Abstract
We aimed to replicate, in a specific athletic event cohort (only track and field) and in two different ethnicities (Japanese and East European, i.e. Russian and Polish), original findings showing the association of the angiotensin-II receptor type-2 gene (AGTR2) rs11091046 A>C polymorphism with athlete status. We compared genotypic frequencies of the AGTR2 rs11091046 polymorphism among 282 track and field sprint/power athletes (200 men and 82 women), including several national record holders and Olympic medallists (214 Japanese, 68 Russian and Polish), and 2024 control subjects (842 men and 1182 women) (804 Japanese, 1220 Russian and Polish). In men, a meta-analysis from the two combined cohorts showed a significantly higher frequency of the C allele in athletes than in controls (odds ratio: 1.62, P=0.008, heterogeneity index I2=0%). With regard to respective cohorts, C allele frequency was higher in Japanese male athletes than in controls (67.7% vs. 55.9%, P=0.022), but not in Russian/Polish male athletes (61.9% vs. 51.0%, P=0.172). In women, no significant results were obtained by meta-analysis for the two cohorts combination (P=0.850). The AC genotype frequency was significantly higher in Russian/Polish women athletes than in controls (69.2% vs. 42.1%, P=0.022), but not in Japanese women athletes (P=0.226). Our results, in contrast to previous findings, suggested by meta-analysis that the C allele of the AGTR2 rs11091046 polymorphism is associated with sprint/power track and field athlete status in men, but not in women.
Keywords: Renin-angiotensin system, AGTR2, Track and field, Sprint, Power, Physical performance
INTRODUCTION
The renin-angiotensin system (RAS) is a key regulator of blood pressure and fluid homeostasis [1, 2]. The circulating angiotensinogen (AGT), produced by the liver, is cleaved by renin to yield angiotensin I, and further cleaved to angiotensin II by the angiotensin-converting enzyme (ACE). Angiotensin II is the major effector molecule of the RAS, acting via angiotensin II type 1 receptor (AGTR1) and type 2 receptor (AGTR2), as a potent vasoconstrictor and muscle growth factor [2, 3, 4]. AGTR2 mediates the effects of angiotensin II on cellular differentiation and growth. It is generally reported to have opposite effects of those mediated by AGTR1, e.g. vasodilatation, nitric oxide (NO) release and inhibition of proliferation and growth [2]. Two polymorphisms in the RAS genes, AGT Met235Thr (rs699) and ACE I/D (rs4340), have already been reported to be related to physical performance [5-9].
Mustafina et al. [10] recently found a novel genetic polymorphism in the RAS genes that seems to be a candidate explaining variability in athletic performance phenotypes: an A>C polymorphism (rs11091046) in the AGTR2 gene of the X chromosome, located at Xq22-q23, has been shown to be associated with muscle fibre type composition in physically active Russian men, as well as with athletic status and physical performance in Russian and Polish populations. The C allele of the AGTR2 rs11091046 polymorphism seems to be associated with a higher proportion of slow-twitch muscle fibres, endurance athlete status and aerobic performance; and the A allele seems to be associated with a higher percentage of fast-twitch fibres and power-oriented disciplines. Nevertheless, these results are not totally unequivocal since the C allele prevalence was also found to be higher in male strength athletes compared with control subjects. As no other information has been published on the association between the AGTR2 rs11091046 polymorphism and sprint/power performance, whether the Mustafina et al. results could be extrapolated to other ethnicities remains to be elucidated. This is a question of interest because differences among the findings of studies in the field of genetics and athletic performance are partly attributable to the different sizes and ethnic/geographic origin of the study populations. Therefore, it is necessary to validate the aforementioned evidence by replication in large, independent and event-specific cohorts of athletes from different ethnicities. To replicate the previous findings, we compared genotypic frequencies of the AGTR2 rs11091046 A>C polymorphism among Japanese, Russian and Polish sprint/power track and field athletes and ethnically matched controls.
MATERIALS AND METHODS
All athletes were sprint/power track and field competitors who participated in sprint events up to 400 m, jumping events, and throwing events. National athletes were defined as those who were competitive at national level competitions in their respective country, while international athletes were defined as those who had competed at major international competitions. All the participants were informed of the purpose and methods of the study, and each of them provided written informed consent for participation. The study was approved by ethics committees of Juntendo University, Nippon Sport Science University, and the National Institute of Health and Nutrition in Japan; and the ethics committees of St Petersburg University and the Pomeranian Medical University. This study was conducted, for each cohort, in accordance with the Declaration of Helsinki for Human Research.
Subjects
Japanese cohort: Consisted of 214 athletes (56 women): 42 international; 172 national. The control group consisted of 804 healthy subjects (593 women) living in the Tokyo area. Athletes and controls belong to the same Eastern-Asian descent.
Russian/Polish cohort: Comprised 68 athletes (26 women): 61 international; 7 national. The control group consisted of 1220 healthy subjects (589 women) from Russia and Poland. Athletes and controls were all Caucasians of Eastern-European descent.
Genotyping
DNA samples were obtained from saliva, buccal swabs or venous blood and extracted as previously described [9-11]. For the Japanese cohorts, the AGTR2 rs11091046 polymorphism was genotyped at Juntendo University, Chiba, Japan, using a Real-Time Thermocycler in the end-point analysis mode (LightCycler 480, Roche Applied Science, Mannheim, Germany) with the TaqMan SNP genotyping assay method (Assay ID: C___1841568_10). Allelic discrimination analysis was performed with LightCycler 480 SW software version 1.5.1.62 (Roche Applied Science, Mannheim, Germany). The Russian/Polish cohort was genotyped using allelic discrimination assays with TaqMan probes (Applied Biosystems, Carlsbad, California, USA) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, California, USA) or by PCR on a Tercyk ‘multicanal amplificator’ (DNA Technology, Moscow, Russia) and restriction enzyme digestion or by the use of HumanOmni1-Quad BeadChips (Illumina Inc, USA), as previously described [10].
Statistical analysis
The SPSS statistical package version 20.0 for Windows was used to perform all statistical evaluations. Intergroup genotype frequency differences were tested by Pearson’s χ2 test of independence. AGTR2 rs11091046 is situated in the sexual X chromosome; males are hemizygous and contribute a single allele and females contribute two alleles to the genotype, and thus comparisons were made separately by gender. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to estimate the degree of contribution of the AGTR2 rs11091046 polymorphism to athlete status. Meta-analysis was conducted using the Review Manager (RevMan) computer program (version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, http://tech.cochrane.org/revman). The DerSimonian and Laird random-effects models were used to calculate weighted OR of athletes with the C allele. The test of overall effect was assessed using the Z score. Heterogeneity, which assesses the consistency of the results of studies in meta-analyses, of OR between studies was assessed using the I 2 statistic. The level of significance was set at P<0.05.
RESULTS
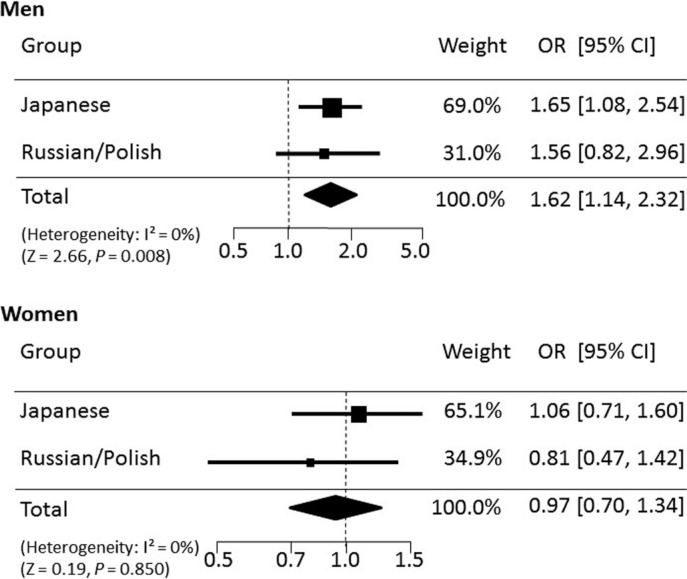
Table 1 shows the genotype frequencies of the AGTR2 rs11091046 polymorphism in the two ethnic groups. Meta-analysis showed that in the pooled model (Japanese + Russian/Polish populations), C allele frequency was significantly higher in male athletes (national and international) than in controls (OR: 1.62, 95% CI: 1.14-2.32; P=0.008) (see Figure 1), and in the same direction (although non-significantly) in only male international athletes vs. controls (OR: 1.52, 95% CI: 0.94-2.44; P=0.090 ). The C allele frequency was also significantly higher in Japanese male athletes than in controls (67.7% vs. 55.9%, OR: 1.65, 95% CI: 1.08 – 2.54, P=0.022), but not in the Russian/Polish population (OR: 1.56, 95% CI: 0.82-2.96) (Table 1, Figure 1). In women, no significant results were found by meta-analysis (OR: 0.97, 95% CI: 0.70-1.34, P=0.850) (Figure 1). Nevertheless, the AC genotype frequency was significantly higher in Russian/Polish women athletes than in controls (69.2% vs. 42.1%, P=0.022), but not in the Japanese population (P=0.226) (Table 1). No significant results were found comparing only international athletes in women.
TABLE 1.
Genotype/allele frequency distribution of the AGTR2 rs11091046 polymorphism, among the two ethnic cohorts. (Because AGTR2 is located on the X chromosome, men are hemizygous and contribute a single allele to the genotype).
| Men | Alleles distribution |
||||
|---|---|---|---|---|---|
| A | C | χ2 P value | |||
| Japanese | Athletes (n=158) | 51 (32.3%) | 107 (67.7%) | 0.022* | |
| Controls (n=211) | 93 (44.1%) | 118 (55.9%) | |||
| Russian/Polish | Athletes (n=42) | 16 (38.1%) | 26 (61.9%) | 0.172 | |
| Controls (n=631) | 309 (49.0%) | 322 (51.0%) | |||
| Women |
Genotypes distribution |
||||
| AA | AC | CC | χ2P value | ||
| Japanese | Athletes (n=56) | 4 (7.1%) | 31 (55.4%) | 21 (37.5%) | 0.226 |
| Controls (n=593) | 81 (13.7%) | 268 (45.2%) | 244 (41.1%) | ||
| Russian/Polish | Athletes (n=26) | 4 (15.4%) | 18 (69.2%) | 4 (15.4%) | 0.022* |
| Controls (n=589) | 140 (23.8%) | 248 (42.1%) | 201 (34.1%) | ||
FIG. 1.
Results of the meta-analysis on two ethnic cohorts for the association between the AGTR2 rs11091046 polymorphism and sprint/power athlete status. C allele frequency in controls was set as the reference (if OR>1, C allele frequency is higher in athletes). OR: odd ratio, CI: confidence interval.
DISCUSSION
Our main finding was that the C allele of the AGTR2 rs11091046 A>C polymorphism was more frequently distributed in sprint/power male athletes than in controls by meta-analysis presenting zero heterogeneity, in a relatively large cohort including two ethnic backgrounds as well as in a Japanese population independently. However, no significant results were found by meta-analysis in women.
Mustafina et al. [10] previously reported that the C allele frequency of the AGTR2 rs11091046 polymorphism was significantly higher in Russian and Polish endurance male and female athletes compared with power athletes and control subjects. Additionally, this allele was found to be associated with a higher proportion of slow-twitch muscle fibres. Nevertheless, they also found that this C allele was more frequent in male strength athletes compared with controls, which is also in agreement with our findings in the present study, obtained by meta-analysis in two ethnicities. The contradiction found here with the previous study for AGTR2 rs11091046 C allele frequency results in men could be explained by the wide range of sport disciplines included in the prior study, which complicated drawing firm conclusions. For this reason, we chose to improve in this study the specificity of our cohorts, restraining them to only track and field athletes. We also aimed to improve the competition level, including in our cohorts the best sprint/power athletes from every country, including several Olympic medallists and national record holders.
In women, no significant results were found by meta-analysis of the two cohorts (Figure 1). Independently, the AC genotype frequency was significantly higher in Russian/Polish athletes than in their corresponding controls (Table 1). These results might suggest that the C allele can be favourable for sprint/power performance in men, and unfavourable for women in Caucasian populations. The differences in results between men and women in the present study could be attributed to gender-specific differences existing in response to stimulation and inhibition of the RAS [12, 13]: differential balance in the pressor and depressor arms of the RAS using different pathways depending on sex, differences in expression levels of the RAS components, as well as several interactions between RAS and sexual hormones [14]. Nevertheless, the results obtained in the present study in women are hard to interpret due to skewed X-chromosomal inactivation: the AGTR2 gene being located within the X chromosome, men are hemizygous and contribute a single allele to the genotype, while women contribute two alleles to the genotype, although one of their X chromosomes is randomly inactivated [15, 16].
The AGTR2 receptor is one of the components of the RAS, where angiotensin II exerts its actions via AGTR1 and AGTR2. Angiotensin II, among several functions, exerts effects on muscle performance, e.g. a direct hypertrophic effect as a skeletal muscle growth factor, enhanced noradrenalin release from the nervous system, and increased capillary density in skeletal muscle, and has already been shown to be associated with strength- and power-related sports [3, 7]. AGTR2 is generally reported to mediate effects opposing and counterbalancing those mediated by AGTR1, which is involved in muscle development regulation, i.e., reducing muscle weight, amount of fibre, and type II fibre area [17].
The rs11091046 polymorphism we analysed is located within the 3′-untranslated region of AGTR2, at a microRNA-response-element position possibly corresponding to the hsa-miR-208a-5p and hsa-miR-208b-5p binding sites (98% context score), even though this polymorphic site is poorly conserved among vertebrates (TargetScanHuman tool, http://www.targetscan.org/ http://www.targetscan.org/). Van Rooij et al. [18, 19] found that miR-208a is encoded by intron 27 of the myosin heavy chain 6 gene (MYH6) in humans and that miR-208b is encoded by intron 31 of the myosin heavy chain 7 gene (MYH7) in mice. Using the DDBJ Blastn bioinformatics tool (DNA Data Bank of Japan, http://blast.ddbj.nig.ac.jp/), we confirmed that has-miR-208b-5p is encoded in the same location of MYH7 in humans. Van Rooij et al. showed miR-208a as required for cardiomyocyte hypertrophy, fibrosis, and expression of MYH7 in response to stress and hypothyroidism in humans [18] and miR-208b to control muscle myosin content, myofibre identity, and muscle performance in mice [19]. Thus, we might suggest that AGTR2 gene products can be influenced partly by miR-208 a- and/or b-5p, and that the C allele of the AGTR2 rs11091046 polymorphism in this region can reduce the miRNA/mRNA binding affinity, leading to an increased number of AGTR2 products playing a possible role in muscle performance, especially among elite sprint athletes. Indeed, as we have already mentioned, AGTR2 being able to down regulate AGTR1 expression and function, which can be related to the regulation of muscle development and specialization [17, 20, 21], we can hypothesize that an increasing level of AGTR2 due to the C allele polymorphism could lead to increased muscle mass and type II muscle fibre type composition. In their previous study, however, Mustafina et al. reported the opposite direction for type II muscle fibre type composition [10]. Nevertheless, in this study the authors only differentiated type I from type II muscle fibres without information about type IIa and IIx subgroups’ distribution, and it is well known that type IIa fibres are associated with both sprint and endurance performance. Therefore, further studies are necessary to draw a firm conclusion on this functional mechanism.
It must be kept in mind that elite athletic status is a complex trait resulting from the interaction of numerous phenotypes, involving the combined influence of several genetic variants, each with a significant contribution, as well as the complex interaction of genetic variants that explain individual variations in performance. Further research models in the field of sports genetics should thus account for the polygenic nature of sports related phenotypes. More replication studies are needed in order to specify the genetic combinations required to become an elite athlete and to improve athletic performance and in order to determine how these combinations can differ among ethnicities.
CONCLUSIONS
In summary, the C allele of the AGTR2 rs11091046 A>C polymorphism was associated with sprint/power track and field athlete status in men, assessed by meta-analysis of athletes from two different ethnicities. However, further replication and functional studies are necessary to confirm these findings.
Acknowledgements
This work was supported in part by grants from the Grant-in-Aid for Scientific Research program of the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant No. 15H03081 to N.F.; DNA sample collection and genotyping of Japanese athletes and controls); by a grant-in-aid for scientific research from the Ministry of Health, Labor, and Welfare of Japan (to M.M.; DNA sample collection of controls) and by grants from the Russian Science Foundation (Grant No. 17-15-01436: “Comprehensive analysis of the contribution of genetic, epigenetic and environmental factors in the individual variability of the composition of human muscle fibers”; DNA sample collection and genotyping of Russian athletes and controls). H.Z. and E.M. were recipients of Grant-in-Aid for JSPS Fellow from the Japan Society for the Promotion of Science.
Conflicts of Interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Leckie BJ. Targeting the renin-angiotensin system: what’s new? Curr Med Chem Cardiovasc Hematol Agents. 2005;3(1):23–32. doi: 10.2174/1568016052773298. Epub 2005/01/11. [DOI] [PubMed] [Google Scholar]
- 2.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264(3):224–36. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones A, Woods DR. Skeletal muscle RAS and exercise performance. Int J Biochem Cell Biol. 2003;35(6):855–66. doi: 10.1016/s1357-2725(02)00342-4. [DOI] [PubMed] [Google Scholar]
- 4.Johnston AP, Baker J, De Lisio M, Parise G. Skeletal muscle myoblasts possess a stretch-responsive local angiotensin signalling system. J Renin Angiotensin Aldosterone Syst. 2011;12(2):75–84. doi: 10.1177/1470320310381795. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery HE, Marshall R, Hemingway H, Myerson S, Clarkson P, Dollery C, et al. Human gene for physical performance. Nature. 1998;393(6682):221–2. doi: 10.1038/30374. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Gallego F, Santiago C, Gonzalez-Freire M, Yvert T, Muniesa CA, Serratosa L, et al. The C allele of the AGT Met235Thr polymorphism is associated with power sports performance. Appl Physiol Nutr Metab. 2009;34(6):1108–11. doi: 10.1139/H09-108. [DOI] [PubMed] [Google Scholar]
- 7.Puthucheary Z, Skipworth JR, Rawal J, Loosemore M, Van Someren K, Montgomery HE. The ACE gene and human performance: 12 years on. Sports Med. 2011;41(6):433–48. doi: 10.2165/11588720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Eynon N, Hanson ED, Lucia A, Houweling PJ, Garton F, North KN, et al. Genes for elite power and sprint performance: ACTN3 leads the way. Sports Med. 2013;43(9):803–17. doi: 10.1007/s40279-013-0059-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Mikami E, Chiu LL, A DEP, Deason M, Fuku N, et al. Association analysis of ACE and ACTN3 in elite Caucasian and East Asian swimmers. Med Sci Sports Exerc. 2013;45(5):892–900. doi: 10.1249/MSS.0b013e31827c501f. [DOI] [PubMed] [Google Scholar]
- 10.Mustafina LJ, Naumov VA, Cieszczyk P, Popov DV, Lyubaeva EV, Kostryukova ES, et al. AGTR2 gene polymorphism is associated with muscle fibre composition, athletic status and aerobic performance. Exp Physiol. 2014;99(8):1042–52. doi: 10.1113/expphysiol.2014.079335. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi N, Miyamoto-Mikami E, Murakami H, Nakamura T, Min SK, Mizuno M, et al. ACTN3 R577X genotype and athletic performance in a large cohort of Japanese athletes. Eur J Sport Sci. 2015:1–8. doi: 10.1080/17461391.2015.1071879. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1220–6. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- 13.Hilliard LM, Sampson AK, Brown RD, Denton KM. The „his and hers” of the renin-angiotensin system. Curr Hypertens Rep. 2013;15(1):71–9. doi: 10.1007/s11906-012-0319-y. [DOI] [PubMed] [Google Scholar]
- 14.Kuroski de Bold ML. Estrogen, natriuretic peptides and the renin-angiotensin system. Cardiovascular research. 1999;41(3):524–31. doi: 10.1016/s0008-6363(98)00324-1. [DOI] [PubMed] [Google Scholar]
- 15.Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–3. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 16.Plath K, Mlynarczyk-Evans S, Nusinow DA, Panning B. Xist RNA and the mechanism of X chromosome inactivation. Annu Rev Genet. 2002;36:233–78. doi: 10.1146/annurev.genet.36.042902.092433. [DOI] [PubMed] [Google Scholar]
- 17.Zempo H, Suzuki J, Ogawa M, Watanabe R, Isobe M. A different role of angiotensin II type 1a receptor in the development and hypertrophy of plantaris muscle in mice. J Appl Genet. 2016;57(1):91–7. doi: 10.1007/s13353-015-0291-8. [DOI] [PubMed] [Google Scholar]
- 18.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science (New York, NY) 2007;316(5824):575–9. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 19.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662–73. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, et al. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens. 2012;30(6):1176–84. doi: 10.1097/HJH.0b013e3283532099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin XQ, Fukuda N, Su JZ, Lai YM, Suzuki R, Tahira Y, et al. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension (Dallas, Tex : 1979) 2002;39(5):1021–7. doi: 10.1161/01.hyp.0000016179.52601.b4. [DOI] [PubMed] [Google Scholar]