Abstract
Despite extensive research efforts, drug addiction persists as a largely unmet medical need. Perhaps the biggest challenge for treating addiction is the high rate of recidivism. While many factors can promote relapse in abstinent drug users, the contribution of stress is particularly problematic, as stress is uncontrollable and pervasive in the lives of those struggling with addiction. Thus, understanding the neurocircuitry that underlies the influence of stress on drug seeking is critical for guiding treatment. Preclinical research aimed at defining this neurocircuitry has, in part, relied upon the use of experimental approaches that allow visualization of cellular and circuit activity that corresponds to stressor-induced drug seeking in rodent relapse models. Much of what we have learned about the mechanisms that mediate stressor-induced relapse has been informed by studies that have used the expression of the immediate early gene, cfos, or its protein product, Fos, as post-mortem activity markers. In this review we provide an overview of the rodent models used to study stressor-induced relapse and briefly summarize what is known about the underlying neurocircuitry before describing the use of cfos/Fos-based approaches. In addition to reviewing findings obtained using this approach, its advantages and limitations are considered. Moreover, new techniques that leverage the expression profile of cfos to tag and manipulate cells based on their activity patterns are discussed. The intent of the review is to guide the interpretation of old and design of new studies that utilize cfos/Fos-based strategies to study the neurocircuitry that contributes to stress-related drug use.
Keywords: Stress, Addiction, Relapse, cfos, Fos, Reinstatement
1. Introduction
Drug addiction persists as a critical public health issue in the United States that imposes tremendous economic, medical, and social burdens. As of 2014, an estimated 21.5 million Americans (8.5% of the US population; NSDUH) abused illicit drugs. A major challenge to the treatment of drug addiction is its chronically relapsing nature. Despite advances in understanding the neurobiology of drug addiction, effective treatment is lacking and the risk for relapse once addicts abstain from drug use is very high, with some estimates of relapse risk close to 70%, depending on the abused drug (Brandon et al., 2007; Shah et al., 2006). Unfortunately, there are few FDA-approved medications for the treatment of substance use disorders (SUDs) and none for psychostimulants, thus highlighting a crucial unmet need.
The primary triggers for drug craving and relapse are re-exposure to the abused drug, exposure to drug-associated cues and context, and the onset of stress. Among these triggers, stress is particularly problematic, as it is pervasive in the lives of most individuals who are battling addiction, and, unlike other relapse-inducing stimuli, it is often unavoidable in daily life. For this reason, understanding the neurocircuitry and neurobiological mechanisms that mediate the contribution of stress to relapse should guide the development of more effective relapse prevention therapies and pharmaceutical interventions.
2. Stress influence on drug and alcohol use: evidence from human studies
The relationship between stress and SUDs is well-documented (Sinha, 2009; Sinha et al., 2011). There is a high incidence of stress-associated neuropsychiatric conditions such as anxiety/mood disorders in drug-dependent populations (Chen et al., 2011; Rounsaville et al., 1991); in 2014, about 8 million Americans were diagnosed with both a mental health disorder and a SUD (SAMHSA). Moreover, there is strong evidence that stressors can induce craving in SUD populations (Constantinou et al., 2010; Fox et al., 2008; McRae-Clark et al., 2011; Sinha et al., 2009, 2006, 1999). Importantly, stressor-induced craving or stress reactivity is predictive of later relapse (Back et al., 2009; Sinha et al., 2006). Additionally, in situations where stress does not directly trigger craving and relapse, it may promote reactivity to other relapse triggers, such as drug-associated cues (Furnari et al., 2015; Moran-Santa Maria et al., 2014; Preston and Epstein, 2011; Preston et al., 2018, 2017, 2009). The neurobiological mechanisms that mediate the contribution of stress to drug-seeking behavior are not well understood. Thus, studies aimed at better defining the underlying neurocircuitry and cellular mechanisms are needed.
3. Pre-clinical models of relapse
3.1. Drug self-administration
Pre-clinical rodent models have provided essential information about the neurocircuitry involved in stressor-induced relapse. One of the most commonly used models incorporates drug self-administration, wherein rodents engage in an operant task (e.g., lever pressing or nose poking), to receive drug delivery. The validity of this model stems largely from the observation that rodents will voluntarily self-administer many of the same drugs that are abused by humans, including psychostimulants, heroin, alcohol, and opiates (Gardner, 2000; Weiss et al., 1992). Relapse-like behavior can be assessed by measuring the reinstatement of responding previously reinforced by the drug following a period of extinction training or forced or voluntary abstinence (Farrell et al., 2018). Many of the same stimuli that induce relapse or craving in humans have been demonstrated to reinstate drug seeking in rodents, including non-contingent priming injections of the drug, exposure to drug-associated cues and contexts, and exposure to stressors (Self and Nestler, 1998; Shaham et al., 2000; Stewart, 2000).
A variety of stressors have been demonstrated to reinstate drug seeking following self-administration, including uncontrollable electric footshock (Buczek et al., 1999; Erb et al., 1996; Lê et al., 1998; Shaham and Stewart, 1995; Shepard et al., 2004), acute food deprivation (Shalev et al., 2003a,b, 2000), forced swim (Conrad et al., 2010), pharmacological “stressors” such as yohimbine (Bremner et al., 1996; Feltenstein and See, 2006; Lê et al., 2005; Shepard et al., 2004) and corticotropin releasing factor (Erb et al., 2006; Lê et al., 2002; Shaham et al., 1997), and cues previously paired with social defeat (Funk et al., 2005). Notably, the complexity of the contribution of stress to drug seeking in humans can also be captured using the self-administration/reinstatement approach. Under self-administration conditions where electric footshock does not directly trigger cocaine seeking following extinction, it can potentiate reinstatement in response to a sub-threshold priming dose of cocaine (Graf et al., 2013; McReynolds et al., 2017, 2016) or drug-associated cues (Buffalari and See, 2009; Feltenstein and See, 2006). Thus, the self-administration/reinstatement approach can be used to study the mechanisms and pathways that mediate triggered drug seeking as well as the potentially non-overlapping processes through which stress interacts with other stimuli to promote relapse.
3.2. Conditioned place preference
A second behavioral approach often used to study relapse using rodents involves conditioned place preference (CPP). CPP involves repeatedly pairing one context with non-contingent administration of a drug while pairing a second context with non-contingent administration of the drug's vehicle. When given a choice, animals will typically demonstrate a preference for (i.e., increase the time spent in) the context previously paired with the drug. This preference can be reduced via extinction training wherein animals are allowed to freely explore all contexts under drug-free conditions and can be reinstated by various stimuli, including stressors (Shalev et al., 2002). As is the case with drug self-administration, a variety of stressors can reinstate drug-induced CPP following extinction, including footshock (Lu et al., 2001, 2000; Sanchez and Sorg, 2001; Wang et al., 2000), forced swim (Carey et al., 2007; Kreibich and Blendy, 2004; Ma et al., 2007; Redila and Chavkin, 2008), restraint (Ribeiro Do Couto et al., 2006; Sanchez et al., 2003), and pharmacological stressors, such as administration of yohimbine (Mantsch et al., 2010) or kappa opioid receptor agonists (Redila and Chavkin, 2008).
3.3. Limitations of rodent models of relapse
Although self-administration and CPP based approaches are commonly used to study relapse-related behaviors, they are not without their limitations (see Mantsch et al., 2016 for more in-depth discussion). For example, most of the experiments that have defined the neurocircuitry and mechanisms that underlie drug seeking using these approaches have relied on extinction-induced suppression of drug-seeking behavior rather than voluntary abstinence. Moreover, most stressors that have been tested in rodent reinstatement experiments classify as physical stressors, such as footshock and forced swim, and may not properly model the type of psychosocial stressors that human drug users encounter (see Mantsch et al., 2016 for more in-depth discussion). Nonetheless, despite their limitations, these models have provided a useful tool for exploration of the neurocircuitry and neurobiological mechanisms that contribute to stressor-induced relapse.
4. The neurocircuitry of stressor-induced reinstatement
Research in a number of laboratories, including ours, has focused on identifying the complex neurocircuitry that underlies the contribution of stress to drug seeking as defined using the reinstatement approach. A brief overview of some of the primary neurocircuitry that has been identified thus far is summarized in Fig. 1 (Mantsch et al., 2016; McReynolds et al., 2014b). However, it is important to recognize that, while putative neurocircuitry has been characterized, the interaction between stress and motivational/reward systems is highly complex and not fully defined. Moreover, the ability of stress to engage neurocircuitry that mediates drug seeking likely varies with context, biological sex, the nature and timing of the stress, and the type of abused drug. Indeed, this complexity and the gaps in our understanding establish a need for experimental approaches that define activity at the cellular and circuit level that corresponds to stressor-induced drug seeking. With this in mind, as we discuss some of the basic neurocircuitry that has been implicated in stressor-induced drug seeking, we will also note research needs that can potentially be addressed using Fos-based approaches.
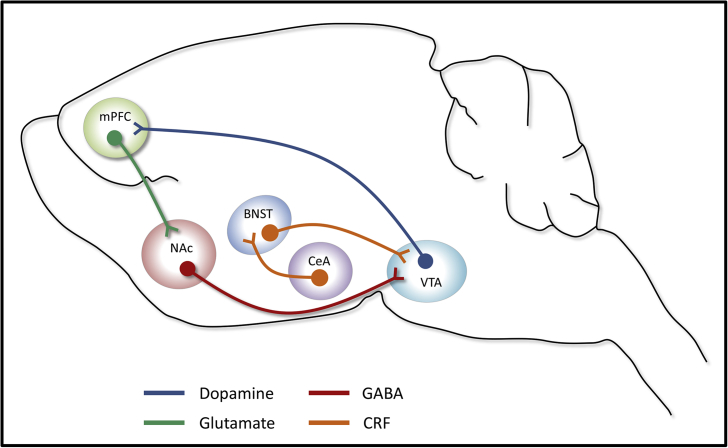
Fig. 1.
Basic neurocircuitry of stressor-induced reinstatement. Stressor-induced reinstatement requires corticotropin-releasing factor (CRF), via activation of CRFR1, and noradrenergic signaling, via activation of β2-adrenergic receptors, in the bed nucleus of the stria terminalis (BNST). CRF in the BNST may arise from the local release of CRF or CRF-containing afferent projections from the central nucleus of the amygdala (CeA). The BNST then sends CRF-containing efferent projections to the ventral tegmental area (VTA) where activation of CRFR1 is critical for stressor-induced reinstatement. The VTA is further regulated by afferent GABAergic projections from the nucleus accumbens (NAc) shell and sends important dopaminergic projections to the medial prefrontal cortex (mPFC). The mPFC then sends a glutamatergic projection to the nucleus accumbens core that is necessary for stressor-, drug-, and drug-associated cue-induced reinstatement.
4.1. Medial prefrontal cortex
The medial prefrontal cortex (mPFC) is ideally positioned to regulate stressor-induced drug seeking, as it is highly responsive to stress, interconnected with both stress- and reward-related circuitry, and important for the control of goal-directed behavior (Cullinan et al., 1995; Diorio et al., 1993; Gourley and Taylor, 2016; McKlveen et al., 2015; Oever et al., 2010). Inhibition of the prelimbic region of the mPFC using either TTX or baclofen/muscimol prevents stressor-induced reinstatement following extinction (Capriles et al., 2003; McFarland et al., 2004). Although a number of stress-responsive systems converge on the mPFC, dopaminergic afferents appear to be particularly important, as antagonism of dopamine D1 receptors in the mPFC blocks stressor-induced reinstatement (Capriles et al., 2003; McFarland et al., 2004; Sanchez et al., 2003). There are a number of open questions regarding the contribution of the mPFC to stressor-induced drug seeking. First, while it is widely assumed that a glutamatergic projection from the prelimbic cortex to the nucleus accumbens core mediates stressor-induced reinstatement, this has not been confirmed and potential contributions of other cortical output pathways have not been ruled out. Second, dopamine D1 receptors are expressed on both GABAergic interneurons and pyramidal neurons in the mPFC (Santana et al., 2009; Vincent et al., 1993), and how exactly dopamine regulates these neuronal populations to induce drug seeking is unknown. Moreover, dopamine effects in the mPFC are mediated through modulation of neurotransmission (Seamans et al., 2001a, 2001b). The sources of D1 receptor-regulated excitatory and inhibitory inputs that contribute to drug seeking have not been well-established. Finally, in addition to the prelimbic cortex, other subregions of the mPFC (e.g., anterior cingulate and orbital frontal cortex) have been implicated in stressor-induced reinstatement (Capriles et al., 2003). Characterization of how this interconnected network of cortical subregions responds to stress and regulates drug seeking is needed.
4.2. Ventral tegmental area
The source of dopamine in the mPFC is neurons that originate in the ventral tegmental area (VTA), a region that is implicated in both reward and stress and may serve as an important integration site for these two processes (Berger et al., 1976; Eden et al., 1987; Moore and Bloom, 1979). As is the case with the prelimbic cortex, inhibition of the VTA via baclofen/muscimol administration prevents stressor-induced reinstatement (McFarland et al., 2004). The VTA is a very heterogenous region, and there is a demonstrated role for glutamatergic (Wang et al., 2005, 2012) and GABAergic (Blacktop et al., 2016) neurotransmission in stressor-induced drug seeking. Moreover, the VTA is regulated by a number of stress-related neuropeptides, including dynorphin (via kappa opioid receptors) and corticotropin-releasing factor (CRF via CRFR1 and CRFR2 receptors), both of which have been implicated in stressor-induced reinstatement (Graziane et al., 2013; Polter et al., 2014; CRF:Blacktop et al., 2011; Chen et al., 2014; Wang et al., 2007). A challenge to understanding the contribution of the VTA to stressor-induced drug seeking is its heterogeneous nature (Lammel et al., 2014). The VTA receives glutamatergic and GABAergic afferents from a number of brain regions (Geisler et al., 2007; Omelchenko and Sesack, 2007), contains both intrinsic interneuron populations and GABAergic projecting neurons, and sends dopaminergic and non-dopaminergic projections throughout the brain (Carr and Sesack, 2000a, 2000b; Margolis et al., 2012; Nair-Roberts et al., 2008; Omelchenko and Sesack, 2009). Moreover, there is evidence that the responsiveness of otherwise similar subgroups of VTA neurons to stress is variable. For example, some VTA dopamine neurons increase their firing rates during periods of stress, while others are inhibited (Brischoux et al., 2009). Similarly, it has been reported that stressors can differentially regulate VTA dopamine neurons according to their efferent projection fields (Deutch et al., 1991; Lammel et al., 2014). Defining which VTA afferents relay relevant stress-related signals to the VTA and understanding which VTA neuronal populations and output pathways are activated to promote drug seeking continue to represent important research questions that can be informed by imaging-based approaches.
4.3. Extended amygdala
The extended amygdala, a complex of structures comprised of the bed nucleus of the stria terminalis (BNST), central amygdala (CeA), and NAc-shell, is critical for processing and integrating stress-related information and plays a role in stressor-induced drug seeking (Koob, 2009; McFarland et al., 2004). The structures that make up the extended amygdala receive dense noradrenergic innervation from the locus coeruleus (LC) and/or medullary cell groups and are highly interconnected with the mesocorticolimbic system (Koob, 2008; Mantsch et al., 2016). Although each of these brain regions sends projections to the VTA (Briand et al., 2010), research has focused largely on the contribution of the BNST to stressor-induced relapse. For this reason, we will focus primarily on the role of the BNST, as an example of a structure in the extended amygdala, in stressor-induced reinstatement.
The BNST is an anatomically complex collection of interconnected subnuclei, some of which are reciprocally connected with the mesocorticolimbic system (Vranjkovic et al., 2017). Sub-regions of the BNST have been implicated in stressor-induced drug seeking (Erb and Stewart, 1999; Vranjkovic et al., 2014; Wang et al., 2006). The BNST is heavily regulated by noradrenaline released from projections that comprise the ventral noradrenergic bundle and arise from medullary cell groups (Aston-Jones et al., 1999). Noradrenergic regulation of the BNST is complex and involves α- and β-adrenergic receptors located at both pre- and post-synaptic sites and the stimulation of local CRF release (Vranjkovic et al., 2017). In particular, β2-adrenergic receptor signaling within the ventral lateral BNST and its regulation of CRF-releasing projections to the VTA appears to be critical for stressor-induced reinstatement (Mantsch et al., 2016; McReynolds et al., 2014a).
Despite our understanding of the contribution of the BNST to stressor-induced drug seeking, there are a number of outstanding research questions. First, the exact phenotype of the BNST-VTA projection that promotes drug seeking is not known. Second, although there is some evidence that a pathway from the CeA to the BNST is important (Erb et al., 2001) the sources of afferent projections into the BNST that are regulated by stress to induce drug seeking are poorly understood. Third, our understanding of the contribution of the complex micro-circuitry and heterogenous cell populations within the BNST, as well as the roles of other neuromodulators and neuropeptides to stressor-induced drug seeking is very limited. While the BNST is a good example of a structure in the extended amygdala with a demonstrated role in stressor-induced reinstatement, it is important to note that both the CeA and the nucleus accumbens shell are also regulated by stress, send projections to the VTA and have been implicated in stressor-induced drug seeking (McFarland et al., 2004; Shaham et al., 2000). Further investigation of how these regions contribute to stressor-induced relapse is needed.
4.4. Summary
Here we have provided a basic overview of some of the neurocircuitry that mediates stressor-induced drug seeking. It is important to note that there are a number of other structures that have been implicated in stressor-induced reinstatement that are not discussed in detail in the current review including the lateral habenula, lateral hypothalamus, ventral pallidum, dorsal/medial raphe nuclei, and insular cortex. It is also clear that we still have much to learn about the neurocircuitry and neurobiological mechanisms that underlies the influence of stress on drug seeking. The remainder of this review will focus on the methodological tools that are available as we further define the neurobiological processes already implicated in stressor-induced drug seeking and identify novel contributing neurocircuits and pathways.
5. Methodological tools used to identify relevant neurocircuitry
The neurocircuitry involved in stressor regulation of drug-seeking behavior has traditionally been defined using two complementary approaches: 1) disrupting the function of circuits; and/or 2) imaging the activity of circuits during or after relevant behaviors.
5.1. Functional manipulations
Historically, the most widely used method to manipulate pathway function is behavioral pharmacology. For example, bilateral, site-specific intracranial administration of the combination of the GABAergic agonists, baclofen and muscimol, or of sodium channel blockers, such as local anesthetics, or tetrodotoxin (TTX), can be used to reversibly inactivate regions of interest prior to reinstatement testing. Likewise, in situations where ligand and receptor systems have already been identified, drugs that interfere with specific signaling systems (e.g., antagonists or inhibitors) can be administered intracranially. To draw conclusions regarding the involvement of pathways in drug seeking, pharmacological disconnection approaches can be applied. Using this approach a pathway is inactivated at its upstream source in one hemisphere and its downstream destination in the other hemisphere resulting in bilateral inhibition of the pathway of interest. This technique is useful when there is minimal decussation and unilateral activation of the pathway is sufficient for behavior, as the control condition is ipsilateral inactivation. This approach is valuable in identifying the role of specific signaling mechanisms within the general circuits involved in stressor-induced reinstatement.
More recently, some of the limitations of behavioral pharmacology to directly test the functional relevance of neurocircuitry involved in stressor-induced reinstatement have been overcome by newer technology such as optogenetics and chemogenetics. Both techniques involve the viral or genetically encoded expression of non-native receptors. In the case of optogenetics, this involves the expression of opsins, which are light-gated ion channels that are activated by specific wavelengths of light. (Fenno et al., 2011). Most commonly, the use of chemogenetics involves the expression of DREADDs (Designer Receptors Exclusively Activated by Designer Drugs), G-protein coupled receptors (hM4Di, hM3Dq, hM3Ds, KORDi) that are activated by biologically inert compounds (Rogan and Roth, 2011). However, there are other chemogenetic tools available, including expression of hM4Di that is predominantly trafficked to the axon, G-protein-coupled receptors that are biased towards β-arrestin signaling, ligand-gated ion channels, and cell-type-specific pharmacology (Atasoy and Sternson, 2018). The advantage of these techniques is the superior spatial resolution and the ability to express these receptors in cell type- and pathway-specific manners. This is accomplished through recombination-dependent expression of the receptors coupled with transgenic or viral expression of Cre recombinase under the control of cell type-specific promoters or through retrograde delivery (Mahler et al., 2014; Smith et al., 2016). Optogenetics provides superior temporal resolution through temporally-limited periods of activation/inactivation of opsins (Fenno et al., 2011) making this technique optimal for discrete behaviors. By contrast, chemogenetics provides a useful alternative as activation/inactivation of circuits with DREADDs occurs over longer time periods with a single treatment that can be administered systemically (Rogan and Roth, 2011). Though there are some issues with the widely used agonist, clozapine-N-oxide (CNO; Gomez et al., 2017; Mahler and Aston-Jones, 2018), there are other commercially available agonists that may mitigate some of the concerns surrounding CNO use (Chen et al., 2015). Both technologies have been critical to the characterization of the neurocircuitry involved in stress-related drug use.
5.2. Imaging neuronal activation
While informative, the manipulation of circuits through either pharmacological or genetic approaches does not necessarily permit determination of the physiological processes that are engaged during, and therefore may encode, drug seeking. Therefore, it is often useful to image the activity of circuits during or after behavior. One primary method is in vivo electrophysiology, which allows for the examination of real time changes in either single unit or population activity within a given brain region in an awake, behaving animal. Traditionally this technique does not conclusively provide information about the phenotypes of the cell populations that drive changes in activation patterns though novel approaches combining cell-type optogenetic stimulation and in vivo electrophysiology mitigate some of those limitations (Roux et al., 2014). Furthermore, there are technical challenges in using this technique to examine circuit activity during reinstatement in response to a variety of stressors including electric footshock and forced swim.
A complementary approach that has been used to assess circuit activation involves ex vivo assessment of immediate early gene (IEG) activation. Although the development of new calcium imaging-based techniques holds much promise for in vivo assessment of circuit activity, most investigation of cellular and pathway function associated with stressor-related drug seeking has utilized this approach. Although assessed ex vivo in sections of brain, the temporal profile of IEG expression allows scientists to draw conclusions regarding activity patterns associated with discrete in vivo manipulations. IEGs and their protein products are expressed rapidly following neuronal activity, with mRNA levels detected as early as 5 min and their protein product detected as early as 15–30 min, depending on the IEG (Kovács, 2008). There are several IEGs with differential time courses for activation and selective cellular localization which provides multiple options when designing experiments, though cfos and Fos expression are the most widely used indicators of neuronal activation. For this reason, the remainder of the review will focus on approaches using Fos expression to image neuronal activation by discussing the activation of neurocircuitry by stress in drug and non-drug experienced animals, advantages and disadvantages of using the Fos imaging approach, and, finally, the use of novel approaches to Fos imaging, and genetic tools using the cfos promoter.
6. Stress-associated neurocircuitry: evidence from Fos imaging
A number of stressors known to reinstate drug seeking also increase Fos in brain regions implicated in drug-seeking behavior, although much of the work to date has been conducted in drug-naïve animals. These brain regions, many of which are included in the putative neurocircuitry for stressor-induced reinstatement as described in Section 4, include the mPFC, dorsal and ventral BNST, dorsal hippocampus, locus coeruleus, paraventricular nucleus of the hypothalamus, and dorsal raphe nuclei, all of which show increased cfos or Fos expression following exposure to multiple reinstating stressors, including forced swim (Briand et al., 2010), electric footshock (Funk et al., 2006, 2003), 30 min restraint (Funk et al., 2006), and systemic administration of yohimbine (Calu et al., 2013; Funk et al., 2006). However, stressor-specific patterns of activation are known. For example, while forced swim (Briand et al., 2010) and yohimbine administration (Funk et al., 2006) increase nucleus accumbens cfos expression, 30 min restraint does not (Funk et al., 2006). Furthermore, increases in cfos expression in the nucleus accumbens shell, but not core, are observed following footshock administration (Funk et al., 2006). It is likely that, in many cases, these differential patterns of activation reflect the nature of the stressor, i.e., physical vs psychosocial vs pharmacological. However, differences in Fos activation may also reflect differences in stressor intensity. This is supported by a recent finding that stressors varying in intensity, such as open field, cat odor, or immobilization stress, elicit differential patterns of Fos activation throughout the brain. Úbeda-Contreras et al. (2018) identified unique patterns of cfos expression that, in some cases, exhibited either a positive or negative relationship with the intensity of the stressor, as assessed by plasma adrenocorticotropic hormone (ACTH) levels, or, in other cases, showed patterns of expression that were independent of stressor intensity. Recognizing which activation responses generalize across stressors and which are stressor-specific is important when interpreting studies aimed at defining neurocircuitry that contributes to stressor-induced relapse.
It is well-established that drug addiction is associated with widespread neuroadaptations that emerge with repeated drug use. For example, consistent with reports that stress reactivity and craving induced by stress imagery is higher in high-frequency vs. low-frequency cocaine users (Fox et al., 2005), stressor-induced reinstatement in rats is heightened following high-intake, long-access cocaine self-administration compared to lower-intake short-access self-administration (Mantsch et al., 2008). For this reason, while much information about stress-related circuitry has been obtained from studies using drug-naïve animals, it is important to understand how that neurocircuitry may differ in animals with drug experience.
While some studies have failed to demonstrate that stressor-induced cfos or Fos expression is different in subjects with a history of drug exposure (Briand et al., 2010), most studies have identified brain regions that display differential activation in drug-experienced compared to drug-naïve animals. Examples of stress-induced Fos activation in drug-experienced, but not drug-naïve, animals include Fos activation in the CeA in response to footshock following chronic cocaine injections (Erb et al., 2004), in the VTA in response to footshock following morphine CPP (Ma et al., 2008), and in the prelimbic cortex in response to acute food deprivation following heroin SA (Shalev et al., 2003a, 2000). Furthermore, even when some Fos activation is observed in drug-naïve animals, this response to stress may be greater in drug-experienced animals. For example, electric footshock increases Fos expression in several stress- and reward-related brain regions to significantly greater levels in rats with a history of ethanol self-administration than in ethanol-naïve rats (Zhao et al., 2006). This is further supported by studies examining cue-induced reinstatement, in which animals with a history of cocaine SA display higher cue reactivity, as assessed by greater Fos activation, in several key brain regions, than drug-naïve animals (Bastle et al., 2012; Kufahl et al., 2009). The effects of prior drug exposure on stressor-induced Fos responses also appears to be brain region-dependent. For example, the BNST, a region that is critical for stressor-induced reinstatement, is consistently activated following stress regardless of drug history (Erb et al., 2004; Ma et al., 2008; Shalev et al., 2003b; Zhao et al., 2006). More work is needed to reconcile differences in regional activation depending on drug history to identify how neurocircuitry involving stress- and reward-related brain regions can be modified by drug experience to promote drug-seeking behavior. Surprisingly, although cfos/Fos imaging has significantly advanced our understanding of the neurobiology that underlies stressor-induced relapse, there are still relatively few studies interrogating relevant neurocircuitry using these approaches.
The neurocircuitry involved in stressor-induced reinstatement of drug seeking as identified by Fos activation is generally supported by human neuroimaging studies. For example, studies using functional magnetic resonance imaging (fMRI) have shown that exposure to personalized stress imagery increases activation of the caudate and dorsal striatum, as defined by an increase in the blood oxygen level-dependent (BOLD) signal, in cocaine-dependent individuals and this increase is associated with stress-induced cocaine craving (Sinha et al., 2005). Additionally, exposure to personalized stress imagery results in increased activation of corticostriatal-limbic circuitry, such as amygdala, caudate-putamen, insula, and anterior cingulate cortex, in cocaine-dependent women compared to healthy controls (Potenza et al., 2012). Furthermore, positron emission tomography (PET) studies using [11C]Raclopride have identified increases in dopamine signaling, and a positive relationship between dopamine signaling and plasma cortisol levels, in the ventral striatum following an amphetamine challenge with or without psychosocial stress (Oswald et al., 2005; Wand et al., 2007). However, there are also clear differences in regional activation as identified by human neuroimaging studies or by rodent Fos activation studies. This is most clear in studies examining the prefrontal cortex. As noted above, stress increases Fos activation in the medial prefrontal cortex of drug-experienced and drug-naïve rodents (Cullinan et al., 1995; Funk et al., 2003; Shalev et al., 2003a,b; Funk et al., 2006; Briand et al., 2010; Schank et al., 2015). In contrast, human neuroimaging studies have consistently identified decreased regional cerebral blood flow or BOLD signal in various subregions of the prefrontal cortex, such as the anterior cingulate cortex (ACC) and ventromedial prefrontal cortex (vmPFC), in response to stress in cocaine- or alcohol-dependent individuals compared to healthy controls (Drexler et al., 2000; Seo et al., 2013; Sinha et al., 2005; Zakiniaeiz et al., 2017) though these patterns may differ in cocaine-dependent women (Potenza et al., 2012). However, it should be noted that the BOLD signal may not reflect overall increased activation of a given brain region as it is not possible to determine whether the BOLD signal is being driven by excitatory or inhibitory neurons (Lauritzen et al., 2012). More work is needed to understand what patterns of the BOLD signal mean for excitation/inhibition within a given brain region and to understand how identified neuronal activation patterns within rodents may inform human drug abuse studies.
7. Utility and caveats of using Fos as a neuronal activity marker
7.1. The immediate early gene cfos
As with any technique, it is necessary to understand the utility and limitations of using Fos as an activity marker to identify relevant neurocircuitry. For this reason, discussion of the advantages and disadvantages of this approach is provided below. The immediate early gene cfos, and its protein product Fos, have been used as activity markers for almost 30 years, and cfos was one of the first identified immediate early genes (IEGs). The major function of Fos protein results from the formation of a heterodimer, via its leucine zipper motif, with members of the Jun protein family to create an inducible transcription factor, activating-protein 1 (AP1), that is involved in the transduction of “later” genes (Curran and Franza, 1988; Morgan and Curran, 1991). Fos is expressed in most cell types in the central nervous system including GABAergic medium spiny neurons and interneurons, glutamatergic, spinal cord, monoaminergic, and neurosecretory neurons (Ceccatelli et al., 1989; Chan et al., 1993; Deutch et al., 1991; Dragunow and Robertson, 1987; Dragunow et al., 1987; Hoffman et al., 1993; Hunt et al., 1987; Leslie et al., 1993; Menétrey et al., 1989; Morgan et al., 1987; Robertson et al., 1992; Szekely et al., 1987). Fos is also expressed in glia (Condorelli et al., 1989; Dragunow and Robertson, 1988b) and astrocytes (Arenander et al., 1989; Hisanaga et al., 1990; McNaughton and Hunt, 1992) though the regulation and function of Fos in these cell types likely differs from that in neurons. For example, depolarization-inducing stimuli that elicit Fos activation in neurons do not induce Fos in astrocytes (Hisanaga et al., 1990). Instead, Fos activation in astrocytes and glia may be the result of cellular mechanisms and signaling involved in differentiation and proliferation of astrocytes, particularly following injury (Dragunow et al., 1990; Hisanaga et al., 1990).
Under basal conditions, cfos mRNA and protein are expressed at very low, barely detectable, levels (Hughes et al., 1992) in most, but not all, brain regions. However, cfos and Fos are rapidly induced in response to various stimuli, including peripheral mechanical and electrical stimulation, pain, inflammation, stress, neuroendocrine and neuropeptide signaling, neuronal depolarization, neurotrophic factors, neurotransmitters, and increases in intracellular Ca2+ levels (Doucet et al., 1990; Gaiddon et al., 1996; Ghosh et al., 1994; Greenberg and Ziff, 1984; Morgan and Curran, 1989; Sheng and Greenberg, 1990; Sheng et al., 1990; Szekely et al., 1987). Following induction, Fos protein primarily accumulates in the nucleus (Condorelli et al., 1989; Dragunow and Robertson, 1988a). The time course of cfos mRNA and protein expression has been established wherein mRNA levels peak between 30 and 60 min and protein levels peak between 1 and 3 h following an acute challenge and this expression returns to low, barely detectable levels within 4–6 h (Chan et al., 1993; Cullinan et al., 1995; Ding et al., 1994; Ikeda et al., 1994; Kovács and Sawchenko, 1996; Sonnenberg et al., 1989). However, it should be noted that there are exceptions to this time course of expression including delayed or prolonged patterns of cfos activation in various brain regions following acute stressors (Cullinan et al., 1995). The low basal level, rapid induction, and transient nature of cfos mRNA and protein expression ideally positions Fos as an activity marker, especially in comparison to other IEGs with longer expression patterns, such as zif268 (Cullinan et al., 1995).
7.2. Utility of the Fos imaging approach
Because cfos mRNA and protein are easy to study and widely used as activity markers, there are a number of studies across which comparisons can be made, assuming the use of the same animal age, sex, species, strain, and time points for quantification of expression. The primary methods used to visualize and analyze cfos mRNA (in situ hybridization), and Fos protein expression (immunohistochemistry), give superb spatial resolution, including analysis of laminar patterns of expression and sub-region specificity in highly heterogenous brain regions. Furthermore, taking advantage of the well-established time course for expression patterns, and the accumulation of Fos protein in the nucleus, a major utility in examining IEG activation is co-localizing the Fos signal with other cellular markers, including those that are cell type-specific, and retrograde tracers (Fig. 2). The ability to concurrently assess Fos protein with cfos mRNA or other IEGs allows examination of activation patterns in response to two different experimental manipulations by exploiting the differential time course and location of mRNA and protein expression. The half-life of cfos mRNA is approximately 15 min (Herdegen and Leah, 1998) and therefore cfos mRNA should return to basal levels when examining expression at a time point chosen for peak protein levels. Therefore, it is possible to, at the same time point, combine immunohistochemistry for nuclear Fos protein expression, which would correspond to earlier exposure to one stimulus, and in situ hybridization for cytosolic cfos mRNA expression, which would correspond to later exposure to a different stimulus (Kovács, 2008; Kovács et al., 2001). For example, a newer, optimized method to concurrently visualize cfos mRNA and Fos protein in the same tissue, tyramide-amplified immunohistochemistry-fluorescence in situ hybridization (TAI-Fish), has been used to identify differential neuronal activation in response to appetitive and aversive stimuli in the same animal (Xiu et al., 2014). A similar version of this technique capitalizes on differential temporal and spatial patterns of IEG mRNA expression termed cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH; Guzowski et al., 1999; Vazdarjanova et al., 2002).
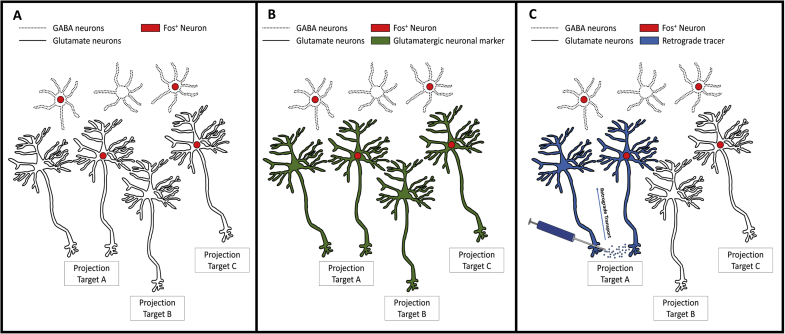
Fig. 2.
Utility of Fos imaging approaches. There are multiple approaches to using Fos as an activity marker and each approach provides different levels of information as detailed below. A) Fos protein expression is predominantly localized to the nucleus (red circles). Fos is expressed in multiple cell types, including GABAergic and glutamatergic neurons, and examination of Fos expression alone will identify multiple cell types and multiple projection targets. B) One method to identify unique patterns of neuronal activation is to co-localize the Fos signal (red) with a cell type-specific marker (green), such as a marker for glutamatergic neurons. Therefore, it is possible to identify neuronal activation of a particular cell type via comparison of the number of neurons that express both Fos and the cell-type specific marker. However, this method still does not provide information about selective activation of efferent projections. C) To identify activation of specific pathways, a retrograde tracer can be used. The retrograde tracer is micro-infused into a specific projection target, is taken up by the axon terminals and is transported back to the cell body. It is possible to then to quantify the co-localization of the retrograde tracer signal (blue) and Fos expression (red) to analyze the pattern of pathway activation. It should be noted that in regions where projection neurons are comprised of multiple cell-types, this method only identifies activation of the specific pathway independent of cell type. To obtain information about cell type- and pathway-specific neuronal activation, it would be necessary to use both a retrograde tracer and a cell type-specific marker in combination with Fos expression. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
As noted above, Fos expression can be co-localized with cell type-specific markers to characterize differential patterns of neuronal activation following an experimental manipulation. This is especially powerful as it allows comparison of patterns of activity in different cell types within the same brain region which is particularly useful when examining heterogenous areas such as the ventral tegmental area. In particular, this approach has been used to distinguish activation of dopaminergic, noradrenergic, glutamatergic and GABAergic neurons following various experimental manipulations (Deutch and Duman, 1996; Ishida et al., 2002a, 2002b; 2001; Ruskin and Marshall, 1997).
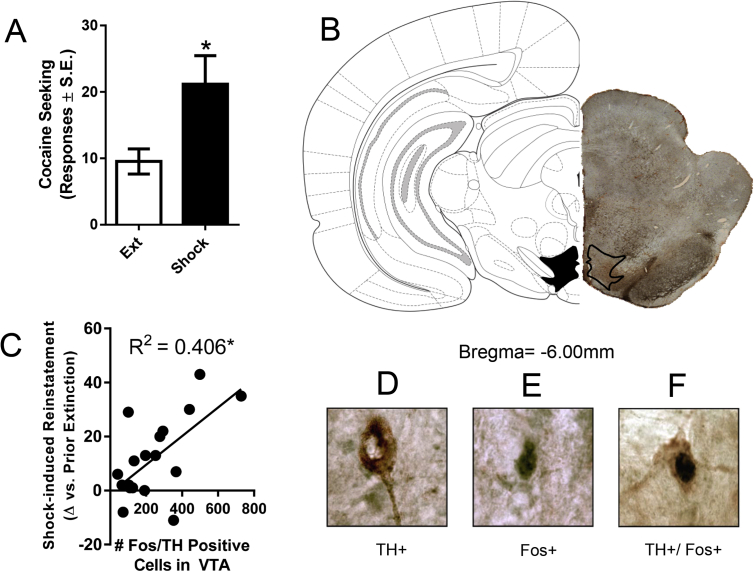
As an example, our lab has used the co-localization approach to identify cell populations in the VTA that are likely involved in the stressor-induced reinstatement of extinguished cocaine seeking. In this study, animals with variable cocaine self-administration history (2 or 6 h SA/day; 0.5 mg/kg/inf), underwent extinction training wherein lever responding decreased as cocaine was no longer available. After reaching extinction criteria (<15 lever presses/2 h), a reinstatement test was given during which a series of intermittent electric footshocks (each footshock: 3 × 0.5 mA, 200 msec duration; average frequency 45 s) was administered over a 15-min period in the self-administration context followed by the reinstatement session wherein lever presses were recorded. Ninety minutes into the reinstatement session, rats were transcardially perfused with 4% PFA and 25-μm sections were taken at the level of the VTA. Sections were processed via immunohistochemistry for expression of tyrosine hydroxylase and Fos with a nickel 3,3′-diaminobenzidine (NiDAB)/3,3′-diaminobenzidine (DAB) stain and the number of VTA dopamine neurons, as defined by TH-expression, that also expressed Fos was calculated. We found that the number of TH/Fos + VTA neurons was positively correlated with cocaine seeking (Fig. 3), suggesting that the activation of dopaminergic neurons in the VTA likely contributes to stressor-induced reinstatement. Similar approaches have been used to demonstrate that restraint stressor-induced reinstatement following cocaine CPP is associated with increased activation of oxytocin neurons in the lateral hypothalamus (Tung et al., 2016) and that yohimbine-induced reinstatement following ethanol self-administration results in increased activation of CRF-, dynorphin- and GABA-positive neurons in the central nucleus of the amygdala (Walker et al., 2017).
Fig. 3.
Stressor-induced reinstatement of cocaine seeking is positively correlated with activation of ventral tegmental area (VTA) dopamine neurons. A) Electric footshock induces significant reinstatement of cocaine seeking, as defined by an increase in the number of lever responses during the reinstatement test as compared to extinction responding, in rats with a variable history of self-administration (2/hr or 6/hr access/day; t (19) = −3.67, p < .05). Data are presented as mean ± SEM. B) Representative image depicting the area within which immunoreactive cells were counted (at −6.0 mm from Bregma). C) The number of cells that were positive for both tyrosine hydroxylase (TH) and Fos expression in the VTA is positively correlated with the magnitude of stress-induced reinstatement (p < .05). D) Representative image of a TH-positive neuron in the VTA. E) Representative image of a Fos-positive neuron in the VTA. F) Representative image of a TH/Fos-positive neuron in the VTA.
Lastly, we and others use co-localization of Fos protein expression and a retrograde tracer to identify relevant neurocircuitry involved in stress and addictive behaviors. Retrograde tracers, such as fluorogold, cholera toxin subunit B (CTb), red and green retrobeads and retrograde viral constructs, are infused into target brain regions, taken up by the axon terminals in that region and transported back to the cell bodies (Köbbert et al., 2000). The tissue is then analyzed for Fos expression, retrograde tracer expression, and co-localization of Fos with the retrograde tracer (Fig. 2) with higher percentages of co-localized neurons indicating greater activation of that particular pathway (Mahler and Aston-Jones, 2012). As an example of this approach, using fluorogold injections into the VTA, Briand et al. (2010) demonstrated that forced swim-induced reinstatement following cocaine CPP is associated with increased activation of vBNST, dBNST, lateral hypothalamus, and infralimbic cortical afferents to the VTA.
Notably, depending on the availability of antibodies or genetically encoded reporters, it is possible to triple-label brain tissue to identify co-localization of Fos, a cell-specific marker, and a retrograde tracer to examine activation of cell type-specific pathways, such as dopaminergic projections from the ventral tegmental area. Furthermore, the newer retrograde tracers, including Alexa fluor-conjugated CTb and retrograde viral constructs expressing different fusion proteins, and red and green retrobeads, enable analysis and comparison of activation of multiple pathways within the same animal. One of the strongest advantages of using Fos as an activity marker is to co-localize expression with these different markers to obtain cell type- and pathway-specific patterns of activation during behavior which, until recently with the development of newer in vivo imaging approaches, has not been feasible. Furthermore, unlike in vivo electrophysiology and the newer in vivo imaging approaches, Fos imaging is not limited to a single brain region and it is possible to examine expression patterns throughout the whole brain. These approaches are relatively simple, fairly inexpensive, and provide a lot of information from a single animal, thus positioning Fos imaging as a useful first-pass approach to identifying activated brain regions and pathways following behavior.
7.3. Caveats to using Fos as a neuronal activity marker
Fos imaging is a useful tool, but there are several limitations and caveats to consider when applying this approach. First, while it is possible to gain insight into neuronal activation from increases in Fos expression, it is important to be careful about interpreting null findings, as the absence of changes in Fos expression does not preclude the presence of relevant changes in neuronal activity. This is best demonstrated by the observation that there are differential patterns of basal expression of IEGs (Cullinan et al., 1995) and differential patterns of IEG activation following a stimulus wherein increases in other IEGs, such as arc, c-jun, and zif268, but not cfos, are observed (Chan et al., 1993; Cullinan et al., 1995; Lucas et al., 2008). Furthermore, it is often assumed that neuronal stimulation will always result in cfos transcription. However, this is not always the case. For example, substantia nigra activity has been measured following kindled seizure induction but corresponding changes in Fos were not observed (Labiner et al., 1993) and there are experimental conditions where a physiological response to HPA axis activation has been detected without corresponding changes in Fos expression in the PVN (Brown and Sawchenko, 1997; Figueiredo et al., 2003). The presence or absence of changes in Fos can depend on many factors, including whether mRNA or protein levels are being examined. Traditionally, it is assumed that changes in mRNA will always lead to changes in protein expression. However, there are multiple factors that can influence protein expression including, but not limited to, post-transcriptional modifications, inefficient translation, rapid mRNA decay or protein degradation and therefore changes in cfos mRNA are not always consistent with levels of Fos protein expression (Hisanaga et al., 1992; Kiessling et al., 1993; Worley et al., 1993). Fos activation only occurs upon pronounced cellular stimulation activation, which accounts for its low basal level of expression in most, but not all, brain regions (Kovács, 2008). Because of this requirement for a robust stimulation, Fos imaging is unlikely to capture subtle changes in neuronal activation in response to weaker “subthreshold” stimuli.
One of the most common approaches for analyzing Fos expression is cell counting. This approach is dependent on low basal levels of Fos and requires expression within a given cell reaching a detection threshold in order to produce a signal. In using this approach, Fos imaging primarily gives an on/off signal and does not provide information about the magnitude of expression within a cell. Thus, if experimental manipulations result in the same number of neurons being activated, but Fos levels within the cell change, the effect will not be captured by cell counting. An alternative approach is a densitometric analysis in which the overall magnitude of the Fos response within a brain region is measured and provides information about changes in levels of mRNA or protein expression. However, if changes in the relevant neurocircuitry only require a small number of neurons, these changes may still not be captured even with densitometric analysis. For example, Liu et al. (2014) discovered that only 5–6% of neurons in the dorsal striatum were activated following an acute methamphetamine injection. Methamphetamine induced a 3–20-fold increase in IEG levels in Fos-expressing neurons that were separated via fluorescence-activated cell sorting (FACS). However, IEG expression was 10-fold lower or undetectable when assessed in unsorted samples. Under these types of conditions, even a densitometric analysis would be unlikely to capture those relevant changes using traditional Fos imaging approaches. A more accurate assessment of mRNA and protein expression changes typically require using a more quantitative approach such as qPCR, for mRNA, and western blotting, for protein, though these approaches lack the spatial resolution as they require bulk tissue dissection. Taken together, a failure of an experiment to influence Fos expression may be a consequence of several interacting factors and it is necessary to be cautious when interpreting null findings.
In addition to identifying the best approach for quantification of Fos expression for a given experiment, it is important to include the correct controls. Studies examining Fos expression following a behavior, should include proper behavioral control groups to accurately determine the specificity of the observed Fos expression patterns to the behavior of interest. Behavioral control groups should closely match the conditions of the experimental group to limit differences to factors other than the experimental manipulation. For example, experiments examining changes in Fos expression following stressor-induced reinstatement in animals with a history of drug self-administration should include control groups of animals that have been surgically implanted with venous catheters and are provided access to saline for self-administration or received yoked saline/vehicle infusions prior to testing for drug seeking. These groups control for effects of a history of surgery/recovery, repeated infusions, and/or continual exposure to the drug context and associated discrete cues. Such control conditions also permit assessment of the effects of an acute stressor on Fos under conditions where reinstatement is not observed, thus allowing for identification of expression patterns unique to stressor-induced drug-seeking behavior.
Beyond behavioral control groups, there are other measures to consider in the design, execution and analysis of a Fos imaging experiment. Typically, when performing in situ hybridization or immunohistochemistry in a brain region, it is important to take series of sections that uniformly span the anterior/posterior (A/P) axis of that brain region. This is important as some studies have identified differential Fos staining patterns along the A/P axis of structures following drug-seeking behavior (Mahler and Aston-Jones, 2012). In addition, in larger experiments where it is not possible for all sections to be labeled in a single “run,” it is important to account for potential variability across batches of labeled tissue. This can be accomplished by ensuring that each batch of sections contains at least one sample from each group so that any variability across batches is not biased towards any particular experimental group.
When using cell count analyses to quantify Fos expression, data are typically presented as either total Fos-immunoreactive (ir) cells or Fos-ir cells/mm2. Both are valid approaches. However, presenting Fos expression as Fos/mm2 allows for better direct comparison across brain regions with different areas. When using a densitometric analysis, the intensity of the signal is the critical measure, so a background signal subtraction method should be used with a clear definition of the parameters reported. Additionally, in all instances, a clear definition of the boundaries for the subregions being targeted for analysis should be reported to ensure reproducibility and allow for comparison across studies. For any quantification of Fos expression, the analysis may be best served by a comparison of Fos staining within the target brain region to a control region unlikely to show changes in Fos to provide more definitive evidence of the specificity of the pattern of expression to the relevant behavior. Finally, as most Fos studies report a single value for each animal, it is important to be clear about how that value was obtained. For example, when there is no gradient along the A/P axis, values may be obtained by averaging densities or counts between hemispheres within a slice, and then possibly averaging those values across multiple sections. This minimizes the likelihood that aberrant values in any individual section from a brain will skew results.
Interpreting a change in Fos expression also requires caution, and it is important to consider the nature of the neuronal activation identified by Fos, as it may not reflect plasticity relevant to behavior. For example, recent work examining neuronal ensembles suggests that many behaviors are mediated by as few as 3% of neurons in a given brain region (Bossert et al., 2011; Cruz et al., 2014). Furthermore, these activated neuronal ensembles may exhibit selective changes in plasticity which are unlikely to be identified using traditional approaches. Whitaker et al. (2017) found that food self-administration resulted in increased intrinsic excitability in Fos-expressing neurons in the prelimbic cortex and decreased excitability in non-Fos expressing neurons via distinct mechanisms. Notably, there are other IEGs that may better reflect plasticity relevant for behavior. One such IEG is arc. The protein product of arc has a demonstrated functional role in synaptic plasticity (Guzowski et al., 2000; Messaoudi et al., 2007) and has been implicated in several cognitive and learning and memory related processes (Guzowski et al., 2000; McIntyre et al., 2005).
Fos imaging approaches, as they have been traditionally used, do not provide information about the fine tuning of circuits, shifts in firing patterns (e.g. tonic to phasic), or changes in the “signal-to-noise” ratio. Notably, in the locus coeruleus (LC), Fos expression is triggered by induction of either phasic or tonic firing (Grant et al., 1992). Thus, a shift in the LC firing pattern from phasic to tonic or vice versa would not be reflected by Fos expression. Similarly, examining Fos expression is unlikely to capture “signal-to-noise” shifts in phasic dopamine release such as those that emerge in response to repeated administration of drugs of abuse (see e.g., Wanat et al., 2009). An important theory of how changes in dopamine firing determine drug seeking posits that the phasic dopamine “signal” elicited by drug-associated cues becomes progressively more salient following repeated drug use in part due to attenuation of the tonic “noise” pattern of firing. As both tonic and phasic activity are associated with the Fos expression, such shifts in firing patterns are unlikely to be captured using Fos-based approaches.
Fos imaging also has poor temporal resolution. Each measurement can only represent a single time point following behavior. Thus, this approach provides the most accurate information when Fos imaging is conducted following a discrete experimental manipulation that occurs within a well-defined time window. However, when Fos expression is examined following more prolonged periods of experimental manipulation, such as those associated with reinstatement, the Fos signal represents at best a rough approximation of neuronal activation as it relates to the experimental time-point of interest (e.g., activity at the start of the reinstatement test session). Interpretation is further complicated by the likelihood that there are some cell populations that are activated in response to the onset of stress, while the activation of other cells may signal stressor termination (Holly and Miczek, 2016; Navratilova et al., 2012; Tanimoto et al., 2004). Furthermore, reliance on a single time point means that there is no pre-experimental measure of basal Fos levels. As a result, comparison of neuronal activation patterns must occur across subjects which can increase experimental variability.
In addition, unlike in vivo electrophysiology and newer in vivo imaging approaches, Fos does not provide real-time assessment of changes in neuronal activity, although it can provide information about changes in a cell type- and pathway-specific manner. Nevertheless, when examining Fos expression independent of co-localization studies, it is important to remember that Fos is expressed in multiple cell types and thus it is not feasible to make conclusions about the cell types or pathways driving the changes in Fos or the connectivity of activated neurons. For example, the activation of GABAergic interneurons in a brain region would produce a positive Fos signal that would likely be associated with an overall inhibition of neuronal outputs from that structure. Even when co-localizing Fos with retrograde tracers, there is still a limitation to the number of pathways that can be examined in a single brain. In sum, Fos imaging is an incredibly useful tool, in so far as the caveats and limitations are taken into consideration. Newer approaches to analyzing Fos expression and the development of novel technology using the cfos promotor may mitigate some of the limitations of the traditional Fos imaging approach.
8. Novel approaches in using Fos signaling
8.1. Towards a “whole-brain” approach to Fos analysis
In spite of the caveats noted in the previous section, there are several new ways to incorporate Fos analysis into behavioral neuroscience research. Automation of image acquisition and cell counting permit investigators to move from investigations of a small set of regions of interest (ROIs) to a whole-brain approach to Fos analysis. Quantification of Fos across a wide range of ROIs can inform how treatments influence the function of macroscale networks, in a way that is similar to the whole-brain analyses used in functional magnetic imaging (fMRI). One straightforward approach is to expand Fos quantification to a set of structures that have known anatomical relationships and compute correlation coefficients between pairs of nodes to generate hypotheses about the function of these pathways in relationship to behavior. For example, George et al. (2012) quantified Fos in ROIs within the prefrontal cortex (PFC), amygdala and hippocampus of alcohol dependent rats. Withdrawal from alcohol was associated with a decrease in correlated activity between the mPFC and the CeA suggesting that alcohol use and abstinence may specifically modify this segment of the neural circuit that controls alcohol consumption (George and Hope, 2017). The Fos results in this example provide a reliable foundation and justification for conducting riskier tract-specific mechanistic experiments to test the involvement of the circuit model in vivo.
8.2. Graph theory-based network analysis
In the example provided above, pair-wise correlations proved to be informative, but the statistical approach did not push the limits of the multivariate statistical approaches that would enable inferences to be drawn from the relationships between Fos across all of the ROIs. The adoption of graph theory-based computational methods over the last several years has led to major advances in understanding how networks within the brain interact as correlates of behavior, cognition and disease (Bullmore and Sporns, 2009). Here the term graph refers to abstract representation of the organization of nodes within a network and the nature of their connections, called edges. Nodes are typically anatomically defined ROIs while edges may be functional, structural, or effective. Functional connectivity between regions exists when neuronal activity in an ROI or a community, a subnetwork of interconnected network nodes, reliably predicts activity in another ROI, ROIs, or communities. Anatomical, or “connectome” data can be used to inform the structural connectivity among ROIs and complement functional inferences. Ideally, functional and structural connectivity provide a framework for investigating the mechanisms by which activity in one community influences the other, called effective connectivity. For more detailed description of the network parameters and underlying computations that can be evaluated with graph theoretical analysis we refer readers to (Rubinov and Sporns, 2010). Graph theoretical analyses are well suited for quantifying components of abstract networks such as the large, multidimensional time-series datasets involved in human neuroimaging studies (Bassett and Sporns, 2017). However, Graph analyses are useful with static data such as social media networks and so can be applied to Fos.
As reviewed above, Fos has been used extensively as a dependent measure in stress research with the vast majority of investigations quantifying Fos in a small set of hypothetically significant ROIs. These studies led to the neural circuit model of stress and drug seeking articulated in Section 4 in which stressor-induced drug seeking is thought to be a consequence of the interactions of specialized brain networks. As the field moves to test and expand the circuity of stress and SUDs, we believe graph theory provides a set of tools that can be applied to neural circuit analysis to generate computational models and hypotheses about specific functions of ROIs and networks. Graph theory was recently applied to a simple fear conditioning experiment which we believe illustrates how well large-scale Fos analysis can set the stage for expansion of our network models. Fos was quantified in 84 ROIs from mice given fear recall test either 1 or 36 days after context-shock conditioning (Wheeler et al., 2013). The recall test evoked strikingly different patterns of network activation, and importantly, different roles for key network nodes as a function of recall time. Following on this initial account, Vetere et al. (2017) first used a computational model to simulate the effect of “lesioning” nodes with high functional connectivity on network organization. The model predicted a linear relationship between node connectivity and importance to in vivo fear consolidation which was tested in vivo with node-specific chemogenetic silencing in later behavioral experiments. The results found, unequivocally, that silencing of higher degree nodes, such as the CA1 hippocampal region, produced the most robust interference with fear consolidation while silencing low degree nodes, such as medial dorsal thalamus had effect. While these results are consistent with prior investigations of fear consolidation, the computational model enabled novel discoveries. Specifically, silencing the lateral septal nucleus or the laterodosal thalamic nucleus, regions never implicated previously in fear consolidation, produced marked impairments in fear recall consistent with their high degree of functional connectivity. To summarize, ROIs identified as putatively critical with graph analysis of Fos immunoreactivity were indeed needed for consolidation of fear memory; importantly, many of these structures had not previously been considered important for this process and so the approach led to a significant advance in understanding.
Another appealing application of Graph theory to brain wide Fos immunoreactivity is to describe how networks implicated in behavioral or cognitive control interact. For example, Graph theory would allow a holistic test, and expansion, of the prevailing model that HPA axis (i.e., PVN and its excitatory inputs) and reward circuits (i.e., mesocorticolimbic DA) interact to control drug-seeking behaviors. Functionally connected subsets of ROIs exhibit “small-world” organization (Sporns, 2013) which facilitates local computation. Small worlds communicate with other and the global network through specialized nodes, termed “hubs.” In the context of stressor-induced relapse, structures like the VTA and BNST are likely to serve as hubs because of the established role in both drug reward and stressor reactivity. Graph theory-based analyses of the stressed brain would identify tracts for hypothesis-based, model driven, intersectional (i.e. tract specific interventions) studies that link functional subnetworks to specific behavioral or biological phenomena which can clarify many of the open questions noted above. This method was recently applied in the Christianson lab to test how exposure to stressful social stimuli influences functional connectivity of emotional and cortical structures with an anatomically defined social decision-making network. The analysis revealed that exposure to stressed conspecifics led to increased functional connectivity in subnetworks consisting of structures previously ascribed to social decision making (O'Connell and Hofmann, 2012) and socioemotional processes (Adolphs, 2009). A few ROIs appeared to be hubs between the subnetworks (e.g., prelimbic prefrontal cortex, insular cortex, and bed nucleus of the stria terminals) and exposure to stressed conspecifics altered how the subnetworks interacted (Rogers-Carter et al., 2018). Specifically, upon exposure to stressed stimuli there were far more functionally connected ROIs than after exposure to naive stimuli; the interpretation is that greater network integration is an important feature of the neural basis for social stress transmission. These two illustrations mirror a major theme in stress research which is to understand how the brain response to acute stressors changes as a consequence of stress chronicity or interaction with drugs of abuse. In light of the neural circuitry of stress and drug seeking summarized in Fig. 1, one can imagine taking a similar approach to investigating brain-wide network activity after different components of drug self-administration and reinstatement procedures to more completely describe how stressors change the way drugs of abuse are processed centrally. Graph theoretical analysis of brain wide connectivity patterns at times of stressor-induced reinstatement, for example, will both test existing ideas about neural control of this phenomenon but also identify new directions for investigation in the form of novel ROIs and circuits that could be targeted for therapeutic development.
8.3. Genetic tools using the cfos promoter
Most of the traditional Fos imaging approaches have required that brain tissue be fixed and have involved identification of activated neurons through in situ hybridization or immunohistochemical approaches. More recently, a series of transgenic cfos fluorescent reporter rodent lines have been established. These lines capitalize on the discrete activation of the cfos promoter to express effector proteins such as EGFP, enhanced green fluorescent protein, or β-galactosidase (β-gal), from the lacZ gene, in strongly activated neurons. The expression of effector proteins lasts for several hours and can then be used as markers of activated neurons for ex vivo electrophysiology or fluorescent-activated cell sorting (FACS) followed by PCR or high throughput sequencing (Cruz et al., 2015, 2013; Lobo, 2009). The presence of these tags allows for the comparison of Fos-expressing and non-Fos expressing neurons which permits subsequent examination of patterns of gene expression or synaptic activity changes in neuronal subpopulations based on whether or not they were activated by an earlier experimental manipulation. Interestingly, there are now also transgenic mice that express an axon-targeted β-gal reporter under control of the cfos promoter which allows for identification of downstream projection fields of activated (Fos-expressing) neuronal populations (Wilson et al., 2002).
More recently, researchers have leveraged the availability of Fos-LacZ transgenic rodent models to functionally regulate cell populations based on their activation patterns, as defined by Fos expression. Using this approach, the drug Daun02 is administered following exposure to an experimental manipulation (e.g., behavioral testing). During testing in Fos-LacZ rats, strongly activated neurons will express β-gal, and, in those neurons, Daun02 will be converted by β-gal to its active product, daunorubicin, which will produce apoptotic cell death (Cruz et al., 2013). As a result, neuronal ensembles that are activated during testing will no longer be functional and their role in behavior can determined upon subsequent testing. Thus, these approaches not only have the capacity to provide a representation of activation patterns that may encode stress and other stimuli, but they permit assessment of the functional relevance of these neuronal populations. One of the surprising findings from these studies is that as few as 3–12% of neurons in a given brain region (defined by the Fos response) may be activated and relevant for context-induced reinstatement of heroin seeking (Bossert et al., 2011; Cruz et al., 2014; Fanous et al., 2012). The ensembles are highly specific as silencing of the neurons in the nucleus accumbens shell activated by a cocaine-associated context attenuates later context-induced reinstatement of cocaine seeking. However, silencing of a similar number of neurons within the nucleus accumbens shell that are activated by a novel non-associated context has no effect on cocaine-seeking behavior (Cruz et al., 2014). This is likewise supported by findings that there are non-overlapping ensembles in the vmPFC for contexts specifically associated with heroin SA or extinction such that silencing of heroin context ensembles, but not extinction context ensembles, attenuates context-induced heroin seeking (Bossert et al., 2011). Furthermore, studies using this approach suggest that drug-associated context and cues may activate different patterns of Fos-expressing neurons (Cruz et al., 2015). These studies have identified novel roles for brain regions in cocaine-seeking behavior and continue to promote re-evaluation of relevant neurocircuitry. To date this approach has not been used to assess stressor-induced drug seeking.
Unfortunately, expression of β-gal or EGFP in these transgenic models is transient, only lasting hours. To address this limitation, TetTag mice have been developed with the goal of tagging activated neurons with a longer-lasting signal. In this model, a tetracycline transactivator (tTA) is expressed under the control of the cfos promoter, but its expression is inhibited in the presence of doxycycline (Dox). A second construct encodes a reporter, such as LacZ, under the control of the tetO promoter, and is not expressed in the presence of Dox. However, in the absence of Dox, strong neuronal activity drives tTA expression which then binds to a tetracycline response element (TRE), that contains the tetO sequence, to induce LacZ expression. Dox is typically provided in the animal's food and the Dox-containing chow is taken away prior to the experimental manipulation (e.g., reinstatement testing) and returned to the animal following completion of the behavioral paradigm. As a result, tTA-driven LacZ expression is limited to neurons strongly activated only in the period in which mice were Dox-free (Reijmers and Mayford, 2009). This signal is longer lasting because the LacZ construct contains a transcriptional feedbook loop that maintains gene expression even in the presence of Dox through the inclusion of a mutant tTA that is Dox-insensitive. A newer version of the Tet Tag approach uses tTA-driven expression of a histone H2B-EGFP fusion protein under the control of the cfos promoter that produces a longer-lasting signal in activated neurons that takes several weeks to degrade (Tayler et al., 2013, 2011). A major advantage of this approach is the ability to examine neuronal activation patterns several days to weeks after they occur. Thus, it is possible to expose animals to a second experimental manipulation, or re-expose to the first manipulation, and examine co-localization of LacZ or EGFP expression with another IEG. For example, this technique has been used to examine reactivation of the circuits engaged during learning in a subsequent memory retrieval test (Reijmers et al., 2007). While potentially powerful, these techniques have been somewhat limited by the long window of opportunity for neuronal labeling such that background levels of expression can be high. As a result, the induction of the reporter in these studies has only been ∼2–4 fold higher than home cage controls (DeNardo and Luo, 2017). Using the Fos promoter to introduce fluorescent transgenes opens the possibility of ex vivo experiments in which populations of tagged neurons are compared to untagged neurons. This was first demonstrated with the fosGFP mouse (Barth et al., 2004). In this experiment, cfos-driven GFP-expressing neurons were targeted using whole-cell recordings, thus permitting characterization of intrinsic, synaptic and circuit properties of neurons that comprised a behaviorally relevant ensemble. In principle, this type of approach should extend to Fos based cell-sorting, Fos based laser capture and dissection, and Fos based single-cell genomics; to our knowledge these have not yet been reported.
Recent innovations have combined the TetTag mouse lines with viral delivery of optogenetic or chemogenetic receptors downstream of the TRE sequence to permit functional manipulation of Fos-defined neuronal ensembles (Garner et al., 2012; Liu et al., 2012; Ramirez et al., 2015, 2013). Furthermore, the TetTag mouse lines have also been combined with the translating ribosome affinity purification (TRAP) method that produces tTA-driven expression of the EGFP-tagged ribosomal protein L10a under the control of the Fos promoter to isolate and identify actively translating mRNAs in activated neuronal populations (Drane et al., 2014). Lastly, a newer technique to functionally manipulate transiently activated neuronal populations involves different TRAP (Targeted Recombination in Active Populations) mouse lines wherein one transgene expresses a tamoxifen-dependent recombinase, CreERT2, under the control of the cfos promoter, and a second transgene or virus expresses a Cre-dependent effector protein (Guenthner et al., 2013). In these mouse models, Cre recombinase is only expressed in strongly activated neurons and recombination only occurs in the presence of tamoxifen. Therefore, it is possible to obtain constitutive expression of an effector protein in neuronal populations activated during an experimental window (Kawashima et al., 2014). However, it should be noted that tamoxifen is a selective estrogen receptor modulator which may be problematic for studies investigating drug-seeking, particularly in female rodents, as estrogen facilitates drug-seeking behavior (Anker and Carroll, 2011; Doncheck et al., 2018). Recently, Sørensen et al. (2016) introduced a synthetic Fos promoter which has very low background activity, resulting in improved sensitivity of tTA to doxycycline. This method, termed RAM (Robust Activity Marking), is also compatible with adeno-associated virus (AAV) gene transfer, and therefore enables targeting of neuronal ensembles in non-murine models, including rats.
The activity-dependent targeting technologies mentioned above are also enhanced by the development of intersectional/combinatorial genetic/viral strategies. The development of cell-type specific Cre recombinase expression through viral delivery or genetic mouse and rat Cre lines has enabled researchers to examine and manipulate cell type-specific subpopulations of activated neurons (Allen and Luo, 2015; Huang and Zeng, 2013; Madisen et al., 2015). In addition, newer viruses have been identified/produced to enable retrograde delivery of Cre recombinase which allows for pathway-specific expression of genetic tools to manipulate neural circuits or label activated neurons (Junyent and Kremer, 2015; Tervo et al., 2016). Furthermore, these techniques continue to evolve beyond the cfos promoter. For example, a newer synthetic promoter has been developed, enhanced synaptic activity-responsive element (E-SARE), that may provide greater sensitivity over models using the Fos promoter (Kawashima et al., 2013). In addition, newer approaches, such as the FLARE technique, have resolved some issues with the long labeling window associated with traditional TetTag methods that require doxycycline. In this approach, a novel transcription factor is activated only in the presence of coincident increases in intracellular Ca2+, indicating neuronal activation, and blue light administration through a fiber optic cannula, theoretically limiting activation of the transcription factor to a more discrete defined period. The transcription factor can then drive expression of a reporter signal such as mCherry, to tag activated neurons, or expression of opsins to later functionally manipulate the activated circuits (Wang et al., 2017). As activity-dependent targeting technologies continue to be refined, neuroscientists will gain an increasingly powerful set of tools to i.) quantify how stress and drug exposure alter activation patterns of selective neuronal ensembles, ii.) determine how they affect cellular and circuit physiology and iii.) manipulate activity-labeled neuronal populations to provide mechanistic insight to their behavioral relevance.
9. Summary
Here we review the current understanding of the neural circuitry through which stressors promote relapse to drug use. More work is needed to identify patterns of neuronal activation within relevant brain regions and pathways in preclinical models of stressor-induced relapse. Toward this end, Fos imaging-based approaches can provide useful tools for the identification of specific patterns of activation, as long as the caveats and limitations of these approaches are understood and taken into consideration. The combination of traditional Fos imaging approaches, computational and genetic techniques, and in vivo imaging techniques, such as calcium imaging, should provide critical information about the neurobiological mechanisms that mediate stressor-induced relapse and therefore inform and/or guide the development of therapeutic interventions and strategies for the management of SUDs.
Declaration of interest
JR Mantsch is a consultant for and stakeholder in Promentis Pharmaceuticals Inc. The authors do not declare any conflicts of interest.
Acknowledgements
Funding: This work was supported by the National Institutes of Health [grant DA015758 to JR Mantsch].
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.05.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adolphs R. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen W.E., Luo L. Intersectional illumination of neural circuit function. Neuron. 2015;85:889–892. doi: 10.1016/j.neuron.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Carroll M.E. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr. Top. Behav. Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Arenander A.T., Vellis J. de, Herschman H.R. Induction of c-fos and TIS genes in cultured rat astrocytes by neurotransmitters. J. Neurosci. Res. 1989;24:107–114. doi: 10.1002/jnr.490240115. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Delfs J.M., Druhan J., Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann. N. Y. Acad. Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Atasoy D., Sternson S.M. Chemogenetic tools for causal cellular and neuronal biology. Physiol. Rev. 2018;98:391–418. doi: 10.1152/physrev.00009.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S., Hartwell K., DeSantis S., Saladin M., McRae-Clark A., Price K., Maria M., Baker N., Spratt E., Kreek M., Brady K. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2009;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A.L., Gerkin R.C., Dean K.L. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J. Neurosci. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Sporns O. Network neuroscience. Nat. Neurosci. 2017;20:353–364. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastle R., Kufahl P., Turk M., Weber S., Pentkowski N., Thiel K., Neisewander J. Novel cues reinstate cocaine-seeking behavior and induce fos protein expression as effectively as conditioned cues. Neuropsychopharmacology. 2012;37:2109. doi: 10.1038/npp.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B., Thierry A.M., Tassin J.P., Moyne M.A. Dopaminergic innervation of the rat prefrontal cortex: a fluorescence histochemical study. Brain Res. 1976;106:133–145. doi: 10.1016/0006-8993(76)90078-0. [DOI] [PubMed] [Google Scholar]
- Blacktop J.M., Seubert C., Baker D.A., Ferda N., Lee G., Graf E.N., Mantsch J.R. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop J.M., Vranjkovic O., Mayer M., Hoof M. Van, Baker D.A., Mantsch J.R. Antagonism of GABA-B but not GABA-A receptors in the VTA prevents stress- and intra-VTA CRF-induced reinstatement of extinguished cocaine seeking in rats. Neuropharmacology. 2016;102:197–206. doi: 10.1016/j.neuropharm.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert J.M., Stern A.L., Theberge F.R., Cifani C., Koya E., Hope B.T., Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat. Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon T.H., Vidrine J.I., Litvin E.B. Relapse and relapse prevention. Annu. Rev. Clin. Psychol. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Krystal J.H., Southwick S.M., Charney D.S. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Briand L., Vassoler F., Pierce R., Valentino R., Blendy J. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F., Chakraborty S., Brierley D.I., Ungless M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.R., Sawchenko P.E. Hypophysiotropic CRF neurons display a sustained immediate-early gene response to chronic stress but not to adrenalectomy. J. Neuroendocrinol. 1997;9:307–316. doi: 10.1046/j.1365-2826.1997.00586.x. [DOI] [PubMed] [Google Scholar]
- Buczek Y., Lê A.D., Wang A., Stewart J., Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl.) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Buffalari D.M., See R.E. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol. Behav. 2009;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Calu D.J., Kawa A.B., Marchant N.J., Navarre B.M., Henderson M.J., Chen B., Yau H.-J.J., Bossert J.M., Schoenbaum G., Deisseroth K., Harvey B.K., Hope B.T., Shaham Y. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J. Neurosci. 2013;33:214–226. doi: 10.1523/JNEUROSCI.2016-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N., Rodaros D., Sorge R.E., Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carey A.N., Borozny K., Aldrich J.V., McLaughlin J.P. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur. J. Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D.B., Sesack S.R. Dopamine terminals synapse on callosal projection neurons in the rat prefrontal cortex. J. Comp. Neurol. 2000;425:275–283. doi: 10.1002/1096-9861(20000918)425:2<275::aid-cne9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Carr D.B., Sesack S.R. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S., Villar M.J., Goldstein M., Hökfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proc. Natl. Acad. Sci. U.S.A. 1989;86:9569–9573. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.K., Brown E.R., Ericsson A., Kovács K.J., Sawchenko P.E. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J. Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.W., Banducci A.N., Guller L., Macatee R.J., Lavelle A., Daughters S.B., Lejuez C.W. An examination of psychiatric comorbidities as a function of gender and substance type within an inpatient substance use treatment program. Drug Alcohol Depend. 2011;118:92–99. doi: 10.1016/j.drugalcdep.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N.A., Jupp B., Sztainberg Y., Lebow M., Brown R.M., Kim J.H., Chen A., Lawrence A.J. Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice. J. Neurosci. 2014;34:11560–11570. doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Choo H., Huang X.-P.P., Yang X., Stone O., Roth B.L., Jin J. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem. Neurosci. 2015;6:476–484. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli D.F., Kaczmarek L., Nicoletti F., Arcidiacono A., Dell'Albani P., Ingrao F., Magrì G., Malaguarnera L., Avola R., Messina A. Induction of protooncogene fos by extracellular signals in primary glial cell cultures. J. Neurosci. Res. 1989;23:234–239. doi: 10.1002/jnr.490230214. [DOI] [PubMed] [Google Scholar]
- Conrad K.L., McCutcheon J.E., Cotterly L.M., Ford K.A., Beales M., Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol. Psychiatr. 2010;68:303–305. doi: 10.1016/j.biopsych.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou N., Morgan C., Battistella S., O'Ryan D., Davis P., Curran H. Attentional bias, inhibitory control and acute stress in current and former opiate addicts. Drug Alcohol Depend. 2010;109:220–225. doi: 10.1016/j.drugalcdep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Cruz F.C., Koya E., Guez-Barber D.H., Bossert J.M., Lupica C.R., Shaham Y., Hope B.T. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat. Rev. Neurosci. 2013;14:743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F.C., Babin K.R., Leao R.M., Goldart E.M., Bossert J.M., Shaham Y., Hope B.T. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J. Neurosci. 2014;34:7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F.C., Javier Rubio F., Hope B.T. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res. 2015;1628:157–173. doi: 10.1016/j.brainres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan W.E., Herman J.P., Battaglia D.F., Akil H., Watson S.J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B.R. Fos and jun: the AP-1 connection. Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- DeNardo L., Luo L. Genetic strategies to access activated neurons. Curr. Opin. Neurobiol. 2017;45:121–129. doi: 10.1016/j.conb.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch A.Y., Duman R.S. The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: cellular localization and pharmacological characterization. Neuroscience. 1996;70:377–389. doi: 10.1016/0306-4522(95)00357-6. [DOI] [PubMed] [Google Scholar]
- Deutch A.Y., Lee M.C., Gillham M.H., Cameron D.A., Goldstein M., Iadarola M.J. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb. Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- Ding Y.Q., Qin B.Z., Li J.S., Mizuno N. Induction of c-fos-like protein in the spinoparabrachial tract-neurons locating within the sacral parasympathetic nucleus in the rat. Brain Res. 1994;659:283–286. doi: 10.1016/0006-8993(94)90894-x. [DOI] [PubMed] [Google Scholar]
- Diorio D., Viau V., Meaney M.J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheck E.M., Urbanik L.A., DeBaker M.C., Barron L.M., Liddiard G.T., Tuscher J.J., Frick K.M., Hillard C.J., Mantsch J.R. 17β-Estradiol potentiates the reinstatement of cocaine seeking in female rats: role of the prelimbic prefrontal cortex and cannabinoid Type-1 receptors. Neuropsychopharmacology. 2018;43:781–790. doi: 10.1038/npp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J.P., Squinto S.P., Bazan N.G. Fos-jun and the primary genomic response in the nervous system. Possible physiological role and pathophysiological significance. Mol. Neurobiol. 1990;4:27–55. doi: 10.1007/BF02935584. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H.A. Generalized seizures induce c-fos protein(s) in mammalian neurons. Neurosci. Lett. 1987;82:157–161. doi: 10.1016/0304-3940(87)90121-2. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H.A. Brain injury induces c-fos protein(s) in nerve and glial-like cells in adult mammalian brain. Brain Res. 1988;455:295–299. doi: 10.1016/0006-8993(88)90088-1. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H.A. Localization and induction of c-fos protein-like immunoreactive material in the nuclei of adult mammalian neurons. Brain Res. 1988;440:252–260. doi: 10.1016/0006-8993(88)90993-6. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Peterson M.R., Robertson H.A. Presence of c-fos-like immunoreactivity in the adult rat brain. Eur. J. Pharmacol. 1987;135:113–114. doi: 10.1016/0014-2999(87)90767-9. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Castro D. de, Faull R.L. Induction of Fos in glia-like cells after focal brain injury but not during wallerian degeneration. Brain Res. 1990;527:41–54. doi: 10.1016/0006-8993(90)91058-o. [DOI] [PubMed] [Google Scholar]
- Drane L., Ainsley J.A., Mayford M.R., Reijmers L.G. A transgenic mouse line for collecting ribosome-bound mRNA using the tetracycline transactivator system. Front. Mol. Neurosci. 2014;7:82. doi: 10.3389/fnmol.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler K., Schweitzer J.B., Quinn C.K., Gross R., Ely T.D., Muhammad F., Kilts C.D. Neural activity related to anger in cocaine-dependent men: a possible link to violence and relapse. Am. J. Addict. 2000;9:331–339. doi: 10.1080/105504900750047382. [DOI] [PubMed] [Google Scholar]
- Eden C.G. Van, Hoorneman E.M., Buijs R.M., Matthijssen M.A., Geffard M., Uylings H.B. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22:849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- Erb S., Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S., Shaham Y., Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl.) 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S., Salmaso N., Rodaros D., Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S., Lopak V., Smith C. Cocaine pre-exposure produces a sensitized and context-specific c-fos mRNA response to footshock stress in the central nucleus of the AMYGDALA. Neuroscience. 2004;129:719–725. doi: 10.1016/j.neuroscience.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Erb S., Petrovic A., Yi D., Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl.) 2006;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Fanous S., Goldart E.M., Theberge F.R., Bossert J.M., Shaham Y., Hope B.T. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J. Neurosci. 2012;32:11600–11609. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.R., Schoch H., Mahler S.V. Modeling cocaine relapse in rodents: behavioral considerations and circuit mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2018 doi: 10.1016/j.pnpbp.2018.01.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein M.W., See R.E. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav. Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Fenno L., Yizhar O., Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo H.F., Bodie B.L., Tauchi M., Dolgas C.M., Herman J.P. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Fox H.C., Talih M., Malison R., Anderson G.M., Kreek M.J., Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Fox H.C., Hong K.-I.A.I., Siedlarz K., Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D., Li Z., Shaham Y., Lê A.D. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122:1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Funk D., Harding S., Juzytsch W., Lê A.D. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl.) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Funk D., Li Z., Lê A.D. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Furnari M., Epstein D.H., Phillips K.A., Jobes M.L., Kowalczyk W.J., Vahabzadeh M., Lin J.-L.L., Preston K.L. Some of the people, some of the time: field evidence for associations and dissociations between stress and drug use. Psychopharmacology (Berl.) 2015;232:3529–3537. doi: 10.1007/s00213-015-3998-7. [DOI] [PubMed] [Google Scholar]
- Gaiddon C., Loeffler J.P., Larmet Y. Brain-derived neurotrophic factor stimulates AP-1 and cyclic AMP-responsive element dependent transcriptional activity in central nervous system neurons. J. Neurochem. 1996;66:2279–2286. doi: 10.1046/j.1471-4159.1996.66062279.x. [DOI] [PubMed] [Google Scholar]
- Gardner E.L. What we have learned about addiction from animal models of drug self-administration. Am. J. Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Garner A.R., Rowland D.C., Hwang S.Y., Baumgaertel K., Roth B.L., Kentros C., Mayford M. Generation of a synthetic memory trace. Science. 2012;335:1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S., Derst C., Veh R.W., Z.-D. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O., Hope B.T. Cortical and amygdalar neuronal ensembles in alcohol seeking, drinking and withdrawal. Neuropharmacology. 2017;122:107–114. doi: 10.1016/j.neuropharm.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O., Sanders C., Freiling J., Grigoryan E., Vu S., Allen C.D., Crawford E., Mandyam C.D., Koob G.F. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Ginty D.D., Bading H., Greenberg M.E. Calcium regulation of gene expression in neuronal cells. J. Neurobiol. 1994;25:294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- Gomez J.L., Bonaventura J., Lesniak W., Mathews W.B., Sysa-Shah P., Rodriguez L.A., Ellis R.J., Richie C.T., Harvey B.K., Dannals R.F., Pomper M.G., Bonci A., Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley S.L., Taylor J.R. Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat. Neurosci. 2016;19:656–664. doi: 10.1038/nn.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.N., Wheeler R.A., Baker D.A., Ebben A.L., Hill J.E., McReynolds J.R., Robble M.A., Vranjkovic O., Wheeler D.S., Mantsch J.R., Gasser P.J. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J. Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S.J., Bittman K., Benno R.H. Both phasic sensory stimulation and tonic pharmacological activation increase Fos-like immunoreactivity in the rat locus coeruleus. Synapse. 1992;12:112–118. doi: 10.1002/syn.890120204. [DOI] [PubMed] [Google Scholar]
- Graziane N.M., Polter A.M., Briand L.A., Pierce R.C., Kauer J.A. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.E., Ziff E.B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Guenthner C.J., Miyamichi K., Yang H.H., Heller H.C., Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–784. doi: 10.1016/j.neuron.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski J.F., McNaughton B.L., Barnes C.A., Worley P.F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski J.F., Lyford G.L., Stevenson G.D., Houston F.P., McGaugh J.L., Worley P.F., Barnes C.A. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T., Leah J.D. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Brain Res. Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hisanaga K., Sagar S.M., Hicks K.J., Swanson R.A., Sharp F.R. c-fos proto-oncogene expression in astrocytes associated with differentiation or proliferation but not depolarization. Brain Res. Mol. Brain Res. 1990;8:69–75. doi: 10.1016/0169-328x(90)90011-2. [DOI] [PubMed] [Google Scholar]
- Hisanaga K., Sagar S.M., Sharp F.R. N-methyl-D-aspartate antagonists block fos-like protein expression induced via multiple signaling pathways in cultured cortical neurons. J. Neurochem. 1992;58:1836–1844. doi: 10.1111/j.1471-4159.1992.tb10060.x. [DOI] [PubMed] [Google Scholar]
- Hoffman G.E., Smith M.S., Verbalis J.G. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Holly E.N., Miczek K.A. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl.) 2016;233:163–186. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.J., Zeng H. Genetic approaches to neural circuits in the mouse. Annu. Rev. Neurosci. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- Hughes P., Lawlor P., Dragunow M. Basal expression of Fos, Fos-related, Jun, and Krox 24 proteins in rat hippocampus. Brain Res. Mol. Brain Res. 1992;13:355–357. doi: 10.1016/0169-328x(92)90219-2. [DOI] [PubMed] [Google Scholar]
- Hunt S.P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Ikeda J., Nakajima T., Osborne O.C., Mies G., Nowak T.S. Coexpression of c-fos and hsp70 mRNAs in gerbil brain after ischemia: induction threshold, distribution and time course evaluated by in situ hybridization. Brain Res. Mol. Brain Res. 1994;26:249–258. doi: 10.1016/0169-328x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Nakamura M., Ebihara K., Hoshino K., Hashiguchi H., Mitsuyama Y., Nishimori T., Nakahara D. Immunohistochemical characterisation of Fos-positive cells in brainstem monoaminergic nuclei following intracranial self-stimulation of the medial forebrain bundle in the rat. Eur. J. Neurosci. 2001;13:1600–1608. doi: 10.1046/j.0953-816x.2001.01520.x. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Hashiguchi H., Takeda R., Ishizuka Y., Mitsuyama Y., Kannan H., Nishimori T., Nakahara D. Conditioned-fear stress increases Fos expression in monoaminergic and GABAergic neurons of the locus coeruleus and dorsal raphe nuclei. Synapse. 2002;45:46–51. doi: 10.1002/syn.10086. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Nakahara D., Hashiguchi H., Nakamura M., Ebihara K., Takeda R., Nishimori T., Niki H. Fos expression in GABAergic cells and cells immunopositive for NMDA receptors in the inferior and superior colliculi following audiogenic seizures in rats. Synapse. 2002;46:100–107. doi: 10.1002/syn.10129. [DOI] [PubMed] [Google Scholar]
- Junyent F., Kremer E.J. CAV-2–why a canine virus is a neurobiologist's best friend. Curr. Opin. Pharmacol. 2015;24:86–93. doi: 10.1016/j.coph.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Kawashima T., Kitamura K., Suzuki K., Nonaka M., Kamijo S., Takemoto-Kimura S., Kano M., Okuno H., Ohki K., Bito H. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat. Meth. 2013;10:889–895. doi: 10.1038/nmeth.2559. [DOI] [PubMed] [Google Scholar]
- Kawashima T., Okuno H., Bito H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front. Neural Circ. 2014;8:37. doi: 10.3389/fncir.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling M., Stumm G., Xie Y., Herdegen T., Aguzzi A., Bravo R., Gass P. Differential transcription and translation of immediate early genes in the gerbil hippocampus after transient global ischemia. J. Cereb. Blood Flow Metab. 1993;13:914–924. doi: 10.1038/jcbfm.1993.114. [DOI] [PubMed] [Google Scholar]
- Köbbert C., Apps R., Bechmann I., Lanciego J.L., Mey J., Thanos S. Current concepts in neuroanatomical tracing. Prog. Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Koob G.F. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács K.J. Measurement of immediate-early gene activation- c-fos and beyond. J. Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Kovács K.J., Sawchenko P.E. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J. Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács K.J., Csejtei M., Laszlovszky I. Double activity imaging reveals distinct cellular targets of haloperidol, clozapine and dopamine D(3) receptor selective RGH-1756. Neuropharmacology. 2001;40:383–393. doi: 10.1016/s0028-3908(00)00163-5. [DOI] [PubMed] [Google Scholar]
- Kreibich A.S., Blendy J.A. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J. Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl P., Zavala A., Singh A., Thiel K., Dickey E., Joyce J., Neisewander J. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labiner D.M., Butler L.S., Cao Z., Hosford D.A., Shin C., McNamara J.O. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J. Neurosci. 1993;13:744–751. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S., Lim B.K., Malenka R.C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M., Mathiesen C., Schaefer K., Thomsen K.J. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage. 2012;62:1040–1050. doi: 10.1016/j.neuroimage.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Quan B., Juzytch W., Fletcher P.J., Joharchi N., Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl.) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Harding S., Juzytsch W., Fletcher P.J., Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J. Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D., Harding S., Juzytsch W., Funk D., Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl.) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Leslie R.A., Moorman J.M., Grahame-Smith D.G. Lithium enhances 5-HT2A receptor-mediated c-fos expression in rat cerebral cortex. Neuroreport. 1993;5:241–244. doi: 10.1097/00001756-199312000-00014. [DOI] [PubMed] [Google Scholar]
- Liu X., Ramirez S., Pang P.T., Puryear C.B., Govindarajan A., Deisseroth K., Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.-R.R., Rubio F.J., Bossert J.M., Marchant N.J., Fanous S., Hou X., Shaham Y., Hope B.T. Detection of molecular alterations in methamphetamine-activated Fos-expressing neurons from a single rat dorsal striatum using fluorescence-activated cell sorting (FACS) J. Neurochem. 2014;128:173–185. doi: 10.1111/jnc.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo M.K. Molecular profiling of striatonigral and striatopallidal medium spiny neurons past, present, and future. Int. Rev. Neurobiol. 2009;89:1–35. doi: 10.1016/S0074-7742(09)89001-6. [DOI] [PubMed] [Google Scholar]
- Lu L., Ceng X., Huang M. Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence in rats. Neuroreport. 2000;11:2373–2378. doi: 10.1097/00001756-200008030-00008. [DOI] [PubMed] [Google Scholar]
- Lu L., Liu D., Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur. J. Pharmacol. 2001;415:203–208. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- Lucas M., Frenois F., Vouillac C., Stinus L., Cador M., Moine C.Le. Reactivity and plasticity in the amygdala nuclei during opiate withdrawal conditioning: differential expression of c-fos and arc immediate early genes. Neuroscience. 2008;154:1021–1033. doi: 10.1016/j.neuroscience.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Ma Y.-Y.Y., Chu N.-N.N., Guo C.-Y.Y., Han J.-S.S., Cui C.-L.L. NR2B-containing NMDA receptor is required for morphine-but not stress-induced reinstatement. Exp. Neurol. 2007;203:309–319. doi: 10.1016/j.expneurol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Ma D.Y., Xu M.Y., Yang H.C., Yang L.Z. Effect of inhibition of the central nucleus of the amygdala and drug experience on the regions underlying footshock-induced reinstatement of morphine seeking. J. Int. Med. Res. 2008;36:992–1000. doi: 10.1177/147323000803600516. [DOI] [PubMed] [Google Scholar]
- Madisen L., Garner A.R., Shimaoka D., Chuong A.S., Klapoetke N.C., Li L., Bourg A., van der Niino Y., Egolf L., Monetti C., Gu H., Mills M., Cheng A., Tasic B., Nguyen T.N., Sunkin S.M., Benucci A., Nagy A., Miyawaki A., Helmchen F., Empson R.M., Knöpfel T., Boyden E.S., Reid R.C., Carandini M., Zeng H. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Aston-Jones G.S. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J. Neurosci. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Aston-Jones G. CNO Evil? Considerations for the use of DREADDs in behavioral neuroscience. Neuropsychopharmacology. 2018;43:934–936. doi: 10.1038/npp.2017.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Vazey E.M., Beckley J.T., Keistler C.R., McGlinchey E.M., Kaufling J., Wilson S.P., Deisseroth K., Woodward J.J., Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat. Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J.R., Baker D.A., Serge J.P., Hoks M.A., Francis D.M., Katz E.S. Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology. 2008;33:814–826. doi: 10.1038/sj.npp.1301464. [DOI] [PubMed] [Google Scholar]
- Mantsch J.R., Weyer A., Vranjkovic O., Beyer C.E., Baker D.A., Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J.R., Baker D.A., Funk D., Lê A.D., Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis E.B., Toy B., Himmels P., Morales M., Fields H.L. Identification of rat ventral tegmental area GABAergic neurons. PLoS One. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K., Davidge S.B., Lapish C.C., Kalivas P.W. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C.K., Miyashita T., Setlow B., Marjon K.D., Steward O., Guzowski J.F., McGaugh J.L. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen J.M., Myers B., Herman J.P. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J. Neuroendocrinol. 2015;27:446–456. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton L.A., Hunt S.P. Regulation of gene expression in astrocytes by excitatory amino acids. Brain Res. Mol. Brain Res. 1992;16:261–266. doi: 10.1016/0169-328x(92)90234-3. [DOI] [PubMed] [Google Scholar]
- McRae-Clark A., Carter R., Price K., Baker N., Thomas S., Saladin M., Giarla K., Nicholas K., Brady K. Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology. 2011;218:49–58. doi: 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Peña D.F., Blacktop J.M., Mantsch J.R. Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress. 2014;17:22–38. doi: 10.3109/10253890.2013.872617. [DOI] [PubMed] [Google Scholar]
- McReynolds J.R., Vranjkovic O., Thao M., Baker D.A., Makky K., Lim Y., Mantsch J.R. Beta-2 adrenergic receptors mediate stress-evoked reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice. Psychopharmacology (Berl.) 2014;231:3953–3963. doi: 10.1007/s00213-014-3535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Doncheck E.M., Vranjkovic O., Ganzman G.S., Baker D.A., Hillard C.J., Mantsch J.R. CB1 receptor antagonism blocks stress-potentiated reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 2016;233:99–109. doi: 10.1007/s00213-015-4092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Doncheck E.M., Li Y., Vranjkovic O., Graf E.N., Ogasawara D., Cravatt B.F., Baker D.A., Liu Q.-S.S., Hillard C.J., Mantsch J.R. Stress promotes drug seeking through glucocorticoid-dependent endocannabinoid mobilization in the prelimbic cortex. Biol. Psychiatr. 2017 doi: 10.1016/j.biopsych.2017.09.024. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menétrey D., Gannon A., Levine J.D., Basbaum A.I. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J. Comp. Neurol. 1989;285:177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- Messaoudi E., Kanhema T., Soulé J., Tiron A., Dagyte G., Silva B. da, Bramham C.R. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J. Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.Y., Bloom F.E. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu. Rev. Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria M.M., McRae-Clark A., Baker N.L., Ramakrishnan V., Brady K.T. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl.) 2014;231:4157–4165. doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.I., Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Morgan J.I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Morgan J.I., Cohen D.R., Hempstead J.L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts R.G., Chatelain-Badie S.D., Benson E., White-Cooper H., Bolam J.P., Ungless M.A. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E., Xie J.Y., Okun A., Qu C., Eyde N., Ci S., Ossipov M.H., King T., Fields H.L., Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc. Natl. Acad. Sci. U.S.A. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell L.A., Hofmann H.A. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- Oever M.C., Van den Spijker S., Smit A.B., Vries T.J. De. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci. Biobehav. Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Omelchenko N., Sesack S.R. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N., Sesack S.R. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald L.M., Wong D.F., McCaul M., Zhou Y., Kuwabara H., Choi L., Brasic J., Wand G.S. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Polter A.M., Bishop R.A., Briand L.A., Graziane N.M., Pierce R.C., Kauer J.A. Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol. Psychiatr. 2014;76:785–793. doi: 10.1016/j.biopsych.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M.N., Hong K.A.I., Lacadie C.M., Fulbright R.K., Tuit K.L., Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am. J. Psychiatr. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K.L., Epstein D.H. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl.) 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K.L., Vahabzadeh M., Schmittner J., Lin J.-L.L., Gorelick D.A., Epstein D.H. Cocaine craving and use during daily life. Psychopharmacology (Berl.) 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K.L., Kowalczyk W.J., Phillips K.A., Jobes M.L., Vahabzadeh M., Lin J.-L.L., Mezghanni M., Epstein D.H. Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology (Berl.) 2017;234:2631–2642. doi: 10.1007/s00213-017-4663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K.L., Kowalczyk W.J., Phillips K.A., Jobes M.L., Vahabzadeh M., Lin J.-L.L., Mezghanni M., Epstein D.H. Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology. 2018;43:859–867. doi: 10.1038/npp.2017.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S., Liu X., Lin P.-A.A., Suh J., Pignatelli M., Redondo R.L., Ryan T.J.J., Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Ramirez S., Liu X., MacDonald C.J., Moffa A., Zhou J., Redondo R.L., Tonegawa S. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522:335–339. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila V.A., Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl.) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers L., Mayford M. Genetic control of active neural circuits. Front. Mol. Neurosci. 2009;2:27. doi: 10.3389/neuro.02.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers L.G., Perkins B.L., Matsuo N., Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B., Aguilar M.A., Manzanedo C., Rodríguez-Arias M., Armario A., Miñarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl.) 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Robertson G.S., Vincent S.R., Fibiger H.C. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Rogan S.C., Roth B.L. Remote control of neuronal signaling. Pharmacol. Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter M.M., Varela J.A., Gribbons K.B., Pierce A.F., McGoey M.T., Ritchey M., Christianson J.P. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat. Neurosci. 2018;21:404–414. doi: 10.1038/s41593-018-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville B.J., Anton S.F., Carroll K., Budde D., Prusoff B.A., Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch. Gen. Psychiatr. 1991;48:43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- Roux L., Stark E., Sjulson L., Buzsáki G. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr. Opin. Neurobiol. 2014;26:88–95. doi: 10.1016/j.conb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ruskin D.N., Marshall J.F. Differing influences of dopamine agonists and antagonists on Fos expression in identified populations of globus pallidus neurons. Neuroscience. 1997;81:79–92. doi: 10.1016/s0306-4522(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Sanchez C.J., Sorg B.A. Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. Brain Res. 2001;908:86–92. doi: 10.1016/s0006-8993(01)02638-5. [DOI] [PubMed] [Google Scholar]
- Sanchez C.J., Bailie T.M., Wu W.-R.R., Li N., Sorg B.A. Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Santana N., Mengod G., Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Schank J.R., Nelson B.S., Damadzic R., Tapocik J.D., Yao M., King C.E., Rowe K.E., Cheng K., Rice K.C., Heilig M. Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology. 2015;99:106–114. doi: 10.1016/j.neuropharm.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Durstewitz D., Christie B.R., Stevens C.F., Sejnowski T.J. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl. Acad. Sci. U.S.A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Gorelova N., Durstewitz D., Yang C.R. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J. Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self D.W., Nestler E.J. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Seo D., Lacadie C.M., Tuit K., Hong K.-I.I., Constable R.T., Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatr. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.G., Galai N., Celentano D.D., Vlahov D., Strathdee S.A. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988-2000. Drug Alcohol Depend. 2006;83:147–156. doi: 10.1016/j.drugalcdep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl.) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Funk D., Erb S., Brown T.J., Walker C.D., Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J. Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shalev U., Highfield D., Yap J., Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl.) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U., Grimm J.W., Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol. Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U., Marinelli M., Baumann M.H., Piazza P.-V.V., Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl.) 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Shalev U., Robarts P., Shaham Y., Morales M. Selective induction of c-Fos immunoreactivity in the prelimbic cortex during reinstatement of heroin seeking induced by acute food deprivation in rats. Behav. Brain Res. 2003;145:79–88. doi: 10.1016/s0166-4328(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M.E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M.E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Shepard J.D., Bossert J.M., Liu S.Y., Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatr. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict. Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Catapano D., O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl.) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R., Lacadie C., Skudlarski P., Fulbright R.K., Rounsaville B.J., Kosten T.R., Wexler B.E. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl.) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Garcia M., Paliwal P., Kreek M.J., Rounsaville B.J. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatr. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R., Fox H.C., Hong K.A., Bergquist K., Bhagwagar Z., Siedlarz K.M. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Shaham Y., Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl.) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.S., Bucci D.J., Luikart B.W., Mahler S.V. DREADDS: use and application in behavioral neuroscience. Behav. Neurosci. 2016;130:137–155. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg J.L., Macgregor-Leon P.F., Curran T., Morgan J.I. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989;3:359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Sørensen A.T., Cooper Y.A., Baratta M.V., Weng F.-J.J., Zhang Y., Ramamoorthi K., Fropf R., LaVerriere E., Xue J., Young A., Schneider C., Gøtzsche C.R., Hemberg M., Yin J.C., Maier S.F., Lin Y. A robust activity marking system for exploring active neuronal ensembles. Elife. 2016;5 doi: 10.7554/eLife.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013;23:162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J. Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Szekely A.M., Barbaccia M.L., Costa E. Activation of specific glutamate receptor subtypes increases C-fos proto-oncogene expression in primary cultures of neonatal rat cerebellar granule cells. Neuropharmacology. 1987;26:1779–1782. doi: 10.1016/0028-3908(87)90132-8. [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Heisenberg M., Gerber B. Experimental psychology: event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- Tayler K.K., Lowry E., Tanaka K., Levy B., Reijmers L., Mayford M., Wiltgen B.J. Characterization of NMDAR-independent learning in the Hippocampus. Front. Behav. Neurosci. 2011;5:28. doi: 10.3389/fnbeh.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler K.K., Tanaka K.Z., Reijmers L.G., Wiltgen B.J. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol. 2013;23:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Tervo D.G., Hwang B.-Y.Y., Viswanathan S., Gaj T., Lavzin M., Ritola K.D., Lindo S., Michael S., Kuleshova E., Ojala D., Huang C.-C.C., Gerfen C.R., Schiller J., Dudman J.T., Hantman A.W., Looger L.L., Schaffer D.V., Karpova A.Y. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L.-W.W., Lu G.-L.L., Lee Y.-H.H., Yu L., Lee H.-J.J., Leishman E., Bradshaw H., Hwang L.-L.L., Hung M.-S.S., Mackie K., Zimmer A., Chiou L.-C.C. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nat. Commun. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Úbeda-Contreras J., Marín-Blasco I., Nadal R., Armario A. Brain c-fos expression patterns induced by emotional stressors differing in nature and intensity. Brain Struct. Funct. 2018;223:2213–2227. doi: 10.1007/s00429-018-1624-2. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A., McNaughton B.L., Barnes C.A., Worley P.F., Guzowski J.F. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J. Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetere G., Kenney J.W., Tran L.M., Xia F., Steadman P.E., Parkinson J., Josselyn S.A., Frankland P.W. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron. 2017;94 doi: 10.1016/j.neuron.2017.03.037. 363–374.e4. [DOI] [PubMed] [Google Scholar]
- Vincent S.L., Khan Y., Benes F.M. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J. Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O., Gasser P.J., Gerndt C.H., Baker D.A., Mantsch J.R. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J. Neurosci. 2014;34:12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O., Pina M., Kash T.L., Winder D.G. The bed nucleus of the stria terminalis in drug-associated behavior and affect: a circuit-based perspective. Neuropharmacology. 2017;122:100–106. doi: 10.1016/j.neuropharm.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.C., Kastman H.E., Krstew E.V., Gundlach A.L., Lawrence A.J. Central amygdala relaxin-3/relaxin family peptide receptor 3 signalling modulates alcohol seeking in rats. Br. J. Pharmacol. 2017;174:3359–3369. doi: 10.1111/bph.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat M.J., Willuhn I., Clark J.J., Phillips P.E. Phasic dopamine release in appetitive behaviors and drug addiction. Curr. Drug Abuse Rev. 2009;2:195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G.S., Oswald L.M., McCaul M.E., Wong D.F., Johnson E., Zhou Y., Kuwabara H., Kumar A. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Wang B., Luo F., Zhang W.T., Han J.S. Stress or drug priming induces reinstatement of extinguished conditioned place preference. Neuroreport. 2000;11:2781–2784. doi: 10.1097/00001756-200008210-00034. [DOI] [PubMed] [Google Scholar]
- Wang B., Shaham Y., Zitzman D., Azari S., Wise R.A., You Z.-B.B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Fang Q., Liu Z., Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl.) 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Wang B., You Z.-B.B., Rice K.C., Wise R.A. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl.) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang M., Wong A., Liu F. Interactions between NMDA and dopamine receptors: a potential therapeutic target. Brain Res. 2012;1476:154–163. doi: 10.1016/j.brainres.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Wang W., Wildes C.P., Pattarabanjird T., Sanchez M.I., Glober G.F., Matthews G.A., Tye K.M., Ting A.Y. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat. Biotechnol. 2017;35:864–871. doi: 10.1038/nbt.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F., Hurd Y.L., Ungerstedt U., Markou A., Plotsky P.M., Koob G.F. Neurochemical correlates of cocaine and ethanol self-administration. Ann. N. Y. Acad. Sci. 1992;654:220–241. doi: 10.1111/j.1749-6632.1992.tb25970.x. [DOI] [PubMed] [Google Scholar]
- Wheeler A.L., Teixeira C.M.M., Wang A.H., Xiong X., Kovacevic N., Lerch J.P., McIntosh A.R., Parkinson J., Frankland P.W. Identification of a functional connectome for long-term fear memory in mice. PLoS Comput. Biol. 2013;9:e1002853. doi: 10.1371/journal.pcbi.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker L.R., Warren B.L., Venniro M., Harte T.C., McPherson K.B., Beidel J., Bossert J.M., Shaham Y., Bonci A., Hope B.T. Bidirectional modulation of intrinsic excitability in rat prelimbic cortex neuronal ensembles and non-ensembles after operant learning. J. Neurosci. 2017;37:8845–8856. doi: 10.1523/JNEUROSCI.3761-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson Y., Nag N., Davern P., Oldfield B.J., McKinley M.J., Greferath U., Murphy M. Visualization of functionally activated circuitry in the brain. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3252–3257. doi: 10.1073/pnas.042701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley P.F., Bhat R.V., Baraban J.M., Erickson C.A., McNaughton B.L., Barnes C.A. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J. Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu J., Zhang Q., Zhou T., Zhou T.T., Chen Y., Hu H. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat. Neurosci. 2014;17:1552–1559. doi: 10.1038/nn.3813. [DOI] [PubMed] [Google Scholar]
- Zakiniaeiz Y., Scheinost D., Seo D., Sinha R., Constable R.T. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clin. 2017;13:181–187. doi: 10.1016/j.nicl.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Dayas C., Aujla H., Baptista M., Martin-Fardon R., Weiss F. Activation of group ii metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the Hippocampus and amygdala. J. Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.