Abstract
Depression is a highly prevalent psychiatric disorder, yet its etiology is not well understood. The validation of animal models is therefore a critical step towards advancing knowledge about the neurobiology of depression. Psychosocial stress has been promoted as a prospective animal model of depression, however, different protocols exist with variable responses, and further investigations are therefore required. We aimed to characterise the behavioural and body weight responses to the social defeat/overcrowding (SD/OC) model and to explore the effects of the antidepressant fluoxetine and the peroxynitrite scavenger, CuII(atsm), therein. Male C57BL/6JArc mice were exposed to a 19 day SD/OC protocol at two levels of aggression, determined by terminating SD bouts after one, or approximately five social defeat postures. This was followed by a battery of behavioural tests including social interaction test (SIT), locomotor activity (LMA), light-dark box test (LDB), saccharin preference test (SPT) and the forced swim test (FST). Mice were dosed daily with vehicle, fluoxetine (20 mg/kg) or CuII(atsm) (30 mg/kg) throughout the protocol. SD/OC increased body weight compared to controls, which was abolished by fluoxetine and attenuated by CuII(atsm). Weight gain specifically peaked during OC sessions but was not affected by either drug treatment. Fluoxetine reduced the number of defeat postures during fight bouts on some days. SD/OC otherwise failed to elicit depression- or anxiety-like behaviour in the tests measured. These data raise questions over the SD/OC model as an etiological model of depression-related behaviours but highlight the potential of this model for investigations into mechanisms regulating binge eating and weight gain under conditions of chronic social stress.
Keywords: Psychosocial stress, Social defeat, Depression, Weight gain, Fluoxetine, CuII(atsm)
1. Introduction
Depression is a debilitating and highly prevalent psychiatric disorder characterised primarily by a depressed mood, loss of interest or pleasure in everyday activities and significant changes in weight or appetite (American Psychiatric Association, 2013). Behind HIV/AIDS, depression is the second largest contributor to the global burden of disease and is predicted to maintain that position through to 2030 (Mathers and Loncar, 2006). Despite this, the etiology of depression remains to be adequately elucidated. Several reasons have contributed to this lack of understanding, including that depression is a complex, heterogeneous disorder with multiple factors contributing to its development (Berton et al., 2012; Krishnan and Nestler, 2008). Furthermore, due to the subjective nature of depression, aspects of its symptomatology have proven challenging to replicate in animal models of the disorder (Cryan and Holmes, 2005; Krishnan and Nestler, 2008; Nestler and Hyman, 2010). The improvement of animal models is therefore an essential step towards advancing the understanding of the neurobiology of depression and the development of effective treatments. For an animal model to be considered valid the symptoms induced must be reasonably analogous to those observed in humans (face validity), the treatments effective in humans must also be effective in the animal model (predictive validity) and the model and human disease should have identical causative factors (construct validity) (McKinney and Bunney, 1969; Slattery and Cryan, 2014). Several rodent models of depression have been developed over the past few decades including chronic mild stress (CMS) (Willner, 1997), olfactory bulbectomy (Harkin et al., 2003), maternal deprivation (Levine et al., 1991; Marco et al., 2009; Schmidt et al., 2011) and chronic restraint stress paradigms (Christiansen et al., 2011; Sadler and Bailey, 2016). However, many of these models either fail to reproduce some of the core symptoms of depression, do not satisfy the aforementioned requirements for a valid animal model, or suffer from poor cross-reliability between laboratories.
An emerging class of animal models of depression utilise psychosocial stress paradigms comprising repeated social defeats. Social defeat models involve placing an animal into the home cage of an aggressive resident, enabling physical defeat and subordination from the defeated animal. Such models have demonstrated central aspects of face, predictive and construct validity (Berton et al., 2006; Tsankova et al., 2006) whereby treatment with clinical antidepressants reversed behavioural and physiological effects induced by the model. Depending on the model, mice may be exposed to the resident aggressor for periods of up to between 10 min (for a detailed protocol see Golden et al., 2011) and 2 h (Savignac et al., 2011b). This increases the opportunity for fight wounds to develop (see Golden et al., 2011; and Savignac et al., 2011b) which potentially contribute pain and inflammation to the psychosocial stress (Pryce and Fuchs, 2017). The social defeat/overcrowding (SD/OC) model is an alternative protocol, where mice are separated by barriers following the initial defeat, therefore reducing the opportunity for injuries to occur, and are also intermittently subjected to unpredictable periods of overcrowding with other defeated mice (Finger et al., 2011, 2012; Reber et al., 2006; Tramullas et al., 2012). Studies utilising this model have demonstrated increased social avoidance in the social interaction test (SIT), increased immobility time in the forced swim test (FST), anxiety-like behaviour in the light-dark box test (LDB) as well as alterations in body weight, protein levels, gene expression and other physiological functions (Finger et al., 2011, 2012; Reber et al., 2006; Tramullas et al., 2012). However, results across laboratories have been varied, calling in to question the reproducibility of the model. Additionally, the predictive validity of this model has yet to be ascertained. Further characterisation of this model using a clinically effective antidepressant would be a crucial step towards its more widespread use in preclinical depression research.
To this end, we aimed to further characterise the SD/OC model in male C57BL/6JArc mice, including analyses of behaviour during SD sessions and a battery of behavioural tests for depression and/or anxiety-like behaviours at the conclusion of the SD/OC protocol. We also aimed to assess the effects of the clinically effective antidepressant fluoxetine, and the potential antidepressant properties of CuII(atsm), in the SD/OC model. CuII(atsm) is a member of the bis(thiosemicarbazone) (BTSC) class of compounds. BTSCs are stable, low molecular weight compounds capable of crossing cell membranes including the blood-brain barrier (BBB) (Fodero-Tavoletti et al., 2010). BTSCs show therapeutic potential in neurodegenerative diseases (Hung et al., 2012; Kenche and Barnham, 2011; Roberts et al., 2014) via mechanisms that include the scavenging of the highly reactive nitrogen free radical, peroxynitrite (reviewed in Mckenzie-Nickson et al., 2016). Recent evidence suggests that depression is associated with neuroinflammatory and oxidative/nitrosative stress mechanisms (Hannestad et al., 2011; Heneka et al., 2014; Hurley and Tizabi, 2013; Maes et al., 2009, 2011; Raedler, 2011). Targeting nitrosative stress has also previously been demonstrated to have antidepressant effects in animal models (Doucet et al., 2013; Harkin et al., 1999; Peng et al., 2012). We therefore sought to assess CuII(atsm) in the SD/OC model due to its anti-neuroinflammatory and anti-nitrosative stress properties.
2. Materials and methods
2.1. Animals
Two separate trials were conducted using male C57BL/6JArc mice (Trial 1: n = 48, 10 weeks of age; Trial 2: n = 40, 9 weeks of age). Mice of various strains (SJL (48% of total bouts fought), Swiss (23%), SJL/BL6 (15%), C57BL/6 (6%), CD1 (5%), Sv129 (3%)) and age (9–52 weeks of age) were used as resident aggressors in Trial 1. In Trial 2 aggressor mice used were almost exclusively of the SJL strain (92%; 17–24 weeks of age). All mice were purchased from Animal Resources Centre (C57BL/6JArc, SJL; Canning Vale, WA, Australia) or bred in-house at The Florey Institute of Neuroscience (Melbourne, Victoria, Australia). All mice involved in the study were single housed for at least one week prior to commencement of their respective trials and remained single housed for the duration of the experiment (except during the overcrowding procedures). Experimental mice were housed in standard open top cages (26.5 × 14 × 12 cm) and aggressor mice in standard transparent cages (29.5 × 16 × 13 cm), both with sawdust bedding and tissue paper nesting material. The holding room was temperature controlled (18.5 ± 1 °C) and under a 12 h light/dark cycle (lights on at 0700 h). Standard rodent food and water was available ad libitum. All experimentation was performed in accordance with the Prevention of Cruelty to Animals Act (2004), under the guidelines of the National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Experimental Purposes in Australia (2013) and approved by The Florey Animal Ethics Committee (AEC number: 15–020). All efforts were made to minimise animal suffering.
2.2. Social defeat/overcrowding (SD/OC) protocol
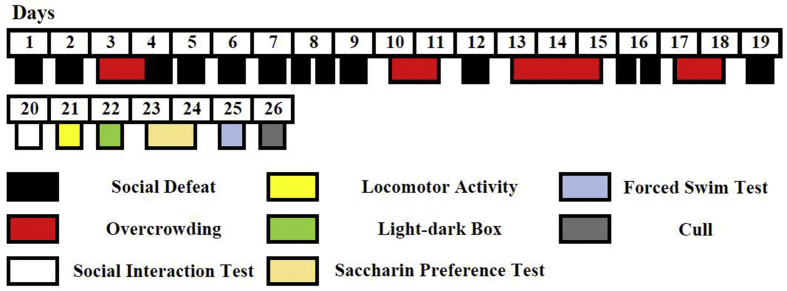
The SD/OC protocol was carried out as previously described (Finger et al., 2011, 2012; Reber et al., 2006). All aggressor mice were screened for aggressive behaviour on at least three individual days prior to the first day of experiments. The mice were exposed to a test C57BL/6 intruder until the first attack followed by defeat posture (Fig. 1) (Miczek et al., 1982), or for a maximum of 10 min. Mice displaying the shortest attack latencies were chosen as aggressors for the trials. Experimental C57BL/6JArc mice were randomly assigned to one of four groups: 1. No SD/OC and treated with vehicle (Control (Con)), 2. Exposed to SD/OC and treated with vehicle (Veh), 3. SD/OC with fluoxetine treatment (Fluox) or 4. SD/OC with CuII(atsm) treatment (Cu(atsm)) (Trial 1: n = 12; Trial 2: n = 10 per group). Control mice remained undisturbed except for daily oral gavaging. SD/OC mice were exposed to a 19 day unpredictable stress protocol consisting of social defeat (SD) and overcrowding (OC) sessions (Fig. 2). SD sessions were carried out once (on days 1, 2, 4–7, 9, 12 and 19) or twice (on days 8 and 16) per day. Experimental mice never encountered the same aggressor mouse more than once. Aggressors were ranked according to latency to attack and pseudorandomly assigned to ensure a balanced and consistent presentation of aggression. SD sessions consisted of placing the experimental mouse into the home cage of the aggressor and allowing interaction until defeat posture/s were displayed by the experimental mouse (Fig. 1). The mice were then separated by a wire mesh barrier for 2 h to allow visual, auditory and olfactory, but not physical contact. After 2 h the barrier was removed and another bout and defeat was allowed to take place. The thirteen SD sessions were thus comprised of two SD bouts each, resulting in a total of 26 SD bouts across the protocol. The number of defeat postures displayed was measured for each of these 26 SD bouts. In Trial 1, SD bouts were terminated following one defeat posture regardless of whether an attack occurred or not. In Trial 2, SD bouts were terminated following at least five defeat postures and definitive attack behaviour from the aggressor. If attacks were particularly ferocious and containing extensive biting behaviour by the aggressor, the SD bout was terminated early. All SD bouts were filmed (side-on, four cages at a time) using a standard video camera. Number and latency of defeat posture and latency to attack in Trial 2 were quantified from video recordings. For the overcrowding protocol all mice from one treatment group were housed together in a standard transparent cage for 24 or 48 h with free access to food and water. No injuries were observed in any of the overcrowded mice. Overcrowding occurred on days 3–4, 10–11, 13–15 and 17–18 (Fig. 2).
Fig. 1.
Mouse defeat posture.
An experimental C57BL/6JArc mouse in characteristic defeat posture.
Fig. 2.
Experimental timeline.
Experimental timeline showing the 19 day SD/OC protocol comprised of repeated social defeats and overcrowding procedures, followed by 6 days of behavioural analysis.
2.3. Drugs
Fluoxetine (Cat No. PHR1394, Sigma Aldrich) or CuII(atsm) (prepared in-house according to Gingras et al., 1962) were administered daily at a dose of 20 mg/kg (Nollet et al., 2012) and 30 mg/kg (Hung et al., 2012), respectively. Standard suspension vehicle (SSV; 0.9% Na-carboxymethylcellulose, 0.5% benzyl alcohol, 0.5% Tween 80®, 0.4% sodium chloride) was used as a vehicle. All drugs were prepared fresh daily and administered by oral gavage at a volume of 4 ml/kg. Gavage was performed daily throughout the SD/OC and behavioural testing period, a total duration of four weeks, in order to encompass both the manifestation of any depression-like changes and the onset of antidepressant action.
2.4. Body weights
All mice were weighed daily prior to gavage sessions. Fluctuations in weight were measured by calculating the difference in weight as a percentage of the initial weight on day 1, or the percentage change from the initial weight for a specified period (Day 1–19, 20–26, 1–26; pre OC-post OC).
2.5. Behavioural analysis
Following the conclusion of the SD/OC model all mice underwent a battery of behavioural tests (Fig. 2). All mice were habituated to the respective behavioural assessment rooms for at least 1 h prior to any behavioural test. With the exception of the saccharin preference test (SPT), which was conducted over a 48 h period, all behavioural testing was conducted between 0900 and 1630 h.
2.5.1. Social interaction test (SIT)
To investigate the influence of the SD/OC model on social avoidance behaviour, the SIT was conducted on the day following the final social defeat. The SIT was carried out as previously described (Finger et al., 2011, 2012). The experimental mouse was placed into a plastic box (29.5 × 35.5 × 22.5 cm) containing an empty (‘no target’) wire mesh cage (9 × 9 x 10.5 cm) positioned against the wall and allowed to explore for 2.5 min. The mouse was returned to its home cage for 1 min, while an unfamiliar mouse (‘target’) was placed inside the wire mesh cage. ‘Target’ mice were SD-naïve males of the Swiss strain in Trial 1 and a SJL in Trial 2. The experimental mouse was then placed into the box and allowed to explore for another 2.5 min. The equipment was cleaned with 80% ethanol and dried between trials. All testing was conducted under red light to reduce interference of general anxiety-like behaviour with social interaction behaviour. All trials were recorded via cameras mounted on the ceiling above the box and evaluated using Top Scan Lite software (Clever Sys, Inc.). Social avoidance behaviour was assessed by measuring the time spent in the interaction zone (IZ) (5 cm wide area around the wire mesh cage) and calculating an interaction ratio (time spent in IZ with ‘target’ present [s]/time spent in IZ with ‘no target’ present [s]). An interaction ratio of less than one was considered indicative of social avoidance behaviour.
2.5.2. Locomotor activity (LMA)
LMA in an open field was assessed by placing mice individually into the locomotor cell (27.3 × 27.3 × 20 cm) and activity monitored for 15 min by a grid of infrared beams. All locomotor cells were cleaned with 80% ethanol and dried between each trial. The data were analysed using Activity Monitor Version 6.02 software (Med Associates, Inc).
2.5.3. Light-dark box test (LDB)
The influence of the SD/OC model on anxiety-related behaviour was examined in the LDB. The LDB was carried out as previously described (Finger et al., 2010, 2011). The LDB was conducted using the locomotor activity cell with a dark compartment insert (13.3 × 26.6 × 18.8 cm) covering half the floor area and a lamp positioned over the light half (934–999 lux). Mice were placed inside the dark compartment facing away from the access door (small archway, 5.3 × 6.6 cm). The duration of the test was 10 min and activity was measured by a grid of infrared beams. The locomotor cell and dark box insert were cleaned with 80% ethanol and dried between trials. The data were analysed using Activity Monitor Version 6.02 software (Med Associates, Inc). The number of transitions between compartments and the time spent in the light side was recorded.
2.5.4. Saccharin preference test (SPT)
The SPT was used to assess aspects of anhedonia. Mice were habituated to drinking from 15 ml Falcon tubes with the tips cut off for several days prior to the testing. Upon testing, mice were presented with two 15 ml Falcon tubes, one containing tap water and the other 0.1% (w/v) saccharin (Merck Millipore), for a period of 48 h. The position of the tubes was exchanged after 24 h to avoid the development of side bias. All tubes were weighed at the 0, 24 and 48 h time points. Saccharin preference was calculated as the weight of saccharin consumed as a percentage of the total fluid (saccharin + water) consumption.
2.5.5. Forced swim test (FST)
The FST was used to evaluate behavioural despair/learned helplessness. The FST was carried out as previously described (Finger et al., 2012; Porsolt et al., 1977). 2 L glass beakers were filled with approximately 1.8 L (14 cm) of water (25 ± 0.5 °C) and mice were individually placed into the water for 6 min. The water was changed between each trial. Behaviour was recorded by a video camera positioned side on to the beaker and analysed using Forced Swim Version 2.00 software (Clever Sys, Inc.). The duration of immobility was recorded during the last 4 min of the 6 min test.
2.6. Statistical analysis
Statistical analyses were performed using the software packages GraphPad Prism (Version 7.02 for windows) or SigmaPlot 12.0. Comparisons between respective ‘target’ and ‘no target’ trials in the SIT were analysed using Student's paired t-test. The body weights were averaged over two day blocks in order to meet the assumptions for parametric testing and analysed using two-way repeated measures analysis of variance (ANOVA), followed by Tukey's post hoc multiple comparisons test. The number of defeat postures displayed and latency to defeat postures were log10 transformed in order to meet the assumptions for parametric testing and analysed using two-way repeated measures ANOVA followed by Tukey's post hoc multiple comparisons test. Latency to attack data did not meet assumptions for parametric testing and were therefore analysed within each SD session using Wilcoxon Signed Rank tests. One aggressor in session one and four on session two were excluded from this analysis since they were not used in both the pre- and post-barrier bouts in these sessions. All other data were analysed using a one-way ANOVA followed by Tukey's post hoc tests. For all analyses, P < 0.05 was considered to be statistically significant. Except for Fig. 5A where medians and 95% confidence intervals (CI) are presented, all data are presented as the mean ± SEM.
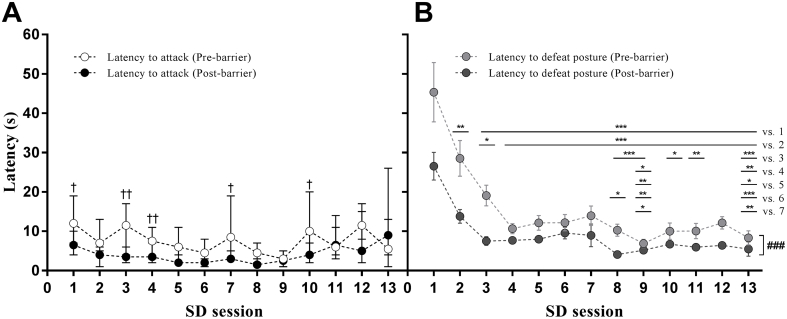
Fig. 5.
Effects of SD/OC on the latencies to attack by residents and to display defeat posture by intruders.
(A) Latency to attack by residents and (B) latency to display defeat posture by intruders in Trial 2. n = 30. In (A) data are medians ± 95% CI; †P < 0.05, ††P < 0.01 for pre- versus post-barrier bouts within session by Wilcoxon Signed Rank test. In (B) data are means ± SEM; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 between SD sessions; ###P < 0.001 for main effect of pre- versus post-barrier bouts exposure to resident, by two-way repeated measures ANOVA on log10 transformed data.
3. Results
3.1. Social defeat sessions
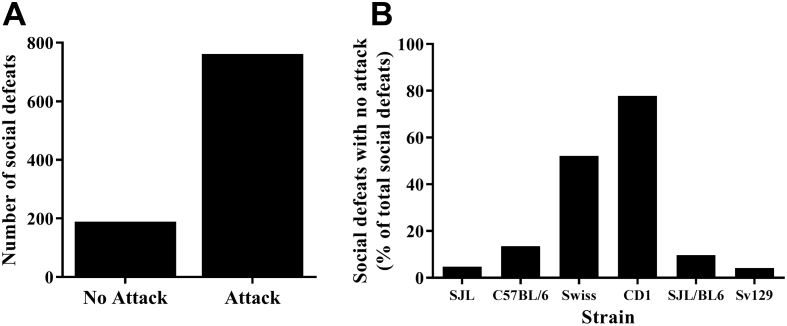
In Trial 1 a substantial number of mice displayed defeat posture without the need to be physically attacked, but rather displayed defeat posture as soon as the aggressor came in close proximity (Fig. 3A). The majority of the SDs presented without an attack were perpetrated by Swiss or CD1 mice (Fig. 3B). SJL mice were observed to be the most reliably aggressive strain and were selected as the predominant aggressors for Trial 2. In Trial 2, SDs were aimed to be terminated following five defeat postures (Fig. 4) and definitive attack behaviour by the aggressor. However, variation in the number of defeat postures displayed occurred due to early termination of SD bouts (as a result of overly ferocious attacks) and the speed of defeat posture presentation.
Fig. 3.
Social defeats elicited with or without an attack.
(A) Distribution of social defeats in Trial 1 where no attack or an attack occurred and (B) the strain of aggressor eliciting the no attack social defeats.
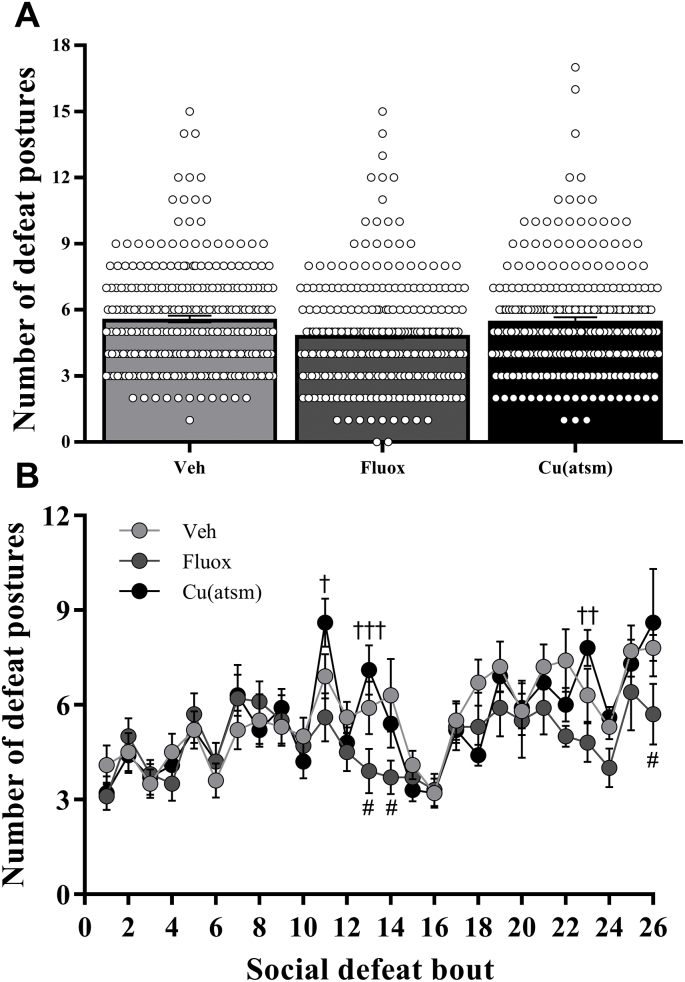
Fig. 4.
Effects of SD/OC on the number of defeat postures displayed.
The number of social defeats displayed per (A) overall treatment group and (B) across time during Trial 2. Data are means ± SEM. n = 10. #P < 0.05 vs. Veh; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. Fluox for respective social defeat number, by two-way repeated measures ANOVA with Tukey's post hoc tests on log10 transformed data.
Two-way repeated measures ANOVA revealed that the number of defeat postures displayed was augmented with increasing number of SD bouts, with a significant interaction between SD bout and drug treatment observed (Treatment: F(2,27) = 1.631, P = 0.2144; SD bout number: F(25,675) = 11.06, P < 0.001; interaction: F(50,675) = 1.491, P = 0.0179; Fig. 4B). Post hoc analysis revealed a significant decrease in the number of defeat postures in the fluoxetine treated mice versus vehicle for SD bouts 13, 14 and 26 (P < 0.05; Fig. 4B) and also a decrease in defeat postures in fluoxetine treated mice compared to CuII(atsm) treated mice for SD sessions 11 (P < 0.05), 13 (P < 0.001) and 23 (P < 0.01; Fig. 4B).
Median attack latency by resident aggressors in Trial 2 was approximately stable across the thirteen SD sessions, with a shorter latency to attack demonstrated in post- compared with pre-barrier bouts, depending on SD session (n = 30 for all sessions: Session 1, Z = −2.055, P < 0.05; Session 3, Z = −3.138, P < 0.01; Session 4, Z = −3.045, P < 0.01; Session 5, Z = −1.823, P < 0.1; Session 7, Z = −2.37, P < 0.05; Session 8, Z = −1.903, P < 0.1; Session 10: Z = −2.14, P < 0.05; Fig. 5A).
The latency to present defeat posture reduced with increasing numbers of SD sessions, particularly in the latter of the two SD bouts (“post-barrier”) within a session (Session: F(12,346) = 25.525, P < 0.001; pre/post barrier: F(1,346) = 105.954, P < 0.001; interaction: F(12,346) = 1.225, P = 0.264; Fig. 5B), but was not affected by treatment (Treatment: F(2,673) = 0.224, P = 0.801; SD session: F(25,673) = 15.587, P < 0.001; interaction: F(50, 673) = 0.846, P = 0.767; data not shown). Note: data from two mice were excluded from the latency analyses: one vehicle-treated mouse in SD bout two which had a latency to defeat posture of 474 s; the other mouse (fluoxetine treatment, SD bout 17) because it did not show a defeat posture before termination of the fight bout due to excessive aggression.
3.2. Body weight
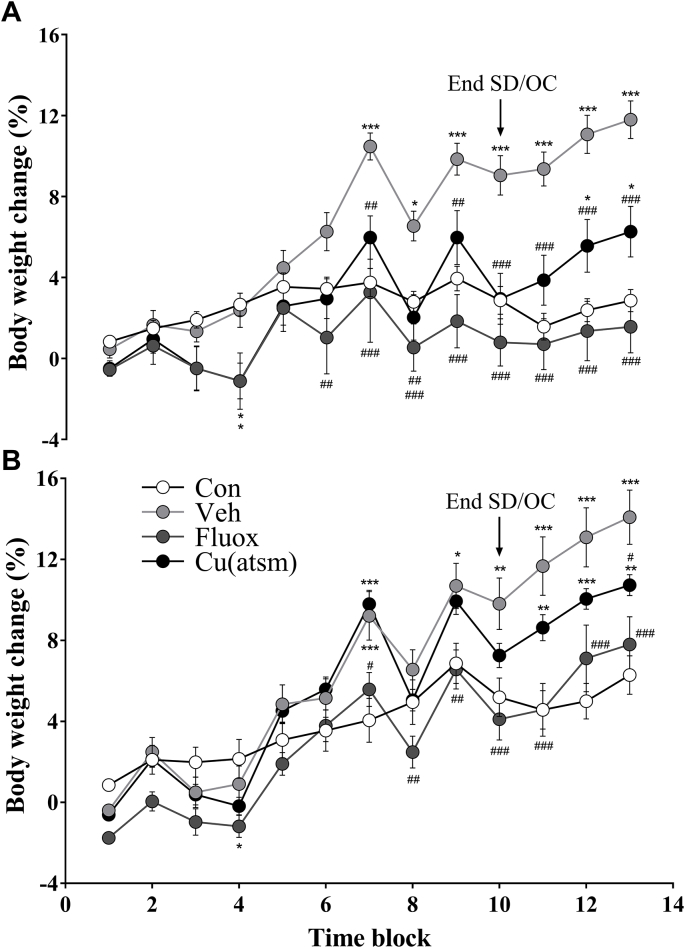
The SD/OC model influenced body weight and was modulated by drug treatment over time (Trial 1: treatment: F(3,43) = 8.18, P = 0.0002; time: F(12, 516) = 52.29, P < 0.001; interaction: F(36, 516) = 9.833, P < 0.001; Fig. 6A; Trial 2: treatment: F(3, 36) = 5.267, P = 0.0041; time: F(12, 432) = 179.0, P < 0.001; interaction: F(36, 432) = 8.972, P < 0.001; Fig. 6B).
Fig. 6.
Effects of SD/OC on body weight across time.
Percentage change in body weight in (A) Trial 1 and (B) Trial 2. Arrowheads represent the end of the SD/OC component of the protocol. Body weights were averaged over two days per time block in order to meet the assumptions for parametric testing. Data are means ± SEM. n = 10–12. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001 vs. Con; #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 vs. Veh within time blocks, by two-way repeated measures ANOVA with Tukey's post hoc tests.
SD/OC – Vehicle treated mice exhibited significant weight gain relative to control mice (Fig. 6). In Trial 1 SD/OC – Vehicle treated mice exhibited an increased body weight gain compared to non-SD controls during SD/OC exposure (F(3, 43) = 9.405, P < 0.05; Fig. 7A), following conclusion of the SD/OC component during behavioural testing (F(3, 43) = 11.73, P < 0.001; Fig. 7B) and throughout the entire trial (F(3, 43) = 18.08, P < 0.001; Fig. 7C). In Trial 2 weight gain in SD/OC – Vehicle treated mice did not differ significantly from controls during SD/OC exposure (Fig. 7D) however, increased weight gain compared to controls was observed following conclusion of the SD/OC component during behavioural testing (F(3, 36) = 6.23, P < 0.01; Fig. 7E) and throughout the entire trial (F(3, 36) = 9.465, P < 0.001; Fig. 7F).
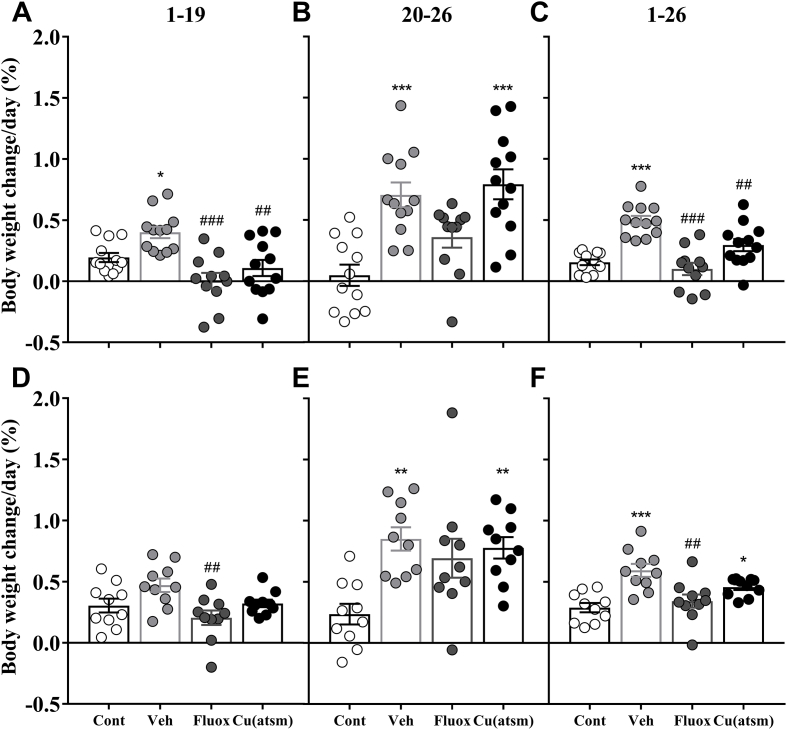
Fig. 7.
Effects of SD/OC on body weight during specific testing components.
Percentage change in body weight in (A, B, C) Trial 1 and (D, E, F) Trial 2. (A, D) The change in weight per day was calculated for the SD/OC component (day 1–19), (B, E) the period following the SD/OC component during behaviour testing (day 20–26) and (C, F) the entire duration of the protocol (day 1–26). Data are means ± SEM. n = 10–12. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001 vs. Con; ##P ≤ 0.01, ###P ≤ 0.001 vs. Veh, by one-way ANOVA with Tukey's post hoc tests.
Fluoxetine treatment inhibited weight gain during SD/OC exposure and attenuated weight gain after SD/OC exposure (Fig. 6). In both trials SD/OC – Fluoxetine treated mice exhibited a decreased body weight gain during SD/OC exposure relative to SD/OC – Vehicle (F(3, 43) = 9.405, P < 0.001; Fig. 7A; F(3, 36) = 4.525, P < 0.01; Fig. 7D). Over SD/OC and behaviour testing together, SD/OC – Fluoxetine treated mice exhibited a decreased body weight gain compared to SD/OC – Vehicle (P < 0.001; Fig. 7C; P < 0.01; Fig. 7F).
Over SD/OC and behaviour testing together, CuII(atsm) treatment attenuated weight gain compared to SD/OC – Vehicle in Trial 1 only (P < 0.01; Fig. 7C). This was primarily due to decreased weight gain during SD/OC exposure compared to SD/OC – Vehicle (P < 0.01; Fig. 7A) whereas no difference between vehicle and CuII(atsm) treated mice was observed following the SD/OC component during behaviour testing (Fig. 7B, E). During this period an increase in body weight gain compared to non-SD controls was observed in both trials (P < 0.001; Fig. 7B; P < 0.01; Fig. 7E), comparable to the increase in weight gain see in vehicle treated mice. Across both trials and all SD/OC groups, the mean weight gain during the 19 day SD/OC component was 0.06 ± 0.01 g/day and during the seven days of behaviour testing was 0.17 ± 0.01 g/day.
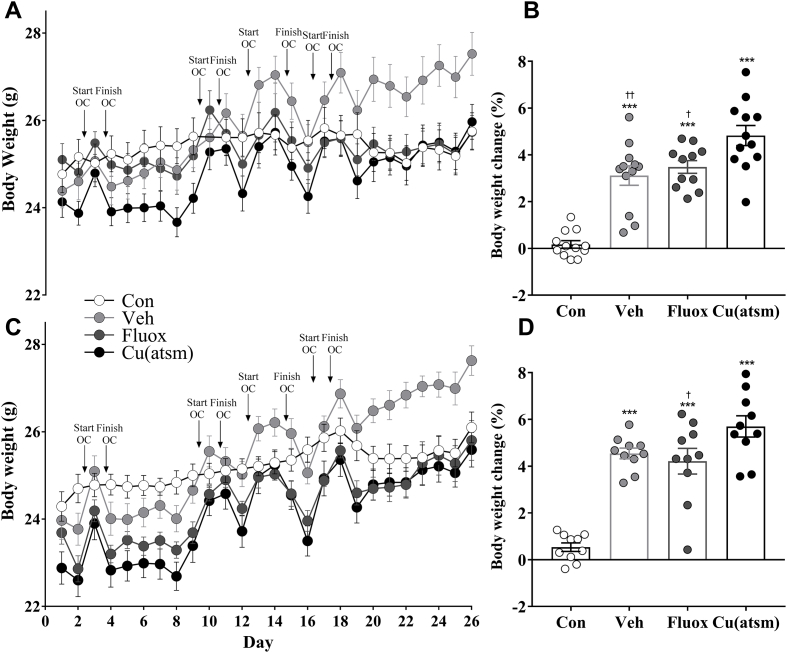
All mice exposed to the SD/OC protocol exhibited peaks in weight associated with the days in which they were overcrowded (Fig. 8A, C). In both trials, all mice exposed to SD/OC exhibited an increased average weight gain across the four OC sessions compared to control mice (F(3, 43) = 33.65, P < 0.001; Fig. 8B; F(3, 36) = 33.65, P < 0.001; Fig. 8D), which remained single housed during these periods. Fluoxetine treated mice exhibited a decreased weight gain during OC sessions compared to CuII(atsm) treated mice (P < 0.05; Fig. 8B; P < 0.05; Fig. 8D), as too did vehicle treated mice for Trial 1 only (P < 0.01; Fig. 8B). Across both trials and all SD/OC groups, the mean weight gain during the OC procedures alone was 0.87 ± 0.04 g/day.
Fig. 8.
Effects of overcrowding procedures on body weight.
Changes in body weight in (A) Trial 1 and (C) Trial 2. The peaks and troughs in body weight were associated with the overcrowding procedures throughout the SD/OC protocol. Arrowheads represent the intervals in which the mice were overcrowded. Bar graphs show the mean percentage weight gain during the four OC sessions for (B) Trial 1 and (D) Trial 2. Control mice remained single housed during these periods. Data are means ± SEM. n = 10–12. ∗∗∗P ≤ 0.001 vs. Con; †P < 0.05, ††P < 0.01 vs. Cu(atsm), by one-way ANOVA with Tukey's post hoc tests.
3.3. Behaviour
3.3.1. SIT
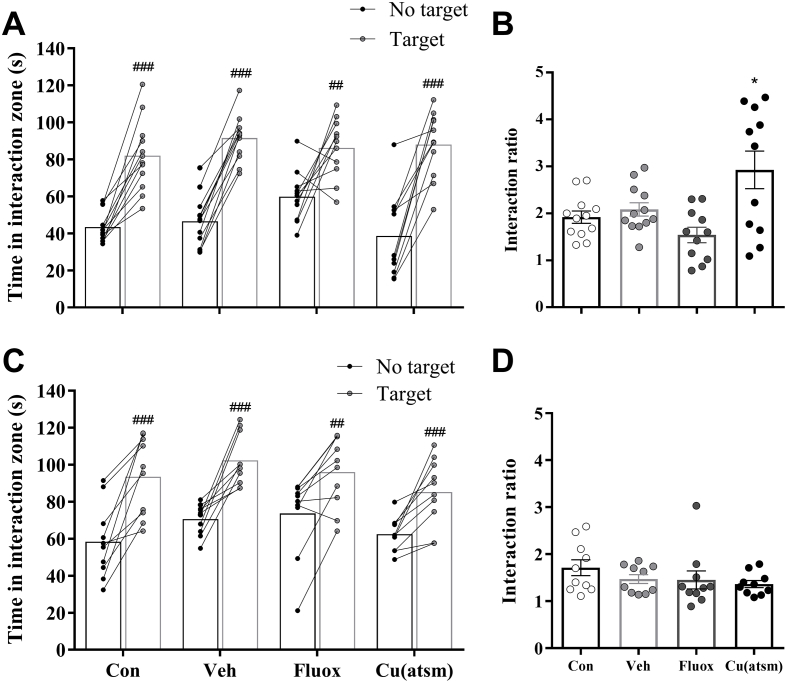
The SD/OC model did not induce social avoidance behaviour in any of the treatment groups in either trial, in contrast, all treatment groups spent significantly more time in the interaction zone with the ‘target’ present compared with their respective trial with ‘no target’ present (Control: P < 0.001, SD/OC – Vehicle: P < 0.001, SD/OC – Fluoxetine: P = 0.0082, SD/OC-CuII(atsm): P < 0.001; Fig. 9A; Control: P = 0.0004, SD/OC – Vehicle: P = 0.0003, SD/OC – Fluoxetine: P = 0.0059, SD/OC-CuII(atsm): P = 0.0009; Fig. 9C). Furthermore, in Trial 1 SD/OC-CuII(atsm) mice exhibited a higher interaction ratio as compared to controls (F(3, 42) = 6.197, P < 0.05; Fig. 9B).
Fig. 9.
Effects of SD/OC on social avoidance behaviour.
Social avoidance behaviour in the social interaction test on day 20 of testing in (A, B) Trial 1 and (C, D) Trial 2. Data are (A, C) time spent in the interaction zone (IZ) with ‘no target’ and ‘target’ and (B, D) the interaction ratio (time spent in IZ with ‘target’ present [s]/time spent in IZ with ‘no target’ present [s]) for Trial 1 and 2 respectively. Data are means ± SEM. n = 10–12. ##P ≤ 0.01, ###P ≤ 0.001 vs. ‘no target’ trial, by Student's paired t-test; ∗P ≤ 0.05 vs. control, by one-way ANOVA with Tukey's post hoc tests.
3.3.2. LMA
No significant difference in the distance travelled (Supp Fig. 1A, E) was observed between groups in either Trial 1 or 2.
3.3.3. LDB
Compared to controls, mice exhibiting anxiety related behaviour were expected to spend less time in the light side of the arena and to make fewer transitions from the dark to light side. SD/OC – Fluoxetine treated mice spent significantly less time in the light area in both trials (F(3, 43) = 5.164, P < 0.01 vs. control; P < 0.05 vs. SD/OC – Vehicle; Supp Fig. 1B; F(3, 36) = 3.254, P < 0.05 vs. control; Supp Fig. 1F). No significant differences were observed for the number of light-dark transitions (data not shown) between groups in either trial.
3.3.4. SPT and FST
No significant difference in saccharin preference was observed in the SPT between groups (Supp Fig. 1C, G). Furthermore, no alterations in immobility time between the groups were seen in the FST in either Trial 1 or 2 (Supp Fig. 1D, H).
4. Discussion
The aims of this study were to evaluate the influence of the SD/OC protocol on the behaviour and body weight of mice, and to further characterise the effects of the antidepressant fluoxetine and copper BTSC compound CuII(atsm) in this model. The principle finding was a reproducible and consistent increase in body weight in mice exposed to the SD/OC protocol, and particularly during OC sessions. Both drug treatments investigated reduced chronic weight gain, but not during OC sessions. In Trial 2 a small effect of fluoxetine on defeat postures was detected during the SD phase, however, the SD/OC model otherwise failed to induce depression- or anxiety-like behaviour in two independent cohorts of mice across a range of behavioural tests, despite the increased aggression administered from Trial 1 to Trial 2.
Social defeat models have been utilised in an attempt to elicit depression/anxiety related etiology with varying degrees of success. Avoidance behaviour following social defeat models is a key behavioural outcome thought to signify social withdrawal (Cryan and Holmes, 2005) and is a common symptom in people with depression (Derntl et al., 2011; Girard et al., 2014). A 10 day social defeat model (SD10) described by Golden et al. (2011) produced significant social avoidance behaviour (Berton et al., 2006; Krishnan et al., 2007; Tsankova et al., 2006; Venzala et al., 2012). However, a concern of this model is the elevated risk of injuries and potentially confounding peripheral inflammation (Golden et al., 2011; Pryce and Fuchs, 2017). The SD/OC model has a shorter SD interaction time therefore reducing the opportunity for injuries to occur, and has also been reported to produce social avoidance behaviour in some (Finger et al., 2011, 2012; Tramullas et al., 2012), but not all studies (Slattery et al., 2012). Social avoidance behaviour, however, was not observed in the current experiments, despite escalating levels of aggression across the two trials – the reasons for this are unclear. Krishnan et al. (2007) subjected 437 C57BL/6 J mice to the SD10 protocol and identified susceptible and unsusceptible subpopulations of responders, with 40–50% of defeated mice showing a preference for interacting with a social target, similar to non-defeated controls. In the present investigation, however, across both trials, none of the 22 vehicle treated C57BL/6JArc mice and only three of the total 65 mice exposed to the SD/OC model exhibited a social interaction ratio of less than one. Differences in the behaviour of substrains of C57BL/6 strains have been reported previously (Matsuo et al., 2010). However, the C57BL/6JArc mice used in the present study were found to be identical to C57BL/6 J from both Jackson (as used in the study by Krishnan et al., 2007) and Charles River laboratories at 1449 single nucleotide polymorphisms in a linkage mapping panel (Zurita et al., 2011). This suggests that substrain genetic differences alone are unlikely to have been a major factor in the resilience seen in intruder mice used in the present study.
It is possible that despite the two escalating levels administered, aggression levels may have been insufficient to produce avoidance or depression-like behaviours in the present study. To date, however, the quantification of injuries, the number of defeat postures and attacks, or aggression intensity are sparsely reported in the literature, making comparisons between models challenging. To address this, we sought to quantify the number of defeat postures expressed per mouse, in order to determine if increasing defeat postures related to subsequent depression-related behaviours. In Trial 1, defeat postures were limited to one display before cessation of SD bouts. We aimed to limit defeat postures to five in the second trial, although the speed with which defeat postures were displayed made precise bout termination challenging, and as a result more defeat postures were commonly displayed. This indicated that intruders were aware of the increased aggression level between trials and responded accordingly. Furthermore, in Trial 2 the number of defeat postures increased with SD sessions, with experimental mice also showing a marked reduction in latency to defeat posture over the first four, but not subsequent sessions. Interestingly, this appeared to be somewhat unrelated to the attack latency of aggressors over the thirteen SD sessions, which was remarkably stable across the entire study. The consistent behaviour of the aggressors also failed to explain the increasing number of defeat postures displayed by intruders over the course of the SD/OC protocol. Within sessions, however, latencies to attack or to show defeat posture in aggressors and intruders respectively, were shorter in the second, post-barrier bouts, indicating the 2 h non-contact exposure effectively intensified aggressive interactions and responses to them. Whether shortened latency to, and increasing number of, defeat postures are evidence of depression-like behaviour or a learned defense mechanism remains to be determined, although the lack of depression-related responses following SD/OC suggests they may primarily represent the latter. However, defeat postures, although largely unaffected by drug treatment, were reduced in fluoxetine-treated mice on three of the 26 SD bouts, suggesting that some contribution from underlying depression-like mechanisms cannot be ruled out.
The specific strain of aggressor mouse used may be a vital aspect of social defeat models. In previous studies employing either the SD/OC or SD10 models, CD1 mice were commonly selected as the aggressors. In screening and Trial 1 of the present study a mixture of strains was utilised and revealed aggression differences amongst them, particularly latency to attack. SJL mice displayed high levels of unprovoked aggression in screening and throughout all SD sessions, reliably exhibiting attack latencies of <5–10 s. This strain is noted for a high expression of aggression and fighting when group housed (Crispens, 1973; Page and Glenner, 1972). Of the 29 SJL mice screened only one exhibited low levels of aggression. On the other hand, Golden et al. (2011) reported that up to half of the screened CD1 mice did not meet the appropriate aggression criteria, a finding confirmed in the present study. The aggression of SJLs, as assessed by attack latency, therefore seems to be an unlikely reason for the lack of depressive-like or social avoidance behaviour observed in intruders. Alternatively, the style of attack may be an important variant in promoting depressive-like behaviour. Subjectively, we observed that Swiss and CD1 mice commonly enveloped the smaller C57BL/6JArc mouse when fighting, using their greater size to their advantage. Bite wounds in these SDs were uncommon. Contrarily, the SJL mice, of comparable size to the C57BL/6JArc mice, exhibited swift, ‘ambush style’ attacks, unexpectedly pouncing at or on the C57BL/6JArc mouse. Bites to the lower back and tail were delivered – typically during running/chasing. Interestingly, this type of aggressive behaviour, despite the reliable ferocity, did not result in avoidance of SJL mice in the SIT. Nor did it result in depressive-like behaviour overall, despite potential conflicts from bite wounds. These data suggest that aggression, defined as attack latency alone, is insufficient to capture the factors required to induce social avoidance behaviour. The precise characteristics of resident aggression that promotes social avoidance behaviour thus remains to be fully determined. Future experiments comparing the aggression style of SJL versus CD1 mice on the behavioural outcomes of victims may therefore prove enlightening. Including the assessment of stress hormone levels in intruders in response to the different styles and levels of aggression of specific aggressor strains would be a valuable addition to such a study.
A main finding of the present study was a consistent increase in body weight in response to the SD/OC protocol. Major alterations in eating patterns and body weight are a common occurrence amongst depressed, anxious or stressed people (Konttinen et al., 2010; Luppino et al., 2010; Ouwens et al., 2009). In the present study, SD stress increased body weight during the SD/OC component, and even more so in the week following stressor exposure. It should be noted that all mice in this study received oral gavage daily, which could have represented an additional stressor. This may have contributed to a slightly lower weight gain in the control (vehicle-treated, no SD/OC) mice (∼4.5%) than expected from reference growth rates (∼11%; Jackson Laboratory, 2018). This amounts to a difference in weight change of ∼0.038 g/day across the 26-day protocol and variations in mice and laboratory conditions could explain this. However, repeated oral gavage performed correctly is not particularly stressful for mice (Arantes-Rodrigues et al., 2012) in comparison with intraperitoneal (IP) injection, as assessed by adrenocorticotropic hormone (ACTH) and cortisol responses (Baek et al., 2015). Furthermore, a number of SD studies have used repeated IP injections for 28–30 days (Beitia et al., 2005; Berton et al., 2006; Tsankova et al., 2006; Venzala et al., 2012) and compromise in weight gain in control mice was not noted in these studies. However, if gavage stress diminished weight gain in the present study, it was controlled for to some degree, since all SD/OC mice also received the same gavage stress and increases in weight relative to the control group were still observed.
In humans, body weight changes in response to stress and/or depression can progress in opposite extremes – substantial increases in food intake and weight are quite common although, lack of appetite and decreased weight are also observed (Stunkard et al., 1990; Weissenburger et al., 1986). Similarly, weight changes in mice exposed to social defeat paradigms are varied. As in the present study, increases in weight during the SD/OC protocol have been previously observed (Finger et al., 2012; Tramullas et al., 2012). However, Reber et al. (2006) reported a decreased weight gain as compared to controls during the 19 day protocol whilst Finger et al. (2011) observed a decrease in body weight in mice on a high-fat diet whereas mice on a low-fat diet exhibited no change. Slattery et al. (2012) found no difference in weight during stressor exposure but observed an increase in weight following stressor cessation. In another social defeat model, Savignac et al. (2011a) found a less intense protocol (more closely resembling SD/OC) resulted in an increase in weight gain whereas the more intense model (more closely resembling SD10) resulted in no change as compared to controls (Savignac et al., 2011a). Goto et al. (2014) also observed an increase in weight gain when employing a less intense version of the SD10 model. Two separate studies found no alteration in weight during the 10 days of defeat but a substantial increase in weight following stressor termination (Venzala et al., 2012, 2013). Savignac et al. (2011b) reported a decrease in weight during their social defeat protocol as did Krishnan et al. (2007). Although these studies show divergent bodyweight changes during social defeat procedures, weight gain following stressor termination is more consistently reported (Melhorn et al., 2010; Razzoli et al., 2011; Slattery et al., 2012), in agreement with the findings from this study. This was postulated to be a protective mechanism to prepare the body for potential future stressors (Slattery et al., 2012).
Specific peaks in weight gain were observed in both present trials which were tightly associated with the overcrowding procedure. During OC sessions defeated mice averaged a body weight increase of 0.87 g per day, a striking difference to the typical male C57BL/6 J growth rate of 0.13 g per day (Jackson Laboratory, 2018). As previously reported (Slattery et al., 2012), a large increase in weight gain was also seen in the present study in the one week period following SD termination however, these only reached a mean of 0.17 g per day (albeit blunted by the protective effects of fluoxetine/CuII(atsm)). The magnitude and rapidity of weight gain and subsequent loss associated with OC sessions suggest a transient hyperphagia. This raises interesting questions regarding the role of the overcrowding procedure as a social stressor. Binge eating was recently recognized as a distinct eating disorder in the DSM V (American Psychiatric Association, 2013), and is associated, amongst other psychiatric disorders, with depression, as well as stress and social distress (Javaras et al., 2008; reviewed in Razzoli et al., 2017; Razzoli et al., 2015). Furthermore, hyperphagia, obesity and insulin resistance have been previously reported in animal models of chronic social defeat (Javaras et al., 2008; Razzoli et al., 2015, 2017). The acute peaks in weight gain caused by OC may pinpoint binge eating behaviour to an acute, defined, social challenge point within the SD/OC model. It is also of interest that neither fluoxetine nor CuII(atsm) attenuated the transient weight gain during OC, perhaps reflective of the findings in humans where selective serotonin re-uptake inhibitors (SSRIs) show limited efficacy for the treatment of binge eating (Ghaderi et al., 2018). OC-induced transient weight gain thus bears further investigation to define underlying neurobiological correlates of relevance to the study of stress-induced binge eating.
Weight gain during the 19 day SD/OC component of the protocol was prevented by fluoxetine whilst CuII(atsm) attenuated it, a finding replicated across both trials. A number of studies have associated antidepressant treatment such as fluoxetine with inhibition of weight gain in humans (Halford et al., 2007; Li et al., 2005; Michelson et al., 1999; Serretti and Mandelli, 2010) and in rodents (Grignaschi and Samanin, 1992; Lightowler et al., 1996; Wong et al., 2005) however, the neurobiological processes behind these changes are not well understood (Kumar et al., 2013). Fluoxetine has been reported to decrease weight gain even in unstressed mice (Yen et al., 1987), and as such may have had direct effects on bodyweight in the present study that were independent of its anti-depressant properties. However, no such effect was observed with CuII(atsm) dosed in non-transgenic C57BL/6 J x C3H/HeJ mice up to 60 mg/kg/day for more than 200 days (McAllum et al., 2013). This is, therefore, the first time that attenuation of stress-induced weight gain has been demonstrated using CuII(atsm) and these data may thus suggest a different mechanism of action between the two compounds in this regard. It also provides support for further investigations into the association between nitrosative stress and aspects of depression such as weight gain (Lopresti and Drummond, 2013; Marazziti et al., 2014).
Despite changes in body weight, SD/OC otherwise failed to elicit depression related behaviour. These results are in agreement with those found by Slattery et al. (2012) who observed no difference in saccharin preference in the SPT, nor immobility time in either the FST or TST following SD/OC. An increase in anxiety-like behaviour was observed in the EPM although this reached significance only nine days after cessation of the SD/OC model (Slattery et al., 2012). A main conclusion of that study was that the SD/OC model induced an anxiogenic, but not depressive, response which only arose in the week following stressor termination (Slattery et al., 2012). In the present study, however, anxiety-related behaviour in the LDB was not influenced by SD/OC as assessed three days after stressor termination. In contrast, Finger et al. (2011) observed a significant decrease in the time spent in the light side of the LDB as well as an increase in immobility time in the FST when compared to controls. However, both these effects were only present in mice on a low fat diet. The finding that chronic fluoxetine treatment did not reduce immobility in the FST in SD/OC mice was unexpected, however, Venzela et al. (2012) observed male C57BL/6 mice exposed to chronic social defeat stress exhibited an increased immobility time compared to controls but this was not altered by fluoxetine treatment, whereas venlafaxine significantly reduced immobility time. Furthermore, it has been reported that C57BL/6 mice are not particularly sensitive to fluoxetine treatment in the FST (Lucki et al., 2001). Together these data suggest that either noradrenergic may be more involved than serotonergic mechanisms in response to SD/OC, or alternatively, that C57BL/6 mice may not be the ideal strain to investigate serotonergic mechanisms in social defeat (Jacobson and Cryan, 2007).
In conclusion, a key aim of this study was to further explore the SD/OC protocol as an etiological animal model of depression; however, depression-like behaviours were not observed in two independent trials with different levels of aggression. The reliable reproduction of complex stress models on depressive-like behaviour remains a focal point for the field, as has recently been highlighted with the related model; CMS (Willner, 2017). In Willner's 2017 investigation few, if any, differences were identified between laboratories that reliably reproduced the CMS model versus those which did not. Nevertheless, possible reasons for reproducibility issues discussed therein included differences in individual experimenters, experimental subjects, stress susceptibility of strains, variation within strains, accuracy of outcomes measures and the severity of stress. We have addressed substrain susceptibility of intruders, severity of stress and individual variations within intruders in the context of the present study. We also identified the importance of the strain and aggression style of resident mice as a possible source of variation, which further research may ultimately prove a valuable discovery tool within SD models. Other factors identified by Willner (2017) may also have played a role in the results of the present study, as in previous studies. However, coupled with comparable results obtained from another study (Slattery et al., 2012) these experiments raise concerns over the reproducibility of the SD/OC protocol as an etiological model of depression across laboratories. Appropriate reporting regarding SD protocols, levels of aggression delivered and responses of victims thereto may prove vital in overcoming issues associated with variability in social defeat stress models. Nevertheless, the SD/OC model reliably elicited stress-induced weight gain; a core symptom of depression in humans. Chronic fluoxetine treatment reversed, and CuII(atsm) attenuated this weight gain, pointing to underlying serotoninergic and peroxynitrite-related mechanisms. The OC component of the SD/OC model may bear further investigation as a platform to study the neurobiology of social stress-induced weight gain and binge eating – a prevalent concern of the modern era. These findings highlight the potential of the SD/OC model to investigate factors affecting weight gain following stressor exposure, and to provide a greater understanding of the mechanisms behind these stress-induced changes.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We thank David J. Hayne for synthesising the CuII(atsm) compound used in this study. We thank Prof. John Cryan and Dr. Beate Finger for their advice regarding the SD/OC model. LHJ is supported by NHMRC Project Grant 1105284 and Alzheimer's Association NIRG-396905. The Florey Institute of Neuroscience and Mental Health acknowledge the support of the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.09.008.
Contributor Information
Kevin J. Barnham, Email: kevin.barnham@florey.edu.au.
Laura H. Jacobson, Email: laura.jacobson@florey.edu.au.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- American Psychiatric Association . American Psychiatric Publishing; Washington, D.C.: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [electronic Resource] 2013. [Google Scholar]
- Arantes-Rodrigues R., Henriques A., Pinto-Leite R., Faustino-Rocha A., Pinho-Oliveira J., Teixeira-Guedes C., Seixas F., Gama A., Colaco B., Colaco A., Oliveira P.A. The effects of repeated oral gavage on the health of male CD-1 mice. Lab. Anim. (NY) 2012;41:129–134. doi: 10.1038/laban0512-129. [DOI] [PubMed] [Google Scholar]
- Baek J.M., Kwak S.C., Kim J.Y., Ahn S.J., Jun H.Y., Yoon K.H., Lee M.S., Oh J. Evaluation of a novel technique for intraperitoneal injections in mice. Lab. Anim. (NY) 2015;44:440–444. doi: 10.1038/laban.880. [DOI] [PubMed] [Google Scholar]
- Beitia G., Garmendia L., Azpiroz A., Vegas O., Brain P.F., Arregi A. Time-dependent behavioral, neurochemical, and immune consequences of repeated experiences of social defeat stress in male mice and the ameliorative effects of fluoxetine. Brain Behav. Immun. 2005;19:530–539. doi: 10.1016/j.bbi.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Berton O., Hahn C.G., Thase M.E. Are we getting closer to valid translational models for major depression? Science. 2012;338:75–79. doi: 10.1126/science.1222940. [DOI] [PubMed] [Google Scholar]
- Berton O., McClung C.A., Dileone R.J., Krishnan V., Renthal W., Russo S.J., Graham D., Tsankova N.M., Bolanos C.A., Rios M., Monteggia L.M., Self D.W., Nestler E.J. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Christiansen S.H., Olesen M.V., Wortwein G., Woldbye D.P. Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav. Brain Res. 2011;216:585–591. doi: 10.1016/j.bbr.2010.08.044. [DOI] [PubMed] [Google Scholar]
- Crispens C.G. Some characteristics of strain Sjl/Jdg mice. Lab. Anim. Sci. 1973;23:408–413. [PubMed] [Google Scholar]
- Cryan J.F., Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Derntl B., Seidel E.M., Eickhoff S.B., Kellermann T., Gur R.C., Schneider F., Habel U. Neural correlates of social approach and withdrawal in patients with major depression. Soc. Neurosci. 2011;6:482–501. doi: 10.1080/17470919.2011.579800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet M.V., Levine H., Dev K.K., Harkin A. Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacology. 2013;38:1575–1584. doi: 10.1038/npp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger B.C., Dinan T.G., Cryan J.F. Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 2010;210:559–568. doi: 10.1007/s00213-010-1858-z. [DOI] [PubMed] [Google Scholar]
- Finger B.C., Dinan T.G., Cryan J.F. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- Finger B.C., Dinan T.G., Cryan J.F. The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrino. 2012;37:729–741. doi: 10.1016/j.psyneuen.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Fodero-Tavoletti M.T., Villemagne V.L., Paterson B.M., White A.R., Li Q.X., Camakaris J., O'Keefe G., Cappai R., Barnham K.J., Donnelly P.S. Bis(thiosemicarbazonato) Cu-64 complexes for positron emission tomography imaging of Alzheimer's disease. J. Alzheimers Dis. 2010;20:49–55. doi: 10.3233/JAD-2010-1359. [DOI] [PubMed] [Google Scholar]
- Ghaderi A., Odeberg J., Gustafsson S., Rastam M., Brolund A., Pettersson A., Parling T. Psychological, pharmacological, and combined treatments for binge eating disorder: a systematic review and meta-analysis. PeerJ. 2018;6 doi: 10.7717/peerj.5113. e5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras B.A., Bayley C.H., Suprunch T. Preparation of some thiosemicarbazones and their copper complexes .3. Can. J. Chem. 1962;40:1053. [Google Scholar]
- Girard J.M., Cohn J.F., Mahoor M.H., Mavadati S.M., Hammal Z., Rosenwald D.P. Nonverbal social withdrawal in depression: evidence from manual and automatic analysis. Image Vis Comput. 2014;32:641–647. doi: 10.1016/j.imavis.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Kubota Y., Tanaka Y., Iio W., Moriya N., Toyoda A. Subchronic and mild social defeat stress accelerates food intake and body weight gain with polydipsia-like features in mice. Behav. Brain Res. 2014;270:339–348. doi: 10.1016/j.bbr.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Grignaschi G., Samanin R. Role of serotonin and catecholamines in brain in the feeding suppressant effect of fluoxetine. Neuropharmacology. 1992;31:445–449. doi: 10.1016/0028-3908(92)90082-z. [DOI] [PubMed] [Google Scholar]
- Halford J.C.G., Harrold J.A., Boyland E.J., Lawton C.L., Blundell J.E. Serotonergic drugs - effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Hannestad J., DellaGioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin A., Kelly J.P., Leonard B.E. A review of the relevance and validity of olfactory bulbectomy as a model of depression. Clin. Neurosci. Res. 2003;3:253–262. [Google Scholar]
- Harkin A.J., Bruce K.H., Craft B., Paul I.A. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 1. Acute treatments are active in the forced swim test. Eur. J. Pharmacol. 1999;372:207–213. doi: 10.1016/s0014-2999(99)00191-0. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- Hung L.W., Villemagne V.L., Cheng L., Sherratt N.A., Ayton S., White A.R., Crouch P.J., Lim S., Leong S.L., Wilkins S., George J., Roberts B.R., Pham C.L., Liu X., Chiu F.C., Shackleford D.M., Powell A.K., Masters C.L., Bush A.I., O'Keefe G., Culvenor J.G., Cappai R., Cherny R.A., Donnelly P.S., Hill A.F., Finkelstein D.I., Barnham K.J. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson's disease. J. Exp. Med. 2012;209:837–854. doi: 10.1084/jem.20112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L.L., Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox. Res. 2013;23:131–144. doi: 10.1007/s12640-012-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Laboratory Body weight information for C57BL/6J (000664) 2018. https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664# Accessed:
- Jacobson L.H., Cryan J.F. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav. Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Javaras K.N., Pope H.G., Lalonde J.K., Roberts J.L., Nillni Y.I., Laird N.M., Bulik C.M., Crow S.J., McElroy S.L., Walsh B.T., Tsuang M.T., Rosenthal N.R., Hudson J.I. Co-occurrence of binge eating disorder with psychiatric and medical disorders. J. Clin. Psychiatr. 2008;69:266–273. doi: 10.4088/jcp.v69n0213. [DOI] [PubMed] [Google Scholar]
- Kenche V.B., Barnham K.J. Alzheimer's disease & metals: therapeutic opportunities. Br. J. Pharmacol. 2011;163:211–219. doi: 10.1111/j.1476-5381.2011.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen H., Mannisto S., Sarlio-Lahteenkorva S., Silventoinen K., Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite. 2010;54:473–479. doi: 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Han M.H., Graham D.L., Berton O., Renthal W., Russo S.J., Laplant Q., Graham A., Lutter M., Lagace D.C., Ghose S., Reister R., Tannous P., Green T.A., Neve R.L., Chakravarty S., Kumar A., Eisch A.J., Self D.W., Lee F.S., Tamminga C.A., Cooper D.C., Gershenfeld H.K., Nestler E.J. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J., Chuang J.C., Na E.S., Kuperman A., Gillman A.G., Mukherjee S., Zigman J.M., McClung C.A., Lutter M. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite. 2013;64:81–88. doi: 10.1016/j.appet.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Huchton D.M., Wiener S.G., Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev. Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Li Z., Maglione M., Tu W., Mojica W., Arterburn D., Shugarman L.R., Hilton L., Suttorp M., Solomon V., Shekelle P.G., Morton S.C. Meta-analysis: pharmacologic treatment of obesity. Ann. Intern. Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- Lightowler S., Wood M., Brown T., Glen A., Blackburn T., Tulloch I., Kennett G. An investigation of the mechanism responsible for fluoxetine-induced hypophagia in rats. Eur. J. Pharmacol. 1996;296:137–143. doi: 10.1016/0014-2999(95)00704-0. [DOI] [PubMed] [Google Scholar]
- Lopresti A.L., Drummond P.D. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2013;45:92–99. doi: 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Lucki I., Dalvi A., Mayorga A.J. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W., Zitman F.G. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatr. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2011;35:676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Marazziti D., Rutigliano G., Baroni S., Landi P., Dell'Osso L. Metabolic syndrome and major depression. CNS Spectr. 2014;19:293–304. doi: 10.1017/S1092852913000667. [DOI] [PubMed] [Google Scholar]
- Marco E.M., Adriani W., Llorente R., Laviola G., Viveros M.P. Detrimental psychophysiological effects of early maternal deprivation in adolescent and adult rodents: altered responses to cannabinoid exposure. Neurosci. Biobehav. Rev. 2009;33:498–507. doi: 10.1016/j.neubiorev.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N., Takao K., Nakanishi K., Yamasaki N., Tanda K., Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllum E.J., Lim N.K., Hickey J.L., Paterson B.M., Donnelly P.S., Li Q.X., Liddell J.R., Barnham K.J., White A.R., Crouch P.J. Therapeutic effects of CuII(atsm) in the SOD1-G37R mouse model of amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2013;14:586–590. doi: 10.3109/21678421.2013.824000. [DOI] [PubMed] [Google Scholar]
- Mckenzie-Nickson S., Bush A.I., Barnham K.J. Bis(thiosemicarbazone) metal complexes as therapeutics for neurodegenerative diseases. Curr. Top. Med. Chem. 2016;16:3058–3068. doi: 10.2174/1568026616666160216155746. [DOI] [PubMed] [Google Scholar]
- McKinney W.T., Jr., Bunney W.E., Jr. Animal model of depression. I. Review of evidence: implications for research. Arch. Gen. Psychiatr. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- Melhorn S.J., Krause E.G., Scott K.A., Mooney M.R., Johnson J.D., Woods S.C., Sakai R.R. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R813–R822. doi: 10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson D., Amsterdam J.D., Quitkin F.M., Reimherr F.W., Rosenbaum J.F., Zajecka J., Sundell K.L., Kim Y., Beasley C.M., Jr. Changes in weight during a 1-year trial of fluoxetine. Am. J. Psychiatr. 1999;156:1170–1176. doi: 10.1176/ajp.156.8.1170. [DOI] [PubMed] [Google Scholar]
- Miczek K.A., Thompson M.L., Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M., Gaillard P., Tanti A., Girault V., Belzung C., Leman S. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology. 2012;37:2210–2221. doi: 10.1038/npp.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens M.A., van Strien T., van Leeuwe J.F. Possible pathways between depression, emotional and external eating. A structural equation model. Appetite. 2009;53:245–248. doi: 10.1016/j.appet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Page D.L., Glenner G.G. Social interaction and wounding in the genesis of “spontaneous” murine amyloidosis. Am. J. Pathol. 1972;67:555–567. [PMC free article] [PubMed] [Google Scholar]
- Peng Y.L., Liu Y.N., Liu L., Wang X., Jiang C.L., Wang Y.X. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J. Neuroinflammat. 2012;9:75. doi: 10.1186/1742-2094-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Fuchs E. Chronic psychosocial stressors in adulthood: studies in mice, rats and tree shrews. Neurobiol Stress. 2017;6:94–103. doi: 10.1016/j.ynstr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler T.J. Inflammatory mechanisms in major depressive disorder. Curr. Opin. Psychiatr. 2011;24:519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Carboni L., Andreoli M., Ballottari A., Arban R. Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav. Brain Res. 2011;216:100–108. doi: 10.1016/j.bbr.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Pearson C., Crow S., Bartolomucci A. Stress, overeating, and obesity: insights from human studies and preclinical models. Neurosci. Biobehav. Rev. 2017;76:154–162. doi: 10.1016/j.neubiorev.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M., Sanghez V., Bartolomucci A. Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front Nutr. 2015;1 doi: 10.3389/fnut.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber S.O., Obermeier F., Straub R.H., Falk W., Neumann I.D. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology. 2006;147:4968–4976. doi: 10.1210/en.2006-0347. [DOI] [PubMed] [Google Scholar]
- Roberts B.R., Lim N.K., McAllum E.J., Donnelly P.S., Hare D.J., Doble P.A., Turner B.J., Price K.A., Lim S.C., Paterson B.M., Hickey J.L., Rhoads T.W., Williams J.R., Kanninen K.M., Hung L.W., Liddell J.R., Grubman A., Monty J.F., Llanos R.M., Kramer D.R., Mercer J.F., Bush A.I., Masters C.L., Duce J.A., Li Q.X., Beckman J.S., Barnham K.J., White A.R., Crouch P.J. Oral treatment with Cu(II)(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2014;34:8021–8031. doi: 10.1523/JNEUROSCI.4196-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler A.M., Bailey S.J. Repeated daily restraint stress induces adaptive behavioural changes in both adult and juvenile mice. Physiol. Behav. 2016;167:313–323. doi: 10.1016/j.physbeh.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Savignac H.M., Finger B.C., Pizzo R.C., O'Leary O.F., Dinan T.G., Cryan J.F. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011;192:524–536. doi: 10.1016/j.neuroscience.2011.04.054. [DOI] [PubMed] [Google Scholar]
- Savignac H.M., Hyland N.P., Dinan T.G., Cryan J.F. The effects of repeated social interaction stress on behavioural and physiological parameters in a stress-sensitive mouse strain. Behav. Brain Res. 2011;216:576–584. doi: 10.1016/j.bbr.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V., Wang X.D., Meijer O.C. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl) 2011;214:131–140. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- Serretti A., Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J. Clin. Psychiatr. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Cryan J.F. The ups and downs of modelling mood disorders in rodents. ILAR J. 2014;55:297–309. doi: 10.1093/ilar/ilu026. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Uschold N., Magoni M., Bar J., Popoli M., Neumann I.D., Reber S.O. Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrino. 2012;37:702–714. doi: 10.1016/j.psyneuen.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Stunkard A.J., Fernstrom M.H., Price A., Frank E., Kupfer D.J. Direction of weight change in recurrent depression. Consistency across episodes. Arch. Gen. Psychiatr. 1990;47:857–860. doi: 10.1001/archpsyc.1990.01810210065009. [DOI] [PubMed] [Google Scholar]
- Tramullas M., Dinan T.G., Cryan J.F. Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress. 2012;15:281–292. doi: 10.3109/10253890.2011.622816. [DOI] [PubMed] [Google Scholar]
- Tsankova N.M., Berton O., Renthal W., Kumar A., Neve R.L., Nestler E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Venzala E., Garcia-Garcia A.L., Elizalde N., Delagrange P., Tordera R.M. Chronic social defeat stress model: behavioral features, antidepressant action, and interaction with biological risk factors. Psychopharmacology (Berl) 2012;224:313–325. doi: 10.1007/s00213-012-2754-5. [DOI] [PubMed] [Google Scholar]
- Venzala E., Garcia-Garcia A.L., Elizalde N., Tordera R.M. Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur. Neuropsychopharmacol. 2013;23:697–708. doi: 10.1016/j.euroneuro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Weissenburger J., Rush A.J., Giles D.E., Stunkard A.J. Weight change in depression. Psychiatr. Res. 1986;17:275–283. doi: 10.1016/0165-1781(86)90075-2. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Reliability of the chronic mild stress model of depression: a user survey. Neurobiol. Stress. 2017;6:68–77. doi: 10.1016/j.ynstr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D.T., Perry K.W., Bymaster F.P. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat. Rev. Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- Yen T.T., Wong D.T., Bemis K.G. Reduction of food-consumption and body-weight of normal and obese mice by chronic treatment with fluoxetine - a serotonin reuptake inhibitor. Drug Dev. Res. 1987;10:37–45. [Google Scholar]
- Zurita E., Chagoyen M., Cantero M., Alonso R., Gonzalez-Neira A., Lopez-Jimenez A., Lopez-Moreno J.A., Landel C.P., Benitez J., Pazos F., Montoliu L. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011;20:481–489. doi: 10.1007/s11248-010-9403-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.