Abstract
Trauma-related disorders of affect and cognition (TRACs) are associated with a high degree of diagnostic comorbidity, which may suggest that these disorders share a set of underlying neural mechanisms. TRACs are characterized by aberrations in functional and structural circuits subserving verbal memory and affective anticipation. Yet, it remains unknown how the neural circuitry underlying these multiple mechanisms contribute to TRACs. Here, in a sample of 47 combat Veterans, we measured affective anticipation using functional magnetic resonance imaging (fMRI), verbal memory with fluorodeoxyglucose positron emission tomography (FDG-PET), and grey matter volume with structural magnetic resonance imaging (sMRI). Using a voxel-based multimodal canonical correlation analysis (mCCA), the set of neural measures were statistically integrated, or fused, with a set of TRAC symptom measures including mild traumatic brain injury (mTBI), posttraumatic stress, and depression severity. The first canonical correlation pair revealed neural convergence in clusters encompassing the middle frontal gyrus and supplemental motor area, regions implicated in top-down cognitive control and affect regulation. These results highlight the potential of leveraging multivariate neuroimaging analysis for linking neurobiological mechanisms associated with TRACs, paving the way for transdiagnostic biomarkers and targets for treatment.
Keywords: Trauma, TBI, PTSD, Multimodal imaging, Canonical correlation
1. Introduction
The neuropsychiatric consequences of traumatic events (e.g., military combat, assault, natural disasters) can manifest as a trauma-related disorder of affect and cognition (TRAC), including posttraumatic stress disorder (PTSD), major depressive disorder (MDD), and mild traumatic brain injury (mTBI). These disorders are highly debilitating and represent a significant burden for mental health clinicians and public policy makers (Hoge et al., 2008; Mac Donald et al., 2011). Moreover, these disorders have a high degree of comorbidity (Goldstein-Piekarski et al., 2016; Stein and McAllister, 2009) — obfuscating clinical decision-making and leading to poor treatment outcomes (Yehuda and Hoge, 2016). Diagnostic comorbidity may indicate that these disorders share underlying neural mechanisms, underscoring the need to provide an objective measure of the converging neural circuitry associated with the enduring effects of trauma (Milham et al., 2017; Woo et al., 2017).
TRACs are commonly linked to aberrations in neural circuits mediating memory processes (Zuj et al., 2016). Indeed, TRACs have been conceptualized as ‘disorders of memory’ (van Marle, 2015), with impairment observed for the encoding and learning phases of verbal memory in list learning tasks (Samuelson, 2011). Meta-analytic and experimental evidence indicates that compared to healthy controls, TRACs have a specific deficit in verbal memory encoding and immediate retrieval relative to visual-spatial memory (Dean and Sterr, 2013; Johnsen and Asbjørnsen, 2008; Mohn and Rund, 2016). Furthermore, pre-deployment verbal memory impairment predicts post-deployment development of TRACs (Marx et al., 2009) and high pre-treatment verbal memory ability on list learning tasks predicts enhanced treatment response (Nijdam et al., 2015; Scott et al., 2017). Verbal memory impairment in TRACs is associated with functional and structural abnormalities across multiple neuroimaging modalities (Bremner et al., 2009). For example, memory impairment has been associated with smaller hippocampal volume using structural magnetic resonance imaging (sMRI) (Monti et al., 2013) and poor verbal memory encoding is associated with low hippocampal neural activity measured by functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) (Bremner et al., 2003). Prefrontal cortex activity, using fMRI and PET, is also associated with verbal memory encoding and immediate recall impairment in individuals with TRACs, including PTSD, mTBI, and MDD (Buchsbaum et al., 2015; McDonald et al., 2012; J. Zhang et al., 2010). Collectively, these results suggest that deficits in verbal memory processes may be a transdiagnostic feature cutting across multiple TRACs and can be observed through several neuroimaging modalities (Bremner et al., 2009; Samuelson, 2011).
There is considerable evidence across fMRI, sMRI, and PET studies that TRACs are associated with aberrations in core affective processing, with neural differences in regions including the dorsolateral prefrontal cortex (dlPFC), anterior insula (aINS), ventromedial prefrontal cortex (vmPFC), hippocampus, and the amygdala compared to healthy controls (Admon et al., 2013; Milad et al., 2009). One such affective process important to trauma-related disorders is affective anticipation (Grupe and Nitschke, 2013; Grupe et al., 2016). Neurobiologically, anticipation of affective information is engaged by the aINS and the extended amygdala (Simmons et al., 2004; Simmons et al., 2006). PTSD is associated with increased aINS and amygdala activity in threat anticipation tasks (Grupe et al., 2016; Simmons et al., 2013). Unmedicated MDD is also associated with increased aINS and decreased prefrontal activity while anticipating aversive pain stimuli, suggesting a deficit in “top-down” regulation of affective responses (Strigo et al., 2013). These findings are consistent with evidence that TRACs are associated with structural connectivity decrements in networks implicated in emotion regulation. However, transdiagnostic aberrations in this circuitry have not always been observed (Glenn et al., 2017; Jovanovic et al., 2010; Reger et al., 2012). These inconsistent findings highlight the need to measure multiple disorders in a single analysis to better understand the circuit dysfunction that cuts across several types of TRACs.
Despite the advances in our understanding of the neural circuitry associated with verbal memory deficits and aversive anticipation abnormalities in TRACs, there is a paucity of research investigating how the relationship between these two mechanisms contribute to TRACs (Zuj et al., 2016). Instead, much of the extant research on TRACs have used a single neuroimaging modality to test a specific circuit hypothesis for a single mechanism. However, focusing on a single neural mechanism (e.g., verbal memory deficits) ignores how the relationship between different mechanisms (e.g., verbal memory processes + affective anticipation) and neural measures (structure and function) mutually contributes to TRACs. One potentially useful approach to address these limitations is to integrate multiple neural measures using multimodal neuroimaging fusion analysis (Calhoun and Sui, 2016; Sui et al., 2012). Multimodal fusion is a class of multivariate analyses that identifies neural patterns from multiple neuroimaging measures by maximizing fit on the shared correlation across features, and can be used to derive transdiagnostic neural profiles of neuropsychiatric disorders (Sui et al., 2013). A multimodal fusion approach has the advantage of potentially identifying complex and high-dimensional relationships between mechanisms that a univariate analytic approach would miss (Sui et al., 2015). Yet, no study to date has attempted this approach to trauma-related disorders.
To this end, we applied a novel voxel-wise multimodal canonical correlation analysis (mCCA) in a sample of Veterans that had completed a verbal memory task of encoding and immediate recall and a threat anticipation task while undergoing FDG-PET and fMRI, respectively. We also collected a high-resolution T1 scan to measure grey matter volume (sMRI). Canonical correlation analysis is a multivariate analysis of correlation, allowing for the measurement of latent canonical variants that represent the maximized linear relationship between multiple sets of variables (Hotelling, 1936). Similar to principal components analysis (PCA) and independent components analysis (ICA), CCA is a form of multivariate data reduction. Whereas, PCA is used to extract the most important features from a single set of variables and ICA derives independent features from a set of variables, CCA is used to extract features from multiple sets of variables such that these multiple features are maximally correlated with each other (Tabachnick and Fidell, 2006). Therefore, CCA has the advantage of explaining variance and novel patterns between multiple sets of features (Härdle & Simar, 2015). CCA has been extended to the analysis of multimodal neuroimaging data (mCCA) to link together multiple neural measures to identify multivariate patterns between brain features and psychiatric disorders (Calhoun and Sui, 2016).
The aim of the current investigation was to test whether multivariate neural patterns linking grey matter volume with neural activity associated with verbal memory functioning (FDG-PET) and threat anticipation (fMRI) mutually contribute to TRACs. Individuals with TRACs have been characterized as having problems with top-down control of affective responses and cognition (Etkin and Wager, 2007; Rive et al., 2013; van der Horn et al., 2016). Therefore, we hypothesized that a multivariate neural circuit would be shared across all trauma-related disorders, particularly in regions of the prefrontal cortex that facilitate top-down control (Goldin et al., 2008; Simmons and Matthews, 2012). We also hypothesized that the amygdala and aINS would be prominent regions expressed across imaging modalities (Liberzon and Abelson, 2016). Using multivariate fusion analyses with multimodal neuroimaging may provide an unprecedented level of understanding of how TRACs are manifested in the brain — paving the way for neuroscientifically informed diagnosis and treatment.
2. Methods
2.1. Participants
To collect a wide array of TRACs, we recruited a sample of 47 male military Veterans from the San Diego Veterans Administration Healthcare System. Two-thirds of the subjects (n = 32) experienced a self-reported combat-related traumatic event, where half of these subjects experienced a self-reported head injury (n = 16). The final third of the subjects (n = 15) did not experience a criterion A trauma during deployment and therefore did not meet criteria for PTSD or mTBI (i.e., Veterans without exposure to blast-related traumatic brain injury and did not meet criteria for deployment-related posttraumatic stress disorder, see Table 1). Subject inclusion and exclusion criteria are provided in the Supplement. The institutional review boards of the VA San Diego Research Service approved all study procedures, and informed consent was obtained from all subjects (Buchsbaum et al., 2015).
Table 1.
Demographics and measures.
| Variable | Mean (SD) & Count |
|---|---|
| Age | 29.6 (5.9) |
| Education | 14.2 (1.2) |
| Ethnicity | |
| Caucasian | 25 |
| Hispanic/Latino | 13 |
| Other | 9 |
| mTBI | 32 |
| Type | |
| Blast | 28 |
| Fall | 6 |
| Vehicle | 6 |
| Other | 4 |
| LOC | |
| Dazed | 28 |
| LOC <1 min | 9 |
| LOC>1–20 min | 5 |
| Don't Recall | 5 |
| CAPS-IV | 40.04 (35.59) |
| BDI-II | 11.07 (10.98) |
Note. mTBI = self-reported mild traumatic brain injury. LOC = self-reported loss of consciousness. CAPS-IV=Clinical Assessment of PTSD Scale. BDI-II=Beck Depression Inventory-II.
2.2. Measuring trauma-related disorders
To assess for trauma-related disorders, we focused on three primary diagnostic or symptom types: mTBI, PTSD symptoms, and depressive symptoms. To measure the presence or absence of mTBI, subjects were first screened using the self-report Brief traumatic brain injury screen (BTBIS). Criteria for a diagnosis of mTBI were next determined using the American Congress of Rehabilitation Medicine guidelines (Harrington et al., 1993). PTSD symptoms were assessed with the Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) (Blake et al., 1995) the gold-standard clinical interview assessing for diagnostic criteria and severity of PTSD. CAPS-IV total score was used to assess PTSD symptom severity. The Beck Depression Inventory version 2 (BDI-II) (Beck et al., 1996) total score was used to assess for symptoms of depression. See Supplemental Methods for more details regarding the psychiatric measures and inclusion/exclusion criteria.
2.3. Tasks
2.3.1. fMRI: cued affective anticipation paradigm
This task (See Supplementary Fig. 1), which has been validated and used in prior studies (Simmons et al., 2004, 2006), combines a simple continuous performance task and a cued stimulus presentation to examine the effects of anticipation. The task has three conditions: (1) a baseline condition, during which the individual is performing the continuous performance task; (2) an anticipation condition, when the stimulus characteristics of the shape changes and signals the impending presentation of a visual stimulus; and (3) the stimulus condition, when the subject is presented with a “pleasant” or “unpleasant” picture. During the baseline condition (4–11 s), a blue square is shown and a medium-range tone (500 Hz) is associated with each trial. For the anticipation condition, subjects are informed that red shapes and a “high-pitched tone” (1000 Hz) predict the consequent presentation of an aversive or “unpleasant” picture, whereas green shapes and a “low-pitch tone” (250 Hz) predict the presentation of a “pleasant” picture. The anticipation condition consists of an 8-s period. The stimulus condition consists of a 2-s presentation of an image, which is shown only once during the task. The images were a combination from the International Affective Picture System (Lang et al., 1999) for the non-aversive (noncombat) images and aversive combat-images that were taken from a photographer accompanying a Marine unit deployed to Iraq (Simmons et al., 2013).
2.3.2. FDG-PET: Serial verbal learning task (SVLT)
During the FDG uptake and prior to FDG-PET acquisition, participants completed the SVLT (see Supplementary Fig. 2). The SVLT is a verbal memory task used to assess the ability to encode and immediately recall verbal information. The SVLT results of these data are reported elsewhere (Buchsbaum et al., 2015). In this task, subjects learn five sets of 16 word lists, where each word was presented sequentially for 1.5 s each. After the 16th word, subjects verbally recalled the word list. Each word list was repeated five times.
2.4. Neuroimaging acquisition and preprocessing
2.4.1. fMRI and sMRI
Imaging experiments were performed on a 3T GE scanner at the UCSD Keck Imaging Center. Each session consisted of an anatomical scan (SPGR; T1-weighted; FOV = 25 cm; matrix = 256 × 256; 176 1 mm sagittal slices; TR = 8 ms; TE = 4 ms; flip = 12°), and a series of BOLD scans (echo planar image [EPI]; T2*-weighted; matrix = 64 × 64; 30 axial slices; 3.43 × 3.43 × 2.6 mm voxels with 1.4 mm gap; TR = 2s; TE = 32 ms; flip = 90°). Subjects performed the cued affective anticipation paradigm that was acquired in 290 repetitions lasting 9 min and 40 s.
2.4.2. fMRI image preprocessing
Standard fMRI image pre-processing and analysis was conducted using AFNI (Cox, 1996). The first two volumes of the EPI images were removed. The remaining images were slice-time and motion corrected. Images were spatially smoothed (6 mm full-width half-maximum) and converted to percent signal change. Second-level analyses were conducted using a general linear model. Two task regressors of interest were entered into the model, one for the unpleasant anticipation period, and one for the pleasant anticipation period. Two regressors of non-interest were entered in for the positive and negative image presentation. These regressors were modeled with the canonical hemodynamic response. Six additional parameters (x, y, z, roll, pitch, yaw) were used as nuisance regressors to adjust EPI intensity changes due to motion artifacts. Constant and linear regressors to account for scanner drift were also included as nuisance regressors. Whole-brain beta-estimates from the unpleasant > pleasant anticipation contrast was then entered into primary analysis (see below).
2.4.3. sMRI image preprocessing
sMRI image processing was conducted using Voxel-Based Morphometry (VBM) within the FSL suite (Douaud et al., 2007) to evaluate grey matter morphometry on a voxel-wise basis. All subjects’ anatomical images were normalized and segmented using the unified segmentation model and tissue probability maps, followed by hidden Markov random field clean-up (Zhang et al., 2001). Jacobian modulation was applied to compensate for the effect of spatial normalization and to restore the original absolute grey matter density in the segmented grey matter images (Good et al., 2001). These modulated images were then smoothed with an 8-mm FWHM smoothing kernel.
2.4.4. FDG-PET acquisition and preprocessing
Each subject had an intravenous line inserted for tracer administration and then rested in a sound-attenuated room until the uptake period began. We administered 5 mCi FDG and the patients performed the serial verbal learning test (Hazlett et al., 2010) which has been used in earlier TBI studies (Buchsbaum et al., 2015). After approximately 32 min in which the SVLT was performed, each patient was positioned in a Siemens/CTI ECAT HR + scanner with the head oriented such that the lowest imaging plane was approximately 1 cm above and parallel to the canthomeatal line. We acquired 63 axial sections spaced 3.5 mm apart with a 15.5-cm field of view (FOV) in 3D mode, with standard 4-mm in-plane spatial resolution (full-width half-maximum, FWHM). Images were acquired at an angle parallel to the canthomeatal plane and reconstructed using a Hann filter (cut-off frequency = 0.5 cycles/pixel) into 128 × 128 pixel images. Four 5-min frames were collected and summed for the full analysis, and split-half images were formed from odd- and even-numbered frames (1 and 3, 2 and 4) for reliability testing. Data were spatially smoothed with Gaussian profile filter of FWHM 3 mm; high-pass temporal filtering with Gaussian-weighted running line detrending was applied to reduce temporal noise (cutoff = 70s).
2.4.5. Multimodal alignment
After preprocessing, each subjects’ imaging modality (fMRI, FDG-PET, sMRI) was aligned to the MNI template using Advanced Normalization Tools (ANTs) (Avants et al., 2011) to better account for linear and morphometric alignment differences between subject and imaging modality. The FDG-PET images were used to create a grey matter mask for subsequent multivariate analyses. All datasets were resampled to 2 × 2x2mm voxels.
2.4.6. Multimodal canonical correlation analysis (mCCA)
A voxel-based mCCA was performed in R (package: CCA). CCA is a multivariate analysis of correlation, allowing for the measurement of latent canonical variant pairs that represent the maximized linear relationship between multiple sets of variables (Hotelling, 1936). Here we applied this approach using multiple measures of trauma-related disorders and multiple neuroimaging modalities (Calhoun and Sui, 2016).
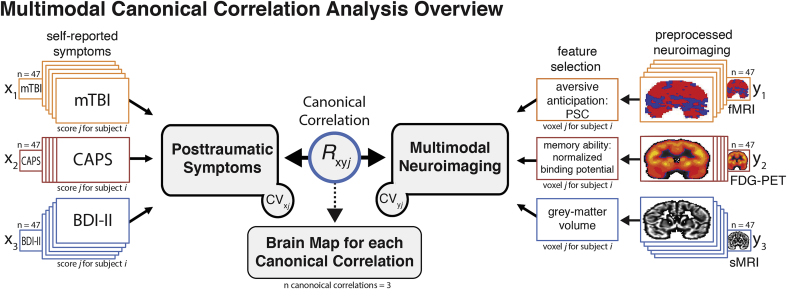
A voxel-wise mCCA approach was used to identify neural regions that reflect the maximized linear relationship between a pair of canonical variants (CV), where one CV is a TRAC feature, and the other feature is a neuroimaging CV. As shown in Fig. 1, for each subject, we defined the TRAC CV by entering three TRAC metrics as separate variables (x1, x2, x3) using presence or absence of a head-injury (mTBI), severity of PTSD symptoms (CAPS-IV total score), and depression severity (BDI-II total score). For the neuroimaging CV, we entered each imaging modality (y1, y2, y3) as separate variables. For the fMRI measure of affective anticipation (y1), we entered the whole-brain beta estimates from the preprocessed unpleasant > pleasant image contrast maps. For the FDG-PET measure of verbal memory functioning, FDG metabolism binding potential-maps were entered (y2). For the sMRI measure of grey-matter volume, voxel-based morphometry (VBM) maps were used (y3). Next, voxel-wise canonical correlation brain maps were derived (orthogonal 1st, 2nd, & 3rd factors), which represent neural circuitry associated with the maximum linear relationship between the two canonical pairs (i.e., correlation of the two CVs), allowing for a voxel-by-voxel integration (or ‘fusion’) across neuroimaging measures and TRACs. For example, through mCCA one can derive where BOLD response, glucose metabolism, and structural volume maximally relate to TRACs. Canonical correlation brain maps were thresholded (p < .001) and conservatively whole-brain corrected (p < .001) for multiple comparisons using AFNI's Clustsim with the spatial autocorrelation function (-acf) to account for the non-Gaussian shape of fMRI data (Cox et al., 2017). We used the -acf parameters from the fMRI blur in Clustsim because it required a larger cluster size to achieve significance (204 voxels) compared to PET (196 voxels) and sMRI (135 voxels).
Fig. 1.
Analysis schematic depicting the multimodal canonical correlation analysis (mCCA). For mCCA, each TRAC measure (x1, x2, x3) and imaging modality (y1, y2, y3) were entered into the model. For the fMRI measure of affective anticipation (y1), we entered the whole-brain beta estimates from the preprocessed unpleasant > pleasant image contrast maps. For the FDG-PET measure of verbal memory functioning, FDG metabolism-maps were entered (y2). For the sMRI measure of grey-matter volume, voxel-based morphometry (VBM) maps were used (y3). On a voxel-by-voxel basis, the mCCA analysis derives latent variables (i.e., canonical variates[CVs]) representing the maximized linear association between the variables on one side and the opposing CV. The output (Rxy) is a voxel-wise brain map of the canonical correlation coefficient between the TRAC CV and the neuroimaging CV.
2.4.7. Fear-network ROIs
We further examined several regions of interest (ROIs) relevant to trauma-related disorders, including the amygdala, anterior insula, and ventromedial PFC, to validate that the mCCA fusion analysis produces strong associations between neuroimaging and trauma-related disorders. Regions of interest (ROI) were anatomically defined using the Harvard-Oxford cortical and subcortical atlases. For more details, see analyses in trauma-related disorder-relevant ROIs are reported in the Supplementary Materials.
For descriptive purposes, we examined the unique and shared contribution between neural measures of cognition and affective anticipation, grey matter volume, and trauma-related disorders. We extracted the pre-processed neuroimaging data from each unimodal neuroimaging modality from ROIs identified from the first canonical correlation brain map. Specifically, we extracted percent signal change from the fMRI measures of the affective anticipation task, FDG metabolism binding potential beta-estimates from the verbal memory task, and grey matter volume from the VBM analysis. Due to the concern of multicollinearity and to avoid making strong interpretations of statistical significance of the individual variables, we don't report inferential statistics but do present data for visual depiction, strength, and direction of the relationships. (e.g. t-values, r-values, p-values).
3. Results
3.1. Fusing multimodal imaging with trauma-related disorders
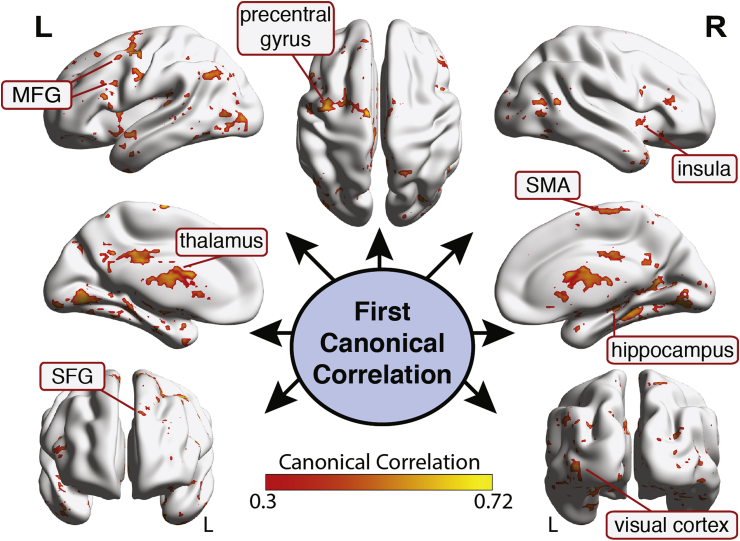
We performed a voxel-wise mCCA to determine how and where the fusion of neural measures of verbal memory, affective anticipation, and grey matter volume, as measured by PET/fMRI/sMRI respectively, related to key TRACs. The aim was to determine the strongest associations between neuroimaging mechanism-measures (fMRI/PET/sMRI) and TRACs (mTBI/PTSD/Depression). The first canonical correlation (Fig. 2) revealed a broad network representing the convergence of verbal memory and anticipatory anxiety neural activation and grey matter volume with TRACs. There was a strong association (R > 0.508, p < .001 cluster-corrected) in the supplemental motor area (SMA) and lateral prefrontal cortices, insula/inferior frontal gyrus (IFG), thalamus, as well as the hippocampus and temporal cortex (See Table 2 for a complete list of clusters identified). These observations indicate a broad set of neural regions that represent the maximized linear integration of verbal memory encoding and recall, affective anticipation processing, and grey matter volume that correlate with the three types of trauma-related disorders. No clusters survived thresholding in the other two canonical correlations (ps > .001; but see Supplemental Materials for sub-threshold brain maps). See Supplementary Figs. 3–5 for effect size (Canonical Correlation R2) brain maps for all three canonical correlations.
Fig. 2.
Results of the voxel-based mCCA for the first canonical correlation associating presence of mTBI, current level of PTSD, and current level of depression with functional MRI, FDG-PET, and structural MRI scans. Clusters represent the 1st canonical correlation that reflects the maximized linear relationship between the trauma-related disorder canonical variant (CV) and the neuroimaging CV (cluster corrected p < .001). Brain maps reflect canonical R values.
Table 2.
Cluster information for the first canonical correlation.
| Region | x | y | z | BA | Volume (voxels) | 1st Canonical Correlation |
|---|---|---|---|---|---|---|
| L Precentral gyrus | −29 | −1 | 47 | 6 | 729 | .523 |
| L Middle frontal gyrus 1 | −38 | 20 | 21 | 9 | 430 | .508 |
| L Middle frontal gyrus 2 | −39 | 57 | 4 | 46 | 239 | .517 |
| R Insula | 37 | 32 | 14 | 13 | 263 | .513 |
| R Supplementary motor area | 8 | −21 | 67 | 6 | 204 | .508 |
| L Rostral superior frontal gyrus | −35 | 43 | −17 | 11 | 202 | .515 |
| R Hippocampus | 36 | −37 | −5 | 36 | 2027 | .519 |
| L Thalamus | −19 | −22 | 2 | – | 11488 | .516 |
| R Rostral superior temporal gyrus | 51 | 9 | −10 | 38 | 418 | .512 |
| R Primary visual cortex | 26 | −81 | 5 | 17 | 388 | .507 |
| L Extrastriate visual cortex | −39 | −71 | −39 | 19 | 221 | .524 |
| L Cerebellum | −36 | −71 | −39 | – | 217 | .504 |
| L Precuneus | −5 | −56 | 25 | 23 | 187 | .472 |
Note. Voxels are 2 mm3 1st Canonical Correlation reflects the maximum linear relationship between the fused neuroimaging modalities and trauma-related disorders of affect and cognition (i.e., shared variance between the two canonical variants). Coordinates are in MNI coordinate space. BA=Broadmann Area.
We also extracted the first canonical correlation from several fear-network ROIs (see Method). We observed a relatively strong integration of functional activity (fMRI and PET) and grey matter volume with TRACs in the amygdala, left amygdala: canonical R = 0.43, p=.002; right amygdala: canonical R = 0.40, p = .005 (See Supplementary Table 1 for all ROIs). The dACC and aINS ROIs showed moderate effects, canonical Rs > 0.36 but did not survive correction for number of ROI correlations ( < 0.05/6 = 0.008). As expected, we observed that the fused neuroimaging modalities performed better at accounting for more variance with trauma-related disorders than any single neuroimaging modality across the ROIs: Fusion R2s > 0.13; unimodal R2s < 0.02 (see Supplementary Table 1 for all ROIs).
3.2. Interpreting the relationship between TRACs and neuroimaging using ROIs identified from the 1st canonical correlation
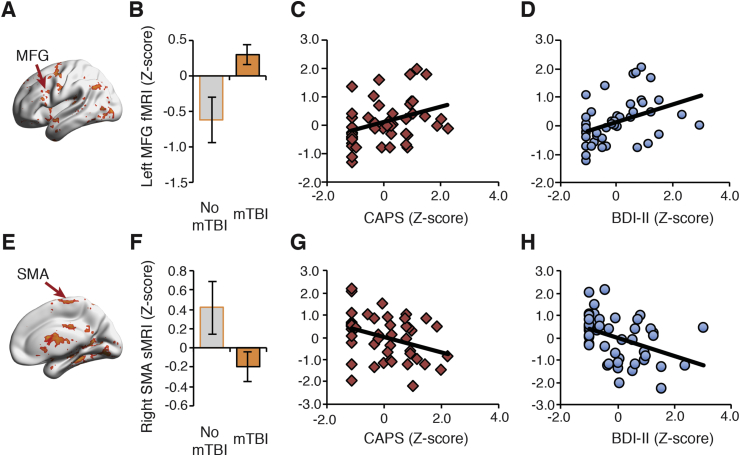
To facilitate interpretation of the first canonical correlation results and for descriptive purposes and not for determination of significance, we extracted the beta-estimates from the fMRI measure of aversive anticipation (aversive > pleasant contrast), glucose metabolism/binding potential from the FDG-PET measure of verbal memory encoding/recall, and grey matter volume from the sMRI measure using ROIs generated from the brain-map of the first canonical correlation. Next, we examined the relationship between neural circuitry and individual trauma-related disorder types. Across all TRACs, two regions revealed a shared latent trauma-related disorder signature depending upon imaging modality: the left middle frontal gyrus (MFG) and the SMA. The left MFG cluster was most evident with the fMRI affective anticipation loading. Whereas, the SMA was found with both neural features associated with affective anticipation and grey matter (fMRI and sMRI). As shown in Fig. 3a, evaluating trauma-related disorders within these clusters, we observed that differences within left MFG fMRI affective anticipation loadings predicted the presence of mTBI (Fig. 3b, bar plot), and was positively associated with PTSD severity (Fig. 3c, second panel) and depressive symptoms (Fig. 3d, third panel). Likewise, we found that the right SMA grey matter measured through sMRI (Fig. 3e), revealed differences between mTBI and no mTBI groups (Fig. 3f, bar plot), and negatively predicted PTSD (Fig. 3g, second panel) and depression severity (Fig. 3h, third panel).
Fig. 3.
Visualization of the first canonical correlation effects in the fMRI and SMA. (A) Aversive anticipation fMRI activity in the left MFG differentiated mTBI from no mTBI patients (B), and was increased with elevated PTSD (C) and depression severity (D). (E) Grey matter volume (sMRI) from a cluster in the right supplementary motor area (SMA) differentiated mTBI from no mTBI patients (F), was negatively associated with PTSD (G) and depression symptoms (H). All ROIs were identified from the first canonical correlation brain map (see Fig. 2). Results are reported for to facilitate interpretation and are for visualization purposes only.
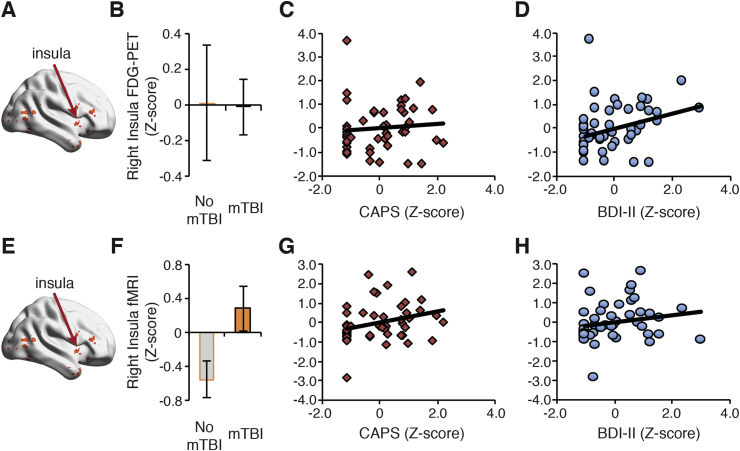
We also explored other important clusters identified from the first canonical correlation brain map. The verbal memory FDG-PET glucose binding potential was positively associated with depression severity in the right insula (Fig. 4a,d) but not for mTBI or PTSD (Fig. 4b,c). Interestingly, this same cluster using affective anticipation fMRI activity revealed a different pattern (Fig. 4b). Specifically, affective anticipation from the right insula (Fig. 4e) was positively associated with the presence of mTBI (Fig. 4f) and to a lesser extent to PTSD symptoms (Fig. 4g), but not depression severity (Fig. 4h). We also identified a large affective anticipation cluster encompassing the thalamus that was particularly important to the presence of mTBI (Supplementary Fig. 6). In addition, affective anticipation in the right hippocampus (Supplementary Fig. 7a-d) was increased in individuals with mTBI and positively associated with PTSD severity, but unrelated to depression. The amygdala ROI was particularly important for mTBI and depression (Supplementary Fig. 8).
Fig. 4.
Visualization of the first canonical correlation effects in the insula. (A) FDG-PET glucose binding potential in the right insula was not associated with mTBI (B) or to PTSD symptoms (C) but was positively associated with depression severity (D). (E) An fMRI-based cluster in the right insula differentiated mTBI from no mTBI patients (F), and was weakly associated PTSD symptoms (G), but not depressive symptoms as measured by the Beck Depression Inventory II (BDI-II) (H). All ROIs were identified from the first canonical correlation brain map (see Fig. 2). Results are reported for to facilitate interpretation and are for visualization purposes only.
4. Discussion
The aim of the current investigation was to integrate neural measures of verbal memory (PET), affective anticipation (fMRI), and grey matter volume (sMRI) via multimodal canonical correlation analysis (mCCA) to better understand the neural circuitry underlying the complex clinical profile associated with TRACs. We identified several brain regions that represented the shared relationship between TRACs and neural measures of cognitive-affective function. From our review of the literature, this is the first study to integrate multiple mechanisms and neural measures using fMRI, FDG-PET, and sMRI with TRACs using a multivariate mCCA analytic approach. These data suggest that leveraging multimodal neuroimaging to identify integrated neural circuitry can provide valuable information associated with the complex neuropsychiatric sequela of TRACs.
TRACs shared cognitive, affective, and grey matter signatures in prefrontal regions, including the MFG, SFG, and SMA. To facilitate interpretation, we show that affective anticipation in the left MFG as measured with fMRI was positively associated with all three TRACs (i.e., presence of mTBI, PTSD symptoms, depression symptoms). The MFG is involved in a vast array of functions, key among these in relation to TRACs is the top-down affective regulation associated with successful cognitive reappraisal of negative information (Goldin et al., 2008; Wager et al., 2008). Increased MFG across all TRACs may suggest an exaggerated recruitment of a top-down control signal to down-regulate periods of affective anticipation that is transdiagnostic to trauma-related disorders (Brinkmann et al., 2017). In addition to the MFG, we demonstrate that SMA is important to TRACS. We observed that SMA structural architecture is another important transdiagnostic region related to TRACs. The SMA is integral to executing explicit-motor plans (Nachev et al., 2007) and resolving emotional conflict (Deng et al., 2014). Indeed, selective serotonin reuptake inhibitors increase SMA activity during emotion regulation and predict treatment success in individuals diagnosed with PTSD (MacNamara et al., 2016). Collectively, these findings are consistent with our hypothesis that prefrontal top-down cognitive-affective neural circuitry is a transdiagnostic neural substrate of trauma-related disorders (Walther et al., 2012).
In addition to prefrontal regions, we found that the insula was a key region underlying neural function and structure in TRACs. The insula is an integral hub in the salience network (Menon and Uddin, 2010), plays a prominent role in integrating cognitive and emotionally salient information (Craig, 2009; Gu et al., 2013), and is important for interoceptive awareness (Critchley et al., 2004). Consistent with this function, increased activity in the insula has been associated with heightened emotional reactivity to internal physiological cues, anticipation of threat (Paulus and Stein, 2010), and cognitive load (Wesley and Bickel, 2014). The pattern observed in the current study could reflect increased cognitive resources devoted to encode and retrieve verbal information or negative affect when cognitively challenged, particularly for elevated depressive symptoms. Alternatively, it may reflect heightened affective responding to anticipating negative stimuli (Simmons et al., 2006).
We also found that the neural signatures in the thalamus and hippocampus were important to TRACs. The thalamus is highly connected to cortical and subcortical brain networks (O'Muircheartaigh et al., 2015), making it well positioned to influence cognitive (Schmitt et al., 2017) and affective processing (Arend et al., 2015). Our observation of thalamic differences in TRACs is consistent with a growing literature showing abnormal functional and structural connectivity in mTBI, PTSD, and MDD patients (Grossman and Inglese, 2016; Wise et al., 2016; Zhang et al., 2016), which is associated with impaired cognitive functioning in these patients (Grossman et al., 2012). We also observed that the right hippocampus was positively associated with TRACs. The hippocampus plays an integral role in episodic and contextual memory (Zeidman and Maguire, 2016), mediates the contextual retrieval of fear extinction (Sotres-Bayon et al., 2006), and is a central component of the fear regulation network via its rich structural and functional connectivity with the amygdala, anterior cingulate cortex, and the ventromedial PFC (Hartley and Phelps, 2010). This has led investigators to suggest that the hippocampus is critical for the pathogenesis of TRACs (Acheson et al., 2012; Liberzon and Abelson, 2016; Maren et al., 2013).
A meta-analysis of neuroimaging studies reported little overlap in the neural signatures of comorbid trauma-related disorders (Simmons and Matthews, 2012). Here, using a multivariate analytic approach, we identified a neuro-mechanistic TRAC profile. Based upon our findings, TRACs are associated with generalized aberrations in a prefrontal network instantiating the top-down control of cognition and affect, potentially contributing to poor executive functioning and emotion regulation (Kohn et al., 2014). This observation extends other work establishing top-down control deficits in mTBI, PTSD, and major depression separately (Etkin and Wager, 2007; Rive et al., 2013; van der Horn et al., 2016) by showing that this deficit is a transdiagnostic marker representing TRACs more broadly. These findings indicate that the dynamic interplay between verbal memory encoding/retrieval, affective anticipation processes, and grey matter may be a key component of TRACs. Through multivariate approaches, we can begin to understand how the integration of multiple mechanisms are manifested in the brain and determine the extent to which linked mechanisms contribute to the manifestation of TRACs.
We had hypothesized that the amygdala would be represented across imaging modalities. Although we did not observe an amygdala cluster through the whole-brain mCCA, we did observe that amygdala was moderately associated with TRACs through the ROI approach. The amygdala is a well-characterized region (Shackman and Fox, 2016) and is implicated in the pathophysiology of comorbid mTBI/PTSD (Bomyea et al., 2017), PTSD alone, (Stevens et al., 2017), and depression (Belzung et al., 2015). One possible reason we did not observe a robust amygdala cluster using the whole-brain mCCA is that our multimodal neuroimaging inputs were not specifically designed to capture amygdala-based processing (Simmons et al., 2011). This raises an important issue in that results from multimodal fusion analyses are inherently dependent upon the tasks and neural metric entered into the analysis. Using task-based neural measures may be helpful for understanding the joint-relationship between relevant mechanistic indices of neuropsychiatric disorders, but the results may not generalize to other mechanisms, tasks, or disorders.
Of course, limitations in our study indicate that important challenges remain. Recruiting larger samples with a wider selection of trauma-related disorders (e.g., substance use, other anxiety disorders) will be necessary, and extending this approach beyond combat Veterans and males will be critical for understanding how the integration of mechanistic neural function and structure represent neuropsychiatric disorders. Another limitation of our CCA approach is the difficulty interpreting individual TRAC disorders or neuroimaging modality-specific variables due to multicollinearity, particularly between mTBI, PTSD, and MDD measures. High relatedness between TRACs underscores the problems inherent to our current neuropsychiatric classification systems (Clark et al., 2017). A challenge for future research will be to apply multimodal neuroimaging fusion with machine learning approaches to develop brain-robust or brain-derived diagnosis and to predict treatment outcomes (Woo et al., 2017). A potential fruitful approach would be to expand the neuroimaging fusion analysis using non-task based neuroimaging modalities (e.g., diffusion MRI, resting state MRI, EEG frontal asymmetry). Using non-task based neural features may increase reliability and generalizability in other samples — a critical necessity for clinical applications (Linden, 2012). For example, network analysis for understanding linked neural structure and function combined with hierarchical clustering or support vector machine learning analysis may provide either better matching to current psychiatric models or potentially lead to new classification and personalized medicine (Calhoun and Sui, 2016).
In conclusion, the results of our study are the first to demonstrate that multimodal fusion of cognition and affective anticipation-based neural circuitry are associated with TRACs, suggestive of generalized dysfunction in top-down control of emotional arousal. Collectively, these data raise the possibility that patterns among distinct mechanisms and neuroimaging modalities may provide novel characterization of the dynamic and multifaceted neural architecture underlying TRACs. This sets the stage for novel prophylactic and precision treatments that target the underlying neural structure and function associated with TRACs.
Author contributions
DMS and ANS performed analysis. DMS, VBR, and ANS wrote the manuscript. ANS, IAS, MSB, and SCM designed study. ADS collected data. All authors discussed results and commented on manuscript.
Competing financial interests
The authors declare no competing financial interests.
Acknowledgements
We thank Alexander Decastro for his assistance with PET data analysis, as well as Elena Kosheleva, Laurel Glockler and Ryan O'Connell for their assistance with data collection. This work was supported in part by the United States Department of Veterans Affairs I01-CX-000816 (IAS), I01-CX000292 & I01-CX000715 (ANS), IK2-CX000864 (ADS), VBR (IO1-BX002558), and (SCM), and from the VA - Center of Excellence in Stress and Mental Health (DMS, ANS, ADS). Writing of this manuscript was partially supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.09.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data Profile
References
- Acheson D.T., Gresack J.E., Risbrough V.B. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology. 2012;62(2):674–685. doi: 10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R., Milad M.R., Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cognit. Sci. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Arend I., Henik A., Okon-Singer H. Dissociating emotion and attention functions in the pulvinar nucleus of the thalamus. Neuropsychology. 2015;29:191–196. doi: 10.1037/neu0000139. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-ii. [Google Scholar]
- Belzung C., Willner P., Philippot P. Depression: from psychopathology to pathophysiology. Curr. Opin. Neurobiol. 2015;30:24–30. doi: 10.1016/j.conb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD Scale. J. Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bomyea J., Matthews S.C., Buchsbaum M.S., Spadoni A.D., Strigo I.A., Simmons A.N. Neural differences underlying face processing in veterans with TBI and co-occurring TBI and PTSD. J. Affect. Disord. 2017;223:130–138. doi: 10.1016/j.jad.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Vythilingam M., Vermetten E., Southwick S.M., McGlashan T., Nazeer A. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatr. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Douglas Bremner J., M. D Neuroimaging in posttraumatic stress disorder and other stress- related disorders. Neuroimaging Clin. 2009;17:809–817. doi: 10.1016/j.nic.2007.07.003.Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann L., Poller H., Herrmann M.J., Miltner W., Straube T. Initial and sustained brain responses to threat anticipation in blood-injection-injury phobia. Neuroimage: Clinic. 2017;13:320–329. doi: 10.1016/j.nicl.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum M.S., Simmons A.N., DeCastro A., Farid N., Matthews S.C. Clusters of low (18)F-fluorodeoxyglucose uptake voxels in combat veterans with traumatic brain injury and post-traumatic stress disorder. J. Neurotrauma. 2015;32:1736–1750. doi: 10.1089/neu.2014.3660. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Sui J. Multimodal fusion of brain imaging data: a key to finding the missing link(s) in complex mental illness. Biol. Psychiatr. Cognit. Neurosci. Neuroimag. 2016;1:230–244. doi: 10.1016/j.bpsc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.A., Cuthbert B., Lewis-Fernández R., Narrow W.E., Reed G.M. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the national institute of mental health's research domain criteria (RDoC) Psychol. Sci. Publ. Interest. 2017;18:72–145. doi: 10.1177/1529100617727266. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel - now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, January;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dean P.J.A., Sterr A. Long-term effects of mild traumatic brain injury on cognitive performance. Front. Hum. Neurosci. 2013;7:30. doi: 10.3389/fnhum.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z., Wei D., Xue S., Du X., Hitchman G., Qiu J. Regional gray matter density associated with emotional conflict resolution: evidence from voxel-based morphometry. Neuroscience. 2014;275:500–507. doi: 10.1016/j.neuroscience.2014.06.040. [DOI] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn D.E., Acheson D.T., Geyer M.A., Nievergelt C.M., Baker D.G., Risbrough V.B. Fear learning alterations after traumatic brain injury and their role in development of posttraumatic stress symptoms. Depress. Anxiety. 2017;34:723–733. doi: 10.1002/da.22642. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatr. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. https://doi.org/S0006-3223(07)00592-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein-Piekarski A.N., Williams L.M., Humphreys K. A trans-diagnostic review of anxiety disorder comorbidity and the impact of multiple exclusion criteria on studying clinical outcomes in anxiety disorders. Transl. Psychiatry. 2016;6:e847. doi: 10.1038/tp.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N.A., Friston K.J., Frackowiak R.S.J. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grossman E.J., Inglese M. The role of thalamic damage in mild traumatic brain injury. J. Neurotrauma. 2016;33:163–167. doi: 10.1089/neu.2015.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E.J., Ge Y., Jensen J.H., Babb J.S., Miles L., Reaume J. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J. Neurotrauma. 2012;29:2318–2327. doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Nitschke J.B. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Wielgosz J., Davidson R.J., Nitschke J.B. Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychol. Med. 2016;46:1885–1895. doi: 10.1017/S0033291716000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Liu X., Van Dam N.T., Hof P.R., Fan J. Cognition-emotion integration in the anterior insular cortex. Cerebr. Cortex. 2013;23:20–27. doi: 10.1093/cercor/bhr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härdle W.K., Simar L. Applied Multivariate Analysis. Springer; Berlin, Heidelberg: 2015. Canonical correlation analysis. [Google Scholar]
- Harrington D.E., Malec J., Cicerone K., Katz H.T. Current perceptions of rehabilitation professionals towards mild traumatic brain injury. Arch. Phys. Med. Rehabil. 1993;74:579–586. doi: 10.1016/0003-9993(93)90155-4. [DOI] [PubMed] [Google Scholar]
- Hartley C.A., Phelps E.A. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett E.A., Byne W., Brickman A.M., Mitsis E.M., Newmark R., Haznedar M.M. Effects of sex and normal aging on regional brain activation during verbal memory performance. Neurobiol. Aging. 2010;31:826–838. doi: 10.1016/j.neurobiolaging.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hotelling H. Relations between two sets of variants. Biometrika. 1936;28:321–377. [Google Scholar]
- Johnsen G.E., Asbjørnsen A.E. Consistent impaired verbal memory in PTSD: a meta-analysis. J. Affect. Disord. 2008;111:74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Jovanovic T., Norrholm S.D., Blanding N.Q., Davis M., Duncan E., Bradley B., Ressler K.J. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress. Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. University of Florida: The Center for Research in Psychophysiology; Gainsville: 1999. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. [Google Scholar]
- Liberzon I., Abelson J.L. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D.E.J. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73:8–22. doi: 10.1016/j.neuron.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Brody D.L. Detection of blast-related traumatic brain injury in U.S. Military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A., Rabinak C.A., Kennedy A.E., Fitzgerald D.A., Liberzon I., Stein M.B., Phan K.L. Emotion regulatory brain function and SSRI treatment in PTSD: neural correlates and predictors of change. Neuropsychopharmacology. 2016;41:611–618. doi: 10.1038/npp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Phan K.L., Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx B.P., Doron-Lamarca S., Proctor S.P., Vasterling J.J. The influence of pre-deployment neurocognitive functioning on post-deployment PTSD symptom outcomes among Iraq-deployed Army soldiers. J. Int. Neuropsychol. Soc. 2009;15:840–852. doi: 10.1017/S1355617709990488. [DOI] [PubMed] [Google Scholar]
- McDonald B.C., Saykin A.J., McAllister T.W. Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imag. Behav. 2012;6:193–207. doi: 10.1007/s11682-012-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Pitman R.K., Ellis C.B., Gold A.L., Shin L.M., Lasko N.B. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatr. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., Craddock R.C., Klein A. Clinically useful brain imaging for neuropsychiatry: how can we get there? Depress. Anxiety. 2017;34:578–587. doi: 10.1002/da.22627. [DOI] [PubMed] [Google Scholar]
- Mohn C., Rund B.R. Neurocognitive profile in major depressive disorders: relationship to symptom level and subjective memory complaints. BMC Psychiatr. 2016;16:108. doi: 10.1186/s12888-016-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J.M., Voss M.W., Pence A., McAuley E., Kramer A.F., Cohen N.J. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 2013;5:41. doi: 10.3389/fnagi.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Wydell H., O'Neill K., Husain M., Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijdam M.J., de Vries G.-J., Gersons B.P.R., Olff M. Response to psychotherapy for posttraumatic stress disorder. J. Clin. Psychiatr. 2015;76:e1023–e1028. doi: 10.4088/JCP.14m09438. [DOI] [PubMed] [Google Scholar]
- O'Muircheartaigh J., Keller S.S., Barker G.J., Richardson M.P. White matter connectivity of the thalamus delineates the functional architecture of competing thalamocortical systems. Cerebr. Cortex. 2015;25:4477–4489. doi: 10.1093/cercor/bhv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger M.L., Poulos A.M., Buen F., Giza C.C., Hovda D.A., Fanselow M.S. Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol. Psychiatr. 2012;71:335–343. doi: 10.1016/j.biopsych.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhé H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Samuelson K. Post-traumatic stress disorder and declarative memory functioning: a review. Dialogues Clin. Neurosci. 2011;13:346–351. doi: 10.31887/DCNS.2011.13.2/ksamuelson. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3182004/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt L.I., Wimmer R.D., Nakajima M., Happ M., Mofakham S., Halassa M.M. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 2017;545:219–223. doi: 10.1038/nature22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.C., Harb G., Brownlow J.A., Greene J., Gur R.C., Ross R.J. Verbal memory functioning moderates psychotherapy treatment response for PTSD-Related nightmares. Behav. Res. Ther. 2017;91:24–32. doi: 10.1016/j.brat.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Fox A.S. Contributions of the central extended amygdala to fear and AnxietyContributions of the central extended amygdala to fear and anxiety. J. Neurosci. 2016;36:8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A.N., Matthews S.C. Neural circuitry of PTSD with or without mild traumatic brain injury: a meta-analysis. Neuropharmacology. 2012;62:598–606. doi: 10.1016/j.neuropharm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Simmons A., Matthews S.C., Stein M.B., Paulus M.P. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. https://doi.org/00001756-200410050-00024 [DOI] [PubMed] [Google Scholar]
- Simmons A.N., Strigo I., Matthews S.C., Paulus M.P., Stein M.B. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol. Psychiatr. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. https://doi.org/S0006-3223(06)00573-7 [DOI] [PubMed] [Google Scholar]
- Simmons A.N., Stein M.B., Strigo I.A., Arce E., Hitchcock C., Paulus M.P. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum. Brain Mapp. 2011;32:1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A.N., Flagan T.M., Wittmann M., Strigo I.A., Matthews S.C., Donovan H. The effects of temporal unpredictability in anticipation of negative events in combat veterans with PTSD. J. Affect. Disord. 2013;146:426–432. doi: 10.1016/j.jad.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F., Cain C.K., LeDoux J.E. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatr. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Stein M.B., McAllister T.W. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am. J. Psychiatr. 2009;166:768–776. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Kim Y.J., Galatzer-Levy I.R., Reddy R., Ely T.D., Nemeroff C.B. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol. Psychiatr. 2017;81:1023–1029. doi: 10.1016/j.biopsych.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo I.A., Matthews S.C., Simmons A.N. Decreased frontal regulation during pain anticipation in unmedicated subjects with major depressive disorder. Transl. Psychiatry. 2013;3:e239. doi: 10.1038/tp.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Adali T., Yu Q., Chen J., Calhoun V.D. A review of multivariate methods for multimodal fusion of brain imaging data. J. Neurosci. Meth. 2012;204:68–81. doi: 10.1016/j.jneumeth.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., He H., Pearlson G.D., Adali T., Kiehl K.A., Yu Q. Three-way (N-way) fusion of brain imaging data based on mCCA+jICA and its application to discriminating schizophrenia. Neuroimage. 2013;66:119–132. doi: 10.1016/j.neuroimage.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Pearlson G.D., Du Y., Yu Q., Jones T.R., Chen J. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol. Psychiatr. 2015;78:794–804. doi: 10.1016/j.biopsych.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B.G., Fidell L.S. BG Tabachnick & LS Fidell (5th ed.) Using Multivariate Statistics. Pearson; Boston, MA: 2006. Canonical correlation. [Google Scholar]
- van der Horn H.J., Liemburg E.J., Aleman A., Spikman J.M., van der Naalt J. Brain networks subserving emotion regulation and adaptation after mild traumatic brain injury. J. Neurotrauma. 2016;33:1–9. doi: 10.1089/neu.2015.3905. [DOI] [PubMed] [Google Scholar]
- van Marle H. PTSD as a memory disorder. Eur. J. Psychotraumatol. 2015;6 doi: 10.3402/ejpt.v6.27633. [DOI] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S., Hugli S., Hofle O., Federspiel A., Horn H., Bracht T. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol. Dis. 2012;47:13–19. doi: 10.1016/j.nbd.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Wesley M.J., Bickel W.K. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol. Psychiatr. 2014;75:435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., Radua J., Nortje G., Cleare A.J., Young A.H., Arnone D. Voxel-based meta-Analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol. Psychiatr. 2016;79:293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Woo C.-W., Chang L.J., Lindquist M.A., Wager T.D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Hoge C.W. The meaning of evidence-based treatments for veterans with posttraumatic stress disorder. JAMA Psychiatr. 2016;73:433–434. doi: 10.1001/jamapsychiatry.2015.2878. [DOI] [PubMed] [Google Scholar]
- Zeidman P., Maguire E.A. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 2016;17:173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imag. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhang J., Mitsis E.M., Chu K., Newmark R.E., Hazlett E.A., Buchsbaum M.S. Statistical parametric mapping and cluster counting analysis of [18F] FDG-PET imaging in traumatic brain injury. J. Neurotrauma. 2010;27:35–49. doi: 10.1089/neu.2009.1049. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen H., Long Z., Cui Q., Chen H. Altered effective connectivity network of the thalamus in post-traumatic stress disorder: a resting-state FMRI study with granger causality method. Appl. Inf. 2016;3:8. doi: 10.1186/s40535-016-0025-y. [DOI] [Google Scholar]
- Zuj D.V., Palmer M.A., Lommen M.J.J., Felmingham K.L. The centrality of fear extinction in linking risk factors to PTSD: a narrative review. Neurosci. Biobehav. Rev. 2016;69:15–35. doi: 10.1016/j.neubiorev.2016.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Profile