Introduction
Purpura fulminans (PF) is a rare life-threatening complication of bacterial sepsis that is characterized by a highly thrombotic subtype of disseminated intravascular coagulation (DIC) and mortality of up to 80%.1 Patients with PF develop a severe deficiency in protein C (PC), a serine protease that is a key endogenous anticoagulant.2 PC is activated at the endothelial surface by thrombin in the presence of the cofactor thrombomodulin (TM). Activated PC (APC) is cytoprotective3 and inhibits thrombin generation by cleaving coagulation factors Va and VIIIa.4 Therefore, TM is a crucial regulatory “switch” governing negative feedback of coagulation. The initiating event in PF is hypothesized to be loss of TM from the endothelial surface in response to infection.5 As a result, conversion of PC to APC is impaired, and coagulation proceeds unchecked.3 Although therapy with PC concentrate has been proposed as a strategy to target the underlying pathophysiologic lesion in PF,6 the belief that endothelial TM loss is an early event in PF that renders infused PC ineffective has limited its widespread adoption.2,5 We report herein data on the kinetics of TM loss in a patient with PF that support the use of supplemental PC in upfront treatment of severe cases.
Case description
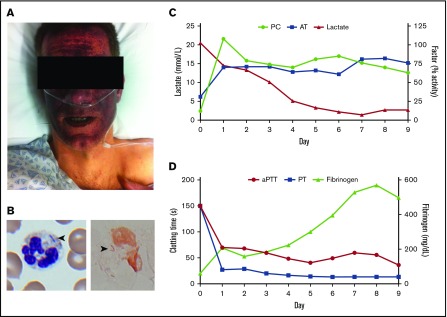
A previously healthy 39-year-old male presented after experiencing 12 hours of sore throat, malaise, and an expanding purpuric rash on his face. Physical examination revealed mask-like purpura with periorbital sparing, scattered purpuric papules on the extremities, and livedo of the hands and feet (Figure 1A; supplemental Figure 1). Initial laboratory studies were consistent with severe DIC and multiorgan system failure (supplemental Table 1). Review of the peripheral blood smear showed neutrophils containing toxic granulations, Döhle bodies, and prominent vacuoles, as well as frequent rod-like Gram-negative basophilic inclusions (Figure 1B). The patient reported owning a new dog and was later noted to have a small puncture wound on his left fourth finger from a dog bite sustained 3 days prior to admission. Based on his history and the findings on peripheral blood smear, a presumptive diagnosis of PF due to Capnocytophaga canimorsus bacteremia was made. C canimorsus is a rare infection but one that carries a very high rate of PF, which occurs in ∼30% to 40% of cases.7,8 Indeed, PF appears to complicate C canimorsus sepsis more frequently on a per-case basis than meningococcal disease, an infection that is broadly understood to be a common cause of PF.
Figure 1.
Initial presentation and clinical course. (A) The patient as he appeared at presentation, following rapid development of a mask-like purpuric rash involving the face. (B) Polymorphonuclear leukocyte containing rod-like basophilic inclusions on Wright’s stain (arrowhead; left panel), and Gram’s stain (arrowhead; right panel). Original magnification ×1000. (C) Plasma levels of PC, antithrombin (AT), and lactate during the first 10 days of hospitalization. (D) Activated partial thromboplastin time (aPTT), prothrombin time (PT), and fibrinogen during the same period. PC and antithrombin concentrates were administered twice daily starting 6 hours after presentation through day 10 of hospitalization.
Shortly after transfer to the medical intensive care unit, the patient experienced cardiac arrest with pulseless electrical activity requiring 10 minutes of cardiopulmonary resuscitation. After reestablishment of spontaneous circulation, he was treated with 100 IU/kg PC concentrate, 3100 IU antithrombin (AT) concentrate, and a 5000-U IV bolus of heparin, followed by a continuous heparin infusion. In addition, he received 40 U of cryoprecipitate and 10 U of fresh-frozen plasma overnight.
The following day, the patient’s admission PC activity level returned at 12% (normal range, 66%-140%), protein S was 30% (normal range, 70%-134%), and AT antigen level was 37% (normal range, 69%-127%). A skin biopsy specimen confirmed the presence of microvascular thrombi (supplemental Figure 2). Although blood cultures remained negative, C canimorsus was detected in the patient’s peripheral blood using a polymerase chain reaction (PCR)–based assay performed at the Centers for Disease Control (see “Methods”). In addition to continued antibiotic therapy, the patient received therapeutic anticoagulation with IV heparin throughout his hospitalization and was administered PC and AT concentrates until hospital day 10, at which point he was able to maintain normal levels without supplementation. Limb necrosis was managed conservatively with meticulous examinations, wound care, and bedside debridement. He steadily improved and was discharged on hospital day 57. He ultimately retained use of all 4 limbs and only required amputation of the 4th and 5th distal phalanges of the left hand (supplemental Figure 3) and 9 of 10 phalanges of both feet. He has since returned to work as a graphic designer.
Methods
Molecular diagnosis of C canimorsus infection
Under research use for diagnostic confirmation, DNA was extracted from a 200-µL blood sample using a QIAamp DNA Mini Kit, according to the manufacturer’s instructions (QIAGEN, Valencia, CA). Two fragments of the 16S rRNA gene were targeted for amplification (supplemental Methods) from the purified DNA using an Expand High-Fidelity PCR System (Roche Diagnostics, Indianapolis, IN). Briefly, each 50-µL reaction contained 10 ng of DNA, 2.5 U of polymerase, 1.5 mM MgCl2, 200 mM deoxynucleoside triphosphates, and 100 nM primers fD1 and R530 (amplicon 1) and F530 and rP2 (amplicon 2). Amplification was performed on an ABI 9700 thermocycler (Applied Biosystems, Foster City, CA) using the following program: 94°C for 5 minutes, followed by 35 cycles of 94°C for 15 seconds, 50°C for 15 seconds, and 72°C for 90 seconds, with a final single extension of 72°C for 5 minutes and then held at 4°C. Amplicons were generated using primers previously described9,10 and were sequenced according to Morey et al11 with a modified set of primers (supplemental Methods).10,12 Sequence data were assembled using Geneious v. 7.0.4 (Biomatters, Auckland, New Zealand), resulting in a trimmed sequence length of 909 base pairs. The resulting sequence was compared with the publicly available database GenBank to identify sequences of related taxa.13 BLAST results indicated matches to clinically relevant strains of C canimorsus sequences with pairwise identities between 99.8% and 98.2%.
Immunohistochemistry and real-time quantitative PCR for TM and E-selectin
This study was approved by the Partners Healthcare Institutional Review Board with a waiver of informed consent because of the use of deidentified discarded specimens obtained in the course of clinical care. The following antibodies were used for immunohistochemistry: E-selectin (Neuromics; M020039), intercellular adhesion molecule 1 (ICAM-1; Sigma; HPA004877), and TM (Abcam; ab6980). Grading of protein expression was performed by an author (O.P.) who was blinded to the time point and source of the specimens. All vessels in skin biopsies were analyzed for the presence of protein staining. Stain intensity and degree of vascular involvement were assessed using an arbitrary scale from 0 (no staining) to 3 (strong staining involving all vessels).
Real-time quantitative PCR for TM and E-selectin
RNA was extracted from formalin-fixed paraffin embedded skin biopsies using a RecoverAll Total Nucleic Acid Isolation Kit, per the manufacturer’s instructions. RNA was quantitated using a NanoDrop 1000 Spectrophotometer. First-strand cDNA synthesis was performed using a SuperScript III kit (Invitrogen), and second-strand synthesis was performed using a NEBNext second strand synthesis kit (New England Biolabs). TaqMan probe and primer sets were obtained for the following genes: E-selectin, TM, GAPDH, and actin (Life Technologies, catalog numbers Hs00950401_m1, Hs00264920_s1, Hs02758991_g1, and Hs01060665_g1, respectively). TaqMan PCR was performed with 1 μL of input DNA using a Prism 7900HT machine and Universal TaqMan Master Mix II (both from Life Technologies), per the manufacturer’s directions. The relative fold difference in E-selectin and TM were calculated compared with the housekeeping gene GAPDH, as previously described, using the ΔΔCt method.
Results and discussion
Response to PC concentrate and anticoagulation
After an initial bolus infusion of PC (100 IU/kg) and AT concentrate (3100 U), these agents were readministered every 12 to 24 hours through day 10 to maintain values >70%, and the patient also received 2 to 4 U of fresh-frozen plasma daily during the first week of hospitalization (see supplemental Table 2 for the relative PC content of different products). Markers of coagulopathy rapidly corrected, followed by improvement in the patient’s lactic acidosis over 7 days (Figure 1C-D). Therapeutic anticoagulation with heparin proved difficult, likely due to the severity of inflammation present.14 Convalescent levels of circulating anticoagulant proteins, checked 86 days after initial presentation, were all within the normal range (PC, 151%; protein S, 115%; AT, 118%).
Expression of endothelial TM
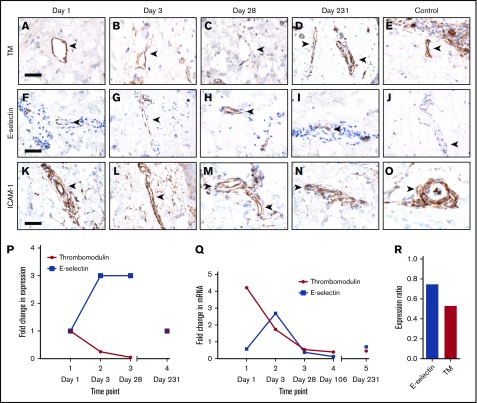
Loss of TM at the endothelial surface is thought to be an early event in PF.5 We performed immunohistochemistry on serial skin biopsy specimens taken on days 0, 3, 28, and 231 (day 0 denotes the first day of presentation) to characterize the kinetics of TM loss (Figure 2A-E). Coagulation-related laboratory results corresponding to each time point are shown in supplemental Table 3. Specimens were also examined for E-selectin (Figure 2F-J) and ICAM-1 (Figure 2K-O), markers of endothelial cell activation and viability, respectively. At presentation, our patient’s endothelial TM protein expression was similar to that of normal control specimens (Figure 2P). Surface TM protein expression remained detectable through the third day of hospitalization before declining to an undetectable level. In contrast, E-selectin protein expression rose threefold between days 1 and 3 and remained elevated throughout the first 28 days of hospitalization, consistent with an ongoing endothelial inflammatory response. Protein expression of TM and E-selectin returned to baseline levels during convalescence (day 231).
Figure 2.
Time course of expression of TM and E-selectin. (A-E) Immunohistochemical staining for TM on blood vessels (arrowheads) in skin biopsy specimens taken at various time points during the patient’s hospitalization (days 1-28) and at convalescence (day 231) compared with a normal control specimen (scale bars, 20 µm). Staining for E-selectin, a marker of endothelial cell activation (F-J), and for ICAM-1, a marker of cell viability (K-O), in the same biopsy specimens. (P) Quantification of the immunohistochemical staining of TM, E-selectin, and ICAM-1 in (A-O), represented as fold change from day 0. Expression of TM and E-selectin returned to baseline at day 231. (Q) TM and E-selectin transcript levels normalized to GAPDH expression at each time point, as determined by real-time quantitative PCR. (R) Quantification of the patient’s convalescent TM and E-selectin transcript levels as a ratio of the transcript levels in a healthy subject.
TM loss is posttranscriptional
Previous in vitro studies have suggested 2 mechanisms for TM loss during inflammation: cleavage of the protein from the endothelial surface5,15 and downregulation of TM gene expression in response to inflammatory cytokines.16 We quantified TM and E-selectin messenger RNA transcript levels in our patient by real-time quantitative PCR of serial skin biopsy specimens (Figure 2Q). We found a 4.2-fold increase in TM transcript levels relative to GAPDH expression at the time of presentation, followed by a gradual decline to baseline levels through day 28. In contrast, E-selectin transcript levels were normal at presentation, increased 2.7-fold by day 3, and decreased thereafter to baseline levels. These data indicate that TM expression is significantly upregulated in response to inflammation and that endothelial TM loss during the early stages of PF is a posttranscriptional event. At convalescence, expression levels of TM and E-selectin in our patient were modestly lower than those of a healthy subject (Figure 2R).
In patients with sepsis complicated by PF, failure of the TM-PC system is hypothesized to be central to the disease process.3,5 This model posits that loss of TM early in the disease course impairs activation of endogenous and therapeutically administered PC. However, small trials and retrospective case series17-20 have consistently suggested that PF patients benefit from supplemental PC, particularly in the prevention of amputations.6 Although results from this single case report should be interpreted with caution, our data support the idea that, during the hyperacute phase of PF, endothelial TM remains intact, and PC is consumed extremely rapidly (a process that requires the presence of functional TM). Endothelial TM loss occurs later and is a secondary event. Therefore, it is overwhelming thrombin generation and the resultant depletion of PC, not the absence of endothelial TM, that is the primary driver of microvascular thrombosis in PF. In our patient, PC concentrate was undoubtedly functioning in concert with other treatments that we administered, and the specific contribution of PC to his recovery is difficult to delineate with certainty. Additionally, due to the lack of sufficient biopsy tissue, we were unable to assay for other endothelial proteins (eg, endothelial PC receptor) that may have played a role in the disease process. We hypothesize that PC supplementation, together with other anticoagulant therapies, served to mitigate the effects of prothrombotic stimuli from the microorganism as antibiotics gradually cleared the infection.
It should be noted that a large randomized trial of recombinant human APC (drotrecogin alfa) in unselected patients with septic shock failed to show benefit.21 The discrepancy between these results and those from smaller experiences that support the use of supplemental PC in PF may be due to the clinical heterogeneity of “severe sepsis,” which includes patients without thrombotic DIC. Clinical trials of PC repletion combined with aggressive anticoagulation in patients with bona fide PF will be required to assess the efficacy of this approach.
In this article, we provide a mechanistic explanation for the therapeutic role of PC concentrate in PF by showing that TM transcription is upregulated in response to infection, and TM protein can persist at the endothelial surface for up to 72 hours after initial presentation in some patients. This observation is consistent with previous work demonstrating that infused PC is rapidly converted to APC in PF patients22 and suggests that supplemental PC can be effective, even after patients present with apparent failure of the TM-PC pathway.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank George Canellos, Robert Mayer, and Ann LaCasce (Dana-Farber Cancer Institute) for support of this project and Sunny Dzik (Massachusetts General Hospital) for providing guidance in the management of this patient.
This work was supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (grant 1K08 HL136840-01) (P.K.B.).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Authorship
Contribution: P.K.B., N.L., R.S., K.E.S., A.S., and J.-A.L. conceived and designed the study and cared for the patient; P.K.B., A.R., O.P., A.B., F.D., J.M., B.H., B.R., and M.C. performed experiments and collected data; and P.K.B. and J.-A.L. drafted the manuscript, which was reviewed by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julie-Aurore Losman, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 422, Boston, MA 02215; e-mail: julieaurore_losman@dfci.harvard.edu.
References
- 1.Lerolle N, Carlotti A, Melican K, et al. . Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188(6):684-692. [DOI] [PubMed] [Google Scholar]
- 2.Powars DR, Rogers ZR, Patch MJ, McGehee WG, Francis RB Jr. Purpura fulminans in meningococcemia: association with acquired deficiencies of proteins C and S. N Engl J Med. 1987;317(9):571-572. [DOI] [PubMed] [Google Scholar]
- 3.Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32(2):69-76. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125(19):2898-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faust SN, Levin M, Harrison OB, et al. . Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345(6):408-416. [DOI] [PubMed] [Google Scholar]
- 6.Smith OP, White B. Infectious purpura fulminans: diagnosis and treatment. Br J Haematol. 1999;104(2):202-207. [DOI] [PubMed] [Google Scholar]
- 7.Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol. 1996;12(5):521-533. [DOI] [PubMed] [Google Scholar]
- 8.Chiappa V, Chang CY, Sellas MI, Pierce VM, Kradin RL. Case records of the Massachusetts General Hospital. Case 10-2014. A 45-year-old man with a rash. N Engl J Med. 2014;370(13):1238-1248. [DOI] [PubMed] [Google Scholar]
- 9.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gee JE, Sacchi CT, Glass MB, et al. . Use of 16S rRNA gene sequencing for rapid identification and differentiation of Burkholderia pseudomallei and B. mallei. J Clin Microbiol. 2003;41(10):4647-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morey RE, Galloway RL, Bragg SL, Steigerwalt AG, Mayer LW, Levett PN. Species-specific identification of Leptospiraceae by 16S rRNA gene sequencing. J Clin Microbiol. 2006;44(10):3510-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wuyts J, Van de Peer Y, Winkelmans T, De Wachter R. The European database on small subunit ribosomal RNA. Nucleic Acids Res. 2002;30(1):183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altschul SF, Madden TL, Schäffer AA, et al. . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young E, Podor TJ, Venner T, Hirsh J. Induction of the acute-phase reaction increases heparin-binding proteins in plasma. Arterioscler Thromb Vasc Biol. 1997;17(8):1568-1574. [DOI] [PubMed] [Google Scholar]
- 15.Ishii H, Uchiyama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemost. 1991;65(5):618-623. [PubMed] [Google Scholar]
- 16.Sohn RH, Deming CB, Johns DC, et al. . Regulation of endothelial thrombomodulin expression by inflammatory cytokines is mediated by activation of nuclear factor-kappa B. Blood. 2005;105(10):3910-3917. [DOI] [PubMed] [Google Scholar]
- 17.Smith OP, White B, Vaughan D, et al. . Use of protein-C concentrate, heparin, and haemodiafiltration in meningococcus-induced purpura fulminans. Lancet. 1997;350(9091):1590-1593. [DOI] [PubMed] [Google Scholar]
- 18.Rintala E, Kauppila M, Seppälä OP, et al. . Protein C substitution in sepsis-associated purpura fulminans. Crit Care Med. 2000;28(7):2373-2378. [DOI] [PubMed] [Google Scholar]
- 19.White B, Livingstone W, Murphy C, Hodgson A, Rafferty M, Smith OP. An open-label study of the role of adjuvant hemostatic support with protein C replacement therapy in purpura fulminans-associated meningococcemia. Blood. 2000;96(12):3719-3724. [PubMed] [Google Scholar]
- 20.Veldman A, Fischer D, Wong FY, et al. . Human protein C concentrate in the treatment of purpura fulminans: a retrospective analysis of safety and outcome in 94 pediatric patients. Crit Care. 2010;14(4):R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranieri VM, Thompson BT, Barie PS, et al. ; PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. [DOI] [PubMed] [Google Scholar]
- 22.de Kleijn ED, de Groot R, Hack CE, et al. . Activation of protein C following infusion of protein C concentrate in children with severe meningococcal sepsis and purpura fulminans: a randomized, double-blinded, placebo-controlled, dose-finding study. Crit Care Med. 2003;31(6):1839-1847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.