Abstract
Backgrounds
More males than females play video games and develop problems with gaming. However, little is known regarding how males and females who game on the Internet may differ with respect to neural responses to gaming cues.
Methods
Behavioral and functional magnetic resonance imaging (fMRI) data were recorded from 40 female and 68 male Internet gamers. This study included three components including participation in a pre-gaming cue-craving task, 30 min of online gaming and a post-gaming cue-elicited-craving task. Group differences were examined at pre-gaming, post-gaming and post- vs pre-gaming times. Correlations between brain responses and behavioral performance were calculated.
Results
Gaming-related cues elicited higher cravings in male vs female subjects. Prior to gaming, males demonstrated greater activations in the striatum, orbitofrontal cortex (OFC), inferior frontal cortex and bilateral declive. Following gaming, male subjects demonstrated greater activations in the medial frontal gyrus and bilateral middle temporal gyri. In a post–pre comparison, male subjects demonstrated greater thalamic activation than did female subjects.
Conclusions
Short-term gaming elicited in males vs females more craving-related activations to gaming cues. These results suggest neural mechanisms for why males may be more vulnerable than females in developing Internet gaming disorder.
Keywords: Internet gaming disorder, sex difference, vulnerabilities, craving
Introduction
Internet gaming disorder (IGD) refers to the problematic and excessive use of online games. IGD is associated with negative health measures including impaired physical and psychological states, social deficits, inefficient work and poor academic performance (Petry and O’Brien, 2013; Petry et al., 2015; Brand et al., 2016; Dong et al., 2017a). The American Psychiatric Association has included IGD in Section III of DSM-5 as warranting more clinical research (American Psychiatric Association, 2013).Diagnostic criteria for IGD were proposed based on other addictions, especially gambling disorder (Petry et al., 2014a) and the existence of formal criteria may facilitate research of IGD.
Online gaming is enjoyable to many with only a small percentage of gamers developing IGD (Griffiths, 2010; King et al., 2011; Kuss and Griffiths, 2012). According to a cross-cultural survey, more than two of three who played games did not report any IGD criteria, with only a small proportion of people qualifying for a diagnosis of IGD (0.3–1.0%; Przybylski et al., 2017). Individuals who play online games regularly without experiencing impaired control or exhibiting cravings for gaming may be defined as demonstrating recreational game use (RGU; Dong et al., 2017a; Dong et al., 2017b).
Most individuals with IGD are male (Dong et al., 2013a; Wang et al., 2016). Although some prior studies have suggested that a minority of female and a majority of male adolescents play videogames (Desai et al., 2010), some recent studies suggest the gap is narrowing. For example, one study found that 87.4% of male and 45.6% of female adolescents in China played online games; however, the proportion of males with IGD was considerably higher in males as compared with that in females (6.3% boys, 2.4% girls; Li et al., 2015). Similar findings have also been observed in Korea (Ha and Hwang, 2014), Taiwan (Koet al.) and elsewhere (Borgonovi, 2016). In addition, gender-related differences in IGD do not appear linked to skill levels in gaming, as studies suggest that female and male gamers have comparable gaming skills (Shen et al., 2016). As there are relatively few females with IGD, some studies have only included male subjects, generating a relative deficit in our understanding of the characteristics of females with IGD (Dong and Potenza, 2016; Weinstein et al., 2017). Such a deficit also extends to video gaming more broadly, although some studies have suggested that non-IGD levels of gaming are linked to negative health measures (e.g. aggression and depression) in females but not in males (Desai et al., 2010). Given that gaming is highly prevalent, understanding gender-related differences in brain and behavioral factors relating to online gaming has important public health implications, as well as potentially providing insight into IGD.
Motivation is an important factor to consider in behaviors and the progression to addiction. Craving reflects a strong motivation, is an important feature of addictions and is included as an inclusionary criterion for substance-use disorders but not for gambling disorder or IGD (American Psychiatric Association, 2013). Craving reflects a motivational state that promotes seeking drugs in drug addictions (Sinha and Li, 2007; Sayette, 2016) and is also relevant to behavioral addictions like gambling (Potenza et al., 2003) and IGD (Dong et al., 2017a). Craving may shift people’s attention toward addiction-related cues (Tiffany, 1990; Sayette, 2016) and influence the evaluation of addiction-related information (Sayette et al., 2010), motivating individuals to pursue immediate satisfaction rather than long-term rewards (Wilson et al., 2014; Balodis and Potenza, 2015; Berridge and Kringelbach, 2015; Piper, 2015; Dong and Potenza, 2016). For these reasons, craving is a target for behavioral therapies like cognitive-behavioral therapy (Potenza et al., 2013). As gender-related differences in the neural correlates of craving involving corticostriatal-limbic circuitry have been observed in both substance and behavioral addictions (Potenza et al., 2012a; Koberet al., 2016), study of these processes as related to gaming warrants direct examination.
Gender-related differences in individuals with RGU may provide insight into those in IGD, as is the case for other behavioral addictions. For example, gender-related preferences in forms of gambling in recreational gambling (Potenza et al., 2006) appear reflected in a gender-specific fashion to gambling problems in problematic gambling (Potenza et al., 2001). Additionally, such gender-related differences have been proposed to relate to gender-related differences in addiction progression (Zakiniaeiz et al., 2016; Zakiniaeiz et al., 2017). Multiple studies have suggested that women may progress more rapidly from behavioral initiation to addiction (Bobzean et al., 2014; Moran-Santa Maria et al., 2014; Becker et al., 2017), with similar findings observed in rats (Hu et al., 2004). Together, these findings highlight the relevance of studying gender-related differences in RGU.
Based on the descriptions above, we sought to investigate in the current study gender-related differences in the neural correlates related to craving when individuals with IGD or RGU are exposed to gaming-related cues and changes in craving that occur following gaming. We first asked individuals with RGU to finish a cue-craving task, in which gaming-related cues could be observed; then they were asked to play online games for about half an hour (one round); after that, the craving measures were assessed again. We then assessed gender-related differences in craving at pre- and post-gaming times and how these differed in post- vs pre-gaming.
Gender-related differences in cues eliciting craving have been reported for other behavioral addictions. For example, men with gambling disorders tend to be triggered by gambling-related cues (e.g. gambling advertisements) whereas women with gambling disorders appear triggered by negative mood states (Grant and Kim, 2002; Hardee et al., 2017). Given that males have been proposed to seek behaviors with addictive tendencies for positive reinforcement motivations whereas females are more likely to do so for negative reinforcement motivations (Zakiniaeiz et al., 2017), we hypothesized that males would show higher cravings than females when viewing the same gaming-related cues and that differences would be observed with respect to increased brain responses in reward system-related (corticostriatal-limbic) brain regions, as has been observed in studies of cue-elicited craving in substance addictions (Potenza et al., 2012b).
In addition, our previous studies have shown that gaming behaviors enhanced IGD subjects’ cravings for gaming-related cues, suggesting that gaming behaviors promoted more gaming craving in IGD, which suggest a possible mechanism underlying craving in and maintenance or progression of IGD (Dong et al., 2017b). Thus, we anticipated that a brief exposure to gaming would increase craving rather than decrease it. We based this belief not only on prior research findings (Dong et al., 2017a), but also experience that for individuals who game on the Internet, gaming sessions may last for hours. As such, a brief exposure was anticipated to elevate craving rather than satisfy it. We also believed that males would be more likely than females to experience cue-induced craving, consistent with findings in substance and gambling addictions (Potenza et al., 2012b;Zakiniaeiz et al., 2017). These differences may relate to tendencies for positive reinforcement motivations being stronger in males and negative reinforcement tendencies stronger in females. Given that we proposed that gaming cues are related to positive motivations, we hypothesized that gaming would enhance greater subjective cravings in male as compared with female subjects and would be related to greater changes in reward system-related brain activations post-gaming as compared with pre-gaming.
Methods and Materials
Ethics
The experiment conforms to the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Human Investigations Committee of Zhejiang Normal University approved this research. All subjects were university students and were recruited through advertisements. All subjects provided written informed consent before scanning.
Participant selection
Data were collected from 68 male subjects and 40 female subjects. Table 1 shows the demographic information from the two groups. All participants were right-handed, had normal or corrected-to-normal vision and completed a structured psychiatric interview (MINI; Lecrubier et al., 1997) that was performed by an experienced psychiatrist and lasted ∼15 min following subjects completing portions of the MINI. All participants were free of Axis-I psychiatric disorders assessed via the MINI. We further assessed depression with the Beck Depression Inventory (BDI; Beck et al., 1961) and only participants scoring less than 5 were included (Table 1).
Table 1.
Demographic information and group differences
| Female N=40 | Male N=68 | T | p | |
|---|---|---|---|---|
| Age (Mean ± s.d.) | 21.62 ± 1.96 | 22.80 ± 2.22 | 2.771 | 0. 007** |
| BDI score (Mean ± s.d.) | 2.31 ± 0.80 | 2.22 ± 0.81 | 0.445 | 0.784 |
| IAT score (Mean ± s.d.) | 33.13 ± 16.05 | 31.32 ± 15.12 | 1.112 | 0.101 |
| DSM-5 score (Mean ± s.d.) | 2.64 ± 2.08 | 2.87 ± 2.01 | 0.561 | 0.576 |
| Game playing per week (h) (Mean ± s.d.) | 12.18 ± 7.22 | 11.99 ± 8.60 | 0.794 | 0.464 |
| Gaming history (months) | 18.32 ± 4.86 | 20.46 ± 5.27 | 1.355 | 0. 093 |
| Self-reported craving | 35.14 ± 6.35 | 34.75 ± 7.49 | 0.824 | 0. 427 |
Note: BDI, Beck Depression inventory; IAT, internet addiction test. **P < 0.01.
Controlling for gaming experience and familiarity
All subjects in the current study had RGU as defined previously (Dong et al., 2017a). Briefly, subjects needed to satisfy that they had been playing online games for a minimum of 1 year without meeting the criteria of IGD (fewer than five of nine of the proposed DSM-5 criteria for IGD) (Petry et al., 2014b) and score less than 50 on Young’s IAT test (IAT, www.netaddiction.com). Individuals with RGU were required to play online games for at least 5 of 7 days in a week and for more than 7 h per week. We only recruited subjects who regularly played League of Legends (LOL; Riot Games, Inc.) and played the game for at least 1 year. Additionally, we measured the subjects’ cravings for gaming in participant selection step using a questionnaire modified from smoking urges (Cox et al., 2001). Anyone who scored higher than 50 was excluded. We referenced their academic performance (as all subjects were university students) to help ensure that they were not addicted.
Materials
The craving-eliciting and comparison cues we used included 120 pictures that were divided into two categories: gaming-related and typing-related pictures (Neutral baseline). A total of 50% of all pictures within each category contained a face and the other half contained a hand. As shown in Figure 1A and B, in gaming-related stimuli, one is displayed playing a game on a computer, with some stimuli showing faces and others showing hands. In the typing-related pictures, background imagery was similar except that somebody is typing rather than gaming.
Fig. 1.
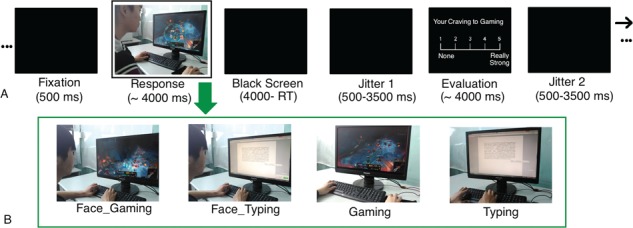
The fMRI task. (A) In one trial of the task, a fixation was presented first for 500 ms, and then a ‘response’ stage lasted for up to 4000 ms. Participants were asked to answer whether or not there were faces in the pictures and press the relevant keys. A jittered black screen was presented for 1500–3500 ms. After this, the evaluation stage followed, in which subjects were asked to evaluate the level of their cravings (0–5) for the relevant behaviors (playing games or typing). This process lasted for up to 4000 ms and was terminated by a button press. After another jitter ranging from 1500–3500 ms, the next trial ensued. (B) Examples of the four types of trials used in the current study. Face_Gaming: Gaming pictures with faces; Face_Typing: typing pictures with faces; Gaming: gaming pictures; Typing: typing pictures.
Controlling for picture complexity
Subjects were instructed to finish a relatively easy cognitive task when seeing the stimuli (identifying the images as being with or without faces). We selected this approach in order that the cognitive task not focus directly on the gaming aspects of the stimuli. According to these criteria, we selected the stimuli used in the current study. All pictures were taken in our laboratory with the same setting (white background, a white desk with a black screen). The genders of individuals in the pictures were also balanced within each category and type. The pictures showed only single sides of faces in order to exclude potential effects of emotions on the faces of individuals in the cues. No accessories were contained in the images of the hands or faces. The only differences between gaming-related stimuli and typing-related stimuli involved the on-screen material.
Cue-reactivity task
Participants were told that they would be paid a guaranteed 50 ¥ (≈$8) for participation and, to encourage their motivation to respond accurately, were told they would be rewarded with additional 0–50 ¥ based on their task performance (Accuracy rates *50).
The cue-reactivity task we used in pre- and post-gaming scans (Figure 1A) has been used previously (Dong et al., 2017a; Dong et al., 2017b) and is described briefly below. In one typical trial, a fixation was presented first for 500 ms and during the response stage, a stimulus was presented with a response needed: participants were asked to answer whether there is a face in the picture and to select ‘yes’ or ‘no’ via button press (1, yes; 2, no; with counter-balancing between subjects). The stimulus turned black after key pressing and lasted for 4000 ms (the response time). A jittered black screen was presented for duration of 1500–3500 ms. No feedback was given about their responses.
In the subsequent evaluation stage, subjects were asked to evaluate the level of their craving [on a scale from 1 (low)–5 (high)] for the relevant stimuli. This process lasted for up to 4000 ms and was terminated by a button press. Another jitter ranging from 1500–3500 ms appeared preceding the next trial. Analyses focused on the ‘response’ stage.
The functional magnetic resonance imaging (fMRI) task at the pre- and post-gaming times was of the same type but differed in content in order to avoid/minimize possible ‘repetition effects’. We designed two copies of the task with different items (Copies A and B). Half of each group of participants participated in an ‘A–B’ sequence and the other half received a ‘B–A’ sequence in their pre- and post-gaming scans.
In the scanner, subjects were asked to play LOL for one round. In general, one round of LOL takes about 20–40 min. To control for time spent in the scanner, subjects were instructed to try to finish the game in 25 min, with notifications provided at 20 and 25 min and termination of the game at 30 min. Using this method, all subjects finished within 30 min (mean ± s.d. = 25.21 ± 2.76; Female: 26.48 ± 2.94; Male: 24.85 ± 2.56; t = 0.423, P > 0.05). To promote subjects’ familiarity with their gaming experiences, participants were asked to log into the game with their own account.
Data collection
Structural images were collected using a T1-weighted three-dimensional spoiled gradient-recalled sequence covering the whole brain (176 slices; repetition time=1700 ms, echo time (TE) = 3.93 ms, slice thickness = 1.0 mm, skip = 0 mm, flip angle = 15, inversion time = 1100 ms, field of view = 240*240 mm, in-plane resolution = 256*256). fMRI was performed on a 3T scanner (Siemens Trio, Erlangen, Germany) with a gradient-echo EPI T2*-weighted-sensitive pulse sequence in 33 slices (interleaved sequence, 3 mm thickness, TR = 2000 ms, TE = 30 ms, flip angle 90°, field of view 220 × 220 mm2, matrix 64 × 64) (Dong et al., 2013b). Stimuli were presented using an Invivo synchronous system (www.invivocorp.com) through a screen in the head coil, enabling participants to view the stimuli. The whole experiment lasted for slightly more than an hour [pre- and post-test (14 min each); T-1 structural (6 min); prepare for scanning and the time between different tasks (6 min); gaming (∼30 min)].
Data pre-processing
The functional data were analyzed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and Neuroelf (http://neuroelf.net), as described previously (DeVito et al., 2012; Krishnan-Sarinet al., 2013). Images were slice-timed, reoriented and realigned to the first volume, with T1-co-registered volumes used to correct for head movements (No head motion difference was found between groups; see details from Supplementary Table S1). Images were then normalized to montreal neurological institute (MNI) space and spatially smoothed using a 6 mm FWHM Gaussian kernel. No subjects were removed from analysis because of head motion (the exclusion criteria were 2 mm in directional movement or 2° in rotational movement during the whole run of the cue-elicited-craving task). A general linear model (GLM) was applied to identify blood oxygen level dependence (BOLD) activation in relation to brain activities. Different types of trials (gaming related, typing related, incorrect or missed) were separately convolved with a canonical hemodynamic response function to form task regressors. The duration of each trial was 4000 ms. The GLMs included a constant term per run. Six head-movement parameters derived from the realignment stage and the age of subjects, game time were included to exclude motion-related variances. A GLM approach was used to identify voxels that were significantly activated for each event during the ‘response’ stage.
Male–female comparisons
In the current study, we focused on three comparisons: gender-related differences at pre-gaming, gender-related differences at post-gaming and gender-related differences in the post- vs pre-gaming comparison. For pre- or post-gaming times, simple t-tests were performed between different subjects (pre/post (male gaming-related stimuli − male typing-related stimuli) − pre/post (female gaming-related stimuli − female typing-related stimuli). The post- vs pre-gaming comparisons were analyzed using a voxel-wise 2*2 (Factor 1, male, female; Factor 2: post- and pre-gaming) repeated-measures analysis. Family-wise error (FWE) thresholds were determined using AlphaSim and all comparisons were corrected using AlphaSim. Significant clusters (FWE corrected, P < 0.001) were thresholded at P < 0.001, two-tailed, uncorrected, with an extent of at least 30 voxels. All of these steps were performed using NeuroElf (Neuroelf.net).
Correlation analyses
We first compared the brain activation between different groups and then took the surviving clusters as region of interest (ROIs) to correlate with behavioral measures. For each ROI, a representative BOLD beta value was obtained by averaging the signal of all the voxels within it.
Results
Behavioral performance
Response times to gaming cues did not differ between males and females at pre-gaming (male, 1252.35 ± 321.15; female, 1154.65 ± 268.77; t = 1.607, P = 0.111) and post-gaming (male, 1147.05 ± 33.44; female, 1106.31 ± 279.38; t = 0.674, P = 0.502) times. As the current task is relatively simple, accuracy within each gender group reached about 100%.
Cue-elicited craving. Gaming-related cues elicited higher cravings in males as compared with females at a trend level prior to gaming (t = 1.811, P = 0.073) and reached significance following gaming (t = 2.322, P = 0.028), although gaming did not result in significantly increased craving from pre- to post-gaming in either males (t = 1.208, P = 0.235) or females (t = 1.238, P = 0.220) and no interaction was found (F = 0.134, P = 0.715; Figure 2).
Fig. 2.
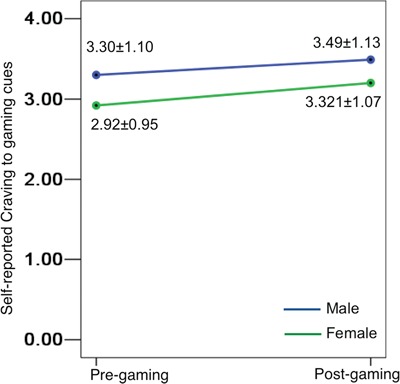
Gaming-related cue-elicited cravings in males and females at pre- and post-gaming times.
Imaging results
Cue-induced brain responses before gaming. Differences in the neural correlates of responses to gaming cues between males and females were calculated. Group differences prior to gaming showed that males demonstrated greater activation in the right striatum, right (OFC), left inferior frontal gyrus, right middle occipital gyrus and bilateral declive (Figure 3A; Table 2). Extracted beta weights from the striatal ROI showed that regional activation was greater in males than in females (Figure 3B) and was positively associated with gaming cue-induced craving in males (r = 0.327, P < 0.006) but not females (r = 0.233, P < 0.172; Figure 3C).
Fig. 3.
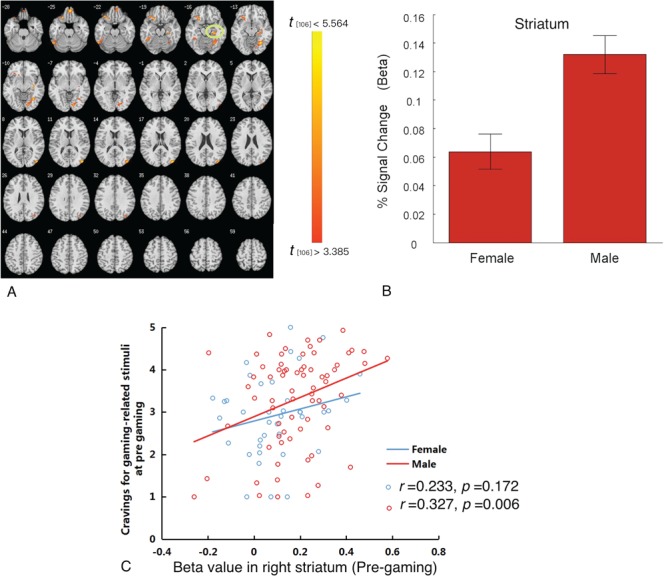
Gender-related differences in craving in response to gaming-related cues prior to gaming. (A) Prior to gaming, males as compared with females demonstrated greater brain activations in the striatum, bilateral declive, OFC and inferior frontal gyrus. (B) Beta weights from the striatal ROI showing cue-elicited activation in males and females. (C) A positive correlation between beta weights from the striatal ROI and subjective craving was observed in males but not females.
Table 2.
Brain responses to gaming-related cues in male as compared with female participants
| Cluster number | x, y, za | Peak intensity | Cluster sizeb | Regionc | Brodmann’s area | |
|---|---|---|---|---|---|---|
| Pre-Gaming | ||||||
| 1 | 42, –84,18 | 4.239 | 72 | R middle occipital gyrus | 19 | |
| 2 | 3,45, –24 | 4.108 | 37 | R OFC | 11 | |
| 3 | –24,24, –24 | 4.094 | 64 | L inferior frontal gyrus | 47 | |
| 4 | 33, –60, –12 | 3.888 | 86 | R declive | ||
| 5 | 33, –18, –9 | 3.787 | 34 | R striatum | ||
| 6 | –57,–60,–24 | 3.518 | 51 | L declive | ||
| Post-Gaming | ||||||
| 1 | 18_15_48 | 4.354 | 32 | R MFG | 32 | |
| 2 | –60, –51, –18 | 3.566 | 58 | L MTG | 37 | |
| 3 | 57, –57,6 | 3.375 | 49 | R MTG | 39 | |
| 4 | –6, –27,42 | 3.287 | 51 | L cingulate gyrus | 31 | |
| (Post-Gaming)–(Pre-Gaming) | ||||||
| 1 | –9,–33, –12 | 4.065 | 46 | L thalamus | ||
| 2 | 9, –93, 9 | 3.452 | 38 | R cuneus | 17 | |
a Peak MNI coordinates.
b Number of voxels. Alphasim correction, P < 0.001; Cluster size > 30 contiguous voxels.
c The brain regions were referenced to the software Xjview (http://www.alivelearn.net/xjview8) and verified through comparisons with a brain atlas.
Cue-induced brain responses after gaming. At post-gaming, male as compared with female participants demonstrated greater activation of the medial frontal gyrus (MFG), left anterior cingulate gyrus and bilateral middle temporal gyrus (MTG) (Figure 4A; Table 2). Beta weights extracted from the medial frontal gyral ROI showed that regional activation was greater in males than in females (Figure 4B) and was positively associated with gaming cue-induced craving in males (r = 0.288, P < 0.016) and females (r = 0.445, P < 0.004; Figure 4C).
Fig. 4.
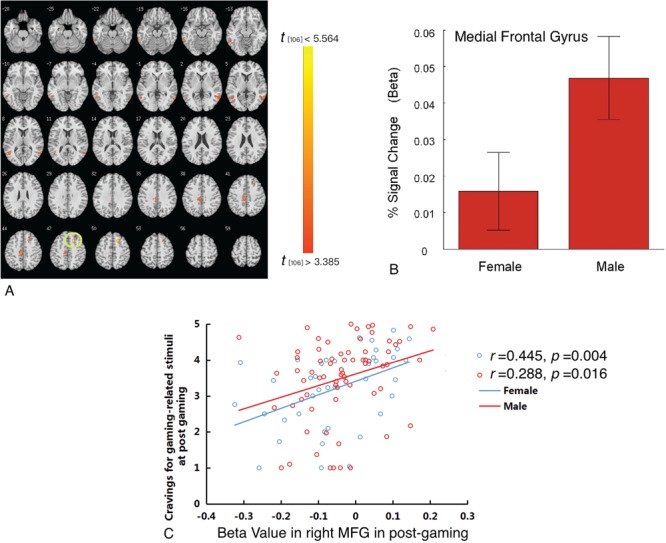
Gender-related differences in craving in response to gaming-related cues after gaming. (A) Following gaming, males as compared with females demonstrated greater brain activations in the MFG and bilateral middle temporal gyrus. (B) Beta weights from the MFG ROI showing cue-elicited activation in males and females. (C) A positive correlation between beta weights from the MFG ROI and subjective craving was observed in males and females.
Cue-induced brain responses at post- vs pre-gaming. The 2*2 (group* post_pre gaming) ANOVA identified the left thalamus and right cuneus, with males showing greater changes in activation from pre- to post-gaming than in females (Figure 5A and B; Table 2). Positive correlations were found between changes in brain activations in the thalamus and cue-induced craving at post-gaming times in males (r = 0.320, P < 0.007) but not females (r = 0.162, P < 0.324; Figure 5C).
Fig. 5.
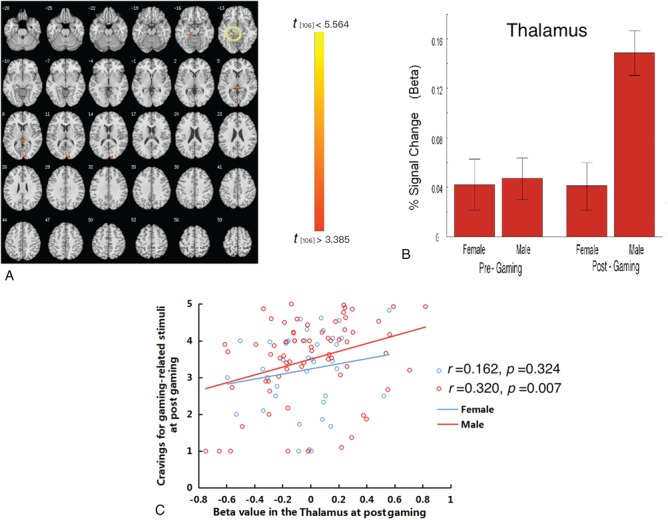
Gender-related differences in craving in response to gaming-related cues following as compared with before gaming. (A) Following gaming as compared with before gaming, males as compared with females demonstrated greater brain activations in the thalamus. (B) Beta weights from the thalamic ROI showing cue-elicited activation in males and females at pre- and post-gaming times. (C) A positive correlation between beta weights from the thalamic ROI and subjective craving post-gaming was observed in males but not females.
Discussion
Our a priori hypotheses were largely supported in males as compared with females with RGU-demonstrated greater craving to gaming cues, particularly following gaming. Furthermore, activation of corticostriatal-limbic circuitry was greater in males than in females in several identified regions and appeared to correlate more consistently with craving responses in males as compared with that in females. The implications of the specific findings are described below.
Gaming cues elicited greater craving in males
Before gaming, males demonstrated greater activation of the right striatum, a region implicated in reward processing (Berridge and Kringelbach, 2015). Activity in the ventral striatum may influence motivated or goal-directed behaviors (Ikemoto et al., 2015; Sayette, 2016) and reward processing including in addictions (Balodis and Potenza, 2015; Cheng et al., 2016; Tobler et al., 2016; Yang et al., 2017). The striatum may also be activated when individuals are exposed to stimuli related to addictions (Balodis et al., 2012; Worhunsky et al., 2014). Studies of IGD subjects also suggest the involvement of the striatum when exposed to gaming cues (Ko et al., 2009; Sun et al., 2012; Liu et al., 2016). In the current study, the greater activation by males as compared with that of females, as well as the positive correlation in males between striatal activation and self-reported craving to cue exposure, suggest that males may be more sensitive to gaming-related cues than females.
The right OFC was also activated more robustly in males as compared with females when they were exposed to gaming-related cues. The OFC may mediate drug-seeking and drug-relapse behaviors in drug addictions (Volkow and Fowler, 2000; Schoenbaum et al., 2006). Greater reactivity in the OFC has been linked to cocaine (Balodis et al., 2016; Kober et al., 2016), methamphetamine (Volkow et al., 2001) and heroin (Volkow and Fowler, 2000) addictions in response to drug-associated cues. In the current study, the brain responses in male subjects (higher OFC activation to gaming-related cues) suggest that they may demonstrate a more addiction-like response to cues that may predispose them to developing IGD. However, this explanation is speculative and needs direct examination, especially in longitudinal studies.
Gaming-related cues activated non-corticostriatal-limbic regions in male as compared with female subjects prior to gaming
Besides the traditional reward-related (corticostriatal-limbic) brain regions identified at pre-gaming time, the declive demonstrated greater activation in males as compared with females when exposed to gaming-related cues. The declive is a part of the cerebellum and has been implicated in automatic motor control, motor adaptation and the acquisition of new motor skills (Ramnani, 2014; Takakusaki, 2017). Sports studies have shown that automatic processing is accompanied by improved performance, with extra attention potentially disrupting optimal performance (Wulf and Prinz, 2001; Wu et al., 2015). When a movement becomes automatic, neural efficiency may be increased by the strengthening of connections within specific neural networks (Balsters and Ramnani, 2011; Wu et al., 2015). In the current study, the higher brain responses in the declive in males when exposed to gaming-related cues suggests that gaming cues may be activating automatic or well-established brain responses for potential game playing, which may relate to greater gaming craving when seeing gaming-related cues. However, alternate possibilities exist, particularly as the declive has been implicated in other functions including reward processing.
Gaming-related cues elicited greater neural responses in male as compared with female subjects at post-gaming
Following gaming, several brain regions in the prefrontal cortex (PFC), including the MFG, demonstrated gender-related differences, with males again showing greater activation than females. Again, these brain regions have been implicated in cue-elicitation studies of drug addictions. PFC activation, including within the MFG, has been reported in individuals with tobacco-, alcohol- and opioid-use disorders (Goldstein and Volkow, 2011; Schmidt et al., 2013). Similarly, PFC activations have also been observed in behavioral addictions. Excessive online gamers demonstrated BOLD activations in medial and dorsolateral PFC when viewing gaming pictures, and these activations were correlated with gaming urges (Ko et al., 2009). PFC activation has also been observed in individuals with gambling disorder (Crockford et al., 2005). Dysfunctional activity in the PFC during response inhibition has been suggested as a hallmark feature of addictions (Goldstein and Volkow, 2011). In the current study, the finding that males as compared with females with RGU showed increased greater PFC activation suggests that RGU males in particular may share this neural feature. However, the significant correlations between regional activation and self-reported craving in both males and females suggest that this region may be important across gender groups for gaming, and possibly transitions to IGD, although those possibilities warrant direct examination.
At post-gaming, higher bilateral MTG activation was also observed in males as compared with that in females. Although the involvement of the MTG was not hypothesized a priori, several possible explanations exist. Studies have suggested a role for the MTG in the retrieval of stored long-term memories, including conceptual knowledge (Brod et al., 2016; Lempert et al., 2017), context memory/recollection and item memory/familiarity (Squire et al., 2007; Meunier and Barbeau, 2013), autobiographical memory (Dede et al., 2016) and episodic memory (Thakralet al). In addition, lesions to this region may lead to severe deficits in memory retrieval (Lau et al., 2008; Binder et al., 2009). At post-gaming, subjects have recently played LOL for about 25 min prior to the exposure to gaming-related cues. Thus, the higher MTG activation in males as compared with females suggests that the gaming-related stimuli may have elicited more gaming-related memories, and this possibility warrants direct examination in future studies.
Gaming and cue-elicited brain activations
From pre- to post-gaming cue exposure, gender-related differences in brain activations were observed across time with respect to thalamic activation. The thalamus acts as a relay which is responsible for reciprocal connections with the cerebral cortex. Thus, it contributes to many functions and behaviors (Schiff, 2008) and has been implicated in motivational drives related to addiction (Chambers et al., 2003). A recent optogenetics study has identified that the thalamus through input to the nucleus accumbens influences opiate withdrawal-induced physical signs and aversive memories (Zhu et al., 2016). In addition, cocaine (Neumann et al., 2016) or methamphetamine (Li et al., 2017) may induce synaptic alternations in thalamus-to-nucleus accumbens projections. In the current study, gaming increased the males’ thalamic responses to gaming cues more than the females’, with the positive correlation between thalamic activation and self-reported cravings during post-gaming exposure in males only suggesting a more prominent role for the thalamus in males than in females. Taken together, the findings suggest a cue-related biological mechanism linked to craving that may be particularly relevant for males. The extent to which that this may represent a biomarker for male vulnerability in developing IGD warrants additional examination.
Limitations
Several limitations warrant mention. First, as gaming pictures were used as stimuli, the evoking effect may not be as strong or robust as for other stimuli, particularly as the pictures are static and most gaming is active. Second, in order to control for the potential effects from different types of games, only one game was used in the current study, and different types of games may generate different effects. Given the popularity of the LOL game at the time of the study, particularly among both males and females and the importance for the experimental design to select appropriately stimulating gaming cues, the current study focused on the LOL. The extent to which the findings extend to other groups engaging predominantly on other Internet games warrants additional study. Third, as all subjects were exposed to gaming, test/retest effects cannot be excluded, although the use of novel stimuli before and after gaming mitigates against this possibility. Fourth, in the current study, the males were older than the females. Although we included age as a covariate in the current study, the age difference warrants further consideration in future studies, with respect to the models being tested in the current study. Fifth, we recruited 40 female participants in the current study. While the number of females is fewer than that of males in the study, 40 subjects are currently considered to constitute a reasonable sample size for a group in fMRI studies. Nonetheless, the gender-related imbalance is also a limitation of the current study. Sixth, by including gaming and typing stimuli that each included or excluded faces, there exists the possibility that social content may vary across cues. As these were balanced across gaming and non-gaming cues, we believe that this aspect that is important for validity checking is controlled for across gaming and non-gaming cues. Future studies should examine the extent to which social context may influence craving and other responses in gaming and IGD. Seventh, there were no significant changes in craving from pre- to post-gaming sessions in either gender group separately or by gender over time and there may be multiple reasons for this observation. First, the smaller number of female participants may have limited our ability to observe such gender-related differences. Second, the assessment that we used to assess craving may have been relatively blunt. We used a 5-level Likert scale (from 1–5), which may be relatively less sensitive than perhaps a 9-point scale. Third, there may be individual differences in craving responses (for example, some individuals may have found that a brief session of gaming may have partially satisfied their craving rather than increased it). This last possibility may speak to the observed correlations which arguably rely more strongly on individual differences in response than group effects per se.
Conclusions
The current study found that (i) gaming-related cues elicited more craving in males with RGU than in females with RGU; (ii) gaming cues activated corticostriatal-limbic brain regions, as well as others, to a greater extent in males than in females; and (iii) brain activations to gaming cues tended to correlate more consistently with craving responses in males as compared with females, particularly in subcortical brain regions. These results suggest potential biological mechanisms for male vulnerability in developing IGD and further examination in longitudinal studies are warranted to investigate this possibility.
Competing interests
The authors report that they have no financial conflicts of interest with respect to the content of this manuscript. Dr Potenza has received financial support or compensation for the following: Dr Potenza has consulted for and advised Ironwood, Lundbeck, INSYS, Shire, RiverMend Health, Opiant/Lightlake Therapeutics and Jazz Pharmaceuticals; has received research support from the NIH, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and Pfizer pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for legal and gambling entities on issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the NIH and other agencies; has edited journals; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. The other authors reported no biomedical financial interests or other potential conflicts of interest.
Significance statement
The current study examined female and male participants who engage in gaming on the Internet. Female and male gamers with similar socio-demographic characteristics participated in a cue-reactivity task before and after a half hour of gaming. Between-gender comparisons of behavioral and neural measures at pre-gaming, post-gaming, post- vs pre-gaming times were made. The findings suggest that short-term gaming elicits in males vs females more craving-related activations to gaming cues. These results suggest possible craving-related neural mechanisms for why males may be more vulnerable than females in developing IGD.
Contributors
G.D. designed the task and wrote the first draft of the manuscript. LW., X.D. collected and analyzed the data and prepared the figures. M.P. contributed in editing, interpretation and revision processes. All authors contributed to and have approved the final manuscript.
Funding
This research was supported by National Science Foundation of China (31371023).
Supplementary Material
Acknowledgements
M.N.P.’s involvement was supported by the National Center for Responsible Gaming. The funding agencies did not contribute to the experimental design or conclusions, and the views presented in the manuscript are those of the authors and may not reflect those of the funding agencies.
References
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn, Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., et al. (2016). Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology, 41(8), 2112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., Stevens M.C., Pearlson G.D., Potenza M.N. (2012). Attending to striatal ups and downs in addictions. Biological Psychiatry, 72(10), e25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Potenza M.N. (2015). Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biological Psychiatry, 77(5), 434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters J.H., Ramnani N. (2011). Cerebellar plasticity and the automation of first-order rules. Journal of Neuroscience, 31(6), 2305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561–71. [DOI] [PubMed] [Google Scholar]
- Becker J.B., McClellan M.L., Reed B.G. (2017). Sex differences, gender and addiction. Journal of Neuroscience Research, 95(1 and 2), 136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Kringelbach M.L. (2015). Pleasure systems in the brain. Neuron, 86(3), 646–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean S.A.M., DeNobrega A.K., Perrotti L.I. (2014). Sex differences in the neurobiology of drug addiction. Experimental Neurology, 259, 64–74. [DOI] [PubMed] [Google Scholar]
- Borgonovi F. (2016). Video gaming and gender differences in digital and printed reading performance among 15-year-olds students in 26 countries. Journal of Adolescence, 48, 45–61. [DOI] [PubMed] [Google Scholar]
- Brand M., Young K.S., Laier C., Wölfling K., Potenza M.N. (2016). Integrating psychological and neurobiological considerations regarding the development and maintenance of specific Internet-use disorders: an Interaction of Person-Affect-Cognition-Execution (I-PACE) model. Neuroscience and Biobehavioral Reviews, 71, 252–66. [DOI] [PubMed] [Google Scholar]
- Brod G., Lindenberger U., Wagner A.D., Shing Y.L. (2016). Knowledge acquisition during exam preparation improves memory and modulates memory formation. Journal of Neuroscience, 36(31), 8103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. (2003). Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry, 160(6), 1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Huang C.C., Ma T., et al. (2016). Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption. Biological Psychiatry, 81(11),918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L.S., Tiffany S.T., Christen A.G. (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research, 3(1), 7–16. [DOI] [PubMed] [Google Scholar]
- Crockford D.N., Goodyear B., Edwards J., Quickfall J., el-Guebaly N. (2005). Cue-induced brain activity in pathological gamblers. Biological Psychiatry, 58(10), 787–95. [DOI] [PubMed] [Google Scholar]
- Dede A.J., Wixted J.T., Hopkins R.O., Squire L.R. (2016). Autobiographical memory, future imagining, and the medial temporal lobe. Proceedings of the National Academy of Sciences of the United States of America, 113(47), 13474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R.A., Krishnan-Sarin S., Cavallo D., Potenza M.N. (2010). Video-gaming among high school students: health correlates, gender differences, and problematic gaming. Pediatrics, 126(6), E1414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito E.E., Worhunsky P.D., Carroll K.M., Rounsaville B.J., Kober H., Potenza M.N. (2012). A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence, 122(3), 228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Hu Y., Lin X. (2013a). Reward/punishment sensitivities among internet addicts: implications for their addictive behaviors. Progress in Neuro-psychopharmacol & Biological Psychiatry, 46, 139–45. [DOI] [PubMed] [Google Scholar]
- Dong G., Hu Y., Lin X., Lu Q. (2013b). What makes internet addicts continue playing online even when faced by severe negative consequences? Possible explanations from an fMRI study. Biological Psychology, 94(2), 282–9. [DOI] [PubMed] [Google Scholar]
- Dong G., Potenza M.N. (2016). Risk-taking and risky decision-making in internet gaming disorder: implications regarding online gaming in the setting of negative consequences. Journal of Psychiatric Research, 73, 1–8. [DOI] [PubMed] [Google Scholar]
- Dong G., Wang L., Du x., Potenza M. (2017a). Gaming increases craving to gaming-related stimuli in individuals with internet gaming disorder. Biological Psychiatry: Cognitive Neuroscience and Imaging, 2, 404–12. [DOI] [PubMed] [Google Scholar]
- Dong G., Wang L., Wu L., Potenza M. (2017b). Cognitive control and reward/loss processing in internet gaming disorder: results from a comparison with recreational internet game-users. European Psychiatry, 44, 30–8. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews. Neuroscience, 12(11), 652–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J.E., Kim S.W. (2002). Gender differences in pathological gamblers seeking medication treatment. Comprehensive Psychiatry, 43(1), 56–62. [DOI] [PubMed] [Google Scholar]
- Griffiths M.D. (2010). The role of context in online gaming excess and addiction: some case study evidence. International Journal of Mental Health and Addiction, 8(1), 119–25. [Google Scholar]
- Ha Y.M., Hwang W.J. (2014). Gender differences in Internet addiction associated with psychological health indicators among adolescents using a national web-based survey. International Journal of Mental Health and Addiction, 12(5), 660–9. [Google Scholar]
- Hardee J.E., Cope L.M., Munier E.C., Welsh R.C., Zucker R.A., Heitzeg M.M. (2017). Sex differences in the development of emotion circuitry in adolescents at risk for substance abuse: a longitudinal fMRI study. Social Cognitive and Affective Neuroscience, 12(6), 965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Crombag H.S., Robinson T.E., Becker J.B. (2004). Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology, 29(1), 81–5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S., Yang C., Tan A. (2015). Basal ganglia circuit loops, dopamine and motivation: a review and enquiry. Behavioural Brain Research, 290, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D.L., Delfabbro P.H., Zajac I.T. (2011). Preliminary validation of a new clinical tool for identifying problem video game playing. International Journal of Mental Health and Addiction, 9(1), 72–87. [Google Scholar]
- Ko C.-H., Yen J.-Y., Chen S.-H., Wang P.-W., Chen C.-S., Yen C.-F. (2011). Evaluation of the diagnostic criteria of Internet gaming disorder in the DSM-5 among young adults in Taiwan. Journal of Psychiatric Research, 53, 103–10. [DOI] [PubMed] [Google Scholar]
- Ko C.H., Liu G.C., Hsiao S., et al. (2009). Brain activities associated with gaming urge of online gaming addiction. Journal of Psychiatric Research, 43(7), 739–47. [DOI] [PubMed] [Google Scholar]
- Kober H., Lacadie C.M., Wexler B.E., Malison R.T., Sinha R., Potenza M.N. (2016). Brain activity during cocaine craving and gambling urges: an fMRI study. Neuropsychopharmacology, 41(2), 628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S., Balodis I.M., Kober H., et al. (2013). An exploratory pilot study of the relationship between neural correlates of cognitive control and reduction in cigarette use among treatment-seeking adolescent smokers. Psychology of Addictive Behaviors, 27(2), 526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss D.J., Griffiths M.D. (2012). Internet gaming addiction: a systematic review of empirical research. International Journal of Mental Health and Addiction, 10(2), 278–96. [Google Scholar]
- Lau E.F., Phillips C., Poeppel D. (2008). A cortical network for semantics: (de)constructing the N400. Nature Reviews. Neuroscience, 9(12), 920–33. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D.V., Weiller E., et al. (1997). The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry, 12(5), 224–31. [Google Scholar]
- Lempert K.M., Speer M.E., Delgado M.R., Phelps E.A. (2017). Positive autobiographical memory retrieval reduces temporal discounting. Social Cognitive and Affective Neuroscience, 12(10), 1584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yu Q., Zhang L., Jin S. (2015). The gender difference on Internet addictive among adolescent: the mediation effect of the differentiation of social and psychological situation in school. Chinese Journal of Clinical Psychology, 23(6), 1044–8. [Google Scholar]
- Li Y., Dong H., Li F., et al. (2017). Microstructures in striato-thalamo-orbitofrontal circuit in methamphetamine users. Acta Radiologica, 58(11), 1378–85. [DOI] [PubMed] [Google Scholar]
- Liu L., Yip S.W., Zhang J.T., et al. (2016). Activation of the ventral and dorsal striatum during cue reactivity in Internet gaming disorder. Addiction Biology, 22(3), 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M.N., Steinberg M.A., McLaughlin S.D., Wu R., Rounsaville B.J., O’Malley S.S. (2001). Gender-related differences in the characteristics of problem gamblers using a gambling helpline. American Journal of Psychiatry, 158(9), 1500–5. [DOI] [PubMed] [Google Scholar]
- Meunier M., Barbeau E. (2013). Recognition memory and the medial temporal lobe: from monkey research to human pathology. Rev Neurol (Paris), 169(6–7), 459–69. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria M.M., Flanagan J., Brady K. (2014). Ovarian hormones and drug abuse. Current Psychiatry Reports, 16(11), 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P.A., Wang Y., Yan Y., et al. (2016). Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology, 41(9), 2399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N.M., O'Brien C.P. (2013). Internet gaming disorder and the DSM-5. Addiction, 108(7), 1186–7. [DOI] [PubMed] [Google Scholar]
- Petry N.M., Rehbein F., Gentile D.A., et al. (2014a). An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction, 109(9), 1399–406. [DOI] [PubMed] [Google Scholar]
- Petry N.M., Rehbein F., Gentile D.A., et al. (2014b). An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction, 109(9), 1399–406. [DOI] [PubMed] [Google Scholar]
- Petry N.M., Rehbein F., Ko C.H., O'Brien C.P. (2015). Internet gaming disorder in the DSM-5. Current Psychiatry Reports, 17(9), 72. [DOI] [PubMed] [Google Scholar]
- Piper M.E. (2015). Withdrawal: expanding a key addiction construct. Nicotine & Tobacco Research, 17(12), 1405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M., Hong K., Lacadie M., Fulbright R., Tuit K.L., Sinha R. (2012a). Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. American Journal of Psychiatry, 169(4), 406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M.N., Balodis I.M., Franco C.A., et al. (2013). Neurobiological considerations in understanding behavioral treatments for pathological gambling. Psychology of Addictive Behaviors, 27(2), 380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M.N., Hong K.I., Lacadie C.M., Fulbright R.K., Tuit K.L., Sinha R. (2012b). Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. American Journal of Psychiatry, 169(4), 406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M.N., Maciejewski P.K., Mazure C.M. (2006). A gender-based examination of past-year recreational gamblers. Journal of Gambling Studies, 22(1), 41–64. [DOI] [PubMed] [Google Scholar]
- Potenza M.N., Steinberg M.A., Skudlarski P., et al. (2003). Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Archives of General Psychiatry, 60(8), 828–36. [DOI] [PubMed] [Google Scholar]
- Przybylski A.K., Weinstein N., Murayama K. (2017). Internet gaming disorder: Investigating the Clinical Relevance of a new phenomenon. American Journal of Psychiatry,174(3), 230–6. [DOI] [PubMed] [Google Scholar]
- Ramnani N. (2014). Automatic and controlled processing in the corticocerebellar system. Progress in Brain Research, 210, 255–85. [DOI] [PubMed] [Google Scholar]
- Sayette M.A. (2016). The role of craving in substance use disorders: theoretical and methodological issues. Annual Review of Clinical Psychology, 12(12), 407–33. [DOI] [PubMed] [Google Scholar]
- Sayette M.A., Schooler J.W., Reichle E.D. (2010). Out for a smoke: the impact of cigarette craving on zoning out during reading. Psychological Science, 21(1), 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N.D. (2008). Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Annals of the New York Academy of Sciences, 1129, 105–18. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Walter M., Gerber H., et al. (2013). Inferior frontal cortex modulation with an acute dose of heroin during cognitive control. Neuropsychopharmacology, 38(11), 2231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G., Roesch M.R., Stalnaker T.A. (2006). Orbitofrontal cortex, decision-making and drug addiction. Trends in Neurosciences, 29(2), 116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C.H., Ratan R., Cai Y.D., Leavitt A. (2016). Do men advance faster than women? Debunking the gender performance gap in two massively multiplayer online games. Journal of Computer-Mediated Communication, 21(4), 312–29. [Google Scholar]
- Sinha R., Li C.S. (2007). Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review, 26(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Wixted J.T., Clark R.E. (2007). Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews. Neuroscience, 8(11), 872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Ying H., Seetohul R.M., et al. (2012). Brain fMRI study of crave induced by cue pictures in online game addicts (male adolescents). Behavioural Brain Research, 233(2), 563–76. [DOI] [PubMed] [Google Scholar]
- Takakusaki K. (2017). Functional neuroanatomy for posture and gait control. Journal of Movement Disorders, 10(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral P.P., Wang T.H., Rugg M.D. (2017). Decoding the content of recollection within the core recollection network and beyond. Cortex, 91(7), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany S.T. (1990). A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review, 97(2), 147–68. [DOI] [PubMed] [Google Scholar]
- Tobler P.N., Preller K.H., Campbell-Meiklejohn D.K., et al. (2016). Shared neural basis of social and non-social reward deficits in chronic cocaine users. Social, Cognitive and Affective Neuroscience, 11(6), 1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Chang L., Wang G.J., et al. (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry, 158(12), 2015–21. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S. (2000). Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex, 10(3), 318–25. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu L., Wang L., Zhang Y., Du X., Dong G. (2016). Impaired decision-making and impulse control in Internet gaming addicts: evidence from the comparison with recreational Internet game users. Addiction Biology, 22(6), 1610–21. [DOI] [PubMed] [Google Scholar]
- Weinstein A., Livny A., Weizman A. (2017). New developments in brain research of internet and gaming disorder. Neuroscience and Biobehavioral Reviews, 75, 314–30. [DOI] [PubMed] [Google Scholar]
- Wilson S.J., Delgado M.R., McKee S.A., et al. (2014). Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cognitive, Affective and Behavioral Neuroscience, 14(4), 1196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky P.D., Malison R.T., Rogers R.D., Potenza M.N. (2014). Altered neural correlates of reward and loss processing during simulated slot-machine fMRI in pathological gambling and cocaine dependence. Drug and Alcohol Dependence, 145, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Liu J., Zhang H., Hallett M., Zheng Z., Chan P. (2015). Attention to automatic movements in Parkinson's Disease: modified automatic mode in the striatum. Cerebral Cortex, 25(10), 3330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G., Prinz W. (2001). Directing attention to movement effects enhances learning: a review. Psychonomic Bulletin & Review, 8(4), 648–60. [DOI] [PubMed] [Google Scholar]
- Yang L.Z., Shi B., Li H., et al. (2017). Electrical stimulation reduces smokers’ craving by modulating the coupling between dorsal lateral prefrontal cortex and parahippocampal gyrus. Social, Cognitive and Affective Neuroscience, 12(8), 1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y., Cosgrove K.P., Mazure C.M., Potenza M.N. (2017). Does telescoping exist in male and female gamblers? Does it matter? Frontiers in Psychology, 8, 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y., Cosgrove K.P., Potenza M.N., Mazure C.M. (2016). Balance of the sexes: addressing sex differences in preclinical research. Yale Journal of Biology and Medicine, 89(2), 255–9. [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Wienecke C.F., Nachtrab G., Chen X. (2016). A thalamic input to the nucleus accumbens mediates opiate dependence. Nature, 530(7589), 219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


