Abstract
Exercise has been shown to reduce the risk of developing Mild Cognitive Impairment and Alzheimer's disease as well as to improve cognition in healthy and cognitively impaired individuals. However, the mechanisms of these benefits are not well understood. The stress hypothesis suggests that the cognitive benefits attributed to exercise may partially be mediated by changes in the cortisol secretion pattern. Chronic stress may increase the risk of AD and exacerbate the cognitive deficits and brain pathology characteristic of the condition while physical activity has been shown to attenuate most of stress consequences and risk factors for AD. Initially, research on the effects of cortisol on cognition and physical activity focused on cortisol levels at one time point but the circadian pattern of cortisol secretion is complex and it is still unclear which aspects are most closely associated with cognitive function. Thus, the aim of this review was to analyze the exercise/stress/cognition hypothesis focusing on the effects of the diurnal cycle of cortisol on cognitive function and physical activity in older adults with and without cognitive impairment.
Keywords: Alzheimer's disease, Exercise, Physical activity, Cognition, Cortisol
1. Introduction
Mild Cognitive Impairment (MCI) represents a higher level of cognitive deterioration than would be expected by aging but yet not meeting the criteria for a diagnosis of dementia (Petersen, 2009) and represents a 10–18% annual risk of converting to probable Alzheimer's Disease (AD) (Petersen, 2009; Risacher et al., 2009). There are several types of MCI, with the amnestic type (aMCI), in which memory loss is the predominant symptom, being more likely to progress to AD (Yaffe et al., 2006).
There are several risk factors for developing AD in addition to genetic factors (Corder et al., 1993). Prevalence increases with age (Querfurth and LaFerla, 2010), cardiovascular conditions (Helzner et al., 2009; Maher and Schubert, 2009; Strachan et al., 2008), oxidative stress (Querfurth and LaFerla, 2010), inflammatory markers (Parachikova et al., 2007), and chronic stress (Rothman and Mattson, 2010). An understanding of these risk factors for the development of counter-strategies to reduce deterioration from MCI to full-blown AD remains a key priority for improving individual and public health outcomes.
Observed links between chronic stress and deterioration of cognitive function provide a focus for investigation as the biological mechanisms linking stress and cognition are increasingly understood (de Quervain et al., 2009; Dedovic et al., 2009; Lupien et al., 2007). The hypothalamic-pituitary-adrenal (HPA) axis is the major endocrine stress axis in humans. Cortisol, released in response to psychosocial and physical threat, affects metabolic, cardiovascular and central nervous systems both acutely and chronically. Basal cortisol secretion is regulated by the hypothalamic suprachiasmatic nucleus (SCN) in response to time of day and external zeitgebers such as light (Buijs and Kalsbeek, 2001). The fine-balanced regulation of stress-induced as well as basal cortisol secretion is essential for the maintenance of homeostasis and health and dysregulation is implicated in many of the risk factors for progression to AD (McEwen, 2000; Tortosa-Martínez and Clow, 2012a; Tsigos and Chrousos, 2002). In addition to exacerbation of these physical risk factors there is evidence of direct pathways linking dysregulation of cortisol secretion and cognitive impairment, which is reviewed here alongside evidence that participation in physical activity attenuates the negative impact of chronic stress and even normalizes cortisol dysregulation, accompanied by improvements in cognition.
Disruption of biological rhythms, including dysregulation of the circadian pattern of cortisol secretion, is associated with poor mental health and is suggested to causative acting via aberrant signaling to peripheral clock genes in the brain (Menet and Rosbash, 2011).
Physical and psychological stressors stimulate activity of the HPA axis which, over a prolonged period of time, can lead to changes in the circadian pattern of cortisol secretion, including reduced dynamic change over time and increased or decreased circulating basal levels. Excessive levels of cortisol over time produce an allostatic overload (McEwen, 2008) with severe health consequences including an increase in cardiovascular conditions (Björntorp, 1997; Nader et al., 2010; Rosmond et al., 1998), inflammation (McEwen, 2008), and oxidative stress (Pajović et al., 2006; Zafir and Banu, 2009); while decreasing levels of Brain Derived Neurotrophic factors (BDNF) (Duman and Monteggia, 2006), hippocampal volumes (Huang et al., 2009; Lupien et al., 1998) and cognitive function (Lucassen et al., 2014; Seeman et al., 1997), all considered risk factors for developing AD.
Thus, chronic stress may increase the risk of AD and exacerbate the cognitive deficits and brain pathology characteristic of the condition (Rothman and Mattson, 2010), including an increased formation of the two main hallmarks of AD, Amyloid β plaques and protein Tau “tangles” (Dong et al., 2008; Green et al., 2006; Jeong, 2006; Lee et al., 2009). Indeed, there is evidence showing that people exposed to chronic stress are 2.7 times more likely to develop AD and to experience more rapid disease progression (Wilson et al., 2006).
In parallel, regular physical activity has been shown to reduce the risk of developing MCI, dementia, and AD (Geda et al., 2010; Hamer and Chida, 2009). Exercise may also improve different cognitive domains, such as memory and executive function, in older adults with MCI (Cammisuli et al., 2017), dementia and AD (Groot et al., 2016). However, the mechanisms mediating these benefits remain unclear. One of the most recent theories, the stress hypothesis, suggests that the cognitive benefits associated with exercise could be mediated by changes in cortisol secretion (Tortosa-Martínez and Clow, 2012b).
Exercise has been shown to attenuate most of the consequences of chronic stress including AD risk factors, with evidence showing positive benefits of exercise for reducing cardiovascular conditions (Mora et al., 2007; Mueller, 2007), inflammation (Cotman et al., 2007), and oxidative stress (Radak et al., 2008); while increasing levels of Brain Derived Neurotrophic Factors (BDNF) (Kramer and Erickson, 2007) and hippocampal volume (Erickson et al., 2009, 2011).
Higher levels of physical activity and fitness seem to improve the ability for coping with stress (Puterman et al., 2011; Rimmele et al., 2007, 2009; Salmon, 2001) but there are some inconsistencies in the literature (Jackson and Dishman, 2006), and there is very limited evidence regarding the effects of exercise on stress in cognitively impaired populations.
Initially, research on the effects of cortisol on cognition and physical activity focused on cortisol levels at one time point (mostly morning levels). However, the circadian pattern of cortisol secretion is complex and it is still unclear which aspects are most closely associated with cognitive function: overall basal levels, indices of dynamic change or both. The cortisol secretory pattern in healthy participants follows a typical circadian rhythm across the day, sharply increasing within 30–45 min after awakening (the cortisol awakening response: CAR), and steadily declining during the remainder of the day, called the diurnal decline (DD) (Edwards et al., 2001).
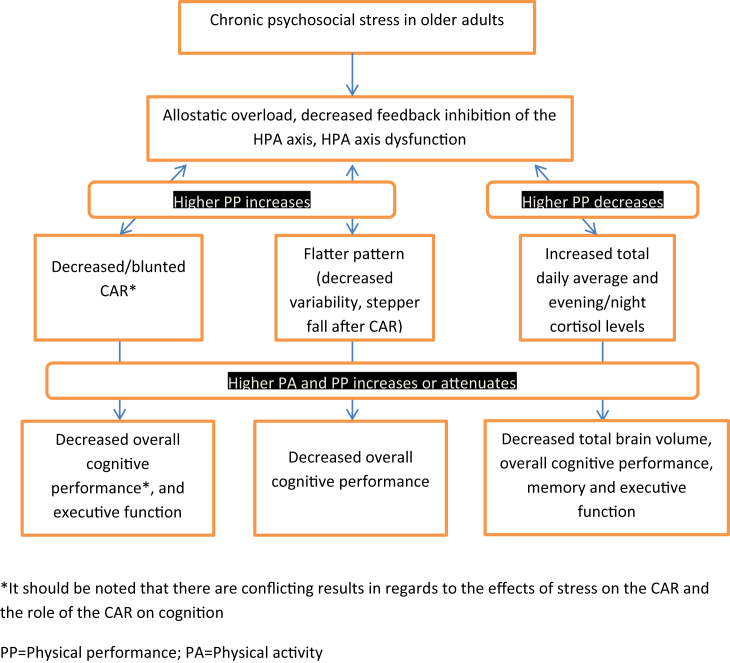
Thus, in this narrative review we will analyze the exercise/stress/cognition hypothesis focusing on the effects of the diurnal cycle of cortisol on cognitive function and physical activity in older adults with and without cognitive impairment (see Fig. 1).
Fig. 1.
Consequences of chronic stress on cognition in older adults and the possible mediating role of physical activity.
2. The diurnal cycle of cortisol and cognitive function
The evidence linking diurnal cortisol secretion levels with cognition in older adults remains mainly at a cross-sectional level (Table 1).
Table 1.
Cross-sectional studies analyzing the relationship between the diurnal cycle of cortisol and cognition in older adults.
| Authors | Sample | Cortisol measurement | Cognitive measurement | Results |
|---|---|---|---|---|
| Almela et al., 2012 | N = 88 (Men: 63.41 ± 4.91; Women: 63.73 ± 3.90) | Waking, 30′, 45′ and 60′ after waking (two consecutive days) | Logical Memory and Auditory Verbal Learning Test, Spatial Span and Spatial Working Memory | ↑ CAR ↓ declarative memory in men and women ↑ CAR ↑ working memory performance only in men |
| Beluche et al., 2010 | N = Men 111 (72.9 ± 4.4); Women 86 (72.8 ± 4.7) | Before cognitive testing (8h40 ± 20′), 15h40 ± 40′, and 21h40 ± 35’. (replicating the times in a quiet day) | Isaacs Set Test; Benton's visual retention test; Trail Making Test B, MMSE | At baseline and in longitudinal analysis, ↑ morning cortisol and a flatter slope were associated with ↓ cognitive performance. |
| Ennis et al., 2016 | N = 56 M = 53.04, SD = 16.94) young (N = 17), middle-aged (N = 21), and older adults (N = 18) | Waking, 30′ later, and then approximately every three hours until 9:17 p.m. on average during 10 consecutive days | Episodic memory (yes-no assocaitive recognition task); Working memory (3-back and Reading Span); Processing speed (Letter Comparison and Pattern Comparison) | A more positive CAR slope was related to better episodic memory regardless of age ↑ waking cortisol ↑ working memory |
| Evans et al., 2011 | N = 50 (73.9 ± 6.9) | Waking, 15′, 30′ and 45’; 3hr, 6hr,9hr, 12hr after waking (two consecutive days) | National Adult Reading Test, the Hopkins Verbal Learning Test (HVLT), Verbal and Semantic Fluency Tests, and Trail-Making Test A and B | ↑ CAR and steeper fall thereafter were assocaciated with ↑ overall cognitive performance |
| Evans et al., 2012 | N = 50 (73.9 ± 6.9) | Waking, 15′, 30′ and 45’ (two consecutive days) | Hopkins Verbal Learning Test (HVLT), and Trail-Making Test A and B | Earlier peak and ↑ CAR were associated with ↑ executive function |
| Geerlings et al., 2015 | N = 4244 (76 ± 5) | The evening before the clinic visit, prior to going to sleep and in the morning 45′ after waking | (1) memory composite score ( modified version of the California Verbal Learning Test); (2) processing speed composite score, (Stroop 1 and 2, the DSST, and the Figure Comparison Test); (3) executive function composite score, (Cambridge Neuropsychological Test Automated Battery Spatial Working Memory test (short version), the Digits Backward test, and Stroop 3) |
↑ evening cortisol was associated with ↓ total brain volume and cognitive performance ↑ levels of morning cortisol were associated with slightly ↑ normal white matter volume and ↑ processing speed and executive functioning, but not with gray matter volume or with memory performance. |
| Hidalgo et al., 2016 | N = 64 (32 men, 64.4 ± 4.2; 32 women (on two consecutive days) | Waking; 30′ and 45′ post-wake; 12am, 4pm, 8pm, and bedtime (two consecutive days) | Logical Memory and Verbal Paired Associates tests Family Pictures test Letter Number Sequencing (LNS) and Digit Span (DS), Spatial Span (SS) | ↑ CAR ↓ verbal (significantly) and visual (marginally) memory performance. No assocaitions with working memory. |
| Kovach et al., 2011 | N = 111 (87 ± 7) older adults with dementia | 30′ after waking, 45′ after breakfast, and 45′ minutes before and after dinner | MMSE and the Cumulative Illness Rating Scale -Geriatric (CIRS-G) | Flatter pattern was associated with ↓ MMSE ↑ Afternoon increase ↑ Illnes burden However, these trends did not reach statistical significance. |
| Lee et al., 2007 | 642 women 325 men (61.1 ± 6.0) | Before, during, and after cognitive testing as well as at visit completion | Language, processing speed, eye-hand coordination, executive functioning, verbal memory and learning, visual memory, and visuoconstruction | ↑ levels of mean cortisol and AUC were associated with ↓ in all cognitive domains but visoconstruction |
| Ouanes et al., 2017 | N = 643 (71.5 ± 4.5) | Waking, 30′ after waking, at 11 a.m. and at 8 p.m. | MMSE; Grober and Buschke Double Memory Test (DMT); DO40 picture naming test, the letter (phonemic) and the category (semantic) fluency tasks; Stroop Test; Consortium to Establish a Registry for Alzheimer's Disease (CERAD); Clinical Dementia Rating (CDR) scale |
↑ AUC ↑ CDR (indicating worse cogitive performance) ↑ AUC ↓ MMSE |
| Peavy et al., 2009 | N = 61healthy older adults (78.5 ± 6.4) 41 with MCI (79.2 ± 5.2). 52 were followed longitudinally (78.6 ± 5.4) | Waking; 30′ post-wake; 2pm, 4pm, and bedtime | Mattis Dementia Rating Scale Immediate and delayed recall from Wechsler Memory Scale—Revised List learning and retention from the California Verbal Learning Test Verbal and visual material from the Dementia Rating Scale memory subscale. |
↑ cortisol levels were associated with ↓ cognitive decline only in subjects with MCI |
| Wolff et al., 2002 | N = 14 young (27.0 ± 2.1); 28 healthy older adults (68.6 ± 1.2); 16 with MCI (70.9 ± 2.0) | 9 am, 11 am, 2 pm, 4 pm, 9 pm, and 11 pm | GDS, MMSE, Wechsler Memory Scale Revised (paired associates and digit span) and the Guild Memory test (paragraph recall) | ↑ average cortisol levels were associated with ↓ imediate paragraph recall only for people with MCI |
Abnormal cortisol secretory patterns include both overall basal levels such as high morning (Beluche et al., 2010; Geerlings et al., 2015) and evening levels (Geerlings et al., 2015); high daily average (Lee et al., 2007) and Area Under the Curve (AUC) (Lee et al., 2007; Ouanes et al., 2017); and flattening of the cortisol diurnal pattern (Beluche et al., 2010; Evans et al., 2011; Kovach et al., 2011) have been associated with worse cognitive performance in healthy older adults.
Although the role of the CAR in cognitive performance remains controversial emerging evidence from carefully controlled studies suggests that larger CARs are associated with better executive function, both between and within individuals. Some studies suggest that a higher CAR indicates better overall cognition (Evans et al., 2011) or executive function (Evans et al., 2012) in healthy older adults. This in line with the idea that the CAR is an adaptive response, increasing in anticipation of daily activities at awakening (Adam et al., 2006; Stalder et al., 2010). The magnitude of the CAR may decrease when impaired prospective recall precludes this anticipatory response and it seems to predict progression from healthy aging to MCI, although not to dementia (Peavy et al., 2012). Similarly, Oosterholt et al. (2015) showed that in clinical burnout an attenuated CAR was associated with poorer performance on the updating component of the ‘3-back’ task, and an increased frequency of cognitive failures although a similar relationship was not shown in healthy adults (Ennis et al., 2016). However, in a 50 day case-study of a healthy young male, days with a greater CAR predicted better same morning executive function (Law et al., 2015). This finding is again consistent with the marked state variation in the CAR and the proposal that the CAR prepares one for the day ahead (Law et al., 2013). A similar state relationship between same day larger CARs and plasticity of the motor cortex has also been demonstrated (Clow et al., 2014).
The CAR is reported to be related with some hippocampal functions such as long term memory and spatial orientation (Wolf et al., 2005) with no CAR observed in individuals with unilateral and bilateral hippocampal lesions (Buchanan et al., 2004), nor in those with severe global amnesia (Wolf et al., 2005). A larger CAR has also been associated with increased hippocampal volume in healthy young men (Pruessner et al., 2007). In an experimental manipulation, Rimmele et al. (2010) demonstrated that pharmacologic suppression of the CAR using metyrapone (a cortisol synthesis inhibitor) impaired memory for prior-day learnt text and imagery in a free recall task. In a sample of elderly participants without dementia, Geerlings et al. (2015) linked higher levels of cortisol at 45-min post-awakening with better processing speed and executive functioning, and slightly increased white matter. The results implicate a possible role of the CAR due to the timing of the cortisol assessment.
However, other studies have found an inverse correlation between the CAR and declarative and verbal memory (Almela et al., 2012; Hidalgo et al., 2016). Interestingly enough (Almela et al., 2012), found that the CAR was positively associated with working memory but only in men. This research group suggested that the CAR might be related differently to cognitive domains dependent on the hippocampus (i.e., declarative memory), than to those that are more dependent on prefrontal cortex functioning such as executive functioning (i.e., working memory). It is also possible that there is an inverted U-shaped relationship between the CAR and cognition (Moriarty et al., 2014), which might partially explain the discrepancies found in the literature.
There are only two studies examining the relationship between the diurnal cycle of cortisol and cognition in people with cognitive impairment. Wolf et al. (2002) found, with a sample of young, healthy older adults and people diagnosed with MCI, that a high average of cortisol levels indicated a worse performance in the paragraph recall, but only in those with MCI. This study did not include a CAR measurement and the first morning sample was collected at 9:00 without controlling for waking time. Also the cognitive assesment was performed within three months of the day of the salivary collection, which could bias the results in the case of people with MCI.
On the opposite side, Peavy et al. (2009) found that high average of cortisol levels were associated with a decrease rate of cognitive decline in subjects with MCI and not in healthy older adults. This is a surprising result, although it is possible that the method of measurement had an influence on the results. The researchers calculated the cortisol average based on five samples taken over the course of one day, including awakening and 30 min after awakening. High CAR levels could influence the total cortisol average leading to misintrepretation of the results.
In summary, although the exact relationship between cognitive function and the diurnal cycle of cortisol is not yet clarified, current evidence seems to indicate that a more dynamic cortisol secretion pattern, as opposed to a flatter profile, represents a healthier profile and a better adaption response to cognitive decline. A parallel could be established here with Heart Rate Variability (Evans et al., 2011), a dynamic autonomic measure closely related to stress, in which a higher variability is indicative of better cardiovascular health (Thayer et al., 2010) and better cognitive functioning (Thayer et al., 2009).
3. Exercise and the diurnal cycle of cortisol
Studies conducted with healthy older adults have shown a positive relationship between a more dynamic cortisol secretion pattern and physical performance but not physical activity levels (Table 2).
Table 2.
The influence of physical performance and physical activity levels on the diurnal cycle of cortisol in older adults.
| Authors | Sample | Cortisol measurement | Physical activity measurement | Results |
|---|---|---|---|---|
| Gardner et al., 2011 | N = 1143 (73.4 ± 4.17) | Phase 5. Waking, 30’ post-wake, 2pm, 10pm (on two consecutive days) | Get up and go test and Flamingo test | ↑ Night-time cortisol levels ↓ Walking speed ↓ Balance ↑ Diurnal drop ↑ Walking speed |
| Gardner et al., 2013a, b | 6 cohort studies: Boyd Orr; Caerphilly Prospective Study (CaPS); Hertfordshire Cohort Study (HCS); MRC National Survey of Health and Development (NSHD); Longitudinal Ageing Study Amsterdam (LASA); and the Whitehall II (WHII) study Ages: between 50 and 92 |
Different protocols including morning levels, CAR, and rest of the day | Walking speed; Chair rises; standing balance; grip strength | ↑ morning cortisol, ↑ diurnal drop, ↑ CAR, ↓ night time cortisol were associated with ↑ walking speed ↑ Diurnal drop ↑ Chair rises ↑ Night time cortisol ↓ balance No associations with grip strength |
| Heaney et al., 2014 | N = 36, 18 men, 18 women (70.7 ± 5.66) | Awakening; 30’ post-wakIN; 3hr, 6hr, 9hr, and 12hr after waking | Physical activity scale from the West of Scotland Twenty-07 Study | No differences in cortisol measurements but ↓ levels of corstisol:DHEA ratio for those who engaged in 1hr per week of physical activity but only in the high stress group |
| Kumari et al., 2010 | N = 2802 (60.9 ± 5.9) | Waking, 30’ post-waking; 2,5hr, 8hr, and 12hr after waking; and bedtime | Walking speed over 8 feet course | Those with a ↓ CAR, ↑ diurnal cortisol and flatter pattern showed ↓ walking speed |
| Lucertini et al., 2015 | N = 22 men 68.13 ± 1.28 | 30’ post-awake, 12.00, 15.00, 18.00, 21.00, 24.00 h | Rockport Walking test | ↓ basal cortisol, especially in the evening and midnight, and ↓ area-under-the-curve for total daily cortisol levels in High Fit individuals |
| Pulopulos et al., 2016 | N = 86 (64.92 ± 3.93) | Waking; 30’ post-awake; 45′ post-wake; |
Walking speed: 10 m at usual speed and as fast as possible | ↑ CAR ↑ Walking speed |
| Strahler et al., 2010 | N = 26 young adults (24.6 ± 2.0), 33 older adults (62.2 ± 6.7), 27 younger ballroom dancers (21.1 ± 4.4) and 31 older ballroom dancers (60.2 ± 6.8) |
Waking; 30’ post-wake; 11am, 3pm, 8pm | To be included, dancers had to dance at least twice per week and compete one a month | ↑basal salivary α-amylasa ↓ amount of physical activity and aging but no effects for cortisol measurements. |
| Sousa et al., 2017 | Kingston (N = 81; 69.0 ± 2.4) Saint-Hyacinthe (N = 81; 68.3 ± 2.5) Tirana (N = 57; 69.4 ± 3.4) Manizales (N = 90; 68.9 ± 2.7) | Waking, 30′ and 60’ post-waking; 3pm, and bedtime | Short Physical Performance Battery | ↓ cortisol peak (30’ post-waking), ↑ cortisol bedtime, and ↓ ratio between these two indices indicated ↓ physical performance |
Physical performance was measured by different tests such as the six minute walk test and the Rockport test to assess cardiovascular performance, chair rises for lower body strength, grip strength, the timed get up and go test for dynamic balance and agility, the flamingo test for balance, and walking speed tests for assessing gait. One study (Sousa et al., 2017) used the Short Performance Battery (Guralnik et al., 1994), which combines the results of gait speed, chair stand and balance tests. Better performance in these tests are indicative of better overall health and physical functioning.
Higher morning, evening and night-time cortisol levels have been associated with lower walking speed and balance (Gardner et al., 2011; Gardner et al., 2013a; b), as well as worse cardiovascular (Lucertini et al., 2015) and overall physical performance (Sousa et al., 2017). Similarly, higher diurnal levels and AUC have been associated with lower walking speed and worse cardiovascular performance (Kumari et al., 2010; Lucertini et al., 2015). A lower diurnal cortisol drop and a flatter pattern, indicate lower walking speed (Gardner et al., 2011; Gardner et al., 2013a; b; Kumari et al., 2010) and higher lower body strength (Gardner et al., 2013a; b). In the three studies analyzing the relationship between the CAR and physical performance, a higher magnitude of the CAR indicated faster walking speed (Gardner et al., 2013a; b; Kumari et al., 2010; Pulopulos et al., 2016).
High levels of physical activity and physical performance have been shown to produce positive effects on brain plasticity and regional gray matter volume, especially in the hippocampus and the prefrontal areas (Erickson et al., 2013). This is of relevance not only because these areas are responsible for relevant cognitive functions, such as memory and executive function, but also because they are associated with the control of the cortisol feedback inhibition mechanism (McEwen and Morrison, 2013). Thus, it is plausible that exercise yields converging benefits in cognition and the cortisol secretion pattern (Lucertini et al., 2015). However, most evidence is based on cross-sectional studies and with healthy older adults. There are very few studies analysing the effects of exercise on the diurnal cortisol secretion pattern and cognitive function with a cognitively impaired sample, and none of them includes participants with Alzheimer's disease. To the best of our knowledge, there are only three published studies in this regard, one cross-sectional (Dijckmans et al., 2017) and two exercise interventions (Baker and Frank, 2010; Tortosa-Martinez et al., 2015), which include a sample of older adults with aMCI.
4. The influence of exercise on cognition through changes in the diurnal cycle of cortisol
At a cross-sectional level, Dijckmans et al. (2017) showed that a greater variance in cortisol levels across the day from morning to evening was associated with better physical performance and overall cognition in a sample of healthy older adults (N = 30) and people with MCI (N = 30). No relationship was found between the CAR and cognition or physical performance. Walking speed and the Six Minute Walk Test (6MWT) were significantly correlated with both cognition and cortisol levels, indicating a link between the three variables, although this relationship did not reach statistical significance.
Baker and Frank (2010) showed that a three-month high intensity aerobic exercise program improved executive function in older adults with amnestic MCI, which was more pronounced for women than for men. Furthermore, within the exercise group, cortisol levels decreased in women while increased in men. However, this study used a one time-point cortisol measure (ranging from 8:00 to 10:00), without controlling for time of awakening. Considering the circadian pattern of cortisol, this might not be the most accurate measure.
Tortosa-Martinez et al. (2015) analyzed the effects of a three-month moderate intensity aerobic exercise program in older adults with amnestic MCI on cognition and the diurnal cycle of cortisol secretion. In this study, at baseline, better physical performance, as measured with the 6MWT and the Timed get up and go test (TGUG) was associated with a higher peak of cortisol at 30 min and a subsequent drop from this peak at noon. Also better performance in the 6MWT was associated with lower cortisol values at 21:00. No correlations were found with the AUC or the CAR. These data would again indicate that more dynamic cortisol secretion pattern would be indicative of better physical performance.
After the intervention, there was a significant increase in the drop between the peak of cortisol at 30 min after awakening and the subsequent drop from this peak to noon in the exercise group. The exercise group also showed a tendency for an increased peak of cortisol at 30 min (p = 0.068) and of the magnitude of the CAR (p = 0.069). Thus, almost the same aspects of the diurnal cortisol profile found to be related to fitness at baseline were improved with aerobic exercise. An unexpected result was that total cortisol values as measured by the total mean or the AUC were not significantly changed with exercise.
At the same time, the exercise group improved executive function performance, while memory remained unchanged. Other studies have found positive changes in memory in people with MCI after six months of exercise training (Lautenschlager et al., 2008; Nagamatsu et al., 2013). Thus, it is possible that longer periods of training could result on memory improvements and different changes in the cortisol secretion pattern, such as total lower levels of diurnal cortisol.
5. Conclusion, limitations and future research
The available evidence supports the stress hypothesis, implicating that the cognitive benefits associated with exercise could be at least partially mediated by changes in the diurnal cortisol secretion pattern to a more dynamic profile. These benefits are probably mediated by the effects of exercise on the hippocampus and the prefrontal cortex, both implicated on cognition and the regulation of the diurnal pattern of cortisol. However, most studies included healthy older adults and have analyzed this phenomenon partially, focusing either on the effects of physical activity on cortisol or the effects of the diurnal cycle of cortisol on cognition, but not all variables at the same time. Furthermore, there are limitations on methodological factors for assessing cortisol patterns, and especially the CAR, that should be noted. For example, obtaining samples from at least two consecutive days and if possible six days is highly recommended. Similarly, objective control of the sampling time accuracy and adherence as well as adequate participant instructions and sampling protocols are also relevant. Also a minimum of three sampling points are needed for assessing the CAR: on awakening, 30 min and 45 min (Stalder et al., 2015). Many of the studies analyzed in this review fail to meet this guidelines.
Thus, more research is needed to test the stress hypothesis including randomized controlled trials analyzing the effects of exercise on the diurnal cortisol secretion pattern and cognition, with cognitively impaired participants (aMCI and AD) and using carefully controlled protocols of cortisol sampling. It would also be of high interest adding measures of the effect of exercise on the hippocampus and the prefrontal cortex.
Acknowledgements
We thank Dr. Angela Clow for her valuable contribution to this work.
Contributor Information
J. Tortosa-Martínez, Email: juan.tortosa@ua.es.
C. Manchado, Email: Carmen.manchado@ua.es.
J.M. Cortell-Tormo, Email: jm.cortell@ua.es.
I. Chulvi-Medrano, Email: ivan.chulvi@ua.es.
References
- Adam E.K., Hawkley L.C., Kudielka B.M., Cacioppo J.T. Day--to--day dynamics of experience-- - cortisol associations in a population- based sample of older adults. Proc Natl Acad Sci U S A. Nov. 2006;7 doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almela M., van der Meij L., Hidalgo V., Villada C., Salvador A. The cortisol awakening response and memory performance in older men and women. Psychoneuroendocrinology. 2012;37:1929–1940. doi: 10.1016/j.psyneuen.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Baker L.D.L., Frank L. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307.Effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beluche I., Carriere I., Ritchie K., Ancelin M.L. A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychol. Med. 2010;40:1039–1049. doi: 10.1017/S0033291709991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björntorp P. Obesity. Lancet. 1997 doi: 10.1016/S0140-6736(97)04503-0. [DOI] [Google Scholar]
- Buchanan T.W., Kern S., Allen J.S., Tranel D., Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biol. Psychiatr. 2004;56:651–656. doi: 10.1016/j.biopsych.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Buijs R.M., Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat. Rev. Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Cammisuli D.M., Innocenti A., Franzoni F., Pruneti C. Aerobic exercise effects upon cognition in Mild Cognitive Impairment: a systematic review of randomized controlled trials. Arch. Ital. Biol. 2017;155:54–62. doi: 10.12871/000398292017126. [DOI] [PubMed] [Google Scholar]
- Clow A., Law R., Evans P., Vallence A.-M., Hodyl N. a, Goldsworthy M.R., Rothwell J.R., Ridding M.C. Day differences in the cortisol awakening response predict day differences in synaptic plasticity in the brain. Stress. 2014;17:219–223. doi: 10.3109/10253890.2014.905533. [DOI] [PubMed] [Google Scholar]
- Corder E., Saunders A., Strittmatter W., Schmechel D., Gaskell P., Small G., Roses A., Haines J., Pericak-Vance M. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. 80-. [DOI] [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007 doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- de Quervain D.J.F., Aerni A., Schelling G., Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front. Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Duchesne A., Andrews J., Engert V., Pruessner J.C. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dijckmans B., Tortosa-Martínez J., Caus N., González-Caballero G., Martínez-Pelegrin B., Manchado-Lopez C., Cortell-Tormo J.M., Chulvi-Medrano I., Clow A. Does the diurnal cycle of cortisol explain the relationship between physical performance and cognitive function in older adults? Eur. Rev. Aging Phys. Act. 2017;14 doi: 10.1186/s11556-017-0175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Yuede C.M., Yoo H.-S., Martin M.V., Deal C., Mace A.G., Csernansky J.G. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatr. 2006 doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Edwards S., Evans P., Hucklebridge F., Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/S0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Ennis G.E., Moffat S.D., Hertzog C. The cortisol awakening response and cognition across the adult lifespan. Brain Cognit. 2016;105:66–77. doi: 10.1016/j.bandc.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Gildengers A.G., Butters M.A. Physical activity and brain plasticity in late adulthood. Dialogues Clin. Neurosci. 2013;15:99–108. doi: 10.4081/ar.2012.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Hu L., Morris K.S., White S.M., Wójcicki T.R., McAuley E., Kramer A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., Wojcicki T.R., Mailey E., Vieira V.J., Martin S.A., Pence B.D., Woods J.A., McAuley E., Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P., Hucklebridge F., Loveday C., Clow A. The cortisol awakening response is related to executive function in older age. Int. J. Psychophysiol. 2012;84:201–204. doi: 10.1016/j.ijpsycho.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Evans P.D., Fredhoi C., Loveday C., Hucklebridge F., Aitchison E., Forte D., Clow A. The diurnal cortisol cycle and cognitive performance in the healthy old. Int. J. Psychophysiol. 2011;79:371–377. doi: 10.1016/j.ijpsycho.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Gardner M.P., Lightman S., Sayer A.A., Cooper C., Cooper R., Deeg D., Ebrahim S., Gallacher J., Kivimaki M., Kumari M., Kuh D., Martin R.M., Peeters G., Ben-Shlomo Y. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: an individual participant meta-analysis. Psychoneuroendocrinology. 2013;38:40–49. doi: 10.1016/j.psyneuen.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M.P., Lightman S., Sayer A.A., Cooper C., Cooper R., Deeg D., Ebrahim S., Gallacher J., Kivimaki M., Kumari M., Kuh D., Martin R.M., Peeters G., Ben-Shlomo Y., Halcyon Study Team Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: an individual participant meta-analysis. Psychoneuroendocrinology. 2013;38:40–49. doi: 10.1016/j.psyneuen.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M.P., Lightman S.L., Gallacher J., Hardy R., Kuh D., Ebrahim S., Bayer A., Ben-shlomo Y. Diurnal cortisol patterns are associated with physical performance in the caerphilly prospective study. Int. J. Epidemiol. 2011;40:1693–1702. doi: 10.1093/ije/dyr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda Y.E., Roberts R.O., Knopman D.S., Christianson T.J., Pankratz V.S., Ivnik R.J., Boeve B.F., Tangalos E.G., Petersen R.C., Rocca W.A. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch. Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings M.I., Garcia M.E., Harris T.B. 2015. Salivary Cortisol, Brain Volumes, and Cognition in Community-dwelling Elderly without Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.N., Billings L.M., Roozendaal B., McGaugh J.L., LaFerla F.M. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J. Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot C., Hooghiemstra A., Raijmakers P., Van Berckel B., Scheltens P., Scherder E., Van der Flier W., Ossenkoppele R. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res. Rev. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Guralnik J.M., Simonsick E.M., Ferrucci L., Glynn R.J., Berkman L.F., Blazer D.G., Scherr P.A., Wallace R.B. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994 doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Hamer M., Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol. Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- Heaney J.L., Carroll D., Phillips A.C. Physical activity, life events stress, cortisol, and DHEA in older adults: preliminary findings that physical activity may buffer against the negative effects of stress. J. Aging Phys. Act. 2014;22:465–473. doi: 10.1123/japa.2012-0082. [DOI] [PubMed] [Google Scholar]
- Helzner E.P., Luchsinger J.A., Scarmeas N., Cosentino S., Brickman A.M., Glymour M.M., Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch. Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo V., Almela M., Pulopulos M.M., Salvador A. Memory performance is related to the cortisol awakening response in older people, but not to the diurnal cortisol slope. Psychoneuroendocrinology. 2016;71:136–146. doi: 10.1016/j.psyneuen.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Huang C.-W., Lui C.-C., Chang W.-N., Lu C.-H., Wang Y.-L., Chang C.-C. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. J. Clin. Neurosci. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Jackson E.M., Dishman R.K. Cardiorespiratory fitness and laboratory stress: a meta-regression analysis. Psychophysiology. 2006 doi: 10.1111/j.1469-8986.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- Jeong Y.H. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer's disease model. Faseb. J. 2006 doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- Kovach C.R., Woods D.L., Logan B.R., Raff H. Diurnal variation of cortisol in people with dementia: relationship to cognition and illness burden. Am. J. Alzheimers. Dis. Other Demen. 2011;26:145–150. doi: 10.1177/1533317510397329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A.F., Erickson K.I. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cognit. Sci. 2007 doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kumari M., Badrick E., Sacker A., Kirschbaum C., Marmot M., Chandola T. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology. 2010;35:1091–1099. doi: 10.1016/j.psyneuen.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Lautenschlager N.T., Cox K.L., Flicker L., Foster J.K., van Bockxmeer F.M., Xiao J., Greenop K.R., Almeida O.P., Bockxmeer F.M. Van, Xiao J., Greenop K.R., Almeida O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. J. Am. Med. Assoc. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Law R., Evans P., Thorn L., Hucklebridge F., Clow A. The cortisol awakening response predicts same morning executive function: results from a 50-day case study. Stress. 2015;18:616–621. doi: 10.3109/10253890.2015.1076789. [DOI] [PubMed] [Google Scholar]
- Law R., Hucklebridge F., Thorn L., Evans P., Clow A. State variation in the cortisol awakening response. Stress. 2013 doi: 10.3109/10253890.2013.817552. [DOI] [PubMed] [Google Scholar]
- Lee B.K., Glass T. a, McAtee M.J., Wand G.S., Bandeen-Roche K., Bolla K.I., Schwartz B.S. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch. Gen. Psychiatr. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Kim J. Bin, Seo J.S., Kim T.K., Im J.Y., Baek I.S., Kim K.S., Lee J.K., Han P.L. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J. Neurochem. 2009;108:165–175. doi: 10.1111/j.1471-4159.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- Lucassen P.J., Pruessner J., Sousa N., Almeida O.F.X., Van Dam A.M., Rajkowska G., Swaab D.F., Czéh B. Neuropathology of stress. Acta Neuropathol. 2014 doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucertini F., Ponzio E., Di Palma M., Galati C., Federici A., Barbadoro P., D'Errico M.M., Prospero E., Ambrogini P., Cuppini R., Lattanzi D., Minelli A. High cardiorespiratory fitness is negatively associated with daily cortisol output in healthy aging men. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., de Leon M., de Santi S., Convit A., Tarshish C., Nair N.P., Thakur M., McEwen B.S., Hauger R.L., Meaney M.J. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., Maheu F., Tu M., Fiocco A., Schramek T.E. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cognit. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Maher P.A., Schubert D.R. Metabolic links between diabetes and Alzheimer's disease. Expert Rev. Neurother. 2009 doi: 10.1586/ern.09.18. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008 doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000 doi: 10.1016/S0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013 doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet J.S., Rosbash M. When brain clocks lose track of time: cause or consequence of neuropsychiatric disorders. Curr. Opin. Neurobiol. 2011 doi: 10.1016/j.conb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S., Cook N., Buring J.E., Ridker P.M., Lee I.M. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty A.S., Bradley A.J., Anderson K.N., Watson S., Gallagher P., McAllister-Williams R.H. Cortisol awakening response and spatial working memory in man: a U-shaped relationship. Hum. Psychopharmacol. 2014;29:295–298. doi: 10.1002/hup.2399. [DOI] [PubMed] [Google Scholar]
- Mueller P.J. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin. Exp. Pharmacol. Physiol. 2007 doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Nader N., Chrousos G.P., Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metabol. 2010 doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu L.S., Chan A., Davis J.C., Beattie B.L., Graf P., Voss M.W., Sharma D., Liu-Ambrose T. Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. J. Aging Res. 2013 doi: 10.1155/2013/861893. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterholt B.G., Maes J.H.R., Van der Linden D., Verbraak M.J.P.M., Kompier M.A.J. Burnout and cortisol: evidence for a lower cortisol awakening response in both clinical and non-clinical burnout. J. Psychosom. Res. 2015;78:445–451. doi: 10.1016/j.jpsychores.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Ouanes S., Castelao E., von Gunten A., Vidal P.M., Preisig M., Popp J. Personality, cortisol, and cognition in non-demented elderly subjects: results from a population-based study. Front. Aging Neurosci. 2017;9:1–9. doi: 10.3389/fnagi.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajović S.B., Pejić S., Stojiljković V., Gavrilović L., Dronjak S., Kanazir D.T. Alterations in hippocampal antioxidant enzyme activities and sympatho-adrenomedullary system of rats in response to different stress models. Physiol. Res. 2006;55:453–460. doi: 10.33549/physiolres.930807. [DOI] [PubMed] [Google Scholar]
- Parachikova A., Agadjanyan M.G., Cribbs D.H., Blurton-Jones M., Perreau V., Rogers J., Beach T.G., Cotman C.W. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol. Aging. 2007;28:1821–1833. doi: 10.1016/j.neurobiolaging.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy G.M., Jacobson M.W., Salmon D.P., Gamst A.C., Patterson T.L., Goldman S., Mills P.J., Khandrika S., Galasko D. The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Dis. Assoc. Disord. 2012;26:260–266. doi: 10.1097/WAD.0b013e3182389a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy G.M., Salmon D.P., Jacobson M.W., Hervey A., Gamst A.C., Wolfson T., Patterson T.L., Goldman S., Mills P.J., Khandrika S., Galasko D. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am. J. Psychiatr. 2009;166:1384–1391. doi: 10.1176/appi.ajp.2009.09040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.C. Early diagnosis of Alzheimer's disease: is MCI too late? Curr. Alzheimer Res. 2009;6:324–330. doi: 10.2174/156720509788929237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner M., Pruessner J.C., Hellhammer D.H., Bruce Pike G., Lupien S.J. The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatr. Res. 2007;155:1–10. doi: 10.1016/j.pscychresns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Pulopulos M.M., Puig-Perez S., Hidalgo V., Villada C., Salvador A. Cortisol awakening response and walking speed in older people. PLoS One. 2016;11:1–12. doi: 10.1371/journal.pone.0152071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E., O'Donovan A., Adler N.E., Tomiyama A.J., Kemeny M., Wolkowitz O.M., Epel E. Physical activity moderates effects of stressor-induced rumination on cortisol reactivity. Psychosom. Med. 2011;73:604–611. doi: 10.1097/PSY.0b013e318229e1e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth H.W., LaFerla F.M. Alzheimer's disease: mechanism of disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1016/B978-0-12-803699-0.00045-1. [DOI] [PubMed] [Google Scholar]
- Radak Z., Chung H.Y., Koltai E., Taylor A.W., Goto S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008 doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Meier F., Lange T., Born J. Suppressing the morning rise in cortisol impairs free recall. Learn. Mem. 2010;17:186–190. doi: 10.1101/lm.1728510. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Seiler R., Marti B., Wirtz P.H., Ehlert U., Heinrichs M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34:190–198. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Zellweger B.C., Marti B., Seiler R., Mohiyeddini C., Ehlert U., Heinrichs M. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology. 2007;32:627–635. doi: 10.1016/j.psyneuen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Risacher S., Saykin A., Wes J., Shen L., Firpi H., McDonald B. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr. Alzheimer Res. 2009;6:347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R., Dallman M.F., Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1998;83:1853–1859. doi: 10.1210/jc.83.6.1853. [DOI] [PubMed] [Google Scholar]
- Rothman S.M., Mattson M.P. Adverse stress, hippocampal networks, and Alzheimer's disease. NeuroMolecular Med. 2010 doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin. Psychol. Rev. 2001 doi: 10.1016/S0272-7358(99)00032-X. [DOI] [PubMed] [Google Scholar]
- Seeman T.E., Mcewen B.S., Singer B.H., Albert M.S., Rowe J.W. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J. Clin. Endocrinol. Metab. 1997;82:2458–2465. doi: 10.1210/jc.82.8.2458. [DOI] [PubMed] [Google Scholar]
- Sousa A.C.P. de A., Marchand A., Garcia A., Gomez J.F., Ylli A., Guralnik J.M., Zunzunegui M.V., Guerra R.O. Cortisol and physical performance in older populations: findings from the international mobility in aging study (IMIAS) Arch. Gerontol. Geriatr. 2017;71:50–58. doi: 10.1016/j.archger.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Stalder T., Evans P.D., Hucklebridge F., Clow A. Associations between psychosocial state variables and the cortisol awakening response in a single case study. Psychoneuroendocrinology. 2010;35:209–214. doi: 10.1016/j.psyneuen.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Stalder T., Kirschbaum C., Kudielka B.M., Adam E.K., Pruessner J.C., Wüst S., Dockray S., Smyth N., Evans P., Hellhammer D.H., Miller R., Wetherell M.A., Lupien S.J., Clow A. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2015;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Strachan M.W.J., Reynolds R.M., Frier B.M., Mitchell R.J., Price J.F. The relationship between type 2 diabetes and dementia. Br. Med. Bull. 2008;88:131–146. doi: 10.1093/bmb/ldn042. [DOI] [PubMed] [Google Scholar]
- Strahler J., Berndt C., Kirschbaum C., Rohleder N. Aging diurnal rhythms and chronic stress: distinct alteration of diurnal rhythmicity of salivary α-amylase and cortisol. Biol. Psychol. 2010;84:248–256. doi: 10.1016/j.biopsycho.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Hansen A.L., Saus-Rose E., Johnsen B.H. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009 doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Yamamoto S.S., Brosschot J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010 doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Tortosa-Martínez J., Clow a. Does physical activity reduce risk for Alzheimer's disease through interaction with the stress neuroendocrine system? Stress. 2012;15:243–261. doi: 10.3109/10253890.2011.629323. [DOI] [PubMed] [Google Scholar]
- Tortosa-Martínez J., Clow A. Does physical activity reduce risk for Alzheimer's disease through interaction with the stress neuroendocrine system? Stress. 2012;15:243–261. doi: 10.3109/10253890.2011.629323. [DOI] [PubMed] [Google Scholar]
- Tortosa-Martinez J., Clow A., Caus-Pertegaz N., Gonzalez-Caballero G., Abellan-Miralles I., Saenz M.J. Exercise increases the dynamics of diurnal cortisol secretion and executive function in people with amnestic mild cognitive impairment. J. Aging Phys. Activ. 2015;23:550–558. doi: 10.1123/japa.2014-0006. [DOI] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G.P. Journal of Psychosomatic Research. 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress; pp. 865–871. [DOI] [PubMed] [Google Scholar]
- Wilson R.S., Arnold S.E., Schneider J.A., Kelly J.F., Tang Y., Bennett D.A. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wolf O.T., Convit A., Thorn E., de Leon M.J. Salivary cortisol day profiles in elderly with mild cognitive impairment. Psychoneuroendocrinology. 2002;27:777–789. doi: 10.1016/s0306-4530(01)00079-8. [DOI] [PubMed] [Google Scholar]
- Wolf O.T., Fujiwara E., Luwinski G., Kirschbaum C., Markowitsch H.J. No morning cortisol response in patients with severe global amnesia. Psychoneuroendocrinology. 2005;30:101–105. doi: 10.1016/j.psyneuen.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Yaffe K., Petersen R.C., Lindquist K., Kramer J., Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement. Geriatr. Cogn. Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- Zafir A., Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12:167–177. doi: 10.1080/10253890802234168. [DOI] [PubMed] [Google Scholar]