Abstract
Interpersonal sensorimotor synchronization (interpersonal SMS) is the foundation of complex human social interaction. Previous studies primarily focused on the individual cognitive processes of interpersonal SMS. However, all individuals compose an entire interaction system with emerged holistic properties during interpersonal SMS. Therefore, we proposed the `holistic cognitive and neural processes’ of interpersonal SMS and defined quantitative measurements that included Holistic Correction Gain (HCG), Holistic Timekeeper Variance (HTV) and Holistic Motor Variance (HMV) based on linear error correction model and inter-brain couplings obtained by hyperscanning technique. We performed a joint-tapping experiment including bidirectional and unidirectional conditions using functional near-infrared spectroscopy (fNIRS) hyperscanning to evaluate effects of these holistic processes on synchronization performance. We found that the dyads’ performance highly correlated with the integrated effect of holistic cognitive processes in both conditions. Each holistic cognitive process played different roles in interpersonal SMS. HCG was critical to maintain synchronization. HTV related to mentalizing others’ behavior. Holistic neural process, the inter-brain coupling of right prefrontal cortex (PFC), was significantly different between bidirectional and unidirectional conditions, which suggested the existence of neural markers at holistic level in interpersonal SMS.
Keywords: interpersonal sensorimotor synchronization, linear error correction model, holistic view, fNIRS hyperscanning, inter-brain neural coupling
Introduction
Interpersonal sensorimotor synchronization (interpersonal SMS) is a temporal coordinated movement among individuals that is prevalent in our social life (Repp and Keller, 2008), and it may relate to the evolution of music and language (Merker et al., 2009). This ability to coordinate with others is fundamental to establish more complex and flexible social interactions (Newman-Norlund et al., 2007; Konvalinka et al., 2010; Hasson et al., 2012; Kim, 2015), which strengthen social bonding and prosocial behavior (Cummins et al., 2005; Hove and Risen, 2009; Kirschner and Tomasello, 2010; Valdesolo et al., 2010; Cohen et al., 2013).
There are two main approaches to study SMS. Information-processing approach aims to clarify internal cognitive processes and involved brain areas of SMS. The main method to describe cognitive processes of synchronization is the linear synchronization model (Vorberg and Wing, 1996; Vorberg and Schulze, 2002; Repp, 2005; Repp and Keller, 2008; Repp and Su, 2013; van der Steen and Keller, 2013; Keller et al., 2014; van der Steen et al., 2015; Elliott et al., 2016). Dynamic system approach uses the available mathematical framework to deal with coupled systems and stochastic processes, based on the definition of a state space, differential and integral calculus, probability, etc. (Haken et al., 1985; Schöner and Kelso, 1988; Kelso, 1995; Jirsa and Kelso, 2005; Tognoli et al., 2007; Righetti et al., 2009; Kelso et al., 2013; Dumas et al., 2014; Tognoli and Kelso, 2015) (See Repp, 2005 for discussion about the two approaches). In the present study, we focus on understanding internal cognitive processes underlying interpersonal SMS using the information-processing approach.
In social psychology and neuroscience research, there was a major shift from isolated to interacting individuals (Hasson et al., 2012; Konvalinka and Roepstorff, 2012; Chatel-Goldman et al., 2013). Research of interpersonal SMS shifted its focus from strictly controlled virtual interpersonal SMS, e.g. one person synchronizing with an adaptive metronome (Repp and Keller, 2008; Fairhurst et al., 2013, 2014), to real interpersonal SMS, e.g. joint tapping and music ensemble performance (Konvalinka et al., 2010; Wing et al., 2014). The experimental situation of interpersonal SMS has become increasingly realistic, but studies primarily focus on `individual’s’ cognitive processes. For example, studies from information-processing approach primarily focused on individual adaptation processes, which are keys to achieving and maintaining interpersonal SMS. Vorberg and Wing proposed a linear phase correction model scheme for a single person to describe the temporal correction process in synchronization with a stable periodic metronome (Vorberg and Wing, 1996). This scheme was extended to describe an individual’s adaptation in multi-person synchronization (Vorberg, 2005; Wing et al., 2014; Jacoby et al., 2015). Researchers used an adaptive metronome and found that musically trained individuals coordinated with different kinds of adaptive metronomes even when it was uncooperative (Repp and Keller, 2008). Wing et al. demonstrated that the optimal error correction of a group member in N-person synchronization task was 1/N. Musicians in a real string quartet synchronization exhibited near-optimal correction even though each musician could not reach optimal correction (Wing et al., 2014). Honisch et al. used a six-person limb-swing synchronization task and found that individuals flexibly adapted different adaptation strategies due to different perceived cues, and timekeeper variance and motor variance differed between different tasks and roles (Honisch et al., 2016). Nowicki et al. found that error correction was not the only strategy of mutual adaptive timing. Individuals exhibited a greater temporal assimilation tendency than error correction when they performed a coordination task in alternation (Nowicki et al., 2013). Individuals’ other psychological processes (e.g. prediction ability and auditory imagery) also influence interpersonal SMS (Keller and Appel, 2010; Pecenka and Keller, 2011).
Many single-person synchronization studies provide helpful evidence to understand the neural mechanisms of interpersonal SMS. The striato–thalamo–cortical system was involved in the timing process, and the coupling of motor and sensory (e.g. auditory) areas engaged in rhythm perception. The cerebellum was critical to prediction and error correction, and activation of prefrontal and parietal areas was found in complex SMS tasks due to the high cognitive control demand (Repp and Su, 2013; Keller et al., 2014). Notably, the development of the hyperscanning technique (Montague, 2002) exposed inter-brain coupling as a new discovery to understand the underlying neural processes of interpersonal SMS. When people coordinated with others, their brain oscillated more synchronously than during non-coordination period (Lindenberger et al., 2009), especially in alpha-mu band (Dumas et al., 2010, 2012; Naeem et al., 2012), even when they were unconscious about the synchronization (Yun et al., 2012). Prefrontal areas [especially the right prefrontal cortex (PFC)] of two interactive individuals exhibited synchronization during the joint-tapping task (Funane et al., 2011; Cui et al., 2012; Holper et al., 2012; Cheng et al., 2015; Baker et al., 2016; Pan et al., 2017). These findings suggested that neural markers of interpersonal SMS exist not only at intra-brain level but also at inter-brain level.
An individual’s processes would influence the performance of interpersonal SMS, but we suggest that these processes do not reflect the entire story. Social interaction processes are performed by all individuals together but not by any individual alone. One important aspect to improve our understanding of this process is to illuminate individual psychological processes and individual characteristics. The other inevitable aspect is to regard individuals as an entire system and examine the system’s holistic characteristics. The simplest two-person interaction may be used as an example. Two individuals involved in a social interaction form a `two-in-one’ system (Koike et al., 2015). This interactive dyad emerges new properties, such as a common goal, shared attention, common effort, synchronized behavior and synchronized neural oscillations, which do not exist in any individual in the absence of interaction. The sense of interacting individuals may shift from self-agency to joint agency (a sense of `we-ness’), which indicates that the psychological boundary between self and others blurs and a sense of `feeling they are one’ emerges (Pacherie, 2012). Researches at individual level are insufficient because this situation cannot explain all of the subtle mechanisms of online social interaction (Chatel-Goldman et al., 2013; Liu and Pelowski, 2014) and may omit the interpersonal effect of social interaction (Konvalinka and Roepstorff, 2012). Therefore, researches at the holistic interaction system level are indispensable to comprehend the cognitive and neural mechanisms of interpersonal SMS.
We used a two-person joint-tapping task with a steady tempo as an example to provide a holistic view of interpersonal SMS. Vorberg (2005) had extended linear error correction model from a single person synchronizing with a steady metronome (Vorberg and Wing, 1996; Vorberg and Schulze, 2002) to a person synchronizing with an adaptive metronome that simulates a two-person situation. Inspired by Vorberg’s work, we proposed the `holistic cognitive and neural processes’ and defined indexes as quantitative measurements: (i) Holistic Correction Gain (HCG), which evaluated a dyad’s holistic effort to correct synchronization errors; (ii) Holistic Timekeeper Variance (HTV), which evaluated the noise of a dyad’s `holistic internal timekeeper’; (iii) Holistic Motor Variance (HMV), which evaluated the noise of a dyad’s `holistic motor execution system’; and (iv) inter-brain neural couplings, which were obtained by hyperscanning technique, described an interactive two-brain system at the holistic neural level. Specific description is available in the Methods part.
In the present study, we conducted a real-person joint-tapping experiment to evaluate effects of holistic cognitive and neural processes on synchronization performance. Dyads performed a joint-tapping task in unidirectional and bidirectional conditions (Konvalinka et al., 2010), and their brain activities were simultaneously recorded using functional near-infrared spectroscopy (fNIRS) hyperscanning. Previous studies demonstrated that synchronization performance in bidirectional condition was better than in unidirectional condition. We hypothesized that the reason for this performance difference was that holistic error correction was more sufficient in bidirectional condition than in unidirectional condition. We also hypothesized that different performances of dyads in the same condition would be explained by integrated effects of the dyad’s all holistic cognitive processes. To explore the effect of each holistic cognitive process on synchronization performance, each holistic behavioral index would be compared between conditions; and correlation of holistic cognitive processes and empathy trait/holistic neural processes would be calculated because interpersonal SMS, as a basic form of social interaction, requires mentalizing ability that can be measured by empathy scale and inter-brain couplings (Cummins et al., 2005; Babiloni et al., 2012; Cui et al., 2012). At neural level, we hypothesized that inter-brain neural coupling (especially coupling of right PFC) would be higher in bidirectional condition than in unidirectional condition because bidirectional condition was more cooperative than unidirectional condition (Cui et al., 2012; Jiang et al., 2012).
Methods
Definition and quantitative measurements of holistic cognitive and neural processes
We used the simplest interpersonal SMS form, two-person synchronization without tempo change, as an example.
Holistic cognitive processes
According to linear phase correction model that was proposed by Vorberg (Vorberg and Wing, 1996; Vorberg and Schulze, 2002) to describe single person synchronizing to metronome and extend to two-person synchronization (Vorberg, 2005), the individual cognitive processes in two-person synchronization mainly contain internal timekeeper, phase correction and motor execution (Figure 1A). To achieve and maintain synchronization at a given tempo, each individual needs an internal timekeeper to produce predefined tempo and predict the onset of next tap, and then the predicted tap is executed by motor system. The actual taps inevitably have synchronization errors due to noise presented in the timekeeper and motor systems. Thus, each individual must utilize the feedback of actual taps to adjust his internal timekeeper to reduce synchronization errors. The parameters of individual cognitive processes can be estimated using linear phase correction model
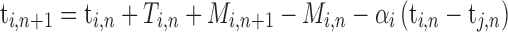 |
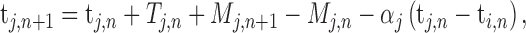 |
(1) |
Fig. 1.
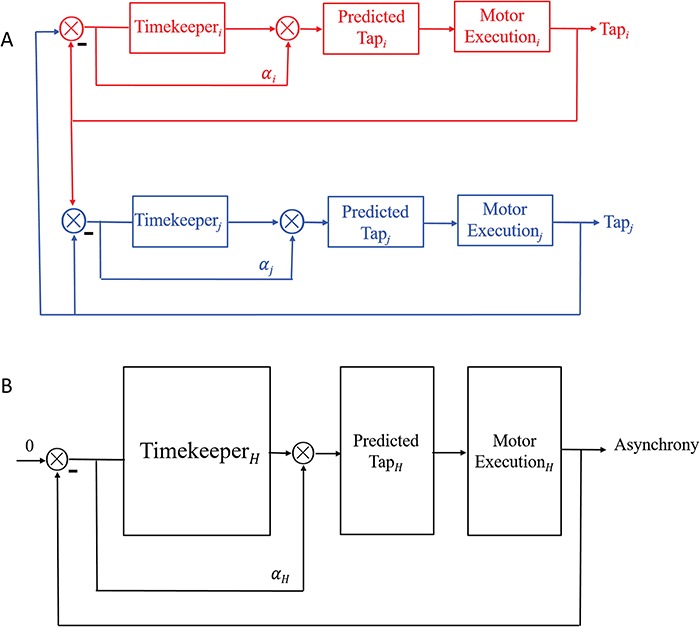
(A) Underlying individual cognitive processes of two-person synchronization. Subscripts i and j represent two persons. (B) A conceptual sketch of `two-in-one’ system from holistic view that shows holistic cognitive processes in two-person synchronization. Subscript H means `Holistic’.
where the variables t, T and M represent the tap onset event time, timekeeper interval and motor delay, respectively (Figure 2A). The phase correction gain 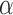 is a fixed proportion of the last asynchrony representing the degree of correction (Figure 2B). The subscripts i and j represent two persons, and n indicates the number of taps. We used this model to first estimate individuals’ phase correction gains
is a fixed proportion of the last asynchrony representing the degree of correction (Figure 2B). The subscripts i and j represent two persons, and n indicates the number of taps. We used this model to first estimate individuals’ phase correction gains 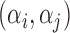 , individuals’ timekeeper variances
, individuals’ timekeeper variances 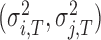 and individuals’ motor variances
and individuals’ motor variances 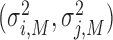 .
.
Fig. 2.
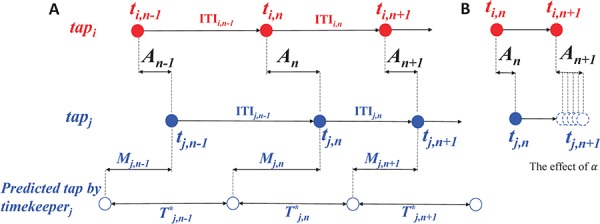
Linear phase correction model. Red and blue solid circles represent the timing of two persons’ tap (i, j represent two person). (A) ITI means inter-tap interval (e.g. 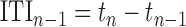 ). A means Asynchrony that is the time difference of two persons’ corresponding taps (e.g.
). A means Asynchrony that is the time difference of two persons’ corresponding taps (e.g. 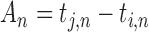 ). When a person (e.g. j) performs rhythmic tapping, he has an internal timekeeper (blue hollow circles) to remember and maintain the rhythm that is represented by T. In real tapping, there are inevitably asynchronies due to noise (e.g. Noise of timekeeper and motor execution). Thus, the person has to adjust T to correct the asynchronies. T* represents the adjusted T and
). When a person (e.g. j) performs rhythmic tapping, he has an internal timekeeper (blue hollow circles) to remember and maintain the rhythm that is represented by T. In real tapping, there are inevitably asynchronies due to noise (e.g. Noise of timekeeper and motor execution). Thus, the person has to adjust T to correct the asynchronies. T* represents the adjusted T and 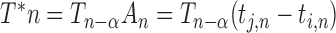 . M means motor delay of action execution. (B) The effect of different α. The dashed blue circles represent possible
. M means motor delay of action execution. (B) The effect of different α. The dashed blue circles represent possible 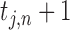 according to different α.
according to different α.
What factors would affect the performance of two-person synchronization? According to our holistic view, we proposed the interacting two partners form a `two-in-one’ system. Underlying the system, `holistic cognitive processes’ would decide the dyad’s performance. As shown in Figure 1B, the system has `holistic timekeeper’, `holistic motor execution’ and `holistic error correction’. To quantitatively measure these `holistic cognitive processes’, we were inspired by Vorberg’s work. The working paper of Vorberg (2005) used a two-person SMS model that described the performance of a dyad as follows (we provide a complete derivation of this model in the Supplementary data):
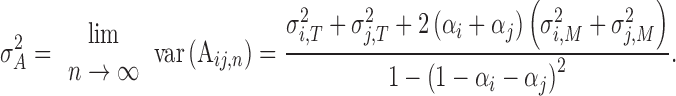 |
(2) |
The variable A represents asynchrony of dyad’s each tapping (Figure 2A) that was defined as the difference of two partners’ tap onset. The
variable 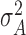 represents the performance of two-person SMS during a sequence of taps. So equation (2) shows all factors that affect the performance. Notably, equation (2) shows that the phase correction gains, the variances of timekeeper intervals and motor delays impact synchronization performance only in the sum form, i.e.
represents the performance of two-person SMS during a sequence of taps. So equation (2) shows all factors that affect the performance. Notably, equation (2) shows that the phase correction gains, the variances of timekeeper intervals and motor delays impact synchronization performance only in the sum form, i.e. 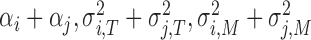 , which suggests that the sums of these parameters, rather than the parameters of a single subject, decide the state of two-person synchronization. Equation (2) supports our holistic view. Therefore, we defined three holistic behavioral indexes to measure `holistic cognitive processes’:
, which suggests that the sums of these parameters, rather than the parameters of a single subject, decide the state of two-person synchronization. Equation (2) supports our holistic view. Therefore, we defined three holistic behavioral indexes to measure `holistic cognitive processes’:
-
(a)
HCG
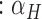
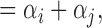 which represents the effort expended in total error correction by the dyad.
which represents the effort expended in total error correction by the dyad. -
(b)
HTV
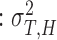
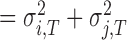 , which indicates the total variance arising from the dyad’s internal timekeeper.
, which indicates the total variance arising from the dyad’s internal timekeeper. -
(c)
HMV:
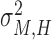
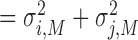 , which indicates the total variance arising from the dyad’s motor delay.
, which indicates the total variance arising from the dyad’s motor delay.
Equation (2) can be rewritten as
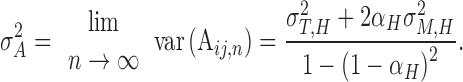 |
(3) |
Therefore, we obtained holistic behavioral indexes to measure the `holistic cognitive processes’ of interpersonal SMS. Further, we proposed holistic space to visually represent holistic behavioral indexes. Take HCG as an example, suppose HTV and HMV are the same. Suppose there are four dyads performing joint-tapping task with stable tempo. Representing individual’s error correction gains in a two-dimensional space (Figure 3A), the figure shows individuals of each dyad have large variance of error correction. However, toward a holistic view of these four dyads, all dyads’ HCGs are equal, which are on the line of HCG = 0.8 (i.e. αH = αP1 + αP2 = 0.8; Figure 3B). These four dyads will have similar performance due to their same error correction (given certain timekeeper and motor noise). These four dyads are similar at holistic level despite the large variance of individuals. We defined this two-dimensional space as holistic space of error correction and defined those lines representing same holistic error correction as equal holistic correction lines (Figure 3C). Holistic space of error correction can be roughly divided into high-correction area and low-correction area. According to the relationship between error correction and performance (Wing et al., 2014), HCGs in the high- or low-correction area (Figure 3D) will lead to a better or worse performance, respectively.
Fig. 3.
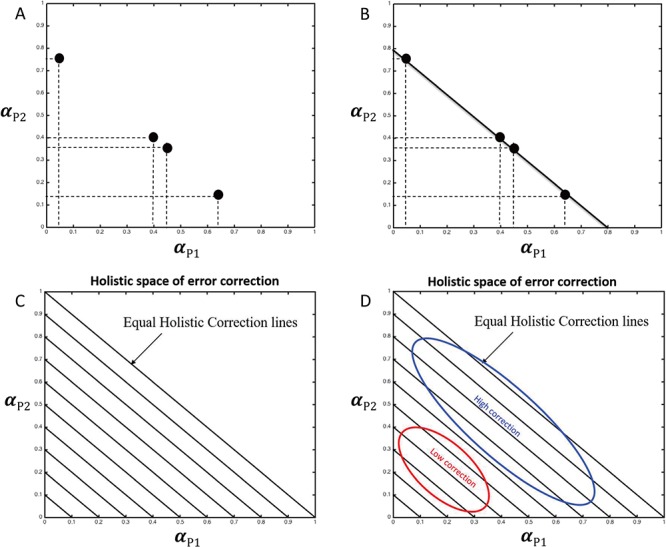
(A) Suppose there were four dyads performing interpersonal SMS with stable tempo. The four black dots represent each dyad’s error correction. (B) The sums of each dyads’ error correction, i.e. HCG, were the same (0.8). (C) and (D) Illustration of `Holistic space of error correction’ and `Equal holistic correction lines’.
Holistic neural processes
Inter-brain neural couplings discovered in hyperscanning studies are appropriate to represent holistic neural processes during interpersonal SMS. In the present study, we used the fNIRS-hyperscanning technique to simultaneously record dyads’ brain activities to assess inter-brain neural couplings during interpersonal SMS.
Participants
Forty-eight right-handed healthy young adults [24 males, mean age 22.77 years (s.d. = 2.19)] were recruited. They randomly comprised 24 same-gender pairs to complete the interpersonal SMS task. All participants had normal hearing and no musical training. Participants in the same pair did not know each other prior to the experiment. The Institutional Review Board at the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University approved this experiment. All participants provided written informed consent. Participants in Figure 4A agreed to the publication of their photographs.
Fig. 4.
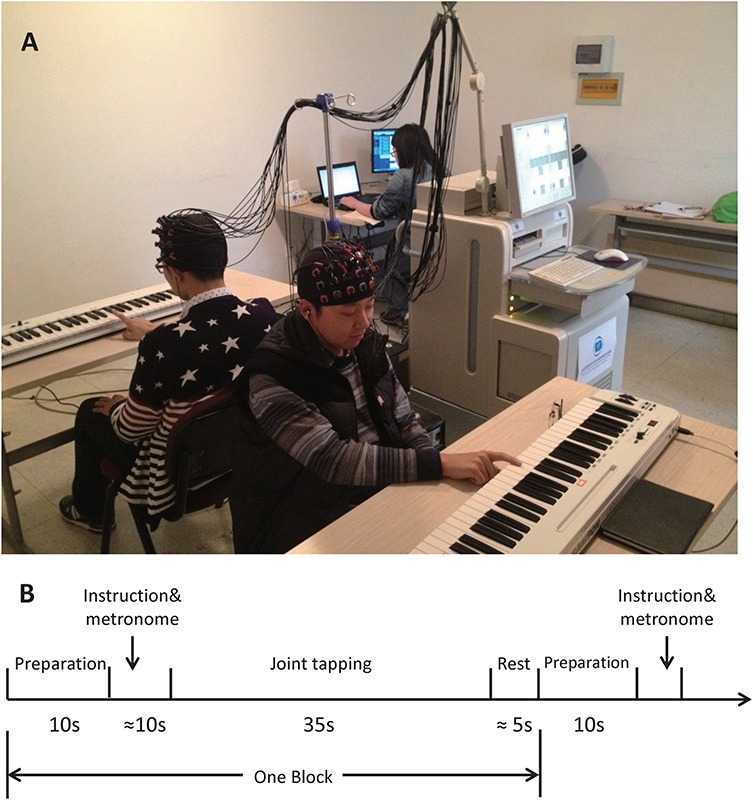
(A) The experimental scene. (B) The experimental procedure.
Procedure
All dyads performed the joint-tapping task on the same key of Musical Instrument Digital Interface (MIDI) keyboards using their index fingers. The task consisted of a bidirectional condition and a unidirectional condition, which were manipulated via auditory feedback in earphones. Dyads heard only their partner’s tapping in bidirectional condition. Leaders in unidirectional condition heard only their own tapping, and followers heard only the leader’s tapping. Each partner of one dyad played the leader and follower once to balance the roles in unidirectional condition. In bidirectional condition, all dyads were instructed to tap synchronously with their partner and to maintain a tapping tempo of 120 beats per minute (bpm) as accurately as possible. In unidirectional condition, leaders were instructed to maintain the 120 bpm tempo, and followers were instructed to tap synchronously with leaders. Participants sat back-to-back to avoid interference of visual information (Figure 4A). A non-interaction condition was included in this task for another study that was not entered into the analysis of the present study.
The procedure of one block (Figure 4B) included 10 s preparation, ≈10 s instruction of condition and hints of metronome (10 beats, 120 bpm) during which participants only heard the hint and got the rhythm, 35 s joint tapping without metronome and ≈6 s rest. There were 16 pseudo-randomized blocks in total (8 blocks of bidirectional condition, 8 blocks of unidirectional condition). All dyads exercised the task prior to the formal experiment. The empathy abilities of all the participants were measured after the experiment using the Interpersonal Reactivity Index (IRI; Davis, 1983).
Apparatus
Behavioral measurement
Participants tapped on two MIDI keyboards (Samson Technologies, New York, USA) that were connected to a computer via a multi-channel audio card. Auditory feedback was sent through the audio card from the computer and received via the earphones. Auditory feedback in different conditions was controlled by different audio tracks in Cubase v5.1(Steinberg, Hamburg, Germany). Output from the keyboards was recorded in Cubase for further behavioral analyses.
Neural measurement
Concentrations of oxygenated hemoglobin (HbO) and deoxygenated hemoglobin of each dyad were measured simultaneously using an ETG-4000 Optical Topography System (Hitachi Medical Co., Tokyo, Japan). Figure 5 shows the customized optode probe sets used for each participant. Eight emitters and 8 detectors comprised 18 channels with 3 cm source-detector separation (DPF695 = 6.51, DPF830 = 5.86). The sampling rate was 10 Hz. Emitters 12 and 17 and detector 18 were placed on Fz, C3 and T3 according to the international 10–20 system (Figure 5).
Fig. 5.
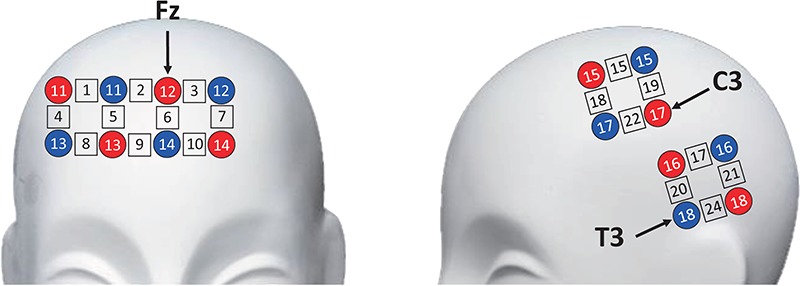
The optode probe sets.
Prefrontal cortex, motor cortex and temporal cortex were covered using 2 × 4, 2 × 2 and 2 × 2 optode probe sets, respectively. These brain areas relate to cognitive, motor and auditory processing, respectively, which are indispensable in the auditory joint-tapping task (Repp and Su, 2013; Keller et al., 2014). Specifically, probe sets on the PFC are asymmetric to cover the medial prefrontal cortex (mPFC) and the right dorsal lateral prefrontal cortex (DLPFC), which are involved in mentalizing (Cui et al., 2012). Probe sets for each dyad were examined and adjusted to guarantee consistency of positions between two partners. Examination and adjusting were also performed across dyads.
Analysis
Four dyads were removed after data checking because of bad behavioral performance and/or problematic neural signal recording. The remaining 20 dyads were analyzed for behavioral and neural indexes.
Behavioral analysis
Behavioral analyses included pre-processing, performance measurement, holistic behavioral indexes computing and integrated effect of holistic cognitive processes.
Pre-processing
The onset of each single tap of dyads was extracted from Cubase files for behavioral analyses using customized MATLAB (MathWorks, Natick, USA) scripts. Tapping data were pre-processed as follows: (i) because the two partners may exhibit different reaction times to `start’ and `stop’ instructions, the first and last one or two taps were removed from each block in order to guarantee the same number of taps for both partners; (ii) to recover missing taps, interpolation was used for the single missing tap according to the average time of its adjacent two taps; (iii) blocks containing continuous multiple missing taps were removed; and (iv) blocks containing a mismatched number of taps after pre-processing (i) and (ii) were removed. Fifty-six of the total 320 blocks (8 blocks per condition *2 conditions * 20 pairs) were removed, and the 264 blocks of 20 dyads were further analyzed.
Performance measurement
Synchronization performance was evaluated using asynchrony (i.e. synchronization error). Smaller asynchronies indicate better performance. Asynchronies are the onset time differences of the two players’ corresponding taps. Asynchronies were computed as Asy = Onsetplayer1 − Onsetplayer2. Mean values and variations of asynchronies were computed as performance indexes. Mean values were computed using the absolute value of asynchronies, and variations were computed using the raw value of asynchronies.
Holistic behavioral indexes computing
First, we estimated individual parameters of two partners in bidirectional condition and followers in unidirectional condition using Nori Jacoby’s (Jacoby et al., 2015) estimation method of phase correction for multi-person synchronization1. Parameters of leaders in unidirectional condition were estimated using Wing and Kristofferson’s method (Wing and Kristofferson, 1973; Kampen and Snijders, 2002). Then parameters of holistic cognitive processes were computed according to the definitions, which included HCG (αH), HTV (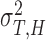 ) and HMV (
) and HMV (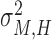 ).
).
Integrated effect of holistic cognitive processes
This analysis aimed to evaluate effect of holistic cognitive processes on synchronization performance. The integrated effect of holistic cognitive processes was computed using equation (3). The integrated effects were firstly compared between conditions. Then, the relationship between integrated effect of holistic cognitive processes and synchronization performance within condition was evaluated by Pearson’s correlation coefficient.
Neural analysis
Channel 6 was discarded because it failed in the adjustment. Only the HbO signal entered into data analyses because it is more sensitive to changes in cerebral blood flow (Hoshi, 2003; Cui et al., 2012; Cheng et al., 2015).
Inter-brain neural coupling
The entire HbO time series of each channel was pre-processed using first and second order detrending and 0–0.2 low-pass filtering. The data of tapping periods in each block were extracted, and the first and last seconds of the tapping period were discarded because brain states at the beginning and at the end of tapping were unstable. Pearson’s correlation coefficients were computed for each dyad’s homologous channels and averaged by four regions of interest (ROIs) which are right PFC (channels 1, 4, 5 and 8), mPFC (channels 2, 3, 9 and 10), motor area (channels 15, 18, 19 and 22) and temporal area (channels 17, 20, 21 and 24). Correlations of the same pair in two sub-conditions of unidirectional condition were averaged as the dyad’s neural coupling in unidirectional condition.
Correlation analysis of holistic cognitive processes, holistic neural processes and empathy trait
Correlation between holistic cognitive processes and holistic neural processes
All dyads’ holistic behavioral indexes (HCG, HTV and HMV) and the four ROIs’ inter-brain neural couplings of the two conditions were normalized to eliminate between-condition variance. Pearson’s correlation coefficients were computed between each holistic behavioral index and each holistic neural index.
Correlation between holistic cognitive processes and empathy trait
Two partners’ holistic scores of IRI were averaged as dyads’ empathy trait scores. Three holistic behavioral indexes were averaged across two conditions. Pearson’s correlation coefficients were computed between the dyads’ empathy scores and condition-averaged HCGs, HTVs and HMVs.
Analysis of individual cognitive and neural processes
To gain a better understanding of how holistic cognitive processes provide extra information on interpersonal SMS than individual processes, we also analyzed (i) the integrated effect of individual cognitive processes, (ii) the relationship between individual cognitive processes and inter-brain couplings, (iii) the relationship between individual cognitive processes and empathy and (iv) intra-brain activation. All of these analyses and results are available in Supplementary data.
Results
Performance of synchronization
Table 1 shows the descriptive statistics of joint-tapping performance in the two conditions. Figure 6 shows the results of paired t-tests. Asynchrony of bidirectional condition was significantly smaller than asynchrony of unidirectional condition (t(19) = −7.55, P < 0.0001) (Figure 6A). Variance of asynchrony of bidirectional condition was significantly smaller than that of unidirectional condition [t(19) = −4.51, P < 0.001] (Figure 6B). These results confirmed the previous finding that dyads performed better in bidirectional condition than in unidirectional condition.
Table 1.
Descriptive statistics of the behavioral results
| Bidirectional condition | Unidirectional condition | |||
|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |
| Asynchrony (ms) | 42.01 | 13.25 | 69.00 | 17.32 |
| Variation of asynchrony (ms2) | 1574.19 | 536.48 | 2562.66 | 1101.66 |
| f(HCG, HTV, HMV)(ms2)1 | 1603.75 | 555.21 | 2079.85 | 1082.13 |
| HCG | 0.63 | 0.20 | 0.37 | 0.09 |
| HTV (ms2) | 1251.06 | 480.70 | 1063.26 | 486.99 |
| HMV (ms2) | 37.33 | 44.26 | 88.32 | 64.24 |
| Inter-tap intervals | 437.89 | 28.47 | 464.85 | 26.38 |
1 f(HCG,HTV,HMV) was computed using equation (3).
Fig. 6.
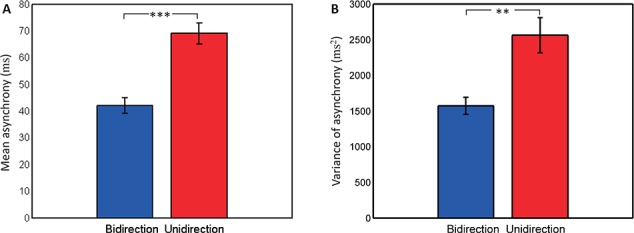
Performance of joint-tapping task. Left: Mean asynchrony. Right: Variance of asynchrony. *P < 0.05, **P < 0.01,***P < 0.001, the same below.
Integrated effect of holistic cognitive processes
According to equation (3), the performance of interpersonal SMS is decided by the integrated effect of three holistic cognitive processes. Figure 7A shows the scatter plot of each dyad’s HCG, HTV and HMV in a three-dimensional space. Each point in this space represents one dyad’s performance in one of the two conditions. Quantitative comparisons of the integrated effect of HCG (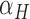 ), HTV (
), HTV (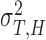 ) and HMV (
) and HMV (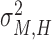 ) are using equation (3). Figure 7B shows the integrated effects of HCG, HTV and HMV in the two conditions. Paired t-test indicated that the integrated effect of three holistic cognitive processes in bidirectional condition was better than in unidirectional condition [t(19) = −2.27, P < 0.05], which was consistent with the performance result of two conditions (Figure 6B). Figure 7C and D shows the relationship between performance and integrated effect of holistic cognitive processes within conditions. The results demonstrated that the performance significantly correlated with the integrated effects of holistic cognitive processes in bidirectional and unidirectional conditions (Figure 7C and D). The Pearson’s correlation coefficient was 0.70 (0.72) of bidirectional (unidirectional) condition.
) are using equation (3). Figure 7B shows the integrated effects of HCG, HTV and HMV in the two conditions. Paired t-test indicated that the integrated effect of three holistic cognitive processes in bidirectional condition was better than in unidirectional condition [t(19) = −2.27, P < 0.05], which was consistent with the performance result of two conditions (Figure 6B). Figure 7C and D shows the relationship between performance and integrated effect of holistic cognitive processes within conditions. The results demonstrated that the performance significantly correlated with the integrated effects of holistic cognitive processes in bidirectional and unidirectional conditions (Figure 7C and D). The Pearson’s correlation coefficient was 0.70 (0.72) of bidirectional (unidirectional) condition.
Fig. 7.
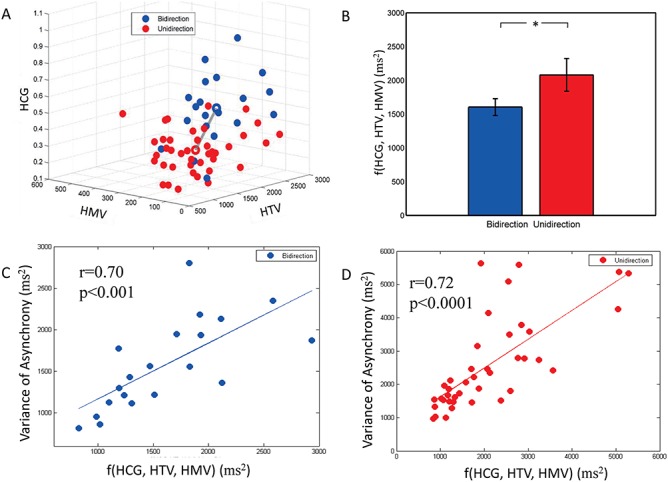
Integrated effects of three holistic cognitive processes. (A) Scatter plot of all dyads’ HCG, HTV and HMV. (B) Integrated effect of HCG, HTV and HMV between conditions. Equation (3) has the notation of f (HCG, HTV, HMV) that represents the integrated effect of HCG, HTV and HMV. (C )and (D) integrated effect of HCG, HTV and HMV within condition.
Effect of each holistic cognitive process
Table 1 shows the descriptive statistics of each holistic cognitive process. Figure 8A shows the scatter plot of HCG in holistic space. It can be roughly seen that the HCG of bidirectional condition (blue dots) is larger than the HCG of unidirectional condition (red dots). We performed paired t-test of HCGs between two conditions. The HCG of bidirectional condition was significantly larger than the HCG of unidirectional condition [t(19) = 6.70, P < 0.0001; Figure 8B], which suggested that dyads made more error corrections in bidirectional condition than in unidirectional condition.
Fig. 8.

Effect of HCG. (A) Scatter plot of HCG in holistic space. (B) Comparison of HCG between two conditions.
Figure 9A shows the scatter plot of HTV in holistic space. Paired t-test showed that the HTV of bidirectional condition was significantly larger than the HTV of unidirectional condition [t(19) = 2.56, P < 0.05], which suggested that the dyads’ total internal timekeeper noises were larger in bidirectional condition than in unidirectional condition (Figure 9B). Correlation analysis showed that HTV had significant negative correlation with empathy trait and inter-brain couplings (Table 2). Figure 9C shows that condition-averaged HTV and the dyads’ empathy scores exhibit significant negative correlation (r = −0.58, df = 18, P < 0.01). This result suggested that the higher the dyads’ empathy ability, the smaller the dyads’ total timekeeper noises. This result suggested that maintaining stable internal timekeepers related to empathy ability. HTV and inter-brain neural coupling of the right PFC were significantly negatively correlated (r = −0.3874, df = 38, P < 0.05; Figure 9D). HTV and inter-brain neural coupling of the motor area were also significantly negatively correlated (r = −0.3468, df = 38, P < 0.05). There were no significant correlations of other holistic cognitive processes and empathy trait/holistic neural processes (Table 2).
Fig. 9.
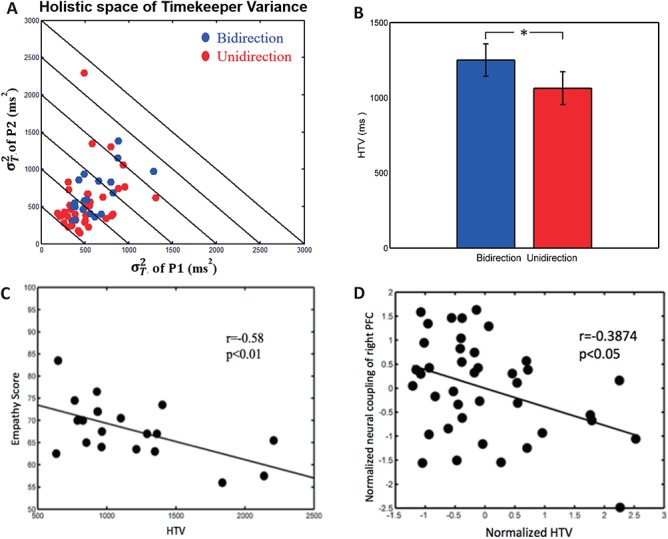
Effect of HTV. (A) Scatter plot of HTV in holistic space. (B) Comparison of HTV between two conditions. (C) Correlation between HTV and empathy trait. (D) Correlations between HTV and inter-brain neural coupling of right PFC.
Table 2.
Correlations between holistic cognitive processes and empathy/holistic neural processes
| Empathy | Inter-brain coupling | ||||
|---|---|---|---|---|---|
| Right PFC | mPFC | Motor area | Temporal area | ||
| HCG | −0.35 | −0.13 | 0.09 | −0.05 | 0.08 |
| HTV | −0.58* | −0.39* | −0.15 | −0.35* | −0.22 |
| HMV | 0.11 | 0.15 | −0.07 | 0.15 | 0.04 |
The HMV of bidirectional condition was significantly smaller than the HMV of unidirectional condition [t(19) = −3.04, P = 0.007], which suggested that the dyads’ total motor noises were smaller in bidirectional condition than in unidirectional condition.
Holistic neural processes
Table 3 lists the descriptive statistics of inter-brain neural coupling. Only neural coupling of the right PFC was significantly different between the two conditions [paired t-test: t(19) = −2.908, P = 0.009; Figure 10]. The couplings of other three ROIs were not significantly different between the two conditions. Paired t-test results of the mPFC, motor and temporal areas were t(19) = −1.505 (P = 0.149), t(19) = −1.822(P = 0.084) and t(19) = −0.400 (P = 0.694), respectively.
Table 3.
Descriptive statistics of inter-brain neural couplings
| Inter-brain coupling(r) | ||||
|---|---|---|---|---|
| Bidirectional condition | Unidirectional condition | |||
| Mean | s.d. | Mean | s.d. | |
| Right PFC | 0.16 | 0.25 | 0.27 | 0.20 |
| mPFC | 0.05 | 0.19 | 0.10 | 0.16 |
| Motor area | 0.08 | 0.19 | 0.16 | 0.16 |
| Temporal area | 0.09 | 0.20 | 0.11 | 0.22 |
Fig. 10.
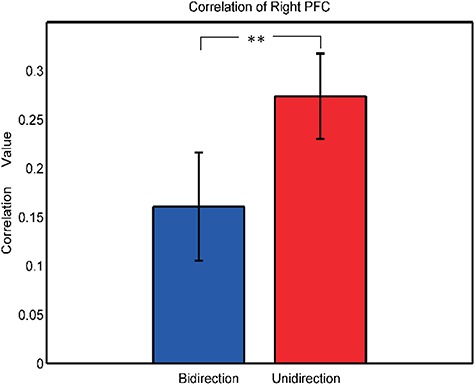
Inter-brain neural couplings of right PFC.
Discussion
The present study proposed a holistic view to understand interpersonal SMS. We proposed the concept of `holistic cognitive and neural processes’ of interpersonal SMS and defined quantitative measurements which included HCG, HTV and HMV based on linear error correction model and inter-brain couplings obtained by hyperscanning technique. We also performed a real-person joint-tapping experiment using fNIRS-hyperscanning technique to evaluate effects of holistic cognitive and neural processes on synchronization performance. Synchronization performance was better in bidirectional condition than in unidirectional condition. This performance difference was due to the integrated effect of holistic cognitive processes. The performance of different dyads within the same condition also strongly correlated with the integrated effect of holistic cognitive processes. Each holistic cognitive process played different roles in interpersonal SMS. HCG mainly affected the synchronization performance. HTV mainly related to mentalizing partner’s state. We also found that the holistic neural process (i.e. inter-brain neural coupling of right PFC) was different between the two conditions. These results will be discussed in sequence as follows.
Synchronization performance in bidirectional condition was more synchronous than in unidirectional condition. This result was consistent with the main findings of previous studies (Konvalinka et al., 2010; Noy et al., 2011; Fairhurst et al., 2013). According to equation (3), synchronization performance is decided by the integrated effect of three holistic cognitive processes. The experiment results validated this effect between and within conditions. The integrated effect of three holistic cognitive processes between conditions (Figure 7B) was consistent with the performance between conditions (Figure 6B). The integrated effect of three holistic cognitive processes significantly positively correlated with performance both in bidirectional/ unidirectional condition (Figure 7C and D). Different dyads exhibited different synchronization performance because dyads, as different interactive systems, exhibited different holistic system characteristics. Dyads made different efforts to correct synchronization errors (HCG) and exhibited different precision degrees of timekeepers (HTV) and different degrees of motor noise (HMV). These differences in holistic cognitive processes integrally produced different synchronization performances.
There was an exceptional finding of synchronization performance’s between-condition difference in Noy’s study (Noy et al., 2011). Participants in a joint improvisation task without musical training performed worse in bidirectional condition than in unidirectional condition. The reason for this result may be that it is too difficult for non-musicians to perform joint improvisation tasks, but imitation can be performed in a leader–follower manner. This phenomenon suggests that the directionality of an interaction and an interactive system’s ability may exhibit an interactive effect. Therefore, we predict that the integrated effect of holistic cognitive processes would be worse in bidirectional condition than in unidirectional condition in difficult synchronization tasks, which exceed the interactive system’s ability. However, this hypothesis requires further validation.
Each holistic cognitive process played different roles in interpersonal SMS. Holistic error correction is the main factor influencing synchronization performance. Dyads in bidirectional condition performed better than in unidirectional condition mainly because they exhibited higher HCG in bidirectional condition (Figure 8). Higher HCG suggested that dyads, as an entire system, corrected more errors in bidirectional condition. In unidirectional condition, leaders performed the self-paced tapping task that can be described by the Wing and Kristofferson’s model. According to the explicit requirement of the model, leaders’ error correction is zero. Thus, synchronization errors were corrected by followers alone in unidirectional condition. In contrast, two partners in bidirectional condition performed error corrections together (average: 0.63), which was better than in unidirectional condition (average: 0.37).
Holistic internal timekeeper is the main cognitive process to maintain tempo and predict onsets of taps. During interpersonal SMS, this process needs to utilize feedback from other’s taps in order to understand and predict other’s taps. Thus, holistic internal timekeeper may relate to the ability of mentalizing. Results showed that HTV significantly negatively correlated with dyad’s empathy trait (Figure 9C). This correlation suggests that the higher a dyad’s empathy trait indicates, the smaller the dyad’s internal timekeeper variance (i.e. more stable internal timekeeper). Maintaining a stable internal timekeeper is beneficial for others to predict following taps and maintain synchronization. A dyad’s high empathy trait may be helpful for maintaining a stable internal timekeeper. HTV also significantly negatively correlated with neural coupling of the right PFC (Figure 9D). This result suggests the smaller the dyad’s internal timekeeper variance (i.e. more stable internal timekeeper), the higher the dyad’s neural coupling of the right PFC. Neural coupling of the PFC may be involved in the mentalizing and prediction of others, which is related to empathy ability. Thus, this correlation was consistent with the previous correlation. However, HTV was greater in bidirectional condition (Figure 9B), which was unbeneficial to maintain synchronization. This result may be due to the enlarged variance of inter-tap intervals (Table 1), which is the cost of greater error correction (Honisch et al., 2016) and may disturb the internal timekeeper, leading to a larger HTV in bidirectional condition. However, the deterioration effect of the HTV appeared counteracted by the promotion effect of HCG and HMV.
Holistic motor system is the executor in interpersonal SMS. The predicted taps of holistic internal timekeeper are implemented by holistic motor system. Noise from motor system is the inevitable source of synchronization errors, but it is not the main factor influencing synchronization performance. The results showed that HMV in bidirectional condition was significantly smaller than HMV in unidirectional condition. Honisch et al. (2016) also found individuals’ motor variances were significantly different between different experimental conditions. It implied that motor variance may be affected by the task. Different aims or different difficulties may influence the fluctuation of motor system then lead to different motor noises.
Different methods of parameter estimation may be another factor that leads to different HMV and HTV between two conditions. Jacoby’s method was used to estimate parameters of dyads in bidirectional condition and followers in unidirectional condition. Wing–Kristofferson’s method was used to estimate parameters of leaders in unidirectional condition. Comparison of Jacoby’s and Wing–Kristofferson’s method is needed.
To gain a better understanding of how holistic cognitive processes provide extra information than individual processes, we also examined the integrated effect of individual processes, explored the relationship between individual processes and inter-brain couplings/empathy and examined intra-brain activations. The integrated effects of individual processes were much smaller than synchronization performance in both conditions (Supplementary Figure S1A). In unidirectional condition, both integrated effect of leaders’ and followers’ parameters were much smaller than synchronization performance (Supplementary Figure S2A). Even significant correlations were found between synchronization performance and integrated effect of individual processes in unidirectional condition (Supplementary Figure S1C) and specifically in followers (Supplementary Figure S2C), it should be noted that synchronization performance is decided by integrated effect of holistic processes at the theoretical level [equation (3)] and the integrated effect of individual processes was far from enough to explain synchronization performance in fact (Supplementary Figure S1A and S2A). Correlation analysis revealed that there were no significant correlations between individuals’ processes and empathy (results were shown in Supplementary data). There were significantly negative correlations between individuals’ timekeeper variance and inter-brain couplings of right PFC and motor area (Supplementary Table S2), which were the same as HTV. To further examine the effect of roles, neither leaders’ nor followers’ processes had significant correlation with inter-brain couplings (Supplementary Table S3). At neural level, no significant difference of intra-brain activations was found between individuals or roles (Supplementary Tables S4–S6). To sum up, holistic cognitive processes could better explain synchronization performance than individual processes, and they had relationship with dyads’ empathy and inter-brain couplings that provided more information than individual processes to understand interpersonal SMS.
The primary neural finding was that holistic neural process was different between bidirectional and unidirectional conditions. Inter-brain neural coupling of right PFC was significantly higher in unidirectional condition than in bidirectional condition (Figure 10). This difference suggests the existence of neural markers at holistic level in interpersonal SMS. Neural coupling of right PFC was reported in cooperative tapping tasks (Cui et al., 2012; Baker et al., 2016; Pan et al., 2017), which may be involved in mentalizing other’s mental states and predicting other’s behavior. However, neural coupling in unidirectional condition, but not in bidirectional condition, exhibited higher synchronization in the present study, which conflicted with the results of Jiang’s study (Jiang et al., 2012) and our prediction. The main reason of this conflict may be task difference. Jiang’s study used a communication task. Bidirectional communication was in a turn-taking way. Each single turn of speaking did not contain all of the information of communication, and the speaker and listener must keep using insufficient information to mentalize the other person’s real meaning and intention. Unidirectional communication allowed speakers to express a piece of relatively complete information. Therefore, language comprehension, rather than mentalizing, was the main process to understand the other person’s meaning in unidirectional communication. Insufficient information led to greater mentalizing processes, which led to more synchronous neural couplings in bidirectional communication than in unidirectional communication. However, every single tap of the two partners in the joint-tapping bidirectional condition was sufficient to discriminate synchronization. A stable tempo also made it easy to predict the partner’s taps. Therefore, understanding and prediction of partner’s behavior in bidirectional joint-tapping primarily relied on auditory discrimination and predefined tempo rather than mentalizing. The unidirectional condition was different because leaders could not hear followers’ tapping. In post-experiment interviews, most participants said that they were always simulating their follower’s tapping in mind when they were leaders. The leader’s behavior was independent for followers, and followers needed to make more effort to predict the leader’s behavior. These processes may explain why neural coupling in unidirectional condition was higher than in bidirectional condition in our study.
The present study has the following limitations. First, interpersonal SMS has different types that include discrete (e.g. finger tapping) and continuous (e.g. limb swing) forms. The present study used a discrete SMS task (i.e. finger tapping) because the information-processing approach mainly models discrete SMS. However, discrete and continuous SMS may have different cognitive processes and neural mechanisms (Repp, 2005; Janzen et al., 2014). How to model and measure holistic cognitive and neural processes of continuous SMS requires further research, for which the dynamic system approach can provide useful modeling methods. Second, the present study only investigated dyads’ finger tapping at a stable tempo for simplification. Jacoby et al. (2015) used linear SMS models for different situations, from single person to multi-person and stable tempo to changing tempo. The present study used the simplest model of interpersonal SMS as an example to evaluate effects of holistic cognitive and neural processes. More complex situations, such as multi-person groups and changing tempo, should be examined further. Lastly, participants in the present study had no musical training experience. Therefore, they could not perform joint-tapping tasks as precisely as people with musical training. As a result, three dyads were excluded from data analyses due to problematic performance, and 17% (56/320) of the blocks of the remaining dyads were excluded due to mismatched taps.
Conclusion
In recent years, studies on the mechanisms of social behavior increasingly focus on real interactions than virtual interactions with computers. Real interactions require researchers to consider all interactive individuals as a whole system and explore emerging characteristics of the interaction system, in addition to focusing on an individual’s cognitive and neural processes. Other research topics have defined several holistic measures of multi-person groups, such as inter-subject correlation, which measured how humans understand the world in the same way (Hasson et al., 2004), and intra-class correlation, which measured within-group coordination during decision making (De Dreu et al., 2016). Toward the holistic view, we proposed the `holistic cognitive and neural processes’, defined quantitative holistic measurements of interpersonal SMS and evaluated effects of these processes using a real two-person interpersonal SMS experiment. We believe that the holistic view is beneficial to understand mechanism of interpersonal SMS and more complex social interaction processes.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 61431002, 61273287, 31221003, 31521063 and 31671077), National Key Basic Research Program of China (973 Program) (Grant 2014CB846100), Program for New Century Excellent Talents in University of Ministry of Education of China (Grant 11-0046), the Major Project of the National Social Science Foundation (Grant 12&ZD228) and the Fundamental Research Funds for the Central Universities.
Supplementary Material
Footnotes
MATLAB script can be found at https://figshare.com/articles/ Parameter_Estimation_of_Linear_Sensorimotor_Synchronization_ Models_Phase_Correction_Period_Correction_and_Ensemble_ Synchronization /1391910
References
- Babiloni C., Buffo P., Vecchio F., et al. (2012). Brains "in concert": frontal oscillatory alpha rhythms and empathy in professional musicians. NeuroImage, 60(1), 105–16. [DOI] [PubMed] [Google Scholar]
- Baker J.M., Liu N., Cui X., et al. (2016). Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Scientific Reports, 6, 26492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatel-Goldman J., Schwartz J.L., Jutten C., Congedo M. (2013). Non-local mind from the perspective of social cognition. Frontiers in Human Neuroscience, 7, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.J., Li X.C., Hu Y. (2015). Synchronous brain activity during cooperative exchange depends on gender of partner: a fNIRS-based hyperscanning study. Human Brain Mapping, 36(6), 2039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Mundry R., Kirschner S. (2013). Religion, synchrony, and cooperation. Religion, Brain & Behavior, 4(1), 20–30. [Google Scholar]
- Cui X. (2012). NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage, 59(3), 2430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins A., Piek J.P., Dyck M.J. (2005). Motor coordination, empathy, and social behaviour in school-aged children. Developmental Medicine and Child Neurology, 47(7), 437–42. [DOI] [PubMed] [Google Scholar]
- Davis M.H. (1983). Measuring individual differences in empathy—evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44(1), 113–26. [Google Scholar]
- Dreu C. K., Gross J., Meder Z., et al. (2016). In-group defense, out-group aggression, and coordination failures in intergroup conflict. Proceedings of the National Academy of Sciences of the United States of America ,113(38), 10524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G., Guzman G. C., Tognoli E., Kelso J. A. S. (2014). The human dynamic clamp as a paradigm for social interaction. Proceedings of the National Academy of Sciences of the United States of America ,111(35), E3726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G., Mario C., Jacqueline N., Jacques M. (2012). Anatomical connectivity influences both intra- and inter-brain synchronizations. PLoS ONE, 7(5), e36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G., Nadel J., Soussignan R., Martinerie J., Garnero L. (2010). Inter-brain synchronization during social interaction. PLoS ONE, 5(8), e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M.T., Chua W.L., Wing A.M. (2016). Modelling single-person and multi-person event-based synchronisation. Current Opinion in Behavioral Sciences, 8, 167–74. [Google Scholar]
- Fairhurst M.T., Janata P., Keller P.E. (2013). Being and feeling in sync with an adaptive virtual partner: brain mechanisms underlying dynamic cooperativity. Cerebral Cortex, 23(11), 2592–600. [DOI] [PubMed] [Google Scholar]
- Fairhurst M.T., Janata P., Keller P.E. (2014). Leading the follower: an fMRI investigation of dynamic cooperativity and leader-follower strategies in synchronization with an adaptive virtual partner. NeuroImage, 84, 688–97. [DOI] [PubMed] [Google Scholar]
- Funane T., Kiguchi M., Atsumori H., Sato H., Kubota K., Koizumi H. (2011). Synchronous activity of two people's prefrontal cortices during a cooperative task measured by simultaneous near-infrared spectroscopy. Journal of Biomedical Optics, 16(7), 077011. [DOI] [PubMed] [Google Scholar]
- Haken H., Kelso J.A.S., Bunz H. (1985). A theoretical model of phase transitions in human hand movements. Biological Cybernetics, 51(5), 347–56. [DOI] [PubMed] [Google Scholar]
- Hasson U., Ghazanfar A.A., Galantucci B., Garrod S., Keysers C. (2012). Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16(2), 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. (2004). Intersubject synchronization of cortical activity during natural vision. Science, 303(5664), 1634–40. [DOI] [PubMed] [Google Scholar]
- Holper L., Scholkmann F., Wolf M. (2012). Between-brain connectivity during imitation measured by fNIRS. NeuroImage, 63(1), 212–22. [DOI] [PubMed] [Google Scholar]
- Honisch J.J., Elliott M.T., Jacoby N., Wing A.M. (2016). Cue properties change timing strategies in group movement synchronisation. Scientific Reports, 6, 19439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y. (2003). Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology, 40(4), 511–20. [DOI] [PubMed] [Google Scholar]
- Hove M.J., Risen J.L. (2009). It's all in the timing: interpersonal synchrony increase affiliation. Social Cognition, 27(6), 949–60. [Google Scholar]
- Jacoby N., Ahissar M., Keller P.E., Tishby N., Repp B.H. (2015). Parameter estimation of linear sensorimotor synchronization models: phase correction, period correction, and ensemble synchronization. Timing & Time Perception, 3(1–2), 52–87. [Google Scholar]
- Janzen T.B., Thompson W.F., Ammirante P., Ranvaud R. (2014). Timing skills and expertise: discrete and continuous timed movements among musicians and athletes. Frontiers in Psychology, 5, 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Dai B., Peng D., Zhu C., Liu L., Lu C. (2012). Neural synchronization during face-to-face communication. Journal of Neuroscience, 32(45), 16064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsa V.K., Kelso J.A.S. (2005). The excitator as a minimal model for the coordination dynamics of discrete and rhythmic movement generation. Journal of Motor Behavior, 37, 35–51. [DOI] [PubMed] [Google Scholar]
- Kampen J., Snijders T. (2002). Estimation of the Wing–Kristofferson model for discrete motor responses. The British Journal of Mathematical and Statistical Psychology, 55(1), 159–68. [DOI] [PubMed] [Google Scholar]
- Keller P.E., Appel M. (2010). Individual differences, auditory imagery, and the coordination of body movements and sounds in musical ensembles. Music Perception, 28(1), 27–46. [Google Scholar]
- Keller P.E., Novembre G., Hove M.J. (2014). Rhythm in joint action: psychological and neurophysiological mechanisms for real-time interpersonal coordination. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369(1658), 20130394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso J.A.S. (1995). Dynamic Patterns: The Self-Organization of Brain and Behavior, Cambridge, MA: MIT Press. [Google Scholar]
- Kelso J.A.S., Dumas G., Tognoli E. (2013). Outline of a general theory of behavior and brain coordination. Neural Networks, 37, 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.Y. (2015). Achieving synchrony: a foundational dimension of intercultural communication competence. International Journal of Intercultural Relations, 48, 27–37. [Google Scholar]
- Kirschner S., Tomasello M. (2010). Joint music making promotes prosocial behavior in 4-year-old children. Evolution and Human Behavior, 31(5), 354–64. [Google Scholar]
- Koike T., Tanabe H.C., Sadato N. (2015). Hyperscanning neuroimaging technique to reveal the "two-in-one" system in social interactions. Neuroscience Research, 90, 25–32. [DOI] [PubMed] [Google Scholar]
- Konvalinka I., Vuust P., Roepstorff A., Frith C.D. (2010). Follow you, follow me: continuous mutual prediction and adaptation in joint tapping. Quarterly Journal of Experimental Psychology, 63(11), 2220–30. [DOI] [PubMed] [Google Scholar]
- Konvalinka I., Roepstorff A. (2012). The two-brain approach: how can mutually interacting brains teach us something about social interaction? Frontiers in Human Neuroscience, 6, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U., Li S.C., Gruber W., Muller V. (2009). Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neuroscience, 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Pelowski M. (2014). A new research trend in social neuroscience: towards an interactive-brain neuroscience. PsyCh journal, 3(3), 177–88. [DOI] [PubMed] [Google Scholar]
- Merker B.H., Madison G.S., Eckerdal P. (2009). On the role and origin of isochrony in human rhythmic entrainment. Cortex, 45(1), 4–17. [DOI] [PubMed] [Google Scholar]
- Montague P.R. (2002). Hyperscanning: simultaneous fMRI during linked social interactions. NeuroImage, 16(4), 1159–64. [DOI] [PubMed] [Google Scholar]
- Naeem M., Prasad G., Watson D.R., Kelso J.A.S. (2012). Electrophysiological signatures of intentional social coordination in the 10-12 Hz range. NeuroImage, 59(2), 1795–803. [DOI] [PubMed] [Google Scholar]
- Newman-Norlund R.D., Noordzij M.L., Meulenbroek R.G., Bekkering H. (2007). Exploring the brain basis of joint action: co-ordination of actions, goals and intentions. Social Neuroscience, 2(1), 48–65. [DOI] [PubMed] [Google Scholar]
- Nowicki L., Prinz W., Grosjean M., Repp B.H., Keller P.E. (2013). Mutual adaptive timing in interpersonal action coordination. Psychomusicology: Music, Mind, and Brain, 23(1), 6–20. [Google Scholar]
- Noy L., Dekel E., Alon U. (2011). The mirror game as a paradigm for studying the dynamics of two people improvising motion together. Proceedings of the National Academy of Sciences of the United States of America, 108(52), 20947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacherie E. (2012). The Phenomenology of Joint Action: Self-Agency vs. Joint-Agency, Cambridge, MA: MIT Press. [Google Scholar]
- Pan Y.F., Cheng X.J., Zhang Z.X., Li X.C., Hu Y. (2017). Cooperation in lovers: an fNIRS-based hyperscanning study. Human Brain Mapping, 38(2), 831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecenka N., Keller P.E. (2011). The role of temporal prediction abilities in interpersonal sensorimotor synchronization. Experimental Brain Research, 211(3–4), 505–15. [DOI] [PubMed] [Google Scholar]
- Repp B.H. (2005). Sensorimotor synchronization: a review of the tapping literature. Psychonomic Bulletin & Review, 12(6), 969–92. [DOI] [PubMed] [Google Scholar]
- Repp B.H., Keller P.E. (2008). Sensorimotor synchronization with adaptively timed sequences. Human Movement Science, 27(3), 423–56. [DOI] [PubMed] [Google Scholar]
- Repp B.H., Su Y.H. (2013). Sensorimotor synchronization: a review of recent research (2006–2012). Psychonomic Bulletin & Review, 20(3), 403–52. [DOI] [PubMed] [Google Scholar]
- Righetti L., Buchli J., Ijspeert A.J. (2009). Adaptive frequency oscillators and applications. The Open Cybernetics & Systemics Journal, 3(2), 64–9. [Google Scholar]
- Schöner G., Kelso J.A.S. (1988). A dynamic pattern theory of behavioral change. Journal of Theoretical Biology, 135, 501–24. [Google Scholar]
- Tognoli E., Kelso J.A.S. (2015). The coordination dynamics of social neuromarkers. Frontiers in Human Neuroscience, 9, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli E., Lagarde J., DeGuzman G.C., Kelso J.A. (2007). The phi complex as a neuromarker of human social coordination. Proceedings of the National Academy of Sciences of the United States of America, 104(19), 8190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdesolo P., Ouyang J., DeSteno D. (2010). The rhythm of joint action: synchrony promotes cooperative ability. Journal of Experimental Social Psychology, 46(4), 693–5. [Google Scholar]
- Steen M.C., Keller P.E. (2013). The ADaptation and Anticipation Model (ADAM) of sensorimotor synchronization. Frontiers in Human Neuroscience, 7, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M.C., Jacoby N., Fairhurst M.T., Keller P.E. (2015). Sensorimotor synchronization with tempo-changing auditory sequences: modeling temporal adaptation and anticipation. Brain Research, 1626, 66–87. [DOI] [PubMed] [Google Scholar]
- Vorberg D. (2005). Synchronization in duet performance: testing the two-person phase error correction model In: 10th Rhythmn Perception and Production Workshop, Alden Biesen, Belgium, July 2–6. [Google Scholar]
- Vorberg D., Schulze H. (2002). Linear phase-correction in synchronization: predictions, parameter estimation, and simulations. Journal of Mathematical Psychology, 46(1), 56–87. [Google Scholar]
- Vorberg D., Wing A.M. (1996). Chapter 4 modeling variability and dependence in timing. Handbook of Perception and Action, 2, 181–262. [Google Scholar]
- Wing A.M., Endo S., Bradbury A., Vorberg D. (2014). Optimal feedback correction in string quartet synchronization. Journal of The Royal Society, Interface, 11(93), 20131125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing A.M., Kristofferson A.B. (1973). Response delays and the timing of discrete motor responses. Perception & Psychophysics, 14(1), 5–12. [Google Scholar]
- Yun K., Watanabe K., Shimojo S. (2012). Interpersonal body and neural synchronization as a marker of implicit social interaction. Scientific Reports, 2, 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


