Abstract
Intense exercise generates an imbalance in the redox system. However, chronic exercise can yield antioxidant adaptations. A few studies with humans have investigated the effects of antioxidant diets on athletes. Therefore we compared the effects of two dietary interventions on oxidative stress in competitive triathletes. Thirteen male triathletes were selected and divided into 2 groups: one that had a regular antioxidant diet (RE-diet) and the other that had a high antioxidant diet (AO-diet). The diet period was 14 days and blood samples were collected before and after this period. The AO-diet provided twice the dietary reference intake (DRI) of α-tocopherol (30 mg), five times the DRI of ascorbic acid (450 mg), and twice the DRI of vitamin A (1800 g), while the RE-diet provided the DRI of α-tocopherol (15 mg), twice the DRI of ascorbic acid (180 mg) and the DRI of vitamin A (900 μg). The oxidative stress parameters evaluated were: thiobarbituric acid reactive substances (TBARS), total reactive antioxidant potential (TRAP), total sulfhydryl, carbonyl, superoxide dismutase (SOD) activity, hydrogen peroxide consumption and glutathione peroxidase (GPx) activity. We observed, after the diet period, an increase in sulfhydryl, TRAP, TBARS and SOD activity, and a decrease in carbonyl levels. However, no changes were found in hydrogen peroxide consumption or GPx activity. We concluded that antioxidant-enriched diets can improve the redox status of triathletes.
Keywords: Athletes, Antioxidants, Oxidative stress, Food, Vitamins
INTRODUCTION
A single session of exhaustive exercise is known to increase reactive oxygen species production in untrained individuals and, consequently, generate oxidative stress [1]. Similarly, athletes of intense aerobic exercise modalities, such as triathlon, could have increased plasmatic oxidative stress [2], which can be related to less or more efficient performance in a field test [3]. However, it has been demonstrated that chronic exercise is associated with a significant reduction in pro-oxidant parameters and an increase in antioxidant capacity [4]. Moreover, antioxidant nutrients intake may support the endogenous antioxidant enzymatic and non-enzymatic defence systems of the athletes, counterbalancing the negative effects of oxidative damage due to free radicals [5]. Interestingly, a previous study from our group showed that triathletes have higher antioxidant enzyme activity when compared to untrained individuals [6].
Athletes regularly participating in up to 40 minutes of acute high-intensity exercise may require higher intakes of exogenous nutrients to increase antioxidant capacity, which can be met through an adequate intake of an antioxidant-rich diet [7]. Studies have evaluated the effect of vitamin supplementation on oxidative stress in athletes, but the results are conflicting [8, 9]. High doses of antioxidants may negatively affect important physiological processes mediated by reactive oxygen species because they may shift from antioxidant to pro-oxidant effects. Despite the possible increased antioxidant capacity with increased vitamin uptake, some studies have demonstrated pro-oxidant effects of these substances in vitro [10] and in vivo [11].
Hence, a prudent recommendation for athletes is to consume vitamins and minerals of natural foods that are rich in antioxidants, such as fruits and vegetables, where natural ratios and proportions are present, acting in synergy to optimize the antioxidant effects, rather than taking supplements [12]. Daily consumption of vegetables is recommended because they are a rich source of vitamins, minerals, and phytochemicals, which exhibit a large number of biological benefits, such as antioxidant activity. Fruit and vegetable intake is correlated with an increase in plasmatic antioxidant concentration related to consumption frequency [13]. However, the recommended daily consumption of fruits and vegetables is rarely reached [14].
Therefore, the purpose of the present study was to compare the effects of two dietary interventions on selected blood markers of oxidative stress in competitive athletes. One diet had a high concentration of antioxidants and the other had a regular one. Our hypothesis is that oxidative damage markers should be reduced and antioxidant biochemical parameters should be increased in the antioxidant rich group.
MATERIALS AND METHODS
Subjects and experimental design
Thirteen male amateur triathletes, engaged in the modality for the last 6 years, participated in this study, and all participants signed an informed consent form. The athletes had not used antioxidant supplements for 4 weeks before and throughout study, which was conducted over 14 days. This study was in accordance with the Declaration of Helsinki and was approved by the Ethical Committee from Universidade Federal do Rio Grande do Sul, Brazil; Luiz Carlos Bombassaro (chairperson); protocol n.2005474.
The participants of this study were randomly assigned to either a regular diet (RE-diet) (n=6) or an antioxidant-rich diet (AO-diet) (n=7). Maximal exercise tests were performed within two weeks before the beginning of treatments. All participants followed specific diet regimens for 14 days and reported to the laboratory after an 8-h overnight fast. Blood samples at rest were obtained (15 ml), before and after 14 days of diet regimens, from the antecubital vein and collected in tubes with EDTA as an anticoagulant. The baseline blood levels were considered controls, which reflected the intake prior to interventions. All participants continued their training sessions and participated in competitions during the study.
Maximal exercise tests
Maximal oxygen uptake (VO2max) was measured using a metabolic chart (CPX/D system; Medical Graphics Corporation; St Paul, MN), and it was assessed breath by breath. Maximal exercise tests were performed on an electromagnetic cycle ergometer (CARDIO2, Medical Graphics Corp., St Louis, USA) and on a treadmill (Quinton Instruments, Seattle, USA) after a gradual protocol until exhaustion. Participants rested for 3 min before starting the test. In the cycle ergometer test, the initial workload was 25 W and it was increased by 25 W every 1 min, whereas in the treadmill test the initial speed was 5 km/h and the work rate was further increased by 0.5 km/h every 20 s. All tests lasted between 8 and 14 min. Heart rate was monitored continuously using a heart rate monitor (POLAR S610). The instruments were calibrated before all tests.
Anthropometric evaluation
Body mass was determined in the athletes without shoes or coats using a calibrated electronic scale and height was measured with a stadiometer. Subcutaneous body fat was assessed using a Lange skin-fold caliper from 7 sites (triceps, pectoral, midaxillary, subscapular, abdominal, suprailiac, quadriceps), and body fat percent was obtained [15].
Dietary intake
The previous dietary intake of the participants was assessed before the study began, using a 3-day food record (excluding weekends, to reflect usual training schedule). Participants were individually instructed on how to record food intakes using standard household measurements (a face-to-face interview). Each participant received a foods guidebook containing photographs showing household utensils and serving sizes. After the food diary had been filled in, a well-trained nutritionist examined it with the participant’s help in order to assess the records and eliminate inconsistencies. Moreover, the nutritionist verified and quantified the food records. All food items consumed were transformed into nutrients using the NUTWIN computer program for dietary analysis (UNIFESP, 1.5 version, 2002, SP, Brazil). The following food characteristics were used: total energy intake (relative to body mass); percentages of total energy from carbohydrates, proteins, and fats; and the dietary intake (total g, mg or mcg) of carbohydrates, proteins (relative to body mass), fats, fatty acids (saturated, mono-unsaturated (MUFA), poly-unsaturated (PUFA)), fibre, iron, β-carotene, ascorbic acid and α-tocopherol.
This study focused on two dietary interventions – RE-diet and AO-diet – for 14 days. Individualized diets were calculated to provide energy according to the training schedule to meet the recommended daily requirements. Macronutrients were in accordance with guidelines for endurance athletes. The diet period was chosen based on previous research that used supplementation with an antioxidant mixture or diets with high/low amounts of fruits and vegetables [7].
The RE-diet consisted of the RDA for α-tocopherol (15 mg), twice the RDA for ascorbic acid (180 mg), and the RDA for vitamin A (900 μg). Vitamins were in accordance with RDA, and did not exceed the upper limit, and the AO-diet consisted of twice the recommended dietary allowance (RDA) for α-tocopherol (30 mg), five times the RDA for ascorbic acid (450 mg), and twice the RDA for vitamin A (1800 μg) [16]. Participants also received: (i) a list of food equivalents for macronutrients; (ii) a list of foods to be avoided and foods to be consumed moderately during the regular diet because of their antioxidant vitamin content; (iii) a table of foods rich in β-carotene, α-tocopherol, and ascorbic acid, as well the number of servings to be consumed during the antioxidant diet. Each participant was allowed to choose fruits and vegetables from the list supplied to them, making it a self-selected diet.
To enhance the adherence to the nutritional advice given throughout the study, every four days during the 14-day diet period, participants were contacted by phone.
Blood collection and sample preparation
Two blood samples were obtained before and after the diet period. Blood samples (15 ml) were collected in the morning (around 8:00 AM) by venous puncture into tubes containing EDTA (ethylene diamine tetra acetic acid) as an anticoagulant. The athletes were instructed to fast for 8 hours before sample collection, and to abstain from exercise before blood collection.
Aliquots from whole blood were obtained through blood count. Blood samples were centrifuged at 1000 X g, 5°C, for 5 minutes for plasma separation. The erythrocyte pellet was washed three times with 0.9% NaCl. The washed cells were lysed with an equal volume of deionized water. The aliquots of plasma and erythrocyte lysate samples were stored at -70°C until analysed.
In plasma carbonyl, total reduced sulfhydryl, uric acid (UA), total radical trapping antioxidant potential (TRAP), and thiobarbituric acid reactive substances (TBARS) were analysed. In erythrocytes, superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities as well as hydrogen peroxide consumption were determined.
Laboratory analyses
Blood count was obtained by using an electronic haematology analyser (Sysmex SE9500).
Enzymatic antioxidant capacity was evaluated in erythrocyte lysate, and included: SOD activity, determined by the inhibition rate of pyrogallol auto-oxidation, with results expressed as units/mg protein [17]; hydrogen peroxide consumption, assessed by tracking the decrease in exogenous hydrogen peroxide absorption at 240 nm, with results expressed as pmol/mg protein [18]; GPx activity, measured by following NADPH oxidation, with results expressed as mmol/min /mg protein [19].
Non-enzymatic blood markers of oxidative stress were evaluated in plasma samples, and included: non-enzymatic antioxidant capacity (TRAP), which was evaluated by the chemiluminescence method, measured using 320 μM Trolox as standard antioxidant, with results expressed as μM Trolox equivalents [20]; the TBARS test, measured as an index of lipid peroxidation, with results expressed as ηmol/mg protein [21]; total reduced sulfhydryl, determined as described by Ellman, with results expressed as ηmol/mg protein [22]; carbonyl groups, measured as described by Levine et al., with results expressed as ηmol/mg protein [23]. To express total protein results, the Lowry method was used [24].
Statistical analyses
The statistical significance of the data was assessed using nonparametric tests. The Mann-Whitney U test was performed to compare groups at baseline (regular vs antioxidant diets). The effects of treatment (group; time before and after diet regimens; and interaction) were estimated using a generalized estimating equation (GEE) followed by Bonferroni’s post-hoc test. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, v.18.0 for Windows). Statistical significance was set at p< 0.05.
RESULTS
The initial characteristics are shown in Table 1. No differences in age, height, body weight, body fat or maximal aerobic power characteristics (VO2max and ventilatory thresholds), training load, and triathlon experience were observed between participants.
TABLE 1.
Baseline physical characteristics, maximal aerobic power and training parameters of participants.
| Parameter | Regular-diet (n=6) | Antioxidant-diet (n=7) | p |
|---|---|---|---|
| Age (years) | 32.0 (27.3-33.8) | 30.0 (25.5-37) | 0.474 |
| Height (m) | 1.74 (1.70-1.77) | 1.78 (1.73-1.83) | 0.317 |
| Body mass (kg) | 73.6 (69.9-80.1) | 76.3 (70.8-81.3) | 0.830 |
| Body fat (%) | 13.0 (9.5-18.5) | 14.1 (11.0-22.0) | 0.522 |
| VO2max treadmill (ml/kg/min) | 59.7 (51.2-68.0) | 58.6 (54.4-60.5) | 0.391 |
| VO2max cycle ergometer (ml/kg/min) | 65.3 (54.9-68.7) | 56.8 (53.4-63.3) | 0.200 |
| Treadmill threshold (% VO2max) | 50.9 (43.6-61.2) | 49.8 (46.4-50.5) | 0.271 |
| Cycle ergometer threshold (% VO2max) | 50.1 (41.0-56.0) | 48.6 (41.0-51.8) | 0.631 |
| Training load (h/week) | 17.2 (11.6-20.6) | 16.7 (14.2-16.8) | 0.390 |
| Training in triathlon (years) | 5.5 (3.3-9.0) | 4.0 (2.8-7.5) | 0.828 |
Note: Data expressed as median (interquartile range (p25-p75)). No differences between groups were observed. Comparisons were tested by Mann-Whitney U test.
Dietetic data are presented in Table 2, which shows the comparisons of nutritional parameters. No differences were observed in energy parameters between groups. At baseline (previous diet), the percentage of carbohydrate was insufficient, and protein was high, for both groups. Both were adjusted for the intervention. The diet groups were adjusted to decrease fat and increase fibre intake. Additionally, antioxidant vitamins showed a great variation between participants in previous diet. Both groups demonstrated an increase in α-tocopherol after the intervention, but β-carotene did not change and ascorbic acid was higher in the AO-diet.
TABLE 2.
Nutritional intake parameters.
| Parameter | Regular-diet (n=6) |
Antioxidant-diet (n=7) |
p |
||||
|---|---|---|---|---|---|---|---|
| Previous diet | Intervention | Previous diet | Intervention | time | group | interaction | |
| Energy (kcal) | 2986 ± 946 | 3070 ± 858 | 3292 ± 817 | 3261 ± 586 | 0.583 | 0.298 | 0.462 |
| Energy (kcal/kg) | 40.9 ± 16.2 | 42.0 ± 15.1 | 46.1 ± 10.8 | 44.8 8.6 | 0.642 | 0.312 | 0.550 |
| Carbohydrate (%) | 55.4 ±7.9 | 60.3 ± 3.6 | 53.1 4.2 | 58.4 ± 3.6 | 0.339 | 0.000 | 0.800 |
| Protein (%) | 18.7 ± 2.9 | 16.5 ± 2.1 | 17.9 5.7 | 16.0 ± 2.9 | 0.756 | 0.005 | 0.978 |
| Protein (g/kg) | 1.89 ± 0.7 | 1.7 ± 0.3 | 1.96 0.2 | 1.8 ± 0.1 | 0.800 | 0.048 | 0.877 |
| Fat (%) | 25.9 ± 6.3 | 23.2 ± 2.1 | 28.9 ± 4.2 | 25.6 3.1 | 0.114 | 0.024 | 0.792 |
| Fiber (g) | 30.0 ± 10.8 | 38.4 ± 9.3 | 23.2 ± 5.9 | 39.8 ± 6.7 | 0.624 | 0.000 | 0.046 |
| α-tocopherol (mg) | 8.2 (4.9-10.8) | 15.5 (14.3-16.4) | 14.1 (7.2-19.6) | 30.6 (29.3-31.2) | 0.000 | 0.000 | 0.074 |
| β-carotene (μg) | 1908 (749-2680) | 889 (847-1016) | 1008 (531-1419) | 1897 (1768-1940) | 0.969 | 0.894 | 0.057 |
| Ascorbic acid (mg) | 181.2 (77.7-253.2)a | 203.6 (195.1-212.6)a | 167.4 (87.8-240.3)a | 483.9 (459.0-498.2)b | 0.090 | 0.006 | 0.029 |
Note: Data expressed as mean ± standard deviation or median (interquartile range (p25-p75)). Different letters mean p<0.05 intragroup.
Haematological parameters are shown in Table 3, with no changes with clinical relevance in any groups, and all were within reference values.
TABLE 3.
Haematological parameters.
| Indices | Regular-diet (n=6) |
Antioxidant-diet (n=7) |
p |
Reference value | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After 14 d | Baseline | After 14 d | group | time | interaction | ||
| Erythrocyte (106/μl) | 4.95±0.39 | 5.08±0.45 | 5.10±0.12 | 5.17±0.12 | 0.439 | 0.002 | 0.427 | 4.5 – 6.1 |
| Hematocrite (%) | 45.22.0 | 46.1±2.3 | 45.4±1.8 | 45.8±1.5 | 0.958 | 0.022 | 0.284 | 39.0 – 50.0 |
| Hemoglobin (g/dl) | 15.3±0.8 | 15.3±0.9 | 15.4±0.6 | 15.4±0.5 | 0.745 | 0.652 | 0.972 | 13.3 – 16.7 |
Note: Data expressed as mean ± standard deviation.
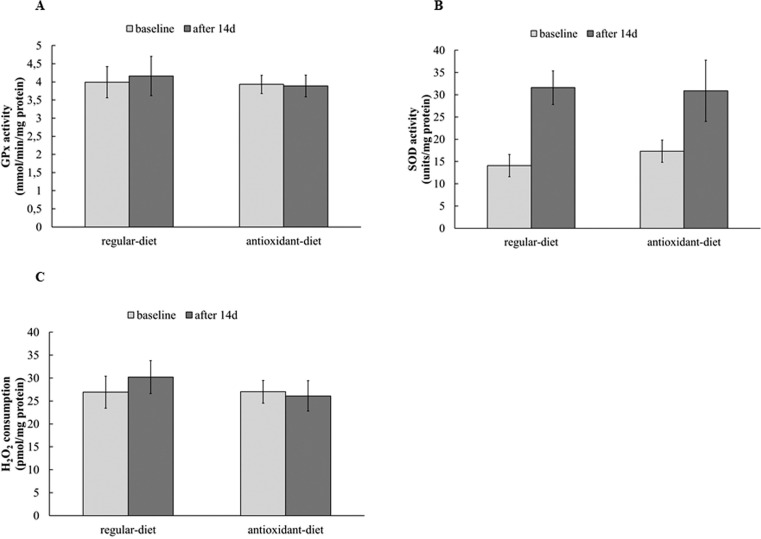
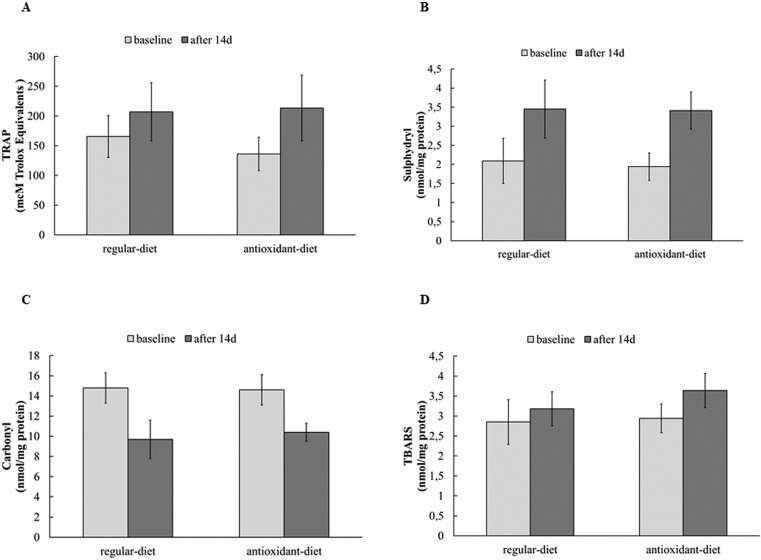
Oxidative stress biomarkers were evaluated by analysing enzymatic antioxidant capacity, which are shown in Figure 1, and non-enzymatic blood markers, which are shown in Figure 2. No differences were found at baseline between the RE-diet and AO-diet, and when comparing the groups after interventions, no significant differences were found. The effects of the 14-day intervention in each group showed no changes in enzymatic antioxidant capacity analysed by hydrogen peroxide consumption, and GPx activity, but SOD activity increased after the RE-diet (Δ=17.5) and after the AO-diet (Δ=13.6). In relation to non-enzymatic blood markers of oxidative stress, when analysed together, both groups presented an increase in TRAP, sulfhydryl and lipid damage (TBARS) after the diets, analysed by GEE (p group>0.05, p time<0.05 and p interaction>0.05). Carbonyl decreased after the diets (p group > 0.05, p time <0.05 and p interaction > 0.05).
FIG.1.
Enzymatic oxidative stress biomarkers.
Note: GPx: glutathione peroxidase (mmol/min/mg prot) (Panel A); SOD: superoxide dismutase (U SOD/mg prot) (Panel B); H2O2 consumption (pmol/mg prot) (Panel C).
Data expressed as mean ± standard deviation. Data are presented as means and SD, analysed by GEE. SOD increased after interventions (p group>0.05, p time<0.05 and p interaction>0.05).
FIG. 2.
Non-enzymatic oxidative stress biomarkers.
Note: TRAP: total antioxidant capacity (μmol/l Trolox) (Panel A); sulfhydryl (nmol/mg prot) (Panel B); carbonyl (nmol/mg prot) (Panel C); TBARS thiobarbituric acid reactive substances (nmol/mg prot) (Panel D). Data expressed as mean ± standard deviation. Data are presented as means and SD, analysed by GEE. TRAP, sulfhydryl and TBARS increased after interventions, and carbonyl decreased after interventions (p group>0.05, p time<0.05 and p interaction>0.05).
DISCUSSION
The present study is an original investigation to verify the effect of an antioxidant-rich diet and of a regular-diet on biomarkers of oxidative stress in triathletes. The main finding of this study was that after 14 days of a regular diet or antioxidant-rich diet, most of the biomarkers of oxidative stress showed positive results. Superoxide dismutase and sulfhydryl increased, and protein damage decreased. Moreover, after the AO diet, with higher fruit and vegetable intakes, the total antioxidant capacity increased 56.7%. However, lipid peroxidation increased after the diets. To date, few human studies have assessed and compared the effects of dietary interventions, with foods instead of supplements, on oxidative stress in competitive athletes.
In relation to the nutritional interventions and habitual intake, vitamin E ingestion in the diet intervention was doubled. Additionally, the dietary intervention provided higher carbohydrate intake than habitual, which was lower than recommendations by ~60%/day. We believe that an adequate intake of carbohydrate proposed to these triathletes does not affect oxidative stress parameters, since our goal was to offer a balanced diet for 14 days.
Interestingly, in the present study an increase in SOD activity was found, probably because even in the group receiving the RE-diet, the amount of micronutrients was increased. This suggests that the athletes’ regular diet has deficiencies, which were corrected by the RE-diet. Two weeks is a short period for expression of an enzyme increase in erythrocytes, which lack the capacity for protein synthesis, but consistent short-term effects, even within hours, on superoxide dismutase activity have been previously reported [25]. The increased SOD activity in erythrocyte agrees with a study on female runners receiving 1 g of vitamin C for 3.3 weeks, compared to placebo [26], and with sprinters receiving green tea extracts for 4 weeks [27]. Additionally, an increase in neutrophil SOD activity in triathletes in response to supplementation with an antioxidant cocktail (vitamins A, C and E) was observed [28]. In the same study, an increase in catalase and GPx activities was observed. However, no changes were detected in GPx activity and hydrogen peroxide consumption (which reflects the activity of several enzymes capable of consuming hydrogen peroxide) in our study, which used foods instead of supplements. It is possible that in erythrocytes, the training adaptations could be more important than antioxidant supplementation effects, whereas lymphocytes show higher sensitivity to this. After 90 days of antioxidant supplementation (250 mg of vitamin E and 15 mg of vitamin A, plus 1 g of vitamin C in the last 15 days) erythrocyte SOD, CAT and GPx activities did not change, but lymphocyte CAT and GPx activities increased [8].
In fact, the literature is conflicting in relation to the effects of antioxidant diet/supplementation adaptations on enzymatic activities, probably due to differences in experimental design, sample, amounts of diet compounds, diet period and other factors. Young et al. found an increase in GPx activity with apple and blackcurrant juice. However, superoxide dismutase and catalase did not show a consistent pattern of change, increasing at lower doses and decreasing at the highest dose [29]. In a model using rats, for a longer period (14 weeks), antioxidant supplementation with α-lipoic acid and α-tocopherol had no significant effect on activities of CAT, GPx and SOD in sedentary animals, while trained animals showed increased GPx and catalase activities [30].
We found an increase in SOD activity after both diet with no changes in GPx activity and hydrogen peroxide consumption. The SOD primary function is to act as an antioxidant agent, removing superoxide radicals through dismutation of the superoxide anion radical (O2•‑) to H2O2. If other enzyme activity does not increase in a proportional way, it is not possible to remove the hydrogen peroxide produced by superoxide dismutation [31]. Thus, this enzymatic imbalance can lead to hydrogen peroxide (H2O2) accumulation, which in order to be removed needs an increase of catalase, peroxiredoxin and/or GPx activities simultaneously to increase SOD activity. We did not observe changes in hydrogen peroxide consumption and GPx activity, which leads us to believe that the amount of H2O2 could be increased. It is important to note that this reactive oxygen species is stable and has high permeability in membranes [32]. This imbalance could play an important role in a likely antioxidant adaptation, since a small increase in reactive species induced by a challenge such as exercise is needed by the cell to increase the antioxidant systems, and it has been demonstrated that nuclear factor E2-related factor 2 (Nrf2), a transcription factor for antioxidant and phase II enzymes, can be activated by H2O2 [33].
After 14 days of RE-diet and AO-diet, a positive effect on TRAP was observed, indicating a higher non-enzymatic antioxidant capacity, which is related to an increased intake of fruits and vegetables, since athletes ate more fruits and vegetables compared to the previous period. Similar results were achieved by Vasankari [34], in a study with antioxidant supplementation (vitamin C and E) in triathletes. It is possible that the antioxidant capacity of plasma is under tight homeostatic control and can be overridden only with acute consumption of large amounts of antioxidant-rich foods or beverages. Other antioxidants besides β-carotene, ascorbic acid, and α-tocopherol are also responsible for the protection provided by fruits and vegetables, such as flavonoids [35]. It is therefore plausible that the beneficial effects of a high intake of fruits and vegetables on the risk of diseases may not result exclusively from the action of antioxidants, such as the well-characterized α-tocopherol and ascorbic acid. Rather, they may result from the action of lesser-known compounds or from the concerted action of a combination of different antioxidants present in these foods [36].
We observed an increase in reduced sulfhydryl after the RE-diet (65%) and AO-diet (75.7%). It is present in proteins and glutathione (GSH), and has been used as a marker of oxidative stress because it is very sensitive to slight changes in reactive species production. Thus, sulfhydryl is considered a cellular redox sensor and a redox buffer, playing a very important cytosolic antioxidant role [37]. Additionally, we observed a decrease in carbonyl levels, which are raised when the cell suffers a pro-oxidative overload [38], after both the RE-diet (34.4%) and AO-diet (28.7%), suggesting an improvement in antioxidant status and indicating that moderate or high amounts of antioxidant constituents of the diets might protect protein from oxidation within the plasma compartment. Acute ingestion of antioxidant-supplemented beverages in cyclists (90 min, 70% VO2max) resulted in a 29% decrease in protein carbonyls compared to a 14% increase in the placebo group [39]. On the other hand, seven days of high juice intake seemed to have a pro-oxidant effect on plasma proteins [29].
Surprisingly, the diets increased TBARS levels, unlike other redox parameters. This method is used as a marker of lipoperoxidation, which is most commonly generated through oxidation by the hydroxyl radical (•OH), the most potent free radical. This reactive oxygen species can be formed via the Fenton reaction, which is favoured by increased levels of hydrogen peroxide and reduced ferrous ion (Fe++) [40]. An imbalance of antioxidant enzymes can increase the hydrogen peroxide, and this imbalance is associated with an increase in vitamin C intake, and can favour the reduction of ferric ion (Fe+++) to Fe++ [12]. Therefore, we believe that it could be one of the mechanisms by which lipoperoxidation increased, since it was demonstrated that vitamin C supplementation increased the resting levels of TBARS [41].
It is important to note that some studies with antioxidant supplementation showed a pro-oxidant effect in endurance athletes [42,43]. Antioxidants can delay muscle repair after exercise, since recovery from exercise is mediated in part by oxidant signalling, and training responses to exercise can also be depressed by antioxidant supplements [44]. Otherwise, mitochondrial biogenesis could be jeopardized by supplements [45]. However, it is important to point out that the doses of the vitamins used in a supplementary way are frequently much higher than those that can be consumed through the diet. Besides that, antioxidant supplementations in general include one to three antioxidants, and in this way cannot act synergistically as antioxidants in foods.
One limitation of the study was the lack of data on the plasma concentrations of antioxidant vitamins. However, increasing the intake of fruits and vegetables through food increases the plasma concentrations of antioxidant nutrients [13].
It is likely that the decreased damage of proteins, better redox state and enhanced antioxidant capacity could be related to cellular adaptations. However, further studies are needed, as well as determination of the mechanism responsible for SOD activation.
CONCLUSIONS
In summary, our data suggest that antioxidant-enriched diets, without supplementation, can improve the redox status of triathletes. We observed decreased damage in proteins (shown by diminished carbonyl levels), a better redox state (shown by increased sulfhydryl) and enhanced antioxidant capacity (shown by raised TRAP).
Conflict of interest
All authors have no conflict of interest to declare.
REFERENCES
- 1.Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules. 2015;5(2):356–377. doi: 10.3390/biom5020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palazzetti S, Richard MJ, Favier A, Margaritis I. Overloaded training increases exercise-induced oxidative stress and damage. Can J Appl Physiol. 2003;28(4):588–604. doi: 10.1139/h03-045. [DOI] [PubMed] [Google Scholar]
- 3.Dékány M, Nemeskéri V, Gyore I, Ékes E, Gógl A, Szots G, Petrekanits M. Physical performance and antioxidant effect in triathletes. Biol Sport. 2008;25(2):101–114. [Google Scholar]
- 4.de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2016 doi: 10.1007/s40279-016-0566-1. [DOI] [PubMed] [Google Scholar]
- 5.Buonocore D, Negro M, Arcelli E, Marzatico F. Anti-inflammatory Dietary Interventions and Supplements to Improve Performance during Athletic Training. J Am Coll Nutr. 2015;34(Suppl 1):62–67. doi: 10.1080/07315724.2015.1080548. [DOI] [PubMed] [Google Scholar]
- 6.Schneider CD, Barp J, Ribeiro JL, Belló-Klein A, Oliveira AR. Oxidative stress after three different intensities of running. Can J Appl Physiol. 2005;30(6):723–734. doi: 10.1139/h05-151. [DOI] [PubMed] [Google Scholar]
- 7.Watson TA, Callister R, Taylor RD, Sibbritt DW, MacDonald-Wicks LK, Garg ML. Antioxidant restriction and oxidative stress in short-duration exhaustive exercise. Med Sci Sports Exerc. 2005;37(1):63–71. doi: 10.1249/01.mss.0000150016.46508.a1. [DOI] [PubMed] [Google Scholar]
- 8.Tauler P, Aguiló A, Gimeno I, Fuentespina E, Tur JA, Pons A. Response of blood cell antioxidant enzyme defences to antioxidant diet supplementation and to intense exercise. Eur J Nutr. 2006;45(4):187–195. doi: 10.1007/s00394-005-0582-7. [DOI] [PubMed] [Google Scholar]
- 9.Taghiyar M, Darvishi L, Askari G, Feizi A, Hariri M, Mashhadi NS, et al. The effect of vitamin C and e supplementation on muscle damage and oxidative stress in female athletes: a clinical trial. Int J Prev Med. 2013;4(Suppl 1):S16–23. [PMC free article] [PubMed] [Google Scholar]
- 10.Zanotto-Filho A, Schröder R, Moreira JC. Xanthine oxidase-dependent ROS production mediates vitamin A pro-oxidant effects in cultured Sertoli cells. Free Radic Res. 2008;42(6):593–601. doi: 10.1080/10715760802144422. [DOI] [PubMed] [Google Scholar]
- 11.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pingitore A, Lima GP, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31(7-8):916–922. doi: 10.1016/j.nut.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Strain JJ, Elwood PC, Davis A, Kennedy O, Coulter J, Fehily A, et al. Frequency of fruit and vegetable consumption and blood antioxidants in the Caerphilly cohort of older men. Eur J Clin Nutr. 2000;54(11):828–833. doi: 10.1038/sj.ejcn.1601101. [DOI] [PubMed] [Google Scholar]
- 14.Moore LV, Thompson FE. Adults Meeting Fruit and Vegetable Intake Recommendations - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):709–713. [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 16.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 17.Marklund S. Pyrogallol autooxidation. Philadelphia: PressBoca Raton; 1985. [Google Scholar]
- 18.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 20.Lissi E, Pascual C, Del Castillo MD. Luminol luminescence induced by 2,2’-Azo-bis(2-amidinopropane) thermolysis. Free Radic Res Commun. 1992;17(5):299–311. doi: 10.3109/10715769209079523. [DOI] [PubMed] [Google Scholar]
- 21.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 22.Riener CK, Kada G, Gruber HJ. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4’-dithiodipyridine. Anal Bioanal Chem. 2002;373(4-5):266–276. doi: 10.1007/s00216-002-1347-2. [DOI] [PubMed] [Google Scholar]
- 23.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 25.Saghir AN, Rickards H, Pall HS. Postprandial changes in superoxide dismutase activity in subjects with Gilles de la Tourette syndrome and controls. Exp Neurol. 1997;144(2):420–422. doi: 10.1006/exnr.1997.6430. [DOI] [PubMed] [Google Scholar]
- 26.Braakhuis AJ, Hopkins WG, Lowe TE. Effects of dietary antioxidants on training and performance in female runners. Eur J Sport Sci. 2014;14(2):160–168. doi: 10.1080/17461391.2013.785597. [DOI] [PubMed] [Google Scholar]
- 27.Jówko E, Długołęcka B, Makaruk B, Cieśliński I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur J Nutr. 2015;54(5):783–791. doi: 10.1007/s00394-014-0757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauler P, Aguiló A, Fuentespina E, Tur JA, Pons A. Diet supplementation with vitamin E, vitamin C and beta-carotene cocktail enhances basal neutrophil antioxidant enzymes in athletes. Pflugers Arch. 2002;443(5-6):791–797. doi: 10.1007/s00424-001-0770-0. [DOI] [PubMed] [Google Scholar]
- 29.Young JF, Nielsen SE, Haraldsdóttir J, Daneshvar B, Lauridsen ST, Knuthsen P, et al. Effect of fruit juice intake on urinary quercetin excretion and biomarkers of antioxidative status. Am J Clin Nutr. 1999;69(1):87–94. doi: 10.1093/ajcn/69.1.87. [DOI] [PubMed] [Google Scholar]
- 30.Marsh SA, Laursen PB, Coombes JS. Effects of antioxidant supplementation and exercise training on erythrocyte antioxidant enzymes. Int J Vitam Nutr Res. 2006;76(5):324–331. doi: 10.1024/0300-9831.76.5.324. [DOI] [PubMed] [Google Scholar]
- 31.Mondola P, Damiano S, Sasso A, Santillo M. The Cu, Zn superoxide dismutase: not only a dismutase enzyme. Front Physiol. 2016;7:594. doi: 10.3389/fphys.2016.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8(3-4):243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 33.Done AJ, Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasankari TJ, Kujala UM, Vasankari TM, Vuorimaa T, Ahotupa M. Increased serum and low-density-lipoprotein antioxidant potential after antioxidant supplementation in endurance athletes. Am J Clin Nutr. 1997;65(4):1052–1056. doi: 10.1093/ajcn/65.4.1052. [DOI] [PubMed] [Google Scholar]
- 35.Harasym J, Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30(5):511–517. doi: 10.1016/j.nut.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998;68(5):1081–1087. doi: 10.1093/ajcn/68.5.1081. [DOI] [PubMed] [Google Scholar]
- 37.Sen CK. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol Cell Biochem. 1999;196(1-2):31–42. [PubMed] [Google Scholar]
- 38.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10(2):389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morillas-Ruiz J, Zafrilla P, Almar M, Cuevas MJ, López FJ, Abellán P, et al. The effects of an antioxidant-supplemented beverage on exercise-induced oxidative stress: results from a placebo-controlled double-blind study in cyclists. Eur J Appl Physiol. 2005;95(5-6):543–-549. doi: 10.1007/s00421-005-0017-4. [DOI] [PubMed] [Google Scholar]
- 40.Winterbourn CC. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013;528:3–25. doi: 10.1016/B978-0-12-405881-1.00001-X. [DOI] [PubMed] [Google Scholar]
- 41.Bryant RJ, Ryder J, Martino P, Kim J, Craig BW. Effects of vitamin E and C supplementation either alone or in combination on exercise-induced lipid peroxidation in trained cyclists. J Strength Cond Res. 2003;17(4):792–800. doi: 10.1519/1533-4287(2003)017<0792:eoveac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Nieman DC, Henson D A, McAnulty SR, McAnulty LS, Morrow JD, Ahmed A, et al. Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc. 2004;36:1328–1335. doi: 10.1249/01.mss.0000135778.57355.ca. [DOI] [PubMed] [Google Scholar]
- 43.McAnulty SR, McAnulty LS, Nieman DC, Morrow JD, Shooter LA, Holmes S, et al. Effect of alpha-tocopherol supplementation on plasma homocysteine and oxidative stress in highly trained athletes before and after exhaustive exercise. J Nutr Biochem. 2005;16:530–537. doi: 10.1016/j.jnutbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Reid MB. Redox interventions to increase exercise performance. J Physiol. 2016;594(18):5125–5133. doi: 10.1113/JP270653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Cabrera MC, Salvador-Pascual A, Cabo H, Ferrando B, Viña J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Rad Biol Med. 2015;86:37–46. doi: 10.1016/j.freeradbiomed.2015.04.006. [DOI] [PubMed] [Google Scholar]