes to a better understanding of the current European ATMP field and identifies issues impacting product development and patient access.
Results
The search for the European Union (EU) ATMP company cohort yielded 13,392 company names, which were checked for duplicates (n = 5,748). Thereafter we excluded non-commercial developers (n = 6,841), those not located in or developing for the EU (n = 208), non-ATMP developers (n = 98), non-developers (n = 14), and several developers for other reasons (n = 212), such as only non-human products, products in preclinical stages, bankrupt, or merged at time of data collection (January 1, 2017). This resulted in a cohort of 271 developers. In total, 38% (n = 101) responded to our survey request. Respondents returned 56 complete and 12 incomplete surveys resulting in 68 developer inputs and a corresponding response rate of 25%. The 33 remaining respondents indicated no interest in participation; reasons given were time constraints or unwillingness to share information. Table 1 displays characteristics of respondents compared with non-respondents. Table 1 shows respondent characteristics did not differ meaningfully from the non-responders, indicating that the responses are representative for the cohort. Detailed product information is included in Table 2. These characteristics were collected in part one of the survey.
Table 1.
Respondent and Non-respondent Characteristics
| Characteristics | Non-respondents, n (%) | Respondents, n (% |
|---|---|---|
| Response Rate | 203 | 68 |
| Respondent (complete) | – | 56 (55) |
| Respondent (incomplete) | – | 12 (12) |
| Not interested respondent | – | 33 (32) |
| Company Size | ||
| SME | 149 (73) | 44 (65) |
| Large company | 54 (27) | 24 (35) |
| Geography | ||
| United Kingdom | 36 (18) | 16 (24) |
| Germany | 33 (16) | 11 (16) |
| United States | 28 (14) | 5 (7) |
| France | 23 (11) | 7 (10) |
| The Netherlands | 16 (8) | 8 (12) |
| Other (Europe) | 64 (32) | 19 (30) |
| Other (rest of the world) | 3 (1) | 2 (3) |
| ATMP Type (Total) | ||
| GTMPs | 80 (40) | 31 (46) |
| Cell-based medicinal products | 121 (59) | 36 (53) |
| Combined ATMPs | 2 (1) | 1 (1) |
| No. of ATMPs in Development | ||
| 1 | 89 (44) | 33 (49) |
| >1 | 114 (56) | 35 (51) |
CTMP, cell therapy medicinal product; GTMP, gene therapy medicinal product; SME, small- and medium-sized enterprise (1–249 employees).22
Table 2.
Survey Respondent Product Characteristics
| Characteristics | Respondents, n (%) |
|---|---|
| Product Development Stage (Total) | 126 |
| Early clinical (phases I–II) | 91 (72) |
| Late clinical (phase III) | 16 (13) |
| Regulatory approval | 7 (6) |
| Commercialization | 12 (10) |
| Intended Therapeutic Area | |
| Oncology | 36 (29) |
| Ophthalmology | 19 (15) |
| Hematology | 18 (14) |
| Orthopedics and skeletal | 12 (10) |
| Immunology | 9 (7) |
| Gastroenterology | 8 (6) |
| Cardiovascular | 8 (6) |
| Neurology | 5 (4) |
| Dermatology | 4 (3) |
| Other | 7 (6) |
| Pediatric Indication | |
| Yes (<18 years) | 51 (40) |
| No (≥18 years) | 75 (60) |
| Orphan Indication | |
| Yes | 69 (55) |
| No | 57 (45) |
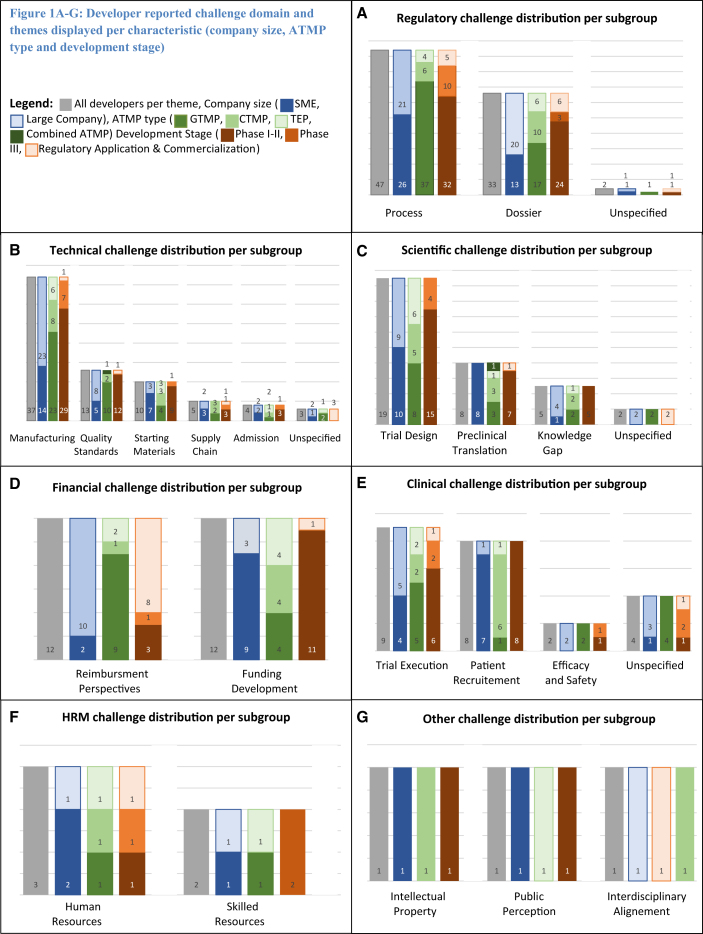
The survey yielded 243 challenge descriptions. After classification, the top three challenge domains were regulatory (34%), technical (30%), and scientific (10%). After further classification of the domains into themes, the top three themes were country-specific requirements (16%), manufacturing (15%), and trial design (8%). A detailed overview of challenge domains, themes, and frequencies is displayed in Table 3. In Figures 1A–1G, themes are presented per subgroup. The results will hereafter be reported per topic and placed into context with literature where possible.
Table 3.
Developer-Reported Challenges in European ATMP Development
| n (%) | |
|---|---|
| Regulatory Challengesa | 82 (34) |
| Regulatory Process | 47 (19) |
| Country-specific requirements | 40 (16) |
| Submission pathways | 4 (2) |
| Pre-submission interaction | 2 (0) |
| Product logistics | 1 (0) |
| Regulatory Dossier | 33 (14) |
| Content uncertainty | 16 (7) |
| Meeting information demand | 9 (4) |
| Information relevance | 5 (2) |
| Post-approval commitment | 3 (1) |
| Unspecified | 2 (0) |
| Technical Challenges | 72 (30) |
| Manufacturing | 37 (15) |
| Quality standards | 13 (5) |
| Starting materials | 10 (4) |
| Supply chain | 5 (2) |
| Product admission | 4 (2) |
| Unspecified | 3 (1) |
| Scientific Challenges | 34 (14) |
| Trial design | 19 (8) |
| Preclinical translation | 8 (3) |
| Knowledge gap | 5 (2) |
| Unspecified | 2 (0) |
| Financial Challenges | 24 (10) |
| Reimbursement perspectives | 12 (5) |
| Funding development | 12 (5) |
| Clinical Challenges | 23 (9) |
| Trial execution | 9 (4) |
| Patient recruitment | 8 (3) |
| Efficacy and safety | 2 (9) |
| Unspecified | 4 (17) |
| HRM Challenges | 5 (2) |
| Human resource | 3 (1) |
| Skilled resource | 2 (0) |
| Other Challenges | 3 (1) |
| Intellectual property | 1 (0) |
| Public perception | 1 (0) |
| Interdisciplinary alignment | 1 (0) |
Percentages are rounded off and displayed as fraction of total challenges (n = 243).
Only regulatory themes are split up in subthemes.
Figure 1.
Developer-Reported Challenge Domains and Themes Displayed per Characteristic (Company Size, ATMP Type, and Development Stage)
Regulatory challenge distribution (A), technical challenge distribution (B), scientific challenge distribution (C), financial challenge distribution (D), clinical challenge distribution (E), human resource management challenge distribution (F), and other challenge distribution (G) per subgroup. Gray bars represent all developers per theme: company size (dark blue bars, SME; light blue bars, large company), ATMP type (dark green bars, GTMP; medium green bars, CTMP; light green bars, TEP; very dark green bars, combined ATMP), development stage (dark orange bars, phases I–II; medium orange bars, phase III; light orange bars, regulatory application and commercialization).
European ATMP Field Composition
Of companies that were active on the European market in January 2017, our survey shows 65% are small- and medium-sized enterprises (SMEs) (Table 1), which is higher compared with the small-molecule and biotechnology industry.22 Half of the respondents are located in Western Europe—United Kingdom (24%), Germany (16%), and France (10%)—which matches previous reports.16 Companies primarily based in the United States, but also developing in and for the EU market, accounted for 7%. Most companies developed cell-based medicinal products (53%), followed by GTMPs (46%) and combined ATMPs (1%). Of respondents, 35 (51%) reported developing more than one ATMP, and 10 developers worked on different ATMP types (e.g., GTMP, CTMP, TEP, or combined ATMP) simultaneously. Together, the 68 respondents were developing 126 ATMPs (Table 2). Acknowledging pharmaceutical development follows a funnel shape, Table 2 shows a higher (72%) percentage of respondent products in early clinical development (phases I–II) compared with non-advanced therapy products.23 Also, a high representation of orphan indications (55%) was reported. In line with previous findings, the top three indications were oncology (29%), ophthalmology (15%), and hematology (14%).16 A new finding was the reported high number of pediatric indications (40%).
Multi-level Regulations
Medicinal product regulations in Europe cover a variety of overlaying jurisdictions and authorities. To group challenges in the regulatory domain, we distinguished between two main themes: the process of working toward a European centralized marketing authorization, which accounted for 57% (n = 47) of the challenges, and composing regulatory dossiers, which are needed for authority approval (40%, n = 33). These themes were thereafter further divided into sub-themes. See Table 3 and Figure 1A for detailed theme and characteristics distribution.
On a European level, few direct references were made to EMA procedures such as pre-submission interactions and scientific advice. Companies mentioned more regulatory interactions with EMA compared with non-ATMP product authorization to understand product nature, clinical trial endpoints, and technical specifications. Also, at day 120 of the MA process, a longer list of questions was mentioned. Meeting the regulators information requests was found to be difficult (n = 9, 11%). The data requested often led to more research and associated costs. The regulator interactions were said to be more frequent compared with non-ATMP development but did help in resolving described challenges. No specific regulatory pathways, such as PRIority MEdicines (PRIME) or protocol assistance in case of orphan drugs, were mentioned. European-level regulatory challenges were mentioned only by companies already involved in ATMP development prior to enforcement of Regulation (European Commission [EC]) 1394/2007.
Further, the majority of regulatory challenges were experienced on a member state level. Meeting country-specific requirements (n = 40, 49%) was the most occurring theme in our survey. GTMP developers reported proportionally more regulatory challenges. This was mainly driven by issues with the genetically modified organism (GMO) legislation (n = 27, 33%), affecting GTMPs the most. The GMO legislation was originally intended for the agri-food sector,24 established by the European Commission but interpreted and implemented on member state level. This local interpretation leads to a variety of national, or even local, responsible governing bodies and procedures. Developers experienced compliance to the GMO legislation as resource intensive and confusing, leading to duplicate applications and inspections resulting in time delays and extra resources without a perception of adding apparent patient or product benefit. For cell-based products, specific challenges with regard to customs and transporting of human tissue across member states were encountered (n = 9, 4%).
Multiple descriptions were given of varying levels of authority’s ATMP familiarity and conflicting scientific advice between national competent authorities (NCAs). This was attributed to a lack of ATMP-specific knowledge and inexperience with this specific medicinal product group. Some developers indicated selecting trial locations based on local legislation interpretations and NCA experience with ATMPs. To address member state variance, one developer followed the EMAs Voluntary Harmonization Procedure (VHP) but experienced contradicting health authority feedback. Like the EU level, also on the member state level more frequent authority interactions took place, solving challenges in most cases.
While compiling dossiers (clinical trial application or MA dossiers), uncertainty around desired information by authorities was reported most (n = 16, 20%). This was partially attributed to the lack of ATMP-specific guidelines.
Manufacturing and Quality Assurance
In the literature, complex manufacturing and difficulty in application of pharmaceutical quality control to ATMPs are frequently mentioned.7, 12, 14 In our survey, we have captured these challenges in the technical domain, which proved to be the second largest (n = 73, 30%). See Figure 1B for themes and characteristics distribution in this domain. Within the technical domain, manufacturing (n = 37, 51%) was the most occurring theme, mostly driven by process scale-up (n = 26, 36%). During scale-up, inconsistency issues were reported most, both in cell and gene therapy products. When seeking external help, finding experienced CMOs was difficult (n = 4, 6%). As with the GMO legislation, GMP legislation is interpreted differently across member states (n = 10, 14%). Additional to what the EMA’s Committee for Advanced Therapies (CAT) may require, NCAs may also request information. Additional information on quality standards (n = 13, 18%) was the most requested. This country variance led to confusion and was perceived to result in a patchwork of manufacturing and quality tests. GTMP developers expressed a need for quality guidance regarding potency, dosing, and impurities. A quality standard challenge mentioned specifically by two TEP developers was the need for high volumes of cell product for quality testing. These batches were thereafter unsuitable for patient use. This was found to be unethical because more donor material was needed.
To comply with GMP guidelines, products for medicinal use are required to be manufactured from appropriate level quality starting materials.25 Suppliers providing certified appropriate quality starting materials were reported to be scarce, as well as expensive (n = 10, 14%). In response, some large ATMP developers expanded their in-house testing ability to certify raw materials, meet standards, and decrease supplier dependency. SMEs might not have the resources or means to copy this practice.
Supply chain challenges (n = 5, 7%) were described in the context of the highly personalized nature of ATMPs. Difficulties were caused by short product shelf-life requiring development of new shipping, preservation, and quality-control solutions. One cell therapy developer switched from an autologous to an allogenic product to overcome these issues. Technical challenges were experienced by both SMEs and large companies. Large companies mentioned that they profited from experience gained in non-ATMP development when addressing technical difficulties. The technical challenges were proportionally reported more in early clinical development, compared with late clinical development, and least in the combined regulatory and/or commercialization phase. No difference was noticed in the subanalyses of domains when distinguishing micro-, small-, and medium-sized enterprises within SMEs.
Translational Uncertainties
To test efficacy and safety of any medicinal product, a rigorous scientific package needs to be built. All challenges associated with planning, design, and rationale of this package are captured in the scientific domain, which yielded 34 (14%) descriptions. ATMPs are currently most often developed for rare and previously untreated disease.26 Developing medicinal products for these indications is associated with a specific set of challenges.27 This was reflected in the most recurring scientific theme: trial design (n = 19, 56%). Descriptions revealed underlying issues such as low patient numbers because of the rare disease indication, little disease progression knowledge, as well as challenges associated with the creation and interpretation of endpoints for new indications. SMEs specifically described difficulties in preclinical translation (n = 8, 24%) mentioning the lack of relevant animal models available. In a few cases (n = 5, 15%), high uncertainty was also reflected by regulators’ feedback, resulting in a request for additional fundamental research. More subgroup details are available in Figure 1C.
Financing and Commercialization
Combining the developer-reported high development costs, reimbursement uncertainty, and the observation of ATMP market authorization holders withdrawing their products from the market for commercial reasons, one might expect the financial domain to be in the top of the challenges.15, 28 Yet, the financial domain yielded 24 challenges, only 10% of all provided descriptions. This domain equally covered two themes: uncertainty in reimbursement perspectives (n = 12, 50%) and funding (n = 12, 50%). Reimbursement uncertainty was mentioned most by large companies (n = 10, 42%), whereas SMEs experienced funding their clinical development most challenging (n = 9, 38%). Development stage also influenced experiencing financial challenges, with companies in late development (regulation/commercialization phase) reporting proportionally more financial challenges. See Figure 1D for all subgroup details.
Clinical Implementation and Acceptance
So far, we mostly discussed challenges experienced on a systems level. Although introduction of new treatments in the clinic is often accompanied by practical issues, these issues are included in the clinical domain (n = 23, 9%). Because of the limited penetration of ATMP in routine clinical care, this domain mostly includes challenges related to trial execution (n = 9, 4%). GTMPs reported proportionally more difficulties in executing trials (n = 5, 2%). At trial sites, additional training was needed in gene product handling, compounding, and admission. Also, trial site employees expressed hesitance toward handling GTMPs (n = 2, 1%). Cell-based products had more trouble reaching study enrollment rates than GTMPs, partially caused by orphan disease indications (n = 6, 2%). Subgroup details are displayed in Figure 1E. Practical issues were also mentioned in the context of employee recruitment in the human resource management (HRM) domain. HRM-related challenges (n = 5, 2%) were differentiated in recruiting personnel in general (n = 3, 1%) and recruiting skilled personnel with ATMP-specific knowledge (n = 2, 1%; see also Figure 1F). One SME reported having difficulty acquiring personnel with specific regulatory ATMP experience. Remaining challenges (n = 3, 1%) were included in the other domain (Figure 1G), and mentioned intellectual property (n = 1) and internal interdisciplinary alignment (n = 1).
Discussion
The aim of this research was to identify challenges experienced in European ATMP development by companies. Our survey shows that the European ATMP field is still in early stages of development with a high representation of SMEs (65%)23, 29 and 72% of reported products in the early clinical stages (phases I–II).22 This is the first study in which the challenges of ATMP developers in the EU are systematically collected and quantified. High resemblance is observed between the challenges from our study and earlier literature, which has mainly focused on academic developers.14, 15 Academic developers also experience difficulty with manufacturing processes,4, 5, 6, 7 followed by the application of GMP requirements to cell and gene products,8, 9, 10 complex trial designs,4, 11 heterogeneous national procedures,12 and reimbursement perspectives.15 However, academia and hospitals produce ATMPs at a smaller scale, not for commercial purposes and for national use only, possibly explaining less of a focus on regulatory challenges. Comparing our findings with the few available papers focusing on companies, manufacturing,15 heterogeneous national procedures,14 and hospital exemption13 are mentioned, which are also reflected in our study findings.
Although our study concentrated on developers active within the EU jurisdiction, some of our findings might also be applicable to other jurisdictions, with the exception of described regulatory challenges on multi-layered regulation, which are bound to jurisdictions. Non-authority-bound challenges include technical, scientific, and clinical challenges, which are most likely to also be experienced outside of the EU. More research is needed to test this hypothesis.
Reimbursement of ATMPs is frequently mentioned as a major hurdle, both from a developer and health technology assessment (HTA) body point of view.28, 30, 31 In our survey, financial challenges represented only 10% of responses, of which 5% specifically address reimbursement perspectives. It is likely that this low percentage can be explained by the early development stages of the ATMP field and high SME representation. Large companies experience more financial security and are therefore able to plan. They are also more likely to have experience in non-ATMP development and are aware of the preparations needed to acquire reimbursement.32 On top of that, the manufacturing of ATMPs is considered to be more expensive by nature and is expected to pose pressure on healthcare budgets.30 Combining the active ATMP pipelines with the prospect of healthcare budget constraints, sustainable ATMP reimbursement will become the next major challenge in this field if not already a reality. Companies should therefore address commercialization of their ATMP early in development. New payment models should be considered and their applicability to ATMPs explored. The potential curability of chronic diseases might shift from long-term and predictable treatment costs to one-off high upfront payments. To address this, a potential for annuity payment models is mentioned in the literature to alleviate these one-time budget constraints.31
Taking a closer look at the reported domains and company size, our survey suggests both SMEs and large companies experience multiple challenges with regard to ATMP-specific regulation and manufacturing. Interpreting the challenge descriptions, large companies seem to be more successful in bringing products to the market, probably by utilizing their non-ATMP development expertise and resources. From the literature it is known that SMEs in general face more challenges with manufacturing, regulatory requirements, and development funding than large companies.33 SMEs are often considered to be highly innovative compared to large companies.34 Most ATMPs on the market are products of large companies collaborating with SMEs or public partners. Examples of products from collaborations of large companies and smaller (public) partners are Strimvelis (autologous CD34+ enriched cell fraction containing CD34+ cells transduced with retroviral vector encoding for the human adenosine deaminase deficiency [ADA] cDNA sequence), Imlygic (talimogene laherparepvec), MACI (autologous cultured chondrocytes), and Holoclar (ex vivo expanded autologous human corneal epithelial cells containing stem cells). Collaborations between large companies and SMEs or academia are a way to move ATMP development forward, in which small partners develop innovative assets and large companies provide financial security and development experience.35 Our study did not query the origin of companies or products, for example, academic spin-off, partnerships, or independent. No difference was observed when further subdividing the SME group into micro-, small-, and medium-sized enterprises in our challenge analyses, perhaps because of small sample size. It would be interesting to incorporate this information in future research because this may influence the challenges experienced.
A key initiative facilitating ATMP development was the adoption of European ATMP legislation (Regulation [EC] 1394/2007). This legislation was the first to define ATMPs and established the Committee for Advanced Therapies (CAT) within the EMA. The CAT is responsible for assessing quality, safety, and efficacy of advanced therapy products. An active approach from European regulators was called for by Maciulaitis et al.5 as early as 2012 describing several pro-active initiatives by the CAT, such as focus groups and workshops. Although most challenges in our survey were experienced in the regulatory domain, the EMA was only mentioned incidentally, and only by developers who have been active in the field before or around adoption of the (EC) 1397/2007 regulation. The majority of the regulatory challenges were experienced on member state level, often attributed to differences in experience and ATMP familiarity between NCAs. The diverse EU landscape was perceived as complex to navigate by both EU and non-EU companies, both in our survey as well as in the literature.36 Several initiatives have started to address member state variance, such as efforts to harmonize GMP requirements and GMO legislation.37 From the developers’ perspective, respondents indicated that seeking frequent and early interactions with EU and NCAs helps attenuate regulatory challenges. Also, building internal ATMP regulatory and manufacturing expertise contributes to addressing challenges.
Taking a broader perspective and combining the high number of identified challenges in the regulatory and manufacturing domain with the high number of smaller and less experienced developers, the sketched situation seems to resemble the early days of biotechnology. This suggest that some of the identified challenges have a more generic character and are non-ATMP specific.18, 20 Similarly, the orphan drugs and new indication linked challenges in the scientific domain are also not ATMP specific. Each individual factor—developing new technologies, development for orphan indications, and new disease areas—adds complexity to the clinical development process. A lesson from early biotechnology innovations we could apply is that gaining experience with new technologies and societal adoption takes time. After the first biologicals entered clinical use, it took 20 to 30 years for these products to become widely available and viable.38 Today, protein-based therapies represented 6 of the global top 10 pharmaceutical products.39 A similar finding is reported by Plagnol et al.,16 who investigated barriers in commercialization of regenerative medicine in the United Kingdom by interviewing leading industrialists. They claim that experienced barriers such as scaling up, lack of experienced people, and lack of business models are also experienced by entrepreneurs in other non-biotech sectors. Our survey was not designed to make a clear distinction between ATMP-specific issues and challenges correlated with an emerging field, new manufacturing techniques, or novel and orphan indications. However, it seems likely that developers active in the ATMP field do experience challenges due to a combination of factors of which not all are ATMP specific. A considerable proportion of challenges is driven by novelty of the field, new and orphan indications, and scientific and technical uncertainties. To test this statement, future research should include exploration of the root causes of the identified experienced challenges. Other considerations to include in future research are challenges experienced in preclinical development and how this may affect challenges downstream.
Our survey provides a snapshot of a rapidly changing commercial European ATMP field. The 271 developers we identified at the start of this study are very likely to change over time. Mergers, acquisitions, and bankruptcies may have occurred even in the short time after this cohort was compiled. Also, our cohort may not include 100% of active ATMP developers. By designing a comprehensive search strategy, we aimed to identify a clear majority of all ATMP developers in Europe. Nonetheless, this is the first comprehensive overview of ATMP companies operating in Europe and identification of their challenges. Previous studies described either incidental challenges (e.g., manufacturing, GMP) or covered a single development phase.13, 14, 15
The high similarity between respondents and non-respondents, and the overlap of our findings with peer-reviewed literature, gray literature, conferences, and workshops2, 15, 40, 41, 42 suggest the reported challenges are likely to be representative for the full cohort. However, we included only commercial developers currently involved in development. This may cause selection bias. Consequently, our results may underestimate developer-experienced challenges. Future analyses should include non-commercial and unsuccessful developers. Although our sample might be small, the quantification helps rank and prioritize identified challenges. Another future consideration is identification of factors that positively influenced ATMP development. Regulations also evolve over time: shortly after we completed data collection, a new ATMP-specific GMP guideline was released.43 A renewed Clinical Trial Application guideline is expected in early 2019. With these new guidances in place, this research can be considered as a baseline measure. It can be used for periodic (re)assessments of the ATMP landscape, following products as they advance through the medicines life cycle, evaluating the influence of regulatory change, scientific advancement, and other factors.
Materials and Methods
We established a cohort of EU ATMP commercial developers. Identified ATMP commercial developers were invited to participate in a survey to systematically collect experienced challenges during clinical development, from first-in-human trials onward, as well as developer and product characteristics.
Cohort Construction
We searched public-accessible databases for company names, EMA SME registry, EUDRACT, Clinicaltrials.gov (sponsor and/or collaborators), and Web of Science (funding agencies), using a comprehensive search query (Table S1). The search was limited to the years 2005–2015. Next, we collected member lists from the largest European (bio)pharmaceutical industry associations. We also searched speaker and attendee affiliations of EMA’s Committee for Advanced Therapies (CAT) reports, ATMP-related conferences, EMA and Innovative Medicines Initiative (IMI) stakeholder meetings, and EMA and IMI public consultations from 2009 to 2016 (Table S2). Lastly, we invited ATMP companies to participate in our research by circulating an open call (Figure S1) on biotechnology associations and society websites in March 2017, as well as announcing the invitation in direct member communications.
Developers were added to the cohort if they met the following inclusion criteria: (1) involved in ATMP (GTMP, CTMP, TEP, or combined ATMP) development as defined by ATMP Regulation (EC) 1394/2007 from January 2005 onward, (2) developer is still active in January 2017, (3) develops ATMPs for human use, (4) is established in or developing for at least 1 of the 28 EU member states, (5) is a commercial entity, and (6) had ATMPs in development of which at least one was in clinical development.
Data Collection
Public data (company websites, annual reports, literature, conference presentations) were used to collect basic cohort characteristics for the full cohort of companies, including company size, geographic location, and types of ATMP products under development. ATMP types were grouped into three categories: GTMP, cell-based medicinal products (combining CTMPs and TEPs), and combined ATMPs.
After cohort construction, we collected contact details of individual employees via public association member lists, conference attendance lists, LinkedIn, and Google search. We targeted senior management officials linked to development in the organization. In large companies we targeted senior managers, department heads, or directors, whereas in SMEs we targeted (vice) presidents, CEOs (chief executive officers), CFOs (chief financial officers), or CMOs (chief marketing officers). Via the survey, detailed developer and product characteristics were collected, as well as challenge descriptions.
Survey Design
The survey consisted of two parts. In the first part, developer and product characteristics were collected using multiple-choice questions. This part contained questions on developer location, number of employees, founding year, and expertise. It also included, for a maximum of three products, ATMP product-specific questions such as classification, intended indication, target population, development stage and time, regulatory pathways used, and utilized regulatory and/or health technology assessment (HTA) body services. In the second part, we asked for experienced development challenges using open text boxes. Each respondent was asked to describe the two biggest challenges experienced per product and per development stage (early clinical development [phases I–II], late clinical development [phase III], regulatory approval, and product commercialization). The introduction to survey part two is included in Figure S2. Respondents were asked to classify challenges in pre-specified domains (clinical, financial, human resource management [HRM], regulatory, scientific, technical, and other challenges). Domain definitions are listed in Figure S2. Prior to survey distribution, content validity was checked by the European Federation of Pharmaceutical Industries and Associations/European Biopharmaceutical Enterprises (EFPIA/EBE) Advanced Therapies joint working group and via a face-to-face interview with a two-person panel consisting of a small CTMP developer and large GTMP developer. The working group and panel provided feedback about flow, question relevance, and missing topics.
In March 2017, an e-mail invitation was subsequently distributed among the cohort via a SurveyMonkey link (https://www.surveymonkey.com/; Palo Alto, CA, USA). The invitation described study objectives, survey contents, and how the data would be handled to maintain the anonymity of respondents. The survey link could be forwarded internally in case multiple departments worked on product development. Recipients were reminded every 2 weeks via e-mail and finally once by telephone before the end of data collection in June 2017.
Data Analysis
Characteristics and challenges were exported from the online survey environment into Microsoft Excel 2016. Missing developer and product characteristics of respondents, due to incomplete responses, were collected through a secondary public domain data search. All challenges (coded and non-coded) were checked for correct classification, according to definitions set in Figure S2 by two Utrecht University researchers (R.M.T.t.H. and A.M.H.). A challenge was assumed to fit only one domain. In ambiguous cases, challenges were added to domains most closely matching the underlying cause. Classification discrepancies were discussed until consensus.
Within each domain, challenges were further categorized into themes, using thematic content analysis methodology: after detailed data familiarization, emerging trends were labeled, reviewed, and eventually defined into mutually exclusive themes.44 The following themes were created within the domains: clinical (trial execution, patient recruitment, efficacy, and safety), financial (funding development, reimbursement perspectives), HRM (human resources, skilled resources), regulatory (process toward filing dossier, dossier compilation), scientific (trial design, preclinical translation, knowledge gap), technical (manufacturing, quality standards, starting materials, supply chain, admission), and other.
Author Contributions
Conceptualization, J.H. and O.H.K.; Validation, J.H. and A.M.H.; Formal Analysis, R.M.T.t.H.; Writing – Original Draft, R.M.T.t.H. and J.H.; Writing – Review & Editing, A.M.H., A.W.B., H.G.M.L., and O.H.K.; Project Administration, A.W.B.; Funding Acquisition, J.H., A.W.B., and O.H.K.
Conflicts of Interest
The authors have no conflicts of interest.
Acknowledgments
This research has been performed within the context of the Escher platform for regulatory innovation that resides under the umbrella of the Dutch not-for-profit organization Lygature (https://www.lygature.org/). The project was supported by an unrestricted research grant from the European Federation of Pharmaceutical Industries and Associations (EFPIA) and its specialized group European Biopharmaceutical Enterprises (EBE). We gratefully acknowledge all respondents for their contribution.
Footnotes
Supplemental Information includes two figures and two tables and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.10.003.
Supplemental Information
References
- 1.European Parliament and the Council of the European Union. (2007). Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Official Journal of the European Union, L 324/121. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:0121:0137:en:PDF.
- 2.Hidalgo-Simon, A. (2017). The EMA view: advanced therapies in Europe. https://www.ebe-biopharma.eu/wp-content/uploads/2017/12/2017-AHS-pesentation-6th-EBE-Annual-Regulatory-Conference-5-Dec-17.pdf..
- 3.Pearce K.F., Hildebrandt M., Greinix H., Scheding S., Koehl U., Worel N., Apperley J., Edinger M., Hauser A., Mischak-Weissinger E. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy. 2014;16:289–297. doi: 10.1016/j.jcyt.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Hanna E., Rémuzat C., Auquier P., Toumi M. Advanced therapy medicinal products: current and future perspectives. J. Mark. Access Health Policy. 2016;4:31036. doi: 10.3402/jmahp.v4.31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciulaitis R., D’Apote L., Buchanan A., Pioppo L., Schneider C.K. Clinical development of advanced therapy medicinal products in Europe: evidence that regulators must be proactive. Mol. Ther. 2012;20:479–482. doi: 10.1038/mt.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wilde S., Guchelaar H.-J., Zandvliet M.L., Meij P. Clinical development of gene- and cell-based therapies: overview of the European landscape. Mol. Ther. Methods Clin. Dev. 2016;3:16073. doi: 10.1038/mtm.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Codinach M., Blanco M., Ortega I., Lloret M., Reales L., Coca M.I., Torrents S., Doral M., Oliver-Vila I., Requena-Montero M. Design and validation of a consistent and reproducible manufacture process for the production of clinical-grade bone marrow-derived multipotent mesenchymal stromal cells. Cytotherapy. 2016;18:1197–1208. doi: 10.1016/j.jcyt.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Viganò M., Giordano R., Lazzari L. Challenges of running a GMP facility for regenerative medicine in a public hospital. Regen. Med. 2017;12:803–813. doi: 10.2217/rme-2017-0051. [DOI] [PubMed] [Google Scholar]
- 9.Kitala D., Kawecki M., Klama-Baryła A., Łabuś W., Glik J., Kraut M., Misiuga M., Nowak M. The isolation and production of the ready-to-use product (the amniotic stem cell culture) in accordance with Good Manufacturing Practice regulations. Stem Cells Dev. 2017;26:694–707. doi: 10.1089/scd.2016.0198. [DOI] [PubMed] [Google Scholar]
- 10.Galli M. ATMPs for cancer immunotherapy: a regulatory overview. In: Bondanza A., Casucci M., editors. Volume 1393. Humana Press; 2016. pp. 1–9. (Tumor Immunology. Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 11.Pignatti F., Aronsson B., Gate N., Vamvakas S., Wade G., Moulon I., Le Courtois P. The review of drug applications submitted to the European Medicines Evaluation Agency: frequently raised objections, and outcome. Eur. J. Clin. Pharmacol. 2002;58:573–580. doi: 10.1007/s00228-002-0532-8. [DOI] [PubMed] [Google Scholar]
- 12.de Wilde S., Veltrop-Duits L., Hoozemans-Strik M., Ras T., Blom-Veenman J., Guchelaar H.J., Zandvliet M., Meij P. Hurdles in clinical implementation of academic advanced therapy medicinal products: a national evaluation. Cytotherapy. 2016;18:797–805. doi: 10.1016/j.jcyt.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Alliance for Regenerative Medicine. (2017). Position on hospital exemption. https://alliancerm.org/sites/default/files/ARM_position_on_HE_final.pdf..
- 14.Van Wilder P. Advanced therapy medicinal products and exemptions to the Regulation 1394/2007: how confident can we be? An exploratory analysis. Front. Pharmacol. 2012;3:12. doi: 10.3389/fphar.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow D., Ussi A., Migliaccio G. Addressing pressing needs in the development of advanced therapies. Front. Bioeng. Biotechnol. 2017;5:55. doi: 10.3389/fbioe.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plagnol A.C., Rowley E., Martin P., Livesey F. Industry perceptions of barriers to commercialization of regenerative medicine products in the UK. Regen. Med. 2009;4:549–559. doi: 10.2217/rme.09.21. [DOI] [PubMed] [Google Scholar]
- 17.Bisson I., Green E., Sharpe M., Herbert C., Hyllner J., Mount N. Landscape of current and emerging cell therapy clinical trials in the UK: current status, comparison to global trends and future perspectives. Regen. Med. 2015;10:169–179. doi: 10.2217/rme.14.71. [DOI] [PubMed] [Google Scholar]
- 18.Tsiftsoglou A.S., Ruiz S., Schneider C.K. Development and regulation of biosimilars: current status and future challenges. BioDrugs. 2013;27:203–211. doi: 10.1007/s40259-013-0020-y. [DOI] [PubMed] [Google Scholar]
- 19.Shi S. Biologics: an update and challenge of their pharmacokinetics. Curr. Drug Metab. 2014;15:271–290. doi: 10.2174/138920021503140412212905. [DOI] [PubMed] [Google Scholar]
- 20.Shire S.J. Formulation and manufacturability of biologics. Curr. Opin. Biotechnol. 2009;20:708–714. doi: 10.1016/j.copbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 21.International B., Lonza S., Delhi T. Best practices for formulation and manufacturing of biotech drug products. BioPharm International. 2009;22:1–10. [Google Scholar]
- 22.Division, C. (2018). SME Office annual report 2017. https://www.ema.europa.eu/documents/report/small-medium-sized-enterprise-sme-office-annual-report-2017_en.pdf.
- 23.Wong C.H., Siah K.W., Lo A.W. Estimation of clinical trial success rates and related parameters. Biostatistics kxx069. 2018 doi: 10.1093/biostatistics/kxx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Union. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. Official Journal of the European Union L. 2001;106:1–38. [Google Scholar]
- 25.European Economic Community Commission Directive 91/356/EEC of 13 June 1991 laying down the principles and guidelines of good manufacturing practice for medicinal products for human use. Official Journal of the European Union L. 1991;193:30–33. [Google Scholar]
- 26.Abou-El-Enein M., Römhild A., Kaiser D., Beier C., Bauer G., Volk H.D., Reinke P. Good Manufacturing Practices (GMP) manufacturing of advanced therapy medicinal products: a novel tailored model for optimizing performance and estimating costs. Cytotherapy. 2013;15:362–383. doi: 10.1016/j.jcyt.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Pariser A.R., Gahl W.A. Important role of translational science in rare disease innovation, discovery, and drug development. J. Gen. Intern. Med. 2014;29(Suppl 3):S804–S807. doi: 10.1007/s11606-014-2881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsden, G., and Towse, A. (2016). Exploring the Assessment and Appraisal of Regenerative Medicines and Cell Therapy Products: Is the NICE Approach Fit for Purpose? https://www.nice.org.uk/media/default/about/what-we-do/science%20policy%20and%20research/regenerative-medicine-study-march2016-2.pdf.
- 29.Garrun D. Drug Dev. Technol; 2012. Small Players, Big Drugs—Pharmaceutical SMEs Take the Innovative Edge. [Google Scholar]
- 30.Hanna E., Tavella F., Rémuzat C., Auquier P., Toumi M. Market access of Atmps: overview and expected challenges. Value Health. 2015;18:A518–A519. [Google Scholar]
- 31.Jørgensen J., Kefalas P. Annuity payments can increase patient access to innovative cell and gene therapies under England’s net budget impact test. J. Mark. Access Health Policy. 2017;5:1355203. doi: 10.1080/20016689.2017.1355203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul S.M., Mytelka D.S., Dunwiddie C.T., Persinger C.C., Munos B.H., Lindborg S.R., Schacht A.L. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 33.Organisation for Economic Co-operation and Development. (2018). Improving the business environment for SMEs through effective regulation. https://www.oecd.org/cfe/smes/ministerial/documents/2018-SME-Ministerial-Conference-Parallel-Session-1.pdf
- 34.Mengal P., Wubbolts M., Zika E., Ruiz A., Brigitta D., Pieniadz A., Black S. Bio-based industries joint undertaking: the catalyst for sustainable bio-based economic growth in Europe. N. Biotechnol. 2018;40(Pt A):31–39. doi: 10.1016/j.nbt.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Mangematin V., Lemarié S., Boissin J., Catherine D., Corolleur F., Coronini R., Trommetter M. Development of SMEs and heterogeneity of trajectories: the case of biotechnology in France. Res. Policy. 2003;32:621–638. [Google Scholar]
- 36.Coppens D.G.M., De Bruin M.L., Leufkens H.G.M., Hoekman J. Global regulatory differences for gene- and cell-based therapies: consequences and implications for patient access and therapeutic innovation. Clin. Pharmacol. Ther. 2018;103:120–127. doi: 10.1002/cpt.894. [DOI] [PubMed] [Google Scholar]
- 37.Renner, M. (2018). CAT work plan 2018. https://www.ema.europa.eu/documents/work-programme/committee-advanced-therapies-cat-work-plan-2018_en.pdf.
- 38.Hopkins M.M., Martin P.A., Nightingale P., Kraft A., Mahdi S. The myth of the biotech revolution: an assessment of technological, clinical and organisational change. Res. Policy. 2007;36:566–589. [Google Scholar]
- 39.Portal, T.S. Top pharmaceutical products by sales worldwide 2017. https://www.statista.com/statistics/258022/top-10-pharmaceutical-products-by-global-sales-2011/.
- 40.European Medicines Agency. (2017). Issues identified by stakeholders: follow- up from EMA’s ATMP workshop. https://www.ema.europa.eu/documents/other/issues-identified-stakeholders-workshop-multi-stakeholder-advanced-therapy-medicinal-products-atmps_en.pdf.
- 41.European Medicines Agency. (2010). Committee for Advanced Therapies (CAT) Work Programme 2010–2015. https://www.ema.europa.eu/documents/work-programme/committee-advanced-therapies-cat-work-programme-2010-2015_en.pdf.
- 42.Alliance for Regenerative Medicine (ARM), European Biopharmaceutical Enterprises (EBE), European Federation of Pharmaceutical Industries and Associations (EFPIA), and EuropaBio. (2017). Possible solutions to improve the European regulatory procedures for clinical trials with Advanced Therapy Medicinal Products consisting of or containing Genetically Modified Organisms. https://www.ebe-biopharma.eu/wp-content/uploads/2017/09/Position_paper_ARM_EFPIA_EBE_EuropaBio_27Sept17_longversion.pdf.
- 43.European Commission. (2017). Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/2017_11_22_guidelines_gmp_for_atmps.pdf.
- 44.Hennink M., Hutter I., Bailey A. Sage Publications Ltd.; 2011. Qualitative Research Methods. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.