Abstract
Epidemiologic studies have shown that the prevalence of stress-related mood disorders is higher in women, which suggests a different response of neuroendocrine circuits involved in the response to stressful events, as well as a genetic background influence. The aim of this study was to investigate the baseline differences in anxiety-like behaviors of females of two commonly used mice strains. Secondly, we have also aimed to study their behavioral and biochemical alterations following stress. Naïve 3-4 months-old Swiss and C57BL/6 female mice were evaluated in the elevated plus maze (EPM) and in the acoustic startle response (ASR) for anxiety-like behaviors. Besides, an independent group of animals from each strain was exposed to cold-restraint stress (30 min/4 °C, daily) for 21 consecutive days and then evaluated in EPM and in the sucrose consumption tests. Twenty-four hours following behavioral experimentation mice were decapitated and their hippocampi (HP) and cortex (CT) dissected for further Western blotting analysis of glucocorticoid receptor (GR) and glial fibrillary acid protein (GFAP). Subsequent to each behavioral protocol, animal blood samples were collected for further plasma corticosterone analysis. C57BL/6 presented a lower anxiety profile than Swiss female mice in both behavioral tests, EPM and ASR. These phenomena could be correlated with the fact that both strains have distinct corticosterone levels and GR expression in the HP at the baseline level. Moreover, C57BL/6 female mice were more vulnerable to the stress protocol, which was able to induce an anhedonic state characterized by lower preference for a sucrose solution. Behavioral anhedonic-like alterations in these animals coincide with reduced plasma corticosterone accompanied with increased GR and GFAP levels, both in the HP. Our data suggest that in C57BL/6 female mice a dysregulation of the hypothalamus-pituitary-adrenal axis (HPA-axis) occurs, in which corticosterone acting on GRs would possibly exert its pro-inflammatory role, ultimately leading to astrocyte activation in response to stress.
Keywords: Stress-related disorders, Female mice, Anxiety-like behavior, Depression-like behavior, Neuroinflammation, Anhedonia
Highlights
-
•
Swiss and C57BL/6 female mice present basal behavioral differences in anxiety tests.
-
•
Anxiety-like behavior is associated with differential HPA-axis regulation pattern.
-
•
Repeated predictable stress induces anhedonic-like behavior in C57BL/6 female mice.
-
•
Anhedonic-like behavior is associated to HPA-axis alterations and activated astrocyte over expression.
1. Introduction
The occurrence of stress-related disorders is influenced by several factors, namely environment, previous experience, genetic factors and, in special, by gender (Joëls and Baram, 2009). Women are twice more prone to suffer from mood and anxiety disorders than men, as shown by the higher prevalence of anxiety disorders and unipolar depression in USA and also in Sao Paulo city - Brazil (Kessler et al., 2012; Viana and Andrade, 2012). It has been proposed that those variances occur due to biological differences in stress response in between females and males (Bangasser and Valentino, 2012). Genetic factors and their interaction with the environment are equally vital as risk factors for the development of stress-related disorders (Russo et al., 2012).
Animal models are an essential tool for stress-related disorders research since they allow the control of each variable separately and a potential synergism in between them. Genetic influences on rodent behavior have gained special attention after 1990 with the onset of transgenic animal models. Several research groups have pointed to genetic background importance for transgenic animal development and how it could affect the outcome (Paylor and Crawley, 1997; Tarantino et al., 2000; Brooks et al., 2005). Since it was first described by Hans Selye as the “General Adaptation Syndrome”, the physiological and pathological responses to stress have been the focus of many investigations.
Pathological responses to stress (e.g. HPA-axis dysregulation or the failure of ending this response) have been linked to the development of various psychiatric disorders. For instance, it is proposed that depressive disorders are, at least in part, due to maladaptive responses to stress (Gold et al., 2015). In this sense, Swiss and C57BL/6 mice are the most used strains in behavioral and pharmacological screening studies related to stress, anxiety and depression. Nevertheless, although women are the individuals most affected by stress, the majority of the animal studies are performed with male rodents. Following the NIH directive of the use of females in biomedical studies (NOT-OD-15-102, NIH, 2015) there has been an steady increase in the number of studies comparing male and females, where many report differential responses between sexes (for a recent review see Bolea-Alamanac et al., 2018). The literature on anxiety and depression considering female individuals as the target population are still a minority, but they point to the importance of studying female response to different stressors, since their impact seems drastically different (Zhu et al., 2014; Lotan et al., 2017).
The two most commonly used mice strains in behavioral neuroscience research are Swiss and C57BL/6. Male and female C57BL/6 mice have been subject to many anxiety-like behavioral investigations since it is a common background for transgenic mice (Paylor and Crawley, 1997; Tarantino et al., 2000; Brooks et al., 2004, 2005; Mineur et al., 2006). Swiss mice have been widely used for biomedical researches in an array of protocols investigating emotionality (Kawakami et al., 2010) and as subjects of pharmacological screenings (Ribeiro and De Lima, 2002; Texeira et al., 2004; Klein et al., 2014; Galdino et al., 2015). Swiss and C57BL/6 mice have also been used to study mood disorders, as well as depressive-like behavior (Martin and Brown, 2010; Costa et al., 2013; Zhu et al., 2014). Although there is a crescent demand for the behavioral comparisons in between males and females, there is a deeper gap in the literature on the comparison of the possible distinctive behavior of different female strains. Here we aimed to address the comparison between the female mice of Swiss and C57BL/6 strains in predictive tests of anxiety- and depression-related behaviors. Furthermore, we have investigated their capability of responding to a repeated stress situation.
2. Material and methods
2.1. Animals
We used 3-4 month-old Swiss and C57BL/6 female mice in this study. Swiss mice were obtained from the Universidade Federal de Santa Catarina (UFSC) Animal's Facility to our laboratory one-week prior the beginning of experimental procedures. C57BL/6 mice breeding pairs were purchased from Univali (Joinvile, SC, Brazil) and raised in our own colony at the laboratory Animal's Facility. All mice were kept under a 12 h/12 h light/dark cycle (lights on at 7:00 AM) and controlled temperature (22 ± 2 °C). Animals had free access to food and water except during behavioral experiments.
The UFSC Committee for the Care and Use of Laboratory Animals approved all experimental procedures here conducted (CEUA-UFSC—PP0798). Procedures also comply with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH publication No. 80–23, 1996) and the Brazilian Law (#11794/2008). We always conducted behavioral experiments between 12:00 PM and 6:00 PM. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Acoustic startle response (ASR)
The acoustic startle response (ASR) is characterized by a strong muscular contraction. It begins in the head and propagates to the whole body in response to a sudden and intense sensorial stimulus (Szabo, 1965; Blaszczyk and Tajchert, 1996). In this paradigm the reflexive startle response is measured after a loud sound presentation to one subject (Schmid et al., 1995; Walker and Davis, 1997; Plappert and Pilz, 2002). In the present study, mice were allowed to experimental acclimation in our apparatus (Insight®, Ribeirão Preto, SP, Brazil) during 5 min (background noise 65 dB). Then, six stimuli intensities (70, 80, 90, 100, 110 and 120 dB) were randomly presented in a 30 min session. In our protocol, we assessed ASR using ten sound stimuli of each intensity above-mentioned, 60 stimuli in total per session. Inter-stimulus interval varied from 10 s to 20 s.
2.3. Elevated plus maze (EPM)
Forty-eight hours following the ASR, animals were tested in the elevated plus maze (EPM), in order to check further differences between strains. In addition, a separated cohort from both strains was tested in the EPM 48 h following the cold-restraint stress protocol, in order to evaluate the stress influence on the anxiety-like behavior. EPM is based on the approach-avoidance paradigm. Animal is conflicted between exploring the apparatus (new environment) and avoiding the open elevated area of the maze (open arms). Our apparatus is made of grey Plexiglas being two open (30 × 5 cm) and two enclosed arms (30 × 5 × 15 cm). Closed arms walls were made of transparent Plexiglas (Insight®, Ribeirão Preto, SP, Brazil). The maze is elevated 50 cm from the floor and the open arms are surrounded by 4 mm-high edges. Each mouse was placed in the central square (5 × 5 cm) facing an open arm and allowed to explore the maze for 5 min. All sessions were recorded by a video camera. Afterwards, an experienced researcher analyzed a number of variables, namely, time spent in the open arms (globally and on its extremities only), entries into the enclosed arms and, entries into the open arms. Lastly, we also assessed the risk assessment posture, in which the animal stretches its body toward the open arms from the enclosed arms and/or central platform (Rodgers et al., 1997). Besides, rearing, head-dipping behavior, entries into the open arms extremities and, time spent on open arms extremities were also evaluated. In order to avoid sensorial cues, the EPM was cleaned between sessions with ethanol 10 %. All experiments were analyzed utilizing the X-Plo-Rat 1.1.0 freeware (USP, Ribeirão Preto, SP, Brazil). The occupational plot for each group was retrieved from the ANYmaze® software (Stoelting, USA) as a result of the tracking for all of the animals in the group during test session.
2.4. Cold-restraint stress
As aforementioned, an independent group of animals was submitted to this protocol prior to behavioral evaluations. We used 3 cm diameter and 10 cm length PVC tubes to perform the cold-restraint stress protocol. Mice were immobilized in these tubes for 30 min at 4 °C, once a day, during 21 days. This type of protocol is considered an inescapable and predictable physical stress model (De Lima and Rae, 1991).
2.5. Sucrose consumption test
Animals were submitted to sucrose consumption test after the end of the cold-restraint stress protocol. The sucrose consumption test is an indicative of hedonic state. We measured the animal preference for a sucrose solution (0.8 %) over water. Sucrose consumption test began after the last day of cold-restraint stress. On the first test day, mice were isolated and presented with two bottles of tap water. Twenty-four hours later mice were re-exposed to two bottles: one containing water and another 0.8 % sucrose solution in water. In order to avoid localization preferences we reversed bottle spatial location after 24 h. We measured liquid consumption by weighting bottles. Sucrose preference over water ratio was calculated as follow: [sucrose consumption/total liquid consumption] *100] (Tadaiesky et al., 2008).
2.6. Western blotting assay
One day after the EPM test, we collected mice brains and dissected the hippocampi and frontal cortex. These brains regions were selected because of their classic involvement in emotionality and stress response (Shin and Liberzon, 2010). Tissues were stored at −80 °C until the beginning of western analyses. Brain tissue samples were then homogenized in complete radio immunoprecipitation (RIPA) lysis buffer supplemented with protease inhibitors. Equal amounts of protein for each sample (30 μg) were loaded per lane and electrophoretically separated using 12% denaturing polyacrylamide gel electrophoresis (SDS-PAGE). Afterwards, proteins were transferred to nitrocellulose membranes using a Mini Trans-Blot Cell system (Bio-Rad Laboratories Inc., Hercules, CA, USA) following the manufacturer's protocol. The neuroinflammatory process is well characterized for being initiated/regulated by glial cells (Skaper et al., 2018). Here we chose to evaluate the astrocytes, given its connection to depression-like behavior (Naijar et al., 2013), besides the well-characterized stress-related GR receptor (Sotnikov et al., 2014). Immunodetection was carried out using monoclonal mouse anti-GFAP (#3670, 1:1000; Cell Signaling Technology, Danvers, MA, USA) and polyclonal rabbit anti-GR (SAB4501309, 1:500; Sigma-Aldrich, St. Louis, MO, USA) primary antibodies incubated overnight. Following washing, membranes were incubated with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:25000, Cell Signaling, Danvers, MA, USA). Immunocomplexes were visualized using SuperSignal West Femto Chemiluminescent Substrate detection system (Thermo Fischer Scientific, Rockford, IL, USA) in a photo documenter (Bio-Rad Laboratories Inc., Hercules, CA, USA). Loading control and densitometric normalization were performed using monoclonal mouse β-actin primary antibody (sc81178, 1:500; Santa Cruz Biotech. Inc., Santa Cruz, CA, USA). Band density measurements were made using NIH ImageJ 1.36 b imaging software (NIH, Bethesda, MD, USA). Bands were measured as optical density (OD) of protein levels and expressed in arbitrary units (A.U.) of the ratio target protein/β-actin.
2.7. Plasma corticosterone
Mice from independent groups were euthanized following one day or 21 days of cold-restraint stress. Groups were compared to naïve animals (not submitted to the stress protocol), matched by littermate. In a separated procedure room, animals were anesthetized with Isoflurane and decapitated (between 11:00 AM and 3:00 PM to avoid the circadian peak of corticosterone). We collected trunk blood in a micro-tube containing 40 μL of a 10 % heparin solution and then centrifuged it at 7.000 r.p.m. for 10 min. The plasma was collected and stored at −80 °C until the assay was performed. Plasma samples were thawed in ice and diluted 100× in assay buffer. We performed the analysis in duplicates in an ELISA plate following manufacturer's instructions (IBL International GMBH, Germany). Absorbances were read at 450 nm in an Infinite M200 TECAN spectrophotometer and the values calculated based on the standard curve.
2.8. Statistical analysis
Statistical evaluation of ASR (with repeated measures) was performed using one-way ANOVA followed by Newman-Keuls’ post hoc test. Comparisons between C57BL/6 and Swiss mice were carried out using Student's t-test. Stress effects over time on plasma corticosterone, as well as GR and GFAP expression following stress were analyzed by two-way ANOVA followed by Newman-Keuls’ post hoc test. Graphic data were expressed as mean ± SEM. p values lower than 0.05 (p < 0.05) were considered statistically significant. We used Statistica 7.0 (StatSoft, Palo Alto, CA, USA) for statistical analysis and GraphPad Prism 5 (GraphPad Prism, San Diego, CA, USA) for graph plots.
3. Results
3.1. Female mice from different strains presented distinctive anxiety-like behavior and HPA-axis activation
Routinely, scientists around the world use different mice strains for a variety of purposes. Mouse models of distinctive strains are intensely used to primordially study human diseases. Anxiety-, depression- and stress-related disorders are frequently studied in mouse models (Krishnan and Nestler, 2011; Belzung, 2014; Kokras and Dalla, 2014). We questioned whether distinct mice strains would behave differently in common tests used to evaluate anxiety-like behavior.
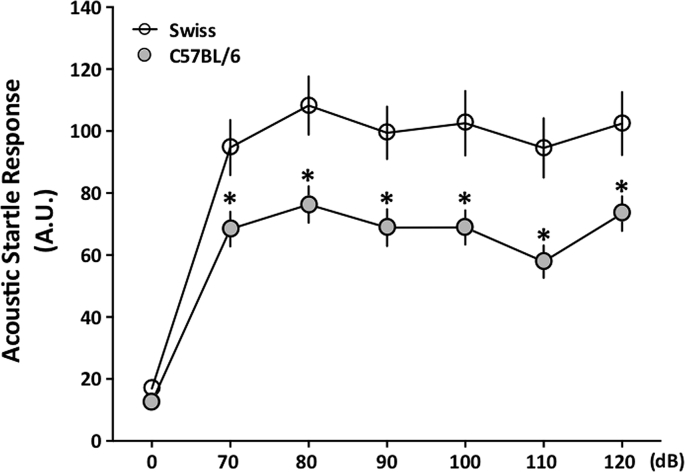
Here we chose to evaluate the two most used mice strains: Swiss and C57BL/6. There is a strain difference at all stimulus intensities and Newman-Keuls post hoc test confirmed differences between all sound intensities in relation to no stimulus (0 dB) for both strains (Fig. 1). On Fig. 1 variance analysis (ANOVA) test indicated overall a difference between the strains [F (1, 54) = 10.929; p = 0.002; Swiss > C57BL/6]. Besides, significant interaction between the indepent factors, strain and stimulus, was observed [F (6, 324) = 4.129, p = 0.0005]. This first result indicated important strain differences affecting anxiety-like behavior in mice.
Fig. 1.
Acoustic startle response of Swiss and C57BL/6 female mice. Behavioral responses of Swiss (clear circles) and C57BL/6 (grey circles) female mice in the acoustic startle response (ASR) at different stimulus intensities varying from 70 to 120 dB. Data are expressed as mean ± S.E.M. and analyzed by a one-way ANOVA with repeated measures followed by the Newman-Keuls´ post hoc test *p < 0.05 when compared to Swiss mice in the same stimulus (n = 24 for Swiss/n = 36 for C57BL/6).
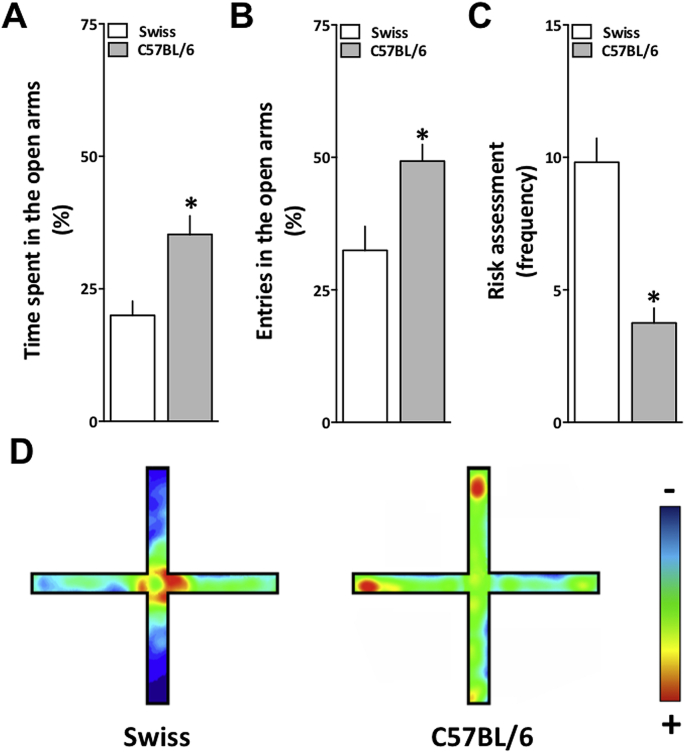
Then we decided to better evaluate this behavior using another task, the Elevated Plus Maze (EPM), which is the most used and recognized task to evaluate anxiety-like behaviors and potential anxiolytic drugs (Griebel and Holmes, 2013). Fig. 2 shows the comparison between Swiss and C57BL/6 female mice behavior in the EPM. Each parameter evaluated in the maze was analyzed using unpaired Student's t-test. C57BL/6 female mice presented significant differences in the exploratory parameters in comparison to Swiss female mice; Namely, an increase in time spent in the open arms [t (30) = - 3.453; p = 0.002] (Fig. 2A), in open arms entries [t (30) = -3.072; p = 0.004] (Fig. 2B), and also in time spent in the open arms extremities [t (30) = -8.383; p < 0.001] (data not shown). Additionally, they presented a decreased risk assessment frequency [t (30) = 3.750; p < 0.001] (Fig. 2C). Differences are highlighted by the representative occupational map of the session for each group (Fig. 2D). Nevertheless, there were no differences between strains in the frequency of enclosed arms entries [t (30) = -0.248; p = 0.805], which is correlated with the locomotor activity (Swiss: 8.90 ± 1.56; C57BL/6: 6.43 ± 1.43). These EPM data further confirmed our ASR previous findings, suggesting that Swiss female mice express more anxiety-like behaviors when compared to C57BL/6.
Fig. 2.
Elevated plus maze exploration by Swiss and C57BL/6 female mice. Behavioral responses of Swiss (white bars) and C57BL/6 (grey bars) female mice in the elevated plus maze (EPM). (A) Percentage of time spent in the open arms. (B) Percentage of entries in the open arms. (C) Frequency of risk assessment behavior. (D) Representative occupational plot of each group generated by ANYmaze®. Vertical arms correspond to the open arms and horizontal to the enclosed arms. The hot colors represent the area most visited by the group and the cold colors, the least. Data are expressed as mean + S.E.M. and analyzed by unpaired Student's t-test *p < 0.05 (n = 10/strain). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
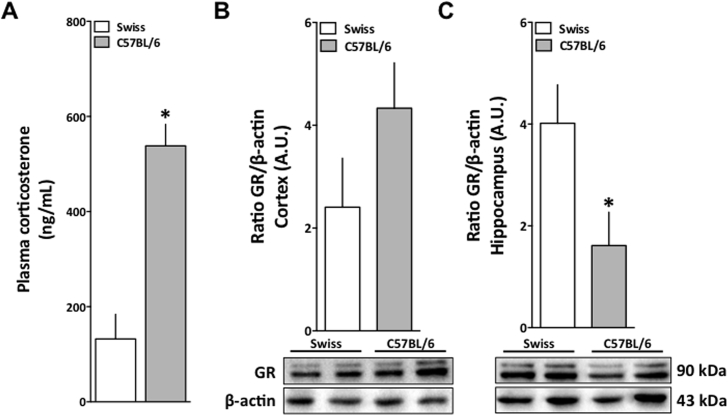
Indeed, anxiety behavior has shown to be regulated by corticoid hormones in different species (Brooke et al., 1994; de Quervain et al., 2011; Sotnikov et al., 2014; Merz and Wolf, 2015). We further questioned whether those animals would have any baseline HPA-axis regulation differences, which in turn could justify the behavioral differences we have reported. Fig. 3 shows data for corticosterone evaluation and GR expression in the cortex and hippocampus of naïve Swiss and C57BL/6 female mice. While C57BL/6 female mice presented augmented corticosterone plasma levels in comparison to Swiss mice [t (5) = 5.64; p = 0.002], they showed a diminished GR expression in the hippocampus [t (10) = 3.39; p = 0.007]. These data could be interpreted as a differential baseline regulation of the HPA-axis between both strains.
Fig. 3.
Plasma corticosterone levels and GR expression in Swiss and C57BL/6 female mice. (A) Plasma corticosterone levels. (B) Graphical plot of GR expression (top) and two western blot representative lanes per group (bottom), in the frontal cortex of Swiss (white bars) and C57BL/6 (grey bars). (C) Graphical plot of GR expression (top) and two western representative lanes per group (bottom), in the hippocampus of Swiss (white bars) and C57BL/6 (grey bars). GR protein (90 KDa) expression was normalized to β-actin (43 KDa) expression for each sample; A.U.: Arbitrary units. Results are expressed as mean + S.E.M. and analyzed by unpaired Student's t-test *p < 0.05 (n = 5/group).
3.2. Stress effects on the anxiety-like behavior of female mice from different strains
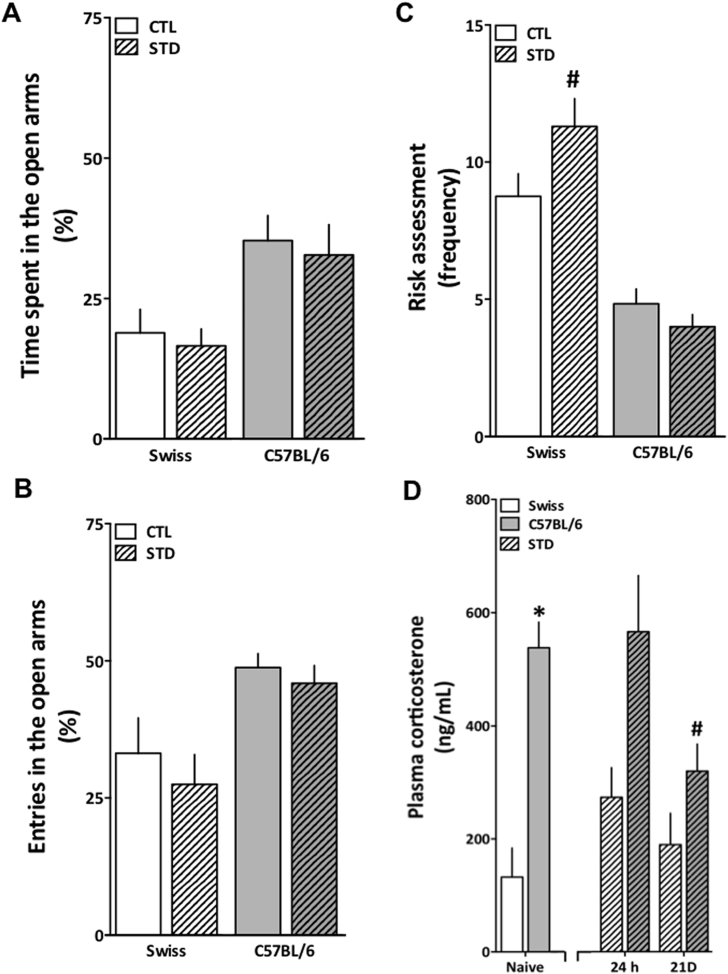
We next questioned whether stress could affect anxiety-like vulnerability in these animals. As we have seen a more pronounced anxiety-like behavior using the EPM test, we decided to test female C57BL/6 and Swiss mice in this task after 21 days of cold-restraint stress. Our cold-restraint stress protocol induced slight behavioral alterations on the EPM test as it can be observed in Fig. 4. Time spent in the open arms was higher in the C57BL/6 strain [F (1,47) = 9.50; p = 0.003] but stress had no influence over this parameter [F (1,47) = 1.38; p = 0.25] (Fig. 4A). Neither the stress protocol [F (1,47) = 0.0007; p = 0.98] nor the strain [F (1,47) = 2.97; p = 0.09] altered the entries in the open arms (Fig. 4B). Swiss animals presented an increased frequency of risk assessment behavior following stress, when compared to C57BL/6 [F (1,47) = 11.49; p = 0.001] (Fig. 4C). No difference between the groups was observed in the locomotion (Swiss CTL: 9.42 ± 0.81; Swiss STD: 10.30 ± 1.02; C57BL/6 CTL: 12.53 ± 0.65; Swiss STD: 13.53 ± 0.83). When we evaluated corticosterone levels following the stress protocol, no statistical differences were detected in the C57BL/6 or in the Swiss animals after 24 h. However, after 21 days of stress, detectable levels of corticosterone in the C57BL/6 samples were significantly lower when compared to their baseline levels [F (2,17) = 3.073; p = 0.053] (Fig. 4D). Based on this set of results, one could argue that following stress corticosterone levels as well as anxiety-like behavior are managed distinctively in both mice strains.
Fig. 4.
Cold-restraint stress effects on EPM behavior and plasma corticosterone in Swiss and C57BL/6 female mice. Mice of both strains were exposed to one (corticosterone) or 21 days (behavior and corticosterone) of cold-restraint stress (30 min daily, 4 °C). (A) Percentage of time spent in the open arms of Swiss mice and C57BL/6 mice. (B) Percentage of entries into the open arms of Swiss mice and C57BL/6 mice. (C) Frequency of risk assessment behavior of Swiss mice and C57BL/6 mice. (D) Plasma corticosterone levels in naïve Swiss and C57BL/6 mice and in mice exposed to one or 21 days of restraint stress. CTL: control group (clear bars); STD: stressed group (streaked bars). 24 h: group submitted to one cold restraint episode of 30 min; 21 D: group submitted to cold restraint stress for 30 min daily, during 21 days. Results are expressed as mean + S.E.M. Data were analyzed by two-way ANOVA followed by Newman-Keuls’ post hoc test (EPM: n = 9–12/group for Swiss mice and n = 15/group for C57BL/6 mice; plasma corticosterone: n = 4/group). *p < 0.05 when compared to Swiss naïve group. #p < 0.05 when compared to C57BL/6 naïve group.
3.3. Stress effects on the hedonic-like behavior of female mice from different strains
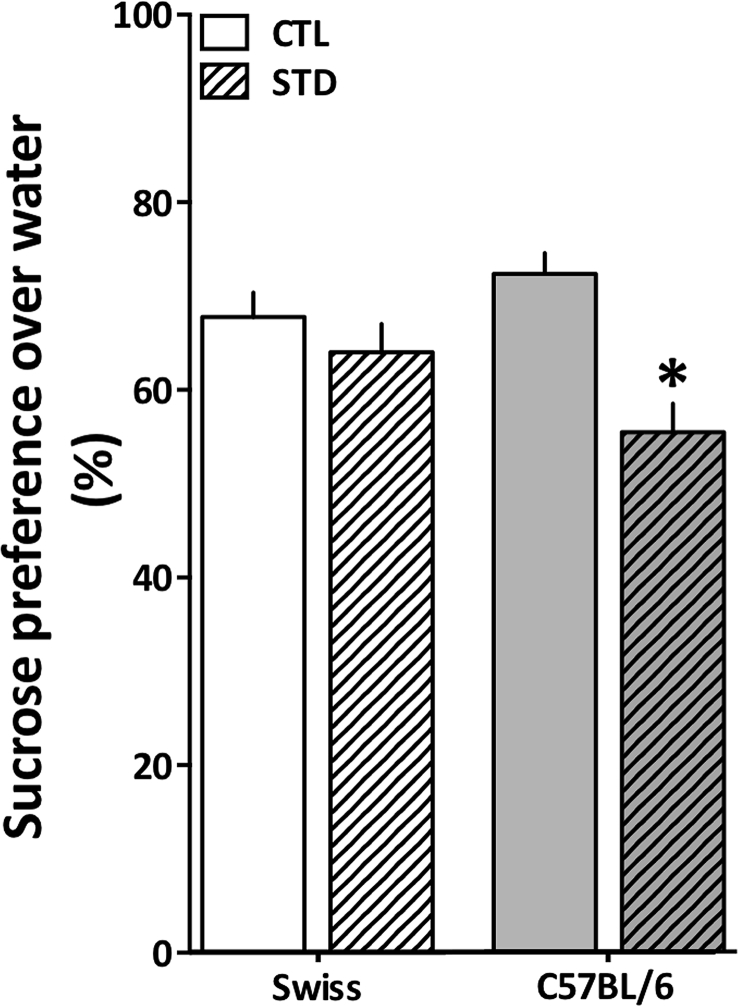
As the animals from different strains seemed to behave differently in tests applied to study anxiety-like behavior, further questions were raised about the ability of those animals to respond in tests used to study depressive-like behavior. Here we used the sucrose preference test, largely employed to evaluate hedonic-like behavior (Sclafani and Clyne, 1987). Anhedonia is proposed as an endophenotype of major depressive disorder (Pizzagalli, 2014) and it is widely used as a reliable experimental measurement due to its response to antidepressant treatment (Papp et al., 1994; Cryan et al., 2002; Willner, 2005). In this test we observed a significant reduction in the preference for sucrose following the stress protocol only in the C57BL/6 strain [F (1, 46) = 5.486, p = 0.02] (Fig. 5). These striking data revealed a different aspect about the C57BL/6 strain, suggesting that those animals are more inclined to show anhedonic-like behavior following stress.
Fig. 5.
Cold-restraint stress effects on sucrose preference in Swiss and C57BL/6 female mice. Swiss (white bars) and C57BL/6 (grey bars) mice were exposed to 21 days of cold-restraint stress (30 min daily, 4 °C) and tested in the sucrose preference over water. CTL: control group (clear bars); STD: stressed group (streaked bars). Results are expressed as mean + S.E.M and analyzed by a two-way ANOVA. *p < 0.05 (n = 9–12/group for Swiss mice and n = 15/group for C57BL/6 mice).
3.4. Evaluation of the glucocorticoid receptor (GR) and GFAP expression in the brain of mice from different strains
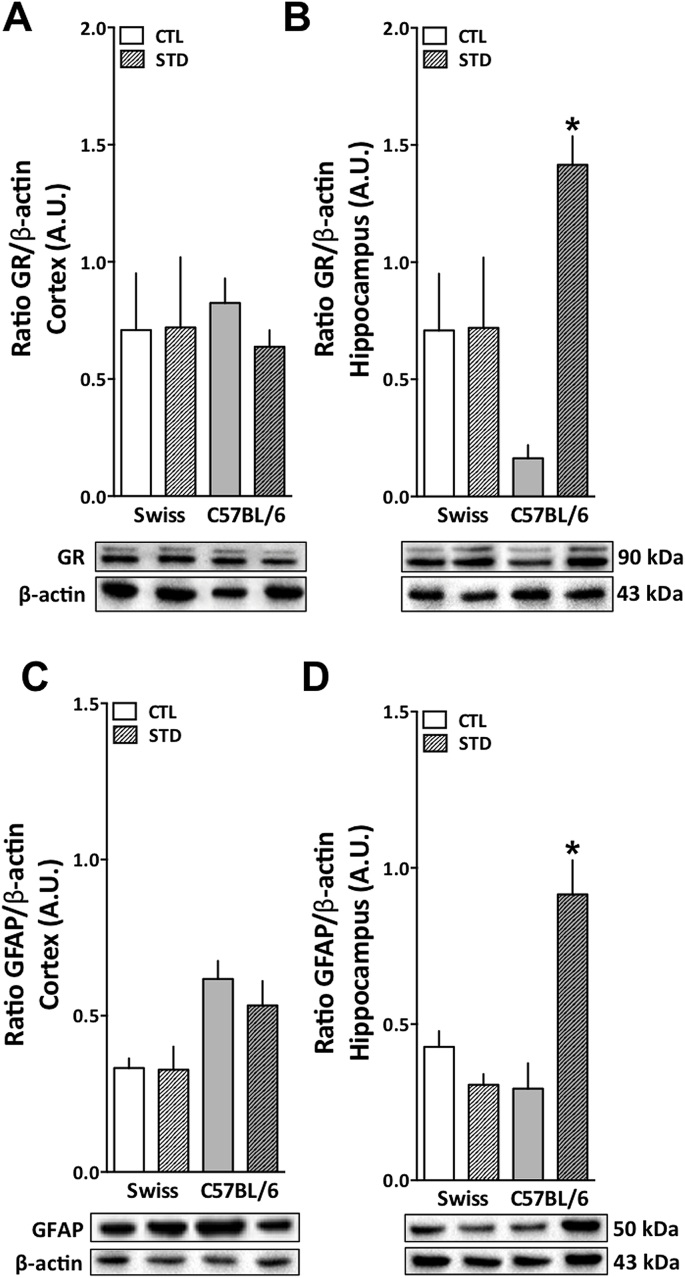
In order to better explain why precisely C57BL/6 female mice appear to be more susceptible to sucrose preference deficits following stress, we conducted biochemical analysis on the brains collected after the behavioral tests. Here we evaluated the potential alterations in GR expression due to the stress protocol utilized. Additionally, recently literature has been connecting depression-like behavior with neuroinflammation (Naijar et al., 2013; Bhattacharya et al., 2016). We evaluated the levels of GFAP protein in these samples, in order to verify one of the important components of the neuroinflammatory process: astrocyte activation/migration. Both, GR [Fig. 6B; F (3,24) = 2.101; p < 0.001] and GFAP [Fig. 6D; F (3,20) = 1.639; p < 0.001] expression were increased in the hippocampus (HP), but not in the cortex (CT) (Fig. 6 A and C) of the C57BL/6 female mice submitted to 21 days of cold-restraint stress. No differences were observed either in the GR or GFAP levels in both brain regions of Swiss mice submitted to the same stress protocol (Fig. 6).
Fig. 6.
Cold-restraint stress effects on GR and GFAP protein expression in the cortex and hippocampus of Swiss and C57BL/6 female mice. Swiss (white bars) and C57BL/6 (grey bars) mice were exposed to 21 days of cold-restraint stress (30 min daily, 4 °C) and euthanized after behavioral testing. Frontal cortex and hippocampus were collected and prepared for Western blotting analysis. GR expression in the (A) frontal cortex and in the (B) hippocampus. GFAP expression in the (C) frontal cortex and in the (D) hippocampus. The western lanes aligned bellow the bars graph show one representative animal of each group. GR (90 KDa) and GFAP (50 KDA) expression were normalized to β-actin (43 KDa) expression of the same samples. CTL: control group (clear bars); STD: stressed group (streaked bars); A.U.: Arbitrary units. Results are expressed as mean + S.E.M. and analyzed by two-way ANOVA followed by Newman-Keuls’ post hoc test. *p< 0.05 (n = 5/group).
4. Discussion
Our results clearly demonstrate fundamental behavioral and biochemical alterations in between the mice strains here studied. Female C57BL/6 mice presented lower levels of anxiety-like behavior as shown in our ASR and EPM results when compared to Swiss female mice paired in age. Female C57BL/6 mice also presented baseline augmented corticosterone plasma levels along with a diminished GR expression in the hippocampus when compared to Swiss female mice. However, Swiss female mice seem to be more resistant to stress than C57BL/6 mice, since following a 21 days stress challenge they did not show any profound anxiety-like or depression-like behavioral alterations. Conversely, C57BL/6 mice exhibited a significant augmented anhedonic-like behavior. Furthermore, here we report that this anhedonic-like behavior coincides with an increased expression of GR and GFAP in the hippocampus of these animals.
Anxiety-like behavior is commonly studied in rodents in order to figure out new drugs that could be useful for humans in the future (Griebel and Holmes, 2013). The ASR is proposed as a tool in the study of emotional conditions because this reflexive response is increased in aversive states (Walker and Davis, 1997; Plappert and Pilz, 2002) and decreased in hedonic states (Schmid et al., 1995). In the EPM, increased exploration of the open arms is interpreted as a reduction in fear-like behaviors, reflecting the reduction of the conflict between avoidance and approach of the aversive area (Carobrez and Bertoglio, 2005). This increased exploration has also been interpreted as an increase in risk-taking behaviors (Cortese et al., 2010; Radhakrishnan et al., 2015).
Although, to our knowledge, there are no direct comparisons between Swiss and C57BL/6 mice strains, several authors have compared the C57BL/6 with other inbred strains; for instance, C57BL/6 mice present a less anxiety-like phenotype than the BALB/cj in the open field and EPM (Brooks et al., 2005; Meziane et al., 2007; Xiao-Lei et al., 2011). Comparisons between inbreed mice strains have been also made using the ASR (Paylor and Crawley, 1997; Willot et al., 2003) and it was found a wide variation between them, suggesting a genetic influence of the startle response. This assumption was reinforced by the identification of 15 QTLs for the ASR in strains derived from C57BL/6J and A/J (Joober et al., 2002).
Here we report that the C57BL/6 female mice demonstrated lower ASR levels and higher EPM exploration than Swiss mice. Our results from the ASR along with our data using the EPM lead us to propose that female C57BL/6 mice present a low anxiety profile when compared with female Swiss mice, which is in accordance to the aforementioned literature about C57BL/6 animals compared to other inbreed mice strains. Our proposition on the anxiety-like behavior in these rodents was raised not only based in behavioral analysis, but also in the plasma corticosterone levels and brain GR expression evaluations, which are important components of the hypothalamic-pituitary-adrenal axis (HPA-axis).
The HPA-axis activation leads to the release of glucocorticoids (GCs) and other hormonal mediators from the adrenal gland. The GCs act through two nuclear receptors: mineralocorticoid (MR) and glucocorticoid (GR). Whereas GCs have a high affinity for MRs and mostly occupy these receptors in physiological levels, they have a low affinity for GRs that are distributed in higher brain structures, namely the hippocampus (Deak, 2008). Here we also address the distinct HPA-axis functionality of Swiss and C57BL/6 female mice. At the baseline level, C57BL/6 presented higher plasma corticosterone concentrations. In agreement with the regulation of the axis by a negative feedback (De Kloet et al., 2005), GR expression in the hippocampus of those animals was drastically decreased. There are mixed reports in the literature regarding the connection between the levels of GR expression and anxiety-like behavior. While many report low GR expression is linked to anxiety (Tronche et al., 1999; Ridder et al., 2005; Zhe et al., 2008), there are studies like the one from Wei et al. (2004) that found that transgenic animals overexpressing GR presented increased anxiety- and depressant-like behavior. Additionally, Sotnikov et al. (2014) reported a higher expression of GR in the pituitary and limbic system of the high anxiety-related mice (HAB) strain, when compared to the normal and low anxiety strains. Thus, it seems to be appropriate to assume that the anxiety-like behavior is associated with a delicate balance/regulation of the HPA-axis function, in which variations to either direction could lead to behavioral alterations. Taken together, our data point to a baseline behavioral difference between Swiss and C57BL/6 female mice, being the later strain less susceptible to present anxiety-like behavior.
Nevertheless, this baseline difference in anxiety-like behavior does not implicate in a differential response to the predictable repeated stress in the EPM. Others have shown that female response to stress is dependent on the kind of stressor (Palanza, 2001; Martin and Brown, 2010; Zhu et al., 2014). The cold-restraint stress used in the present work is the most used protocol to study the impact of stress on the immune system (Glaser and Kiecolt-Glaser, 2005). The physiological response to stress is orchestrated mainly by two systems: the HPA-axis and the sympathetic nervous system (Joëls and Baram, 2009), but several neuroimmune pathways are also activated by stressful events and its effects depend on many factors such as previous experience, strain, age and gender (Hueston and Deak, 2014). It seemed important to address the stress influence on these animals' behaviors, since different mice strains have been widely used to test genetic vulnerability to stress (Tarantino et al., 2000; Jung et al., 2014). Our results indicate that none of the female strains displays any alterations in the EPM parameters following stress submission. In contrast, when using males, other research groups have suggested that Swiss mice are resilient to stress protocols (Yalsin et al., 2008), whereas C57BL/6 mice are vulnerable (Golden et al., 2011; Jung et al., 2014). Then, we decided to verify a putative correlation of our behavioral data following the 21 days cold-restraint stress with the analysis on the corticosterone levels in the same animals.
Interestingly, we demonstrate that both strains are differently affected by cold-restraint stress in terms of corticosterone levels. Swiss mice samples revealed a tiny elevation 24 h after a single exposure to stress and no alterations after 21 days. However, it was found no alterations in the C57BL/6 plasma samples after 24 h, but a significant corticosterone decrease following 21 days of stress. A single exposure to stress in rodents (e.g. foot-shock, restraint in tubes) produces modifications in corticosterone levels (Maier et al., 1986). There is evidence for sexual differences influencing glucocorticoids secretion. Precisely, females have an increased corticosterone secretion in response to restraint stress when compared to males (Kalil et al., 2013; Baab et al., 2014). Besides, Duma et al. (2010) showed a sexual dimorphism in the glucocorticoid response, in which females do not have a strong anti-inflammatory response to glucocorticoids such as males. When the same stimulus is repeatedly presented, there is HPA-axis adjustment, being the glucocorticoid response to the stressor progressively diminished (Bhatnagar et al., 2002), in the same way as seen in our results for C57BL/6 female mice. One could hypothesize that Swiss mice are more sensitive to corticosterone variations following stress because they present lower levels of this hormone at the baseline. On the other hand, C57BL/6 mice already presented profound alterations on corticosterone at the baseline and further GR expression, suggesting an HPA-axis less prone to respond to acute corticosterone variations. Nevertheless, those variations at corticosterone levels seem to be not enough to be translated in behavioral alterations, since we have seen that both strains seem not to be affected by 21 days of stress, at least when evaluated for the anxiety-like behavior.
Although both strains have shown no anxiety-like behavior alterations following predictable repeated stress, C57BL/6 mice presented a depressive-like behavior. Here we chose to evaluate animals utilizing the sucrose preference test. Sucrose preference is used as a measure of anhedonia (Willner, 2005). Different from Swiss mice, C57BL/6 female mice have presented significant reduced preference for the sucrose solution; which could be translated as an anhedonic behavior. Anhedonic behavior in rodents has a high correlation with atypical depressive disorder and melancholic depression (Gold et al., 2015). In stress-related disorders such as melancholic depression, the activities of central glutamate, norepinephrine, central cytokines and pro-inflammatory mediators are significantly and persistently increased (Gold et al., 2015). Classically, glucocorticoids such as corticosterone are considered endogenous anti-inflammatories, but new findings have demonstrated that they can turn into pro-inflammatory mediators depending on the time and releasing concentration (Sorrells and Sapolsky, 2007; Frank et al., 2014). In this sense, inescapable and predictable physical stress (such as the cold-restraint stress here used) could induce pro-inflammatory cytokines, enzymes and nuclear factors expression/release in the brain (e.g. NFkB, iNOS and COX-2; for a review see García-Bueno et al., 2008). Therefore, the same hormones can be responsible for the adaptive and maladaptive response to stress and, in the case of corticosterone; both mechanisms are mediated through the GR (Deak et al., 2015). Although many findings in the literature show a reduction in GFAP expression in response to stress and glucocorticoids (Unemura et al., 2012; Han et al., 2015), growing evidences correlate neuroinflammation and glia activation in response to stress (Deak et al., 2015) and on sickness behavior (Kelly et al., 2018).
Neuroinflammation comprises among other features, an immune activation along with astrocytes and microglial activation and recruitment (Biesmans et al., 2015). Our data show that, in conjunction with depressive-like behavior, there are two major biochemical alterations following predictable and inescapable physical stress in C57BL/6 female mice: an elevated GR expression and an augmented GFAP expression in the hippocampus. The former could be considered an HPA-axis adaptation mechanism due to the above-discussed decreased levels of corticosterone at this time point (Fig. 4D). The later could be a direct effect of corticosterone levels variation in the brain. Others have previously reported increased GFAP expression in the brain following stressful situations (Lambert et al., 2000; Jang et al., 2008; Northrop and Yamamoto, 2012; Diz-Chaves et al., 2013). On the other hand, Araya-Callís et al. (2012) have found a reduced GFAP expression in the hippocampus following chronic social defeat protocol in rats. Likewise, it was demonstrated a GFAP reduction in the periaqueductal grey matter following 6 h daily restraint stress for 3 weeks (Imbe et al. 2012). In both cases, authors used male subjects and correlated GFAP down-regulation with neuroplasticity maintenance. Of note, prior exposure to corticosterone has been show to enhance the neuroinflammatory response (Frank et al., 2007; Kelly et al., 2012). Thus, we suggest that there is a neuronal dysregulation of the HPA-axis, specifically in the C57BL/6 strain, that leads to an increased release of neuroinflammatory cytokines in response to stress (Hueston and Deak, 2014), which in turn could activate astrocytes. In some cases, astroglyosis (i.e. increased GFAP expression) was not directly related to increased neuroinflammation (Kelly et al., 2018). However, evidence in the literature also demonstrated that in females, gonadal hormones influence brain function via an increased number of synaptic neuron-glia connections (Garcia-Segura et al., 1995). In this line of thought, it has been suggested that the estrogen receptor (ER) plays an important role in the expression of astrocytes (Stone et al., 1998). For these reasons, we interpret our GR and GFAP results, and the possible relation in between them, as strain- and sex-dependent. In sum, it is plausible that the stress protocol causes a prolonged HPA-axis dysregulation to the C57BL/6 female mice; thus, being translated in a depressive-like behavior by activating pro-inflammatory pathways in the brain.
Based on our findings, we propose that C57BL/6 female mice have lower anxiety-like behavior than Swiss female mice, which can be related to baseline plasma corticosterone differences in between both strains. Additionally, C57BL/6 female mice are more susceptible to present an anhedonic-like behavior in response to repeated stress, being these changes supported by pro-inflammatory alterations and glucocorticoids action in the hippocampus.
Our data highlight new aspects to the notion that gender and strain are important factors to be considered when scientists study anxiety-like and depression-like behavior, especially when stress is a determinant variable.
Authors' contributions
R.C.N.M. and T.C.M.L. designed the study; R.C.N.M. performed all behavioral experiments; R.C.N.M., M.A.B. and E.C.S.S. performed Western blotting and ELISA experiments; R.C.N.M. and M.A.B wrote the manuscript; T.C.M.L. advised this work offering technical conditions and intellectual support; All of the authors participated in the discussion and elaboration of the manuscript, revised and approved the final version.
Conflicts of interest
Authors declare no actual or potential conflict of interest in this study.
Acknowledgements
Grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, both from Brazil, supported this study. R.C.N. Marchette, E.C.S. Santos and M.A. Bicca were graduate students funded by CNPq, Brazil. T.C.M. de Lima was a recipient of CNPq research grant. Authors truly thank Claudini H. de Pieri and Dr. Flora Lucena for their technical assistance during behavioral protocols. Also, for their technical support during Western blotting and ELISA experiments we thank the Laboratório de Multiusuários em Biologia (LAMEB) staff from UFSC.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.08.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Araya-Callís C. Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology (Berl) 2012;224(1):209–222. doi: 10.1007/s00213-012-2741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baab J.A. Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress. 2014;17(3):224–234. doi: 10.3109/10253890.2014.905534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in molecular and cellular substrates of stress. Cell. Mol. Neurobiol. 2012;32:709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C. Innovative drugs to treat depression: did animal models fail to Be predictive or did clinical trials fail to detect effects? Neuropsychopharmacology. 2014;39(5):1041–1051. doi: 10.1038/npp.2013.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J. Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A. Role of neuroimmunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berl) 2016;233(9):1623–1636. doi: 10.1007/s00213-016-4214-0. [DOI] [PubMed] [Google Scholar]
- Biesmans S. Effect of stress and peripheral immune activation on astrocyte activation in transgenic bioluminescent GFAP-luc mice. Glia. 2015;63(7):1126–1137. doi: 10.1002/glia.22804. [DOI] [PubMed] [Google Scholar]
- Blaszczyk J., Tajchert K. Sex and strain differences of acoustic startle reaction development in adolescent albino Wistar and hooded rats. Acta Neurobiol. Exp. 1996;56:919–925. doi: 10.55782/ane-1996-1199. [DOI] [PubMed] [Google Scholar]
- Bolea-Alamanac B. Female psychopharmacology matters! towards a sex-specific psychopharmacology. J. Psychopharmacol. 2018;32(2):125–133. doi: 10.1177/0269881117747578. [DOI] [PubMed] [Google Scholar]
- Brooke S.M. Dexamethasone resistance among nonhuman primates associated with a selective decrease of glucocorticoid receptors in the hippocampus and a history of social instability. Neuroendocrinology. 1994;60(2):134–140. doi: 10.1159/000126743. [DOI] [PubMed] [Google Scholar]
- Brooks S.P. Behavioral profiles of inbred mouse strains used as transgenic background. I: motor tests. Gene Brain Behav. 2004;3:206–215. doi: 10.1111/j.1601-183X.2004.00072.x. [DOI] [PubMed] [Google Scholar]
- Brooks S.P. Behavioral profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Gene Brain Behav. 2005;4:307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Carobrez A.P., Bertoglio L.J. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005;29(8):1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cortese B.M. Region-specific alteration in brain glutamate: possible relationship to risk-taking behavior. Physiol. Behav. 2010;99(4):445–450. doi: 10.1016/j.physbeh.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A.P. A proposal for refining the forced swim test in Swiss mice. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2013;45:150–155. doi: 10.1016/j.pnpbp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Markou A., Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- De Kloet R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–473. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Lima T.C.M., Rae G.A. Effects of cold-restraint and swim stress on convulsions induced by pentylenetetrazol and electroshock: influence of naloxone pretreatment. Pharmacol. Biochem. Behav. 1991;40(2):297–300. doi: 10.1016/0091-3057(91)90556-h. [DOI] [PubMed] [Google Scholar]
- De Quervain D.J. Glucocorticoids enhance extinction-based psychotherapy. Proc. Natl. Acad. Sci. U.S.A. 2011;108(16):6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T. Immune cells and cytokine circuits: toward a working model for understanding direct immune-to-adrenal communication pathways. Endocrinology. 2008;149:1433–1435. doi: 10.1210/en.2008-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. 2015;18(4):367–380. doi: 10.3109/10253890.2015.1053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav. Immun. 2013;28:196–206. doi: 10.1016/j.bbi.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Duma D. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010;3 doi: 10.1126/scisignal.2001077. ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Galdino P.M. Involvement of monoaminergic systems in the antidepressant-like properties of Lafoensi pacari A. St. Hil. J. Ethnopharmacol. 2015;170:218–225. doi: 10.1016/j.jep.2015.05.015. [DOI] [PubMed] [Google Scholar]
- García-Bueno B., Caso J.R., Leza J.C. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neuroscience and Biobehavioral Reviews. 2008;32:1136–1151. doi: 10.1016/j.neubiorev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L.M. Gonadal hormone regulation of neuronal-glial interactions in the developing neuroendocrine hypothalamus. J. Steroid Biochem. Mol. Biol. 1995;53(1–6):293–298. doi: 10.1016/0960-0760(95)00066-9. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plasticity. 2015;2015:1–11. doi: 10.1155/2015/581976. article ID 581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G., Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013;12(9):667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F., Xiao B., Wen L. Loss of glial cells of the hippocampus in a rat model of post-traumatic stress disorder. Neurochemestry Research. 2015;40(5):942–951. doi: 10.1007/s11064-015-1549-6. [DOI] [PubMed] [Google Scholar]
- Hueston C.M., Deak T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic–pituitary–adrenal axis. Physiol. Behav. 2014;124:77–91. doi: 10.1016/j.physbeh.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Imbe H. Chronic restraint stress decreases glial fibrillary acidic protein and glutamate transporter in the periaqueductal gray matter. Neuroscience. 2012;223:209–218. doi: 10.1016/j.neuroscience.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Jang S. Changes in iNOS, GFAP and NR1 expression in various brain regions and elevation of sphingosine-1-phosphate in serum after immobilized stress. Neurochem. Res. 2008;33(5):842–851. doi: 10.1007/s11064-007-9523-6. [DOI] [PubMed] [Google Scholar]
- Joëls M., Baram T.Z. The neurosymphony of stress. Nature Reviews: Neuroscience. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joober R. Provisional mapping of quantitative trait loci modulating the acoustic startle response and prepulse inhibition of acoustic startle. Neuropsychopharmacology. 2002;27:765–781. doi: 10.1016/S0893-133X(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Jung Y. Strain differences in the chronic mild stress animal model of depression and anxiety in mice. Biomolecules & Therapeutics. 2014;22(5):453–459. doi: 10.4062/biomolther.2014.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil B. Role of sex steroids in progesterone and corticosterone response to acute restraint stress in rats: sex differences. Stress. 2013;16(4):452–460. doi: 10.3109/10253890.2013.777832. [DOI] [PubMed] [Google Scholar]
- Kawakami S.E. Sex-dependent effects of maternal separation on plasma corticosterone and brain monoamines in response to chronic ethanol administration. Neuroscience. 2010;253:55–66. doi: 10.1016/j.neuroscience.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Kelly K.A. Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. Journal of Neurochemestry. 2012;122:995–1009. doi: 10.1111/j.1471-4159.2012.07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K.A. Prior exposure to corticosterone markedly enhances and prolongs the neuroinflammatory response to systemic challenge with LPS. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190546. e0190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Meth. Psychiatr. Res. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein N. Assessment of sedative effects of Passsiflora edulis f. flavicarpa and Passiflora alata extracts in mice, measured by telemetry. Phytother Res. 2014;28(5):706–713. doi: 10.1002/ptr.5043. [DOI] [PubMed] [Google Scholar]
- Kokras N., Dalla C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014;171(20):4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. Animal models of depression: molecular perspectives. Current Topics in Behavior Neuroscience. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert K.G. Activity-stress increases density of GFAP-immunoreactive astrocytes in the rat hippocampus. Stress. 2000;3(4):275–284. doi: 10.3109/10253890009001133. [DOI] [PubMed] [Google Scholar]
- Lotan A. Differential effects of chronic stress in young-adult and old female mice: cognitive-behavioral manifestations and neurobiological correlates. Mol. Psychiatr. 2017 doi: 10.1038/mp.2017.237. [DOI] [PubMed] [Google Scholar]
- Maier S.F. Stressor controllability and the pituitary-adrenal system. Behav. Neurosci. 1986;100(5):669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Martin A.L., Brown R.E. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav. Brain Res. 2010;207:196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Merz C.J., Wolf O.T. Examination of cortisol and state anxiety at an academic setting with and without oral presentation. Stress. 2015;18(1):138–142. doi: 10.3109/10253890.2014.989206. [DOI] [PubMed] [Google Scholar]
- Meziane H. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Gene Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Mineur Y.S., Belzung C., Crusio W.E. Effects of chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Naijar S. Neuroinflammation and psychiatric illness. J. Neuroinflammation. 2013;10(43):1–24. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop N.A., Yamamoto B.K. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. J. Neuroimmune Pharmacol. 2012;7(4):951–968. doi: 10.1007/s11481-012-9391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci. Biobehav. Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Papp M., Klimek V., Willner P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology (Berl) 1994;115(4):441–446. doi: 10.1007/BF02245566. [DOI] [PubMed] [Google Scholar]
- Paylor R., Crawley J.N. Inbreed strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plappert C.F., Pilz P.K.D. Difference in anxiety and sensitization of acoustic startle response between the two inbred mouse strain BALB/cAN and DBA/2N. Gene Brain Behav. 2002;1:178–186. doi: 10.1034/j.1601-183x.2002.10306.x. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A. Sleep deprivation during late pregnancy produces hyperactivity and increased risk taking behavior in offspring. Brain Res. 2015;30(1596):88–98. doi: 10.1016/j.brainres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Ribeiro R.L., De Lima T.C.M. Participation of GABAA receptors in the modulations of experimental anxiety by tachykinin agonists and antagonists in mice. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2002;26(5):861–869. doi: 10.1016/s0278-5846(01)00331-1. [DOI] [PubMed] [Google Scholar]
- Ridder S. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J. Neurosci. 2005;25(26):6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers R.J. Animal models of anxiety: an ethological perspective. Braz. J. Med. Biol. Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Russo S.J. Neurobiology of resilience. Nature Neuroscience Reviews. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Koch M., Schnitzler H.U. Conditioned pleasure attenuates the startle response in rats. Neurobiol. Learn. Mem. 1995;64:1–3. doi: 10.1006/nlme.1995.1037. [DOI] [PubMed] [Google Scholar]
- Sclafani A., Clyne A.E. Hedonic response of rats to polysaccharide and sugar solutions. Neurosci. Biobehav. Rev. 1987;11(2):173–180. doi: 10.1016/s0149-7634(87)80023-4. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper S.D. An inflammation-centric view of neurological disease: beyond the neuron. Front. Cell. Neurosci. 2018;12(72) doi: 10.3389/fncel.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S.F., Sapolsky R.M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikov S. Blunted HPA axis reactivity reveals glucocorticoid system dysbalance in a mouse model of high anxiety-related behavior. Psychoneuroendocrinology. 2014;48:41–51. doi: 10.1016/j.psyneuen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Stone D.J. Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology. 1998;139(7):3202–3209. doi: 10.1210/endo.139.7.6084. [DOI] [PubMed] [Google Scholar]
- Szabo I. Analysis of the muscular action potentials accompanying the acoustic startle reaction. Acta Physica Academiae Scientarium Hungarica. 1965;27:167–178. [Google Scholar]
- Tadaiesky M.T. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson's disease. Neuroscience. 2008;156(4):830–840. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Tarantino L.M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strain. Mamm. Genome. 2000;11:555–564. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- Texeira R.M., Duarte F.S., De Lima T.C.M. Behavioral and immunological effects of substance P in female and male mice. Pharmacol. Biochem. Behav. 2004;79(1):1–9. doi: 10.1016/j.pbb.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Tronche F. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Unemura K. Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J. Pharmacol. Sci. 2012;119:30–39. doi: 10.1254/jphs.12047fp. [DOI] [PubMed] [Google Scholar]
- Viana M.C., Andrade L.H. Lifetime prevalence, age and gender distribution and age-of-onset of psychiatric disorders in the São Paulo metropolitan area, Brazil: results from the São Paulo megacity mental health survey. Rev. Bras. Psiquiatr. 2012;34:249–260. doi: 10.1016/j.rbp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol. Psychiatr. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Wei Q. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101(32):11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willot J.F. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav. Neurosci. 2003;117(4):716–727. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Xiao-Lei A.N. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exp. Anim. 2011;60(2):111–123. doi: 10.1538/expanim.60.111. [DOI] [PubMed] [Google Scholar]
- Yalsin I., Belzung C., Surget A. Mouse strain differences in unpredictable chronic mild stress: a four-antidepressant survey. Behavioral Brain Research. 2008;193:140–143. doi: 10.1016/j.bbr.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Zhe D., Fang H., Yuxiu S. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochemestry and Cytochemestry. 2008;41(4):89–95. doi: 10.1267/ahc.08013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport. 2014;25:1151–1155. doi: 10.1097/WNR.0000000000000243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.