Abstract
Oxytocin has anxiolytic properties whose mechanisms of action are still being identified. DNA methylation in the promoter region of the oxytocin receptor gene (OXTR), an epigenetic modification that putatively reflects a downtuning of the oxytocin system, has previously been implicated in the regulation of fear-related responses through the amygdala. In this study, we attempted to characterize the relationship between methylation of OXTR and anxiogenesis using two distinct endophenotypes: autonomic nervous system activity and subcortical brain structure. In 79 participants, we found that increased OXTR methylation is associated with attenuated resting parasympathetic tone, measured using high-frequency heart rate variability. Further, we found that this relationship is mediated by brain morphology, such that OXTR methylation is associated with increased gray matter of the central amygdala which is, in turn, associated with decreased parasympathetic tone. These results further our understanding of epigenetic regulation of the human oxytocin system and its role in anxiogenesis.
Keywords: OXTR methylation, neuroimaging epigenetics, amygdala, anxiety, parasympathetic nervous system
Introduction
The neurohypophyseal hormone oxytocin is anxiolytic (Neumann and Slattery, 2016) and plays an essential role in human pro-social behaviors (MacDonald and MacDonald, 2010). Administering exogenous oxytocin reduces anxiety and anxious behaviors in rodents (Uvnäs-Moberg et al., 1994; Windle et al., 1997; Rotzinger et al., 2010; Slattery and Neumann, 2010) and humans (Heinrichs et al., 2003; Goodin et al., 2014) and inhibits the concomitant physiological stress response (Grippo et al., 2009; Neumann et al., 2000; de Oliveira et al., 2012). Similarly, humans with higher endogenous levels of oxytocin report less anxiety; this has been observed in both adults (Scantamburlo et al., 2007) and children (Carson et al., 2015). However, researchers are only beginning to elucidate the biological mechanisms—neuroendocrine, autonomic, genetic and epigenetic—through which oxytocin affects anxiety. A more refined understanding of these processes, particularly in how they interact within the same individual, is needed.
Oxytocin’s physiological basis in anxiolysis stems, in part, from its control over the autonomic nervous system. Multiple studies have demonstrated a close connection between oxytocin and the parasympathetic branch of the autonomic nervous system. Administration of oxytocin has been shown to increase high-frequency heart rate variability (HF-HRV; Kemp et al., 2012; Norman et al., 2011), a measure of parasympathetic cardiac control which is consistently attenuated across a range of anxiety disorders (Chalmers et al., 2014). Oxytocin modulates HF-HRV through interactions with the solitary-vagal complex, comprised of the dorsal motor nucleus and the nucleus of the solitary tract. The solitary-vagal complex receives oxytocinergic projections from the paraventricular nucleus of the hypothalamus. Oxytocin receptor activity in the solitary-vagal complex affects cardiac control and has been shown to increase parasympathetic tone by slowing the heart rate during blood pressure challenges (Higa et al., 2002). Notably, oxytocin-mediated changes in cardiac activity have not been observed through the sympathetic branch of the autonomic nervous system (Higa et al., 2002) nor does oxytocin typically affect sympathetic nervous system activity (Gamer and Büchel, 2012), suggesting that oxytocin’s physiological effects stem from exclusive interactions with the parasympathetic nervous system.
Further contributing to this autonomic, anxiolytic pathway is oxytocin’s connection to the central nucleus of the amygdala. The central nucleus regulates the physiological fear response through numerous projections to the brainstem (LeDoux et al., 1988), including the solitary-vagal complex. Central amygdala neural activity is consistently implicated in studies of HF-HRV (Thayer et al., 2012), and lesioning the central amygdala suppresses parasympathetic cardiac activity during stress (Roozendaal et al., 1991). A wealth of research has directly implicated the amygdala as a target of oxytocin-induced anxiolysis. The central amygdala is rich in oxytocin receptors, and it has been shown that oxytocin release in this region contributes to the suppression of fear-conditioned behaviors (Bale et al., 2001; Landgraf and Neumann, 2004; Viviani et al., 2011). In addition, pathways linking the central amygdala to the brainstem, including the solitary-vagal complex, are modulated by oxytocin, and oxytocin administration decreases fear-related behaviors in rodents via these pathways (Viviani et al., 2011). In humans, intranasal oxytocin administration modulates amygdala blood oxygen level dependent (BOLD) response, most often observed as a reduction in amygdala reactivity to aversive stimuli (Kirsch et al., 2005; Domes et al., 2007; Labuschagne et al., 2010). Similarly, this attenuation of amygdala reactivity has also been observed as a function of participants own endogenous (plasma) oxytocin levels (Lancaster et al., 2018). Finally, oxytocin’s amygdalar connection is also reflected in structural differences in the human amygdala: participants with lower endogenous oxytocin levels and carriers of an oxytocin receptor gene (OXTR) single nucleotide polymorphism risk-allele showed increased gray matter density within the amygdala (Tost et al., 2010; Andari et al., 2014; Lancaster et al., 2018), pointing toward an enduring relationship between the oxytocinergic system and the subcortical brain structure.
Many of oxytocin’s effects appear to be mediated by its receptor, encoded by OXTR (Kusui et al., 2001). Importantly, the oxytocin system is partially regulated through epigenetic mechanisms, including DNA methylation. Increased methylation of a DNA methylation control region in the OXTR promoter has been associated with decreased expression of the receptor, reflecting a downtuning of the oxytocin system (Kusui et al., 2001; Gregory et al., 2009). In the same way that differences in plasma oxytocin and genetic variability in OXTR explain anxiogenesis using either a behavioral or neural endophenotype, OXTR methylation is associated with amygdala BOLD response. In a recent paper, greater OXTR methylation in this promoter region (reflecting a dampened oxytocin system) was associated with an increase in participants’ amygdala reactivity to aversive faces (Puglia et al., 2015). However, it remains unclear whether this methylation-associated amygdala dysregulation extends into other biological systems and whether it manifests also in autonomic dysregulation.
In the current study, we sought to better characterize the relationship between epigenetic variability in the oxytocin system and anxiety-related endophenotypes in order to improve our mechanistic understanding of oxytocin’s contribution to anxiolysis. In light of previous work, which has identified relationships between oxytocin, the parasympathetic nervous system and the subcortical brain structure, we expected that greater OXTR methylation would be associated with decreased parasympathetic nervous system activity and increased central amygdala gray matter. Further, given the central amygdala’s autonomic connections to the brainstem, we expected that the gray matter density of the central amygdala might mediate the relationship between OXTR methylation and the parasympathetic nervous system.
Methods
Participants
Structural neuroimaging data, blood samples and cardiovascular data were acquired from 95 participants from the Charlottesville, VA, area (46 males, 49 females; mean age, 21.09; age s.d. = 2.62). Participants in this study were recruited from a larger imaging genetics study and received $75 total for their participation. Eligible participants were Caucasian; between the ages of 18 and 30; had no history of head trauma, seizures or electroconvulsive therapy; were not currently taking stimulants, sedatives or antipsychotic medications; and had no neurological or uncontrolled medical conditions. Neuroimaging and blood collection were performed during one session, and the cardiovascular assessment was completed in a follow-up session on a separate day. All participants provided informed consent as overseen by the University of Virginia Institutional Review Board. Due to equipment failures, usable psychophysiological data were not acquired for 10 participants (1 additional participant experienced an equipment failure mid-experiment, but were included in the analyses due to usable baseline/resting data). Additionally, six participants’ data were excluded due to extreme values, including methylation (two participants) and cardiovascular [HF-HRV/pre-ejection period (PEP); four participants] values which were >2 s.d.s from the mean, leaving a final sample of 79 participants with usable data (35 males, 44 females; mean age, 20.89; age s.d. = 2.40).
Blood collection and DNA extraction
Blood samples were collected at the University of Virginia Fontaine Research Park between 10:00 and 11:00 am. For epigenetic analyses, 8 ml of whole blood were collected in mononuclear cell separation tubes. The tubes were spun at 1800 relative centrifugal force for 30 min, according to product protocol. The Gentra Puregene Blood Kit (Qiagen, Valencia, CA) was then used to lyse mononuclear cells and extract DNA. Extracted DNA was stored at −20°C.
Epigenotyping
The epigenotyping procedures and materials are similar to those employed in Jack et al.(2012). Using Invitrogen kit MECOV50 (Carlsbad, CA), 200 ng of extracted DNA was subject to bisulfite conversion. Subsequently, 20 ng/uL of bisulfite converted DNA was used as a polymerase chain reaction (PCR) template with a Pyromark PCR kit (Qiagen, Valencia, CA) and 0.2 uM/uL primers (5′-TTGAGTTTTGGATTTAGATAATTAAGGATT-3′) and (5′-biotin-AATAAAATACCTCCCACTCCTTATTCCTAA-3′). Three identical PCR machines (C1000 Thermal Cycler, Biorad, Hercules, CA) amplified samples in triplicate. Amplification of the 116bp fragment-containing site −934 within a DNA methylation control region in the OXTR promoter (hg19, chr3:8,810,729-8,810,845) was achieved using the following cycling conditions [step 1: (95°C/15 min)/1 cycle, step 2: (94°C/30 s, 56°C/30 s, 72°C/30 s)/50 cycles, step 3: (72°C/10 min)/1 cycle, step 4: hold at 4°C]. PCR conditions were determined using a set of standards for site −934 at 0, 25, 50, 75 and 100% methylated {theoretical vs experimental Pearson’s correlation of 0.998 [95% confidence interval (95% CI): 0.995–0.999], P < 0.001}. Successful PCR amplification was confirmed using agarose gel electrophoresis for each sample and replicate. Pyrosequencing was performed on a Pyromark Q24 with PyroMark Gold Q24 Reagents (Qiagen, Valencia, CA) using primer (5′-AGAAGTTATTTTATAATTTTT-3′). Samples were randomized to control for plate and run variability. The average deviation from the mean was ±1.58%. Epigenotypes reported are an average of the three replicates.
Anatomical imaging
Structural imaging was performed on a Siemens Magnetom Trio 3T scanner using a 12-channel head coil. High-resolution T1-weighted gradient echo images (MPRAGE, TR = 1900 ms, TE = 2.53 ms, FOV = 250 mm, resolution = 1 × 1 × 1 mm, FA = 9°, 176 total volumes) were acquired for each participant.
Cardiovascular data acquisition
In a subsequent session (mean time elapsed post-scan = 99.98; s.d. = 137.34 days), participants were invited into a laboratory in the University of Virginia Psychology Department to undergo a cardiovascular assessment of autonomic nervous system activity. Participants were asked to avoid consuming caffeine or other stimulants for 3 h prior to the study. All cardiovascular data were recorded using a Bionex impedance cardiograph from MindWare Technologies (Gahanna, OH) using a sampling rate of 1000 Hz. Six spot electrodes were placed across each participant’s thorax to acquire electrocardiogram (ECG) and impedance cardiography data, according to prior recommendations (Qu et al., 1986; Sherwood et al., 1992). Proprietary BioLab software was used for data acquisition and analysis; participants’ physiological data were analyzed in one minute segments, and each segment was visually inspected for artifacts and corrected when applicable.
Resting/baseline and stress task
To collect resting cardiovascular data, participants were instructed to sit in a upright but relaxed position while passively observing nature scenes and sounds for 6 min (prior research has shown that the absence of any stimulus is aversive to participants; Wilson et al., 2014). Data collection commenced when participants indicated they felt sufficiently comfortable and relaxed, and concluded after 6 min had elapsed. For the stress portion of the study, we adapted the math test from the Trier Social Stress Test (Kirschbaum et al., 1993). For a recording duration of 2 min, we asked participants to count down in increments of 13 from 1022 as quickly as possible. When participants made a mistake, they were told to start from the beginning. If they hesitated for >3 s before responding, they were instructed to ``please respond faster.”
Body mass index
Participants’ height and weight were recorded during the second session, and body mass index (BMI) was computed as participants’ height in centimeters divided by their squared weight in kilograms. BMI was not associated with any of our variables of interest and thus is not discussed further.
Statistical analyses
Behavioral data were analyzed using SPSS version 23 (Armonk, NY: IBM Corp.), using two-tailed tests and an alpha criterion of 0.05.
HF-HRV
We estimated parasympathetic nervous activity using HF-HRV from ECG data. Employing the HRV analysis module (Mindware Technologies), the interbeat interval series was estimated using the peak-to-peak distance between the ECG R-waves. This series was detrended and end-tapered, and a Fast Fourier transformation was applied to calculate spectral power. This was then integrated over the high-frequency band of 0.12–0.40 Hz and values were log-transformed to obtain HF-HRV for each participant in units of ms2. This measurement of HF-HRV is also commonly referred to as respiratory sinus arrhythmia, as it corresponds to respiration-linked variance in the heart period from the activity of cardiac vagal neurons (Bertson et al., 1993). Higher HF-HRV reflects greater parasympathetic activity. To validate that our measurement of HF-HRV was unobscured by respiration artifacts, we followed the recommendation of Laborde et al. (2017) and confirmed that participants’ spontaneous respiratory rates fell within the range corresponding to the derivation of HF-HRV. To accomplish this, we estimated participants’ respiration using impedance cardiography (Ernst et al., 1999) and confirmed for each measurement of HF-HRV that participants’ peak respiratory power fell within the 0.12–0.40 Hz frequency band (i.e. a respiratory rate of 7–24 breath cycles/min).
Pre-ejection period
We estimated sympathetic nervous activity using PEP from the first derivative of the impedance waveform (dZ/dt) of the impedance cardiography data. PEP was measured as the time period in milliseconds between the onset of ventricular depolarization (the Q wave from the ECG channel) and the opening of the aortic valve (the B point on the dZ/dt wave). Using the IMP analysis module (Mindware Technologies), the Q wave was automatically pinpointed at the onset of the R wave (Berntson et al., 2004), while a previously validated algorithm estimated the B point as a function of the R-dZ/dt peak interval (Lozano et al., 2007). PEP is an inverse measure of sympathetic activity as a greater time period reflects less sympathetic nervous system activity.
Voxel-based morphometry
FSL-VBM v1.1 (FMRIB software; Douaud et al., 2007) was used to estimate gray matter volume of the central amygdala. Participants’ high resolution structural scans were tissue segmented and non-linearly normalized into Montreal Neurological Institute (MNI) space, creating a study-specific gray matter template. Participants’ native gray matter masks were fitted to the template and multiplied by a Jacobian determinant to correct for the non-linear spatial transformation. These corrected images were then smoothed using a Gaussian kernel with a sigma of 2 mm. Non-parametric permutation testing (n = 5000 permutations) using threshold-free cluster enhancement (P < 0.05, corrected for multiple comparisons) was used to identify gray matter volume differences associated with participants’ OXTR methylation levels. Using methylation as our predictor of interest, we conducted a whole-brain analysis and a priori region of interest (ROI) analysis of the central amygdala using a probabilistic mask of the central amygdala: voxels with >50% probability of belonging to the centromedial nucleus of the bilateral amygdala as specified by the Juelich Histological Atlas (Eickhoff et al., 2005) were included in this mask. Given that prior literature has found differential effects of oxytocin in subregions of the amygdala (Gamer et al., 2010), we conducted a final ROI analysis using a probabilistic mask of the basolateral amygdala to examine whether effects of OXTR methylation on amygdala gray matter were specific to the central nucleus.
Results
Summary statistics for subjective reports, HRV/PEP and OXTR methylation
For both the baseline measurement and the math test stress-elicitation, participants indicated their stress level on a 100-point scale that ranged from 0 (very relaxed) to 100 (very stressed). Participants indicated that they were generally relaxed during baseline/resting measurement (M = 21.19, s.d. = 17.69). Participants’ subjective report of feelings of stress during the math test were modest (M = 58.80, s.d. = 17.69) but were still significantly different from the midpoint, t(75) = 3.02, P = 0.003. Resting HRV (M = 6.79, s.d. = 1.08) was significantly higher than HRV during stress (M = 6.31, s.d. = 1.02), t(77) = 3.79, P = 0.0003. Similarly, resting PEP (M = 125.56, s.d. = 9.85) was significantly greater than PEP during stress (M = 120.38, s.d. = 12.40), t(77) = 5.81, P = 1.31E-7. These results confirm that participants’ parasympathetic activity decreased during stress, while their sympathetic activity increased during stress. Across participants, the average percent methylation at site −934 was M = 46.64 (s.d. = 5.63). Methylation did not correlate with age, r(77) = −0.11, P = 0.352 or differ by sex, t(77) = 1.62, P = 0.110. Furthermore, central amygdala volumes did not correlate with age, r(77) = 0.06, P = 0.583 or differ by sex t(77) = 0.16, P = 0.875. Similarly, HF-HRV and PEP variables did not correlate with age (all Ps > 0.21) or differ by sex (all Ps > 0.09).
OXTR methylation is associated with decreased autonomic nervous system activity
As predicted, OXTR methylation was significantly inversely associated with resting HF-HRV, such that participants with high levels of methylation had lower resting HF-HRV, r(77) = −0.26, P = 0.021 (Figure 1). In contrast, OXTR methylation was not associated with HF-HRV during the stress task, r(76) = –0.07, P = 0.559. Similarly, there was no significant relationship between methylation and PEP at rest, r(77) = –0.04, P = 0.740, or during stress, r(76) = 0.06, P = 0.615.
Fig. 1.
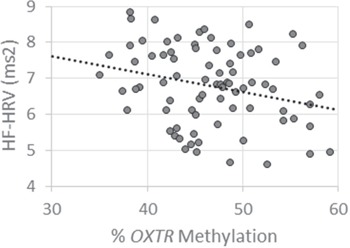
Higher OXTR methylation is associated with lower baseline HF-HRV. Greater methylation levels of OXTR are associated with low resting HF-HRV, measured in milliseconds squared (ms2).
OXTR methylation is associated with increased central amygdala gray matter volume
A priori ROI analysis using a mask of the bilateral central amygdala revealed a significant cluster in the right amygdala (Figure 2A): participants with higher OXTR methylation levels had greater percentage of gray matter in this region (7 contiguous voxels, peak voxel coordinates x = 22, y = −10, z = −8), P = 0.032 (Figure 2B). In a second step, we tested for associations across the whole brain; this exploratory analysis did not reveal any additional associations between OXTR methylation and gray matter density. In a final step, to probe for the specificity of the association with the central amygdala, we reconducted our analysis using a mask of the basolateral amygdala. Similar to the exploratory whole-brain analysis, we did not find any associations with OXTR methylation.
Fig. 2.
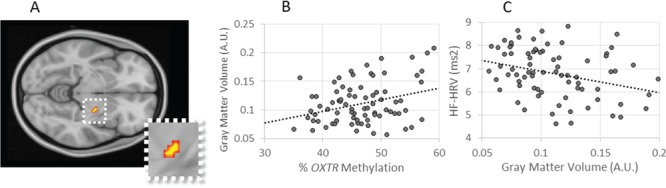
Gray matter density is associated with OXTR methylation and baseline HF-HRV. (A)OXTR methylation positively is associated with gray matter in the right central amygdala, peak voxel xyz = 22, −10, −8, cluster extent = 7 voxels, P = 0.032; cluster displayed on coronal slice at z = −8. (B) Higher percent methylation is associated with increased gray matter volume, AU, arbitrary units.(C) Gray matter volume is in turn associated with lower baseline log HF-HRV, ms2, milliseconds squared.
To test for a relationship between the methylation-associated gray matter differences and our physiological outcomes, we extracted participants’ individual gray matter estimates from the significant cluster identified in the central amygdala. We then used these values to run bivariate correlations. Gray matter densities were significantly inversely associated with HRV at rest, r(77) = −0.32, P = 0.004 (Figure 2C). This negative correlation illustrates that participants with increased central amygdala gray matter densities had decreased resting HF-HRV, which parallels the relationship between gray matter and OXTR methylation. In contrast, gray matter density was not significantly associated with any other physiological measure, i.e. PEP at rest: r(77) = −0.10, P = 0.360; PEP during stress: r(76) = −0.08, P = 0.486; and HF-HRV during stress: r(76) = −0.08, P = 0.488.
OXTR methylation and autonomic nervous system activity is mediated by central amygdala gray matter density
Given the three-way association between OXTR methylation, central amygdala gray matter volume and resting HF-HRV, we tested mediation (model illustrated in Figure 3) using a bias-corrected bootstrapping procedure (Preacher and Hayes, 2008) with 5000 samples. With this procedure, the test of the indirect effect is significant if the 95% CI does not contain zero. Indeed, we observed evidence of mediation (indirect effect = −0.0156; 95% CI, −0.040 to −0.002), such that gray matter density of the central amygdala mediated the relationship between OXTR methylation and HF-HRV.
Fig. 3.
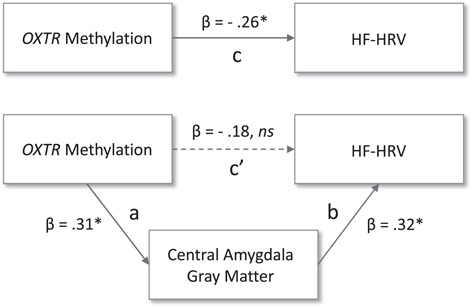
Central amygdala gray matter density mediates the relationship between OXTR methylation and HF-HRV. Figure 3 illustrates the mediational relationship between OXTR methylation, parasympathetic nervous system activity (log HF-HRV) and central amygdala gray matter. Path c reflects the total effect of the predictor on the dependent variable, while path c’ reflects the direct effect and paths ab reflect the indirect effect of the mediator, ns = not significant, * corresponds to a P value smaller than the significance level of 0.05.
Discussion
Our results demonstrate that OXTR methylation is negatively associated with resting parasympathetic tone and that this relationship is mediated by structural differences in the central amygdala. High levels of OXTR methylation have previously been associated with an increase of amygdala reactivity to aversive stimuli (Puglia et al., 2015), a pattern of neural reactivity that is supported by studies investigating the effects of exogenous (Kirsch et al., 2005) and endogenous oxytocin (Lancaster et al., 2018). Because oxytocin signaling has been implicated in anxiolysis, a downtuning of the oxytocin system via DNA methylation of OXTR is assumed to have anxiogenic consequences, but its specific relationship with the autonomic nervous system had not yet been characterized. Here, we provide evidence that epigenetic modification of the human oxytocin system is associated with the parasympathetic branch of the autonomic nervous system and suggest this may occur mechanistically through gray matter density differences in the central amygdala.
Notably, our finding that OXTR methylation predicts amygdala gray matter volume is consistent with our previous research demonstrating that low plasma oxytocin was associated with increased gray matter in the right central amygdala (Lancaster et al., 2018). Here, we have replicated this structural association using OXTR methylation: participants with high OXTR methylation—which is thought to reflect a dampening or reduced efficiency of the endogenous oxytocin system (Kusui et al., 2001)—is associated with increased gray matter in the right central amygdala. This conceptual replication, and the regional specificity of the findings to the central nucleus, reinforces the importance of central amygdala structure in the relationship between oxytocin and negative emotionality. Furthermore, the current findings are generally aligned with our previous work on OXTR methylation and amygdala BOLD response (Puglia et al., 2015). Increased activity within the amygdala (which is likely to contribute to increased gray matter density through dendritic spinogenesis, Keifer et al., 2015; Mitra et al., 2005, and synaptogenesis, Kassem et al., 2013) is a marker of anxious temperament in humans (Schwartz et al., 2003) and non-human primates (Fox et al., 2008; Oler et al., 2010); we have previously shown that participants with high levels of OXTR methylation show increased amygdalar activity to negative stimuli (Puglia et al., 2015). Together, these findings illustrate the synergy of multiple bodily systems (autonomic, oxytocinergic, limbic structure and function) substrative of an anxiety phenotype.
Interestingly, in our sample, OXTR methylation was associated only with the parasympathetic branch of the autonomic nervous system and only at rest. This may indicate that OXTR methylation is not associated with stress-elicited vagal withdrawal but rather with tonic vagal output under baseline conditions. Tonic parasympathetic cardiac control is regulated by vagal preganglionic neurons projecting from the solitary-vagal complex (Machhada et al., 2015), which is innervated by the central nucleus of the amygdala (Saha et al., 2000), a pathway that is modulated by oxytocin (Higa et al., 2002; Viviani et al., 2011). While previous work has shown an oxytocin peptide-mediated increase in vagal outflow under blood pressure challenges (Higa et al., 2002), our results suggest that epigenetic modification of OXTR is more relevant for baseline/tonic vagal efferent action. Porges (1992) and Porges et al. (1996) have argued that fast-acting vagal withdrawal during stress (the `vagal brake’) is evolutionarily adaptive for responding to dynamic threats, enabling the fight or flight response. If vagal withdrawal were epigenetically regulated by the availability of oxytocin receptors, this would limit the ability of the autonomic nervous system to quickly and flexibly alter physiological responses during stress. Instead, our findings suggest that epigenetic influence over the oxytocin system contributes to autonomic function in a manner that is more tonic than dynamic; this is supported by our finding that this relationship is mediated by neural structural differences, which are not transiently occurring.
Future research will be needed to better interpret our findings. For one, we examined putative anxiety endophenotypes (based on prior literature), but we did not link these directly to self-reported anxiety in the current study, nor did we explore the influence of covariates beyond sex and age. Using a more comprehensively phenotyped sample could clarify, for instance, how HF-HRV is linked to anxiogenesis through deficiencies in emotion regulation, as has been previously reported (Thayer and Lane, 2000; Williams et al., 2015). The availability of additional behavioral data could also rule out potential confounds, such as the influence of alcohol consumption on physiology (Quintana et al., 2013) and, thus, should be a goal for subsequent research. Another factor which limits the interpretation of our findings is the current knowledge gap concerning how epigenetic modifications of OXTR reflect functional changes in oxytocin system structure and function in humans. Methylation of the region we assayed on OXTR is known to downregulate transcription of the gene (Kusui et al., 2001; Gregory et al., 2009), but it is unclear whether methylation and resultant changes in expression of OXTRs are distributed across all oxytocin-relevant tissues (Lancaster et al., 2018). There is some evidence (Dadds et al., 2014) that methylation levels are inversely associated with plasma oxytocin levels—potentially implicating a widespread impact on the oxytocin system—but more recent findings suggest this association might be modulated by sex and psychiatric conditions (Rubin et al., 2015). Our understanding of how epigenetic influences on the oxytocin system contribute to anxiogenesis will benefit from future work that addresses these issues.
Conclusion
In this study, we have provided the first evidence that OXTR methylation is associated with tonic parasympathetic function and that this association is mediated by gray matter differences within the central amygdala. These findings highlight the importance of epigenetic regulation of the oxytocin system in anxiety and fear-related processes and suggest a physiological mechanism through which it may operate in humans.
Funding
This work was supported by the National Science Foundation [grant number 1228522] awarded to J.J.C. & J.P.M.
Acknowledgments
We thank Tyler Santander and Travis Lillard for their assistance with data collection and analysis.
References
- Andari E., Schneider F.C., Mottolese R., Vindras P., Sirigu A. (2014). Oxytocin’s fingerprint in personality traits and regional brain volume. Cerebral Cortex, 24(2), 479–86. 10.1093/cercor/bhs328 [DOI] [PubMed] [Google Scholar]
- Bale T.L., Davis A.M., Auger A.P., Dorsa D.M., McCarthy M.M. (2001). CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. The Journal of Neuroscience, 21(7), 2546–52. https://doi.org/21/7/2546[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson G.G., Lozano D.L., Chen Y.-J., Cacioppo J.T. (2004). Where to Q in PEP. Psychophysiology, 41(2), 333–37. 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Bertson G.G., Cacioppo J.T., Quigley K.S. (1993). Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–96. 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Carson D.S., Berquist S.W., Trujillo T.H., et al. (2015). Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Molecular Psychiatry, 20(9), 1085–90. 10.1038/mp.2014.132. [DOI] [PubMed] [Google Scholar]
- Chalmers J.A., Quintana D.S., Abbott M.J.-A., Kemp A.H. (2014). Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Frontiers in Psychiatry, 5, 80 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds M., Moul C., Cauchi A., et al. (2014). Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Development and Psychopathology, 26, 33–40. 10.1017/S0954579413000497. [DOI] [PubMed] [Google Scholar]
- Oliveira D.C.G., Zuardi A.W., Graeff F.G., Queiroz R.H.C., Crippa J.A.S. (2012). Anxiolytic-like effect of oxytocin in the simulated public speaking test. Journal of Psychopharmacology (Oxford, England), 26(4), 497–504. 10.1177/0269881111400642 10.1177/0269881111400642. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Gläscher J., Büchel C., Braus D.F., Herpertz S.C. (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–90. 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., et al. (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain, 130(9), 2375–86. 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K. E., Mohlberg H., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25(4), 1325–1335. 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ernst J.M., Litvack D.A., Lozano D.L., Cacioppo J.T., Berntson G.G. (1999). Impedance pneumography: noise as signal in impedance cardiography. Psychophysiology, 36(3), 333–8Retrieved fromhttp://www.ncbi.nlm.nih.gov/pubmed/10352556. [DOI] [PubMed] [Google Scholar]
- Fox A.S., Shelton S.E., Oakes T.R., Davidson R.J., Kalin N.H. (2008). Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One, 3(7), e2570 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Büchel C. (2012). Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology, 37(1), 87–93. 10.1016/j.psyneuen.2011.05.007 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Büchel C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America, 107(20), 9400–5. 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin B.R., Anderson A.J.B., Freeman E.L., Bulls H.W., Robbins M.T., Ness T.J. (2014). Intranasal oxytocin administration is associated with enhanced endogenous pain inhibition and reduced negative mood states. The Clinical Journal of Pain, 31(9), 757–767. 10.1097/AJP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S.G., Connelly J.J., Towers A.J., et al. (2009). Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Medicine, 7(1), 62 10.1186/1741-7015-7-62 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Trahanas D.M., Zimmerman R.R., Porges S.W., Carter C.S. (2009). Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology, 34(10), 1542–53. 10.1016/j.psyneuen.2009.05.017 10.1016/j.psyneuen.2009.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–98. 10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Higa K.T., Mori E., Viana F.F., Morris M., Michelini L.C. (2002). Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 282(2), R537–45. 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- Jack A., Connelly J.J., Morris J.P. (2012). DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Frontiers in Human Neuroscience, 6, 280 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem M. S., Lagopoulos J., Stait-Gardner T., et al. (2013). Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Molecular Neurobiology, 47(2), 645–61. 10.1007/s12035-012-8365-7 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Keifer O.P., Hurt R.C., Gutman D.A., Keilholz S.D., Gourley S.L., Ressler K.J. (2015). Voxel-based morphometry predicts shifts in dendritic spine density and morphology with auditory fear conditioning. Nature Communications, 6, 7582 10.1038/ncomms8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A.H., Quintana D.S., Kuhnert R.-L., Griffiths K., Hickie I.B., Guastella A.J. (2012). Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PloS One, 7(8), e44014 10.1371/journal.pone.0044014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P., Esslinger C., Chen Q., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25(49), 11489–93. 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. (1993). The `Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/119004. [DOI] [PubMed] [Google Scholar]
- Kusui C., Kimura T., Ogita K., et al. (2001). DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochemical and Biophysical Research Communications, 289(3), 681–6. 10.1006/bbrc.2001.6024 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- Laborde S., Mosley E., Thayer J.F. (2017). Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research – Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Frontiers in Psychology, 8(213). 10.3389/fpsyg.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology, 35(12), 2403–13. 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster K., Goldbeck L., Pournajafi-Nazarloo H., Connelly J.J., Carter C.S., Morris J.P. (2018). The role of endogenous oxytocin in anxiolysis: structural and functional correlates. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(7), 618–25. 10.1016/j.bpsc.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Lancaster K., Morris J.P., Connelly J.J. (2018). Neuroimaging Epigenetics: Challenges and Recommendations for Best Practices. Neuroscience, 370, 88–100. 10.1016/j.neuroscience.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. (2004). Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology, 25(3–4), 150–76. 10.1016/j.yfrne.2004.05.001 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- LeDoux J., Iwata J., Cicchetti P., Reis D. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience, 8(7), Retrieved from http://www.jneurosci.org/content/8/7/2517.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano D.L., Norman G., Knox D., et al. (2007). Where to B in dZ/dt. Psychophysiology, 44(1), 113–9. 10.1111/j.1469-8986.2006.00468.x 10.1111/j.1469-8986.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- MacDonald K., MacDonald T.M. (2010). The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry. UK: Informa UK Ltd London; 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Machhada A., Ang R., Ackland G.L., et al. (2015). Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm, 12(11), 2285–93. 10.1016/j.hrthm.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Jadhav S., McEwen B.S., Vyas A., Chattarji S. (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America, 102(26), 9371–6. 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I.D., Krömer S.A., Toschi N., Ebner K. (2000). Brain oxytocin inhibits the (re)activity of the hypothalamo–pituitary–adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory Peptides, 96(1–2), 31–8. 10.1016/S0167-0115(00)00197-X. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Slattery D.A. (2016). Oxytocin in general anxiety and social fear:aA translational approach. Biological Psychiatry, 79(3), 213–21. 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Norman G.J., Cacioppo J.T., Morris J.S., Malarkey W.B., Berntson G.G., DeVries A.C. (2011). Oxytocin increases autonomic cardiac control: moderation by loneliness. Biological Psychology, 86(3), 174–80. 10.1016/j.biopsycho.2010.11.006 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Oler J.A., Fox A.S., Shelton S.E., et al. (2010). Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature, 466(7308), 864–8. 10.1038/nature09282 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W. (1992). Vagal tone: a physiologic marker of stress vulnerability. Pediatrics, 90(3 Pt 2), 498–504. https://doi.org/1513615. [PubMed] [Google Scholar]
- Porges S.W., Doussard-Roosevelt J.A., Portales A.L., Greenspan S.I. (1996). Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Developmental Psychobiology, 29(8), 697–712. . [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–91. 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Puglia M.H., Lillard T.S., Morris J.P., Connelly J.J. (2015). Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 112(11), 3308–13. 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M., Zhang Y., Webster J.G., Tompkins W.J. (1986). Motion artifact from spot and band electrodes during impedance cardiography. IEEE Transactions on Biomedical Engineering, BME-33(11), 1029–36. 10.1109/TBME.1986.325869. [DOI] [PubMed] [Google Scholar]
- Quintana D.S., McGregor I.S., Guastella A.J., Malhi G.S., Kemp A.H. (2013). A meta-analysis on the impact of alcohol dependence on short-term resting-state heart rate variability: implications for cardiovascular risk. Alcoholism, Clinical and Experimental Research, 37, E23–9. 10.1111/j.1530-0277.2012.01913.x 10.1111/j.1530-0277.2012.01913.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Koolhaas J.M., Bohus B. (1991). Central amygdala lesions affect behavioral and autonomic balance during stress in rats. Physiology & Behavior, 50(4), 777–81. 10.1016/0031-9384(91)90017-I. [DOI] [PubMed] [Google Scholar]
- Rotzinger S., Lovejoy D.A., Tan L.A. (2010). Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides, 31(4), 736–56. 10.1016/j.peptides.2009.12.015 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Rubin L.H., Connelly J.J., Reilly J.L., et al. (2015). Sex and diagnosis-specific associations between DNA methylation of the oxytocin receptor gene with emotion processing and temporal-limbic and prefrontal brain volumes in psychotic disorders. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Batten T.F.C., Henderson Z. (2000). A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience, 99(4), 613–26. 10.1016/S0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G., Hansenne M., Fuchs S., et al. (2007). Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology, 32(4), 407–10. 10.1016/j.psyneuen.2007.01.009 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., Wright C.I., Shin L.M., Kagan J., Rauch S.L. (2003). Inhibited and uninhibited infants ``grown up”: adult amygdalar response to novelty. Science (New York, N.Y.), 300(5627), 1952–3. 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Sherwood A., Royal S.A., Hutcheson J.S., Turner J.R. (1992). Comparison of impedance cardiographic measurements using band and spot electrodes. Psychophysiology, 29(6), 734–41. 10.1111/j.1469-8986.1992.tb02051.x. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Neumann I.D. (2010). Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology, 58(1), 56–61. 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Åhs F., Fredrikson M., et al. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–56. 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–16. 10.1016/S0165-0327(00)00338-4 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tost H., Kolachana B., Hakimi S., et al. (2010). A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences of the United States of America, 107(31), 13936–41 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K., Ahlenius S., Hillegaart V., Alster P. (1994). High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacology Biochemistry and Behavior, 49(1), 101–6. 10.1016/0091-3057(94)90462-6 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Viviani D., Charlet A., Burg E., et al. (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science (New York, N.Y.), 333(6038), 104–7. 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Williams D.P., Cash C., Rankin C., Bernardi A., Koenig J., Thayer J.F. (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in Psychology, 6, 261 10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.D., Reinhard D.A., Westgate E.C., et al. (2014). Just think: the challenges of the disengaged mind. Science, 345(6192), 75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle R.J., Shanks N., Lightman S.L., Ingram C.D. (1997). Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology, 138(7), 2829–34. 10.1210/endo.138.7.5255 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]


