Abstract
Each stem cell resides in a highly specialized anatomic location known as the niche that protects and regulates stem cell function. The importance of the niche in hematopoiesis has long been appreciated in transplantation, but without methods to observe activity in vivo, the components and mechanisms of the hematopoietic niche have remained incompletely understood. Zebrafish have emerged over the past few decades as an answer to this. Use of zebrafish to study the hematopoietic niche has enabled discovery of novel cell–cell interactions, as well as chemical and genetic regulators of hematopoietic stem cells. Mastery of niche components may improve therapeutic efforts to direct differentiation of hematopoietic stem cells from pluripotent cells, sustain stem cells in culture, or improve stem cell transplant.
Introduction
Although stem cells are extremely powerful and have unique capacity for both self-renewal and differentiation in development and regeneration, they have limited function in isolation. A niche is required to integrate organism-level stimuli and regulate stem cell function. First proposed by Schofield in 1978, a niche is a stereotyped microenvironment that protects a stem cell from damage and regulates its behavior.1,2 Niches have specific anatomic locations, structural features, cell types, and extracellular signals that interact with the resident stem cell. These concepts were first hinted at in the mammalian hematopoietic system when hematopoietic stem cells (HSCs) were found to be regionalized within bone marrow and supported by nonhematopoietic cells.3,4
There is great interest in understanding the hematopoietic niche, both for advancing basic science and in treating hematologic disease. Niche signals are key in inducing stem cell formation and maintaining their long-term function, so efforts to derive HSCs in vitro or modulate function in vivo hinge upon recapitulating elements of a niche-like environment. HSC transplant is a curative therapy for a variety of hematologic pathologies, but the process is risky, largely because of low rates of donor stem cell engraftment to the host niche, infection, and graft-versus-host disease. Moreover, among HSCs, there is well-documented heterogeneity in stimuli response and lineage contribution,5-7 although how the niche regulates this is unknown. Recent improvements in deriving HSC-like cells, rejuvenating HSC function, and improving HSC engraftment after transplant have used genes and chemicals uncovered from close study of the niche.8-10
Precisely describing and perturbing the hematopoietic niche has been challenging in mammals because of the inaccessibility of bone marrow and live embryonic tissue. Zebrafish have proven to be an invaluable tool for studying hematopoiesis and the hematopoietic niche in development. A paired mating can produce hundreds of transparent, externally fertilized embryos that are amenable to chemical and genetic screens, as well as high-resolution time-lapse imaging. Generation of transgenic zebrafish is easily accomplished with the use of Tol2 transposase to create reporter lines to specifically label cells of interest or drive expression of genes of interest in candidate cell types.11
These animals develop rapidly, and blood formation and niche colonization can be directly observed within the first few days of development. By 12 hours postfertilization (hpf), the “primitive” wave of hematopoiesis produces erythrocytes and primitive myeloid cells to oxygenate and support the early embryo.12 Soon after, “definitive” hematopoiesis and stem cell production initiates at 26 hpf. Because of the accessibility and visibility of embryos, these processes can be analyzed by gene knockdown or knockout through injection of morpholino antisense oligonucleotides or CRISPR/Cas9 components. Moreover, processes, pathways, and cell–cell interactions discovered in zebrafish are highly conserved in mammals (reviewed by Clements and Traver13), which allows for rapid translation of discoveries in fish to treatments in humans. As a result, zebrafish are now widely used in descriptive and mechanistic studies of the hematopoietic niche.
Sites of definitive hematopoiesis across zebrafish development
Ventral dorsal aorta
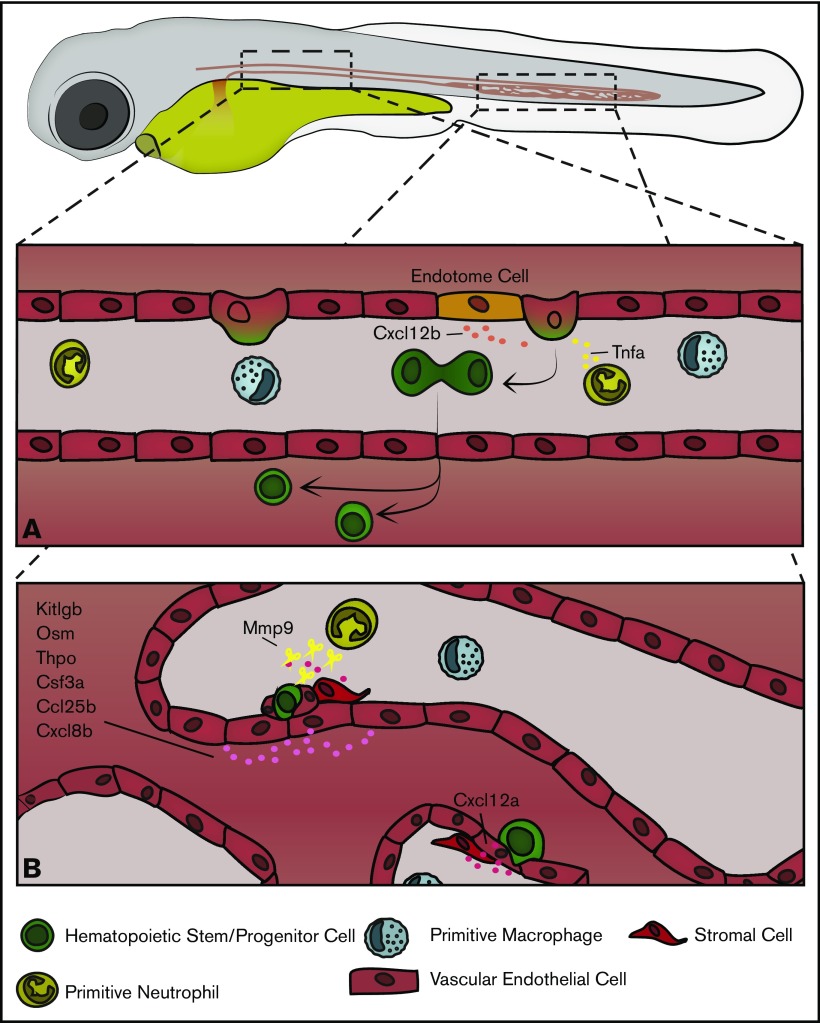
Beginning at around 26 hpf, zebrafish initiate definitive hematopoiesis with the emergence of HSCs that will sustain blood production for life. HSCs emerge from a subpopulation of endothelial cells in the ventral wall of the dorsal aorta. These “hemogenic endothelial cells” round up and bud off, transdifferentiating into primordial hematopoietic stem and progenitor cells (HSPCs) in an endothelial-to-hematopoietic transition.14,15 In zebrafish, new HSPCs emerge ventral to the aorta and must enter into circulation by transmigrating into the cardinal vein. HSPCs can be marked by expression of variety of genes including c-myb, cluster of differentiation 41 (cd41), and runt-related transcription factor 1 (runx1).16-18 The ventral dorsal aorta (VDA) is the functional equivalent mammalian aorta–gonad–mesonephros and comprises what may be termed the initiating hematopoietic niche, both conferring stem cell identity and supporting early maturation. Although only a temporary site of blood production, HSPCs emerging from the VDA interact with a complex milieu of local signals and cell types, occupy the subaortic mesenchyme for several hours, and divide before entering circulation. Critically, nearby vascular endothelial cells, somite-derived endothelial cells, as well as primitive macrophage and neutrophils signal to induce HSPC formation and facilitate their egress into circulation (Figure 1). HSPCs emerge at a rate of around 4.5 cells/h between 28 and 54 hpf and to ultimately give rise to 20 to 30 long-lived HSCs through the process.14,19
Figure 1.
An overview of embryonic hematopoiesis in zebrafish. (A) From 28 to 54 hpf HSPCs are born from endothelial cells in the ventral wall of the dorsal aorta and are induced by a variety of local signals, including Cxcl12b produced by somite-derived “endotome cells” in the dorsal aorta and Tnfa produced by primitive myeloid cells. In zebrafish, HSPCs bud off into the extravascular space ventral to the dorsal aorta, divide, and enter into circulation. (B) From 48 to 96 hpf HSPCs exit circulation and lodge in the CHT, where local signals, including Kitlgb, Osm, thrombopoietin, colony stimulating factor 3a, Ccl25b, Cxcl8b, and Cxcl12a, regulate their trafficking and expansion. Vascular endothelial cells remodel to form a pocket around resident HSPCs, and somite-derived stromal cells anchor HSPCs during CHT occupancy. Primitive neutrophils regulate HSPC egress through secretion of Mmp9, which cleaves locally produced Cxcl12a.
Caudal hematopoietic tissue
After emerging from the VDA, the nascent HSPCs travel to the primary site of embryonic hematopoiesis, the caudal hematopoietic tissue (CHT). Analogous to the mammalian fetal liver, the CHT is a vascular plexus in the ventral region of the tail between the caudal artery and cardinal vein.20 From approximately 48 to 96 hpf nascent HSPCs, following cues from resident endothelial and stromal cells, exit circulation by transmigrating through the vascular endothelium and settle on the abluminal face of the vasculature. HSPCs interface with a series of tissue-resident endothelial cells, stromal cells, and myeloid cells for several hours, expand, and exit into circulation (Figure 1). Cytokines in the CHT will induce both HSC self-renewal and differentiation to committed lymphoid progenitors, which later go on to populate the thymus.18,21 Initially, HSPCs populate more dorsal perivascular spaces in the CHT as sparse, individual cells, but over the course of ∼2 days HSPCs expand and migrate more ventrally as the vasculature of the CHT becomes less complex. By 4 to 5 days postfertilization (dpf) clusters of HSPCs occupy a more ventral region of the CHT concentrated along the cardinal vein, and the number of HSPCs approximately doubles.22
Kidney
Beginning at ∼4 dpf, HSCs begin to seed the adult niche: the kidney. The zebrafish kidney is a highly vascularized flat structure extending along the dorsal body cavity wall of the animal, formed from ventral mesoderm specified in gastrulation.23 The larval zebrafish pronephros contains 2 nephrons with glomeruli fused at the embryo midline, ventral to the dorsal aorta.24 Between 36 and 40 hpf, these glomerular primordia come into contact with the aorta, and begin to express vascular endothelial growth factor 2 and angiopoietin2.24-26 In turn, endothelial cells at the dorsal aorta begin capillary formation and invade the nascent kidney between 40 and 48 hpf.24 Kidney filtration activity begins upon vascularization and fully matures at ∼4 dpf.27 After this point, and especially beginning at 5 dpf, HSCs begin to localize to the pronephros between the tubules of the mesonephros, which eventually makes up the mature adult kidney.20 The kidney is the primary adult hematopoietic niche of zebrafish, akin to the mammalian bone marrow. Here, HSCs are regulated by a cytoarchitecture highly similar to the CHT and are protected from irradiation by an overlying layer of melanocytes.20,28 In the kidney, HSCs reside, self-renew, and differentiate to produce blood for the lifetime of the animal.29
Cells of the hematopoietic niche
Vascular endothelial cells
Vascular endothelial cells line the inside of the entire circulatory system in a single-cell layer and must be transited by HSCs entering or exiting the perivascular niche. Endothelial cells at sites of hematopoiesis are highly specialized to support blood development, and those taken from VDA, CHT, and kidney stroma are able to support hematopoiesis in vitro.30-32 Some endothelial cells in these regions have distinct sites of origin and transcriptional identity.
In the VDA, some endothelial cells are induced to become HSPCs, whereas others regulate this activity. Although not all the pathways regulating HSC induction are known, Notch1 signaling is ultimately critical for hematopoietic specification.33 This is regulated through noncanonical Wnt16 signaling in adjacent somites to induce expression of Notch ligands deltaC and deltaD. These then interact with Notch1 on the surface of vascular precursor cells as they migrate across the somite during formation of the dorsal aorta.34 This interaction ultimately activates a hematopoietic transcriptional program in specified hemogenic endothelium, which includes the transcription factors stem cell leukemia, gata binding protein 2, and runx1.33,35 Notch1 receptor expression in vascular precursors is also regulated by ectopic viral integration site-1 through pAKT signaling.36 Although most endothelial cells in the VDA are derived from a common set of precursors specified from ventral mesoderm in mid-gastrulation, a subset of endothelial cells are derived from a somitic population.23,37,38 These so called “endotome cells” are regulated by the homeobox gene mesenchyme homeobox 1 in the somite, but ultimately migrate to the dorsal aorta, where they are a source of C-X-C motif ligand 12b (cxcl12b) to induce HSC formation from hemogenic endothelium.
The CHT is composed of fenestrated endothelial cells that are loosely adhered to each other, which allows HSCs to squeeze between adjacent endothelial cells to enter and exit the extravascular space, and for gross morphological rearrangement as the embryo develops. CHT endothelial cells express many cytokines that support HSC expansion and differentiation. Kit ligand b (Kitlgb), thrombopoietin, oncostatin M, and colony stimulating factor 3a are all secreted because of a selective endothelial enrichment for the Microphthalmia-associated transcription factor family member: transcription factor EC (Tfec).21,39 Elevating these cytokines through tfec overexpression increases the number of HSPCs in the CHT, whereas tfec−/− embryos fail to maintain HSPCs after emergence from the VDA. Endothelial cell production of Kitlgb and Osm in the CHT directly signal to HSPCs to promote proliferation and inhibit lymphoid differentiation.21
Notably, CHT endothelial cells remodel in association with resident stromal cells to form a pocket around HSCs that have entered the fetal niche.18 This “endothelial cuddling” also occurs in the mammalian fetal liver and may serve to increase local concentration of signaling factors. The transcription factor Krüppel-like factor 6a was recently identified as a crucial mediator of CHT endothelial development and of HSPC lodgment and proliferation within the CHT. In Krüppel-like factor 6a mutants or morpholino knockdowns, CHT complexity and volume are decreased in conjunction with reduced numbers of HSPCs and differentiated blood cells. This is due to direct regulation of expression of chemokine signals chemokine (C-C motif) ligand 25b (ccl25b), and cxcl8b. Ccl25b expressed from endothelial cells interacts with the cognate receptor C-C motif receptor 7 on the surface of HSPCs to promote expansion and migration through the CHT.22 Similarly, CHT endothelial cells express both Cxcl8b and its receptor (Cxcr1) to increase HSPC residency time and the rate of HSPC division.40 This also induces higher expression of cxcl12a, which causes the volume of the CHT to increase, allowing for even greater expansion of the HSC pool.
Stromal cells
Mesenchymal stromal cells are distributed throughout the CHT underlying endothelial cells and may be marked by high expression of cxcl12a.18,41 Analogous to the “CXCL12-abundant reticular cells” found in the mammalian bone marrow,42 these cells contribute to HSPC guidance through the Cxcl12–Cxcr4 signaling axis. HSPC retention and hematopoietic expansion in the CHT is dependent upon resident stromal cells. In the oloca mutant, which has no CHT stromal cells, HSPCs can transiently home to the CHT but are not retained and cannot expand.43 After lodgment and transmigration through the endothelium, HSPCs come into contact with these stromal cells. During CHT residency and endothelial cuddling, stromal cells anchor HSPCs and orient their plane of division; the daughter cell more proximal to the stromal cell remains in place, whereas the daughter cell more distal exits the niche and enters into circulation.18
The nascent polypeptide-associated complex α subunit is required cell autonomously for CHT stromal cell development and survival. These stromal cells are derived from a ventral somitic epithelial population that undergoes an epithelial-to-mesenchymal transition and emigrate into the developing CHT between 22 and 36 hpf just as HSPCs are forming in the VDA.43 The somitic origin for hematopoietic niche stromal cells stands in contrast to the mammalian bone marrow, in which Nestin+ mesenchymal stromal cells are neural crest cell derivatives. It is still unclear what initiating signals induce the epithelial-to-mesenchymal transition and migration of CHT stromal cells. Similar stromal reticular cells have been identified in the kidney but have not been as closely studied.20
Macrophages and neutrophils
Myeloid cells produced in the primitive wave of hematopoiesis play a greater role than previously appreciated in shaping the emergence and expansion of HSPCs through tissue remodeling and inflammatory signaling, but they remain poorly understood. By 30 hpf, primitive macrophages, marked by macrophage expressed 1, are broadly distributed throughout the embryo, including the CHT, but have been specifically noted to infiltrate and patrol the VDA.44-46 This coincides with the initiation of the definitive hematopoietic program. When embryonic macrophages are ablated by liposome-encapsulated clodronate or through targeted expression of bacterial nitroreductase in the presence of metronidazole, there is a significant decrease in HSPCs present in the CHT, suggesting a role for macrophages either in HSPC formation at the VDA or in trafficking to the CHT.46 When pan-myeloid development is blocked with the proto-oncogene b morpholino, there are significantly reduced numbers of cd41+ HSPCs in the VDA at 48 hpf. Injection of the interferon regulatory factor 8 morpholino, which specifically blocks macrophage development and produces higher numbers of neutrophils, produces found higher numbers of cd41+ cells in the VDA at 48 hpf.47 However, when the interferon regulatory factor 8 morpholino was used in another study, the number of cd41+ cells in the CHT was largely unaffected.48 Taken together, these data highlight the still-unresolved roles of macrophages in the early hematopoietic niche; there may be macrophage activity at the VDA, which facilitates HSPC induction and entrance into circulation, or in the CHT, where macrophages could condition the niche or direct HSPC lodgment.
In approaching the roles of neutrophils in HSPC development, several groups have demonstrated roles for inflammatory signaling in the early hematopoietic niches. Signaling by the inflammatory cytokine tumor necrosis factor a (Tnfa) activates Notch1a through jagged1 expression in VDA endothelial cells to activate the hematopoietic program. Although broadly expressed at this stage, tnfa is significantly enriched in primitive neutrophils.47 Additionally, neutrophils express inflammation-responsive matrix metalloproteinases (mmps), which break down extracellular matrix proteins. In a recent study, Theodore et al49 demonstrated that mmp2 and mmp9 are required for HSPC egress from the VDA (mmp2), and CHT (mmp9), and chemical or genetic inhibition results in HSPC accumulation at the VDA subaortic mesenchyme or in the CHT extravascular space. Although mmp2 is broadly expressed in the embryo, mmp9 is highly enriched in primitive neutrophils and regulates levels of Cxcl12 in the CHT. Loss of mmp9 increases HSPC retention in the CHT and produces a hypovascular CHT.
Melanocytes
Recently, a role for pigmented melanocytes was uncovered as a structural component of the adult hematopoietic niche. Melanocytes, derived from migrating neural crest cells, form a UV-protective “umbrella” that covers the dorsal face of the developing kidney beginning at around 48 hpf.28 Zebrafish in the wild inhabit small, clear pools and are exposed to constant UV irradiation from above. Nonpigmented or anesthetized zebrafish oriented with the ventral face up had significantly reduced numbers of HSPCs after UV exposure compared with pigmented fish with dorsal kidney melanocytes. This melanocyte layer over the hematopoietic niche is found in all teleost fish and is present in tadpole stage frogs before hematopoiesis shifts to the bone marrow.28 This highlights the protective role of the niche with hematopoiesis naturally occurring in either densely protective bones or melanocyte shielded kidneys.
Comparison with the murine hematopoietic niche
The use of zebrafish to study HSCs and their niche complements work done in other vertebrate model systems. More specific cells have been enumerated in the murine bone marrow, but overall structures and cell types are shared with zebrafish. Because it is much more laborious to generate transgenic mouse lines, and extremely challenging to perform live imaging in mice, combined approaches using zebrafish and mice have been most successful in demonstrating niche cell function. For example, stromal and endothelial cell populations in the mouse bone marrow and stromal cells in the zebrafish CHT and kidney are not identical but play extremely similar roles producing cytokines, tethering HSCs, and responding to local metalloproteinases.18,21,39,49-54 There are some key differences between the murine and zebrafish niches. HSC birth from aortic endothelial cells occurs in the extravascular space in zebrafish vs the aortic lumen in mice,14,15,55 and there are cell types in the bone marrow niche that have not yet been identified yet in the zebrafish kidney niche including Nestin+ cells and cells of the sympathetic nervous system.56,57
Tools to investigate the stem cell niche
Uncovering regulatory elements of the hematopoietic niche to answer new questions in hematopoiesis and advance therapeutic efforts will require new tools that zebrafish are well-suited for. Heterogeneous behavior among HSCs in lineage contribution and proliferation remains poorly understood, but is generally thought to depend upon regulation by the niche, either in adulthood or during HSC formation.58-60 The flexibility of creating transgenic lines and the power of high resolution live imaging in zebrafish can be combined to approach this with the Zebrabow system.19,61 Zebrabow adopts Brainbow multispectral fluorescent labeling to zebrafish: a ubiquitous promoter drives expression of 3 fluorescent proteins (dTomato, Cyan Fluorescent Protein, Yellow Fluorescent Protein) flanked by unique lox site variants.62,63 Upon stochastic recombination by a tissue-specific Cre of choice, there is expression of 1 fluorophore. This multicolor cassette is duplicated multiple times throughout the genome, and each site undergoes independent recombination. The result is a unique set of color barcodes in each cell, which combine to make a unique composite color that are stably inherited and expressed in all daughter cells. This allows for clonal color labeling of any given population of cells, depending on the choice of Cre driver. Flow cytometry can be used to quantify color size distributions, and live imaging can be used to directly observe developmental events and long-term effects on distinct color clones. This system has already been used in zebrafish hematopoiesis to quantify the numbers of long-lived HSCs initially born in the VDA,19 but could be further used to track individual colors in the VDA or CHT and correlate long-term HSC behaviors with events in the developmental niche.
Similarly, high-powered microscopy could be combined with genomic and epigenetic data to uncover developmental regulation of niche cell types, which could then be related to effects on HSPC induction and expansion. With the rapid proliferation of fluorescent zebrafish reporter lines, lineage-specific gene expression and chromatin accessibility data can be easily collected and combined to generate lineage-specific enhancer/promoter elements. This type of epigenetic analysis has already been used to discover developmentally active enhancers in endothelial cells. When open regions of chromatin identified in ATAC-seq upstream of endothelial-specific genes are coupled to their cognate minimal promoter, they can be used to drive expression of fluorescent reporters under control of native regulatory elements.64 This approach functionally reveals the lineage-specific cis regulatory elements sought after in BAC transgenesis. Because these enhancers are so much smaller than BACS but retain all the information needed for cell-type specificity, they could, in principle, be analyzed to reveal which transcription factor binding sites are crucial to engage lineage-specific gene programs.
Conclusions
As highlighted here, studies of the developing hematopoietic system take great advantage of the strengths of the zebrafish to understand HSC microenvironments. Sites of developmental hematopoiesis in zebrafish are highly accessible and reveal novel cell–cell interactions and cell types. A combination of endothelial cells, stromal cells, primitive myeloid cells, and melanocytes make up the functional zebrafish hematopoietic niche. Produced and sheltered by its surroundings, the HSC is a powerful cell that may be manipulated more effectively as we better understand its environment in situ. Future efforts with new technologies will probe clonal dynamics of hematopoiesis and reveal mechanisms by which the niche regulates HSC behaviors; perturb the microenvironment, which induces HSC formation; and understand the networks that establish and regulate the niche itself. The therapeutic and basic science goals in hematopoietic research depend upon a better understanding of how the niche and stem cell work together.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2R24DK092760-04), National Heart, Lung, and Blood Institute (P01HL131477 and U01HL134812), Alex’s Lemonade Stand Fund (P01HL032262), The Edward P. Evans Foundation (R01CA103846-16A1), and Howard Hughes Medical Institute (1U54DK110805).
Authorship
Contribution: S.J.W. and L.I.Z. wrote the article and prepared figures.
Conflict-of-interest disclosure: L.I.Z. is a founder and stock holder of Fate Therapeutics, CAMP4 Therapeutics, and Scholar Rock. S.J.W. declares no competing financial interests.
Correspondence: Leonard I. Zon, Boston Children's Hospital, 1 Blackfan Circle, Karp 05211, Boston, MA 02115; e-mail: zon@enders.tch.harvard.edu.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1-2):7-25. [PubMed] [Google Scholar]
- 2.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075-1079. [DOI] [PubMed] [Google Scholar]
- 3.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91(3):335-344. [DOI] [PubMed] [Google Scholar]
- 4.Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46(1):65-72. [PubMed] [Google Scholar]
- 5.Benz C, Copley MR, Kent DG, et al. . Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 2012;10(3):273-283. [DOI] [PubMed] [Google Scholar]
- 6.Dykstra B, Kent D, Bowie M, et al. . Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1(2):218-229. [DOI] [PubMed] [Google Scholar]
- 7.Yu VW, Yusuf RZ, Oki T, et al. . Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell. 2016;167(5):1310-1322 e1317. [DOI] [PubMed] [Google Scholar]
- 8.Goessling W, North TE, Loewer S, et al. . Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidi N, Sacma M, Ständker L, et al. . Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J. 2017;36(10):1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimura R, Jha DK, Han A, et al. . Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017;545(7655):432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan KM, Fujimoto E, Grabher C, et al. . The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236(11):3088-3099. [DOI] [PubMed] [Google Scholar]
- 12.Thompson MA, Ransom DG, Pratt SJ, et al. . The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197(2):248-269. [DOI] [PubMed] [Google Scholar]
- 13.Clements WK, Traver D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat Rev Immunol. 2013;13(5):336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112-115. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HF, Traver D, Zhu H, et al. . Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106(12):3803-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North TE, Goessling W, Walkley CR, et al. . Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamplin OJ, Durand EM, Carr LA, et al. . Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160(1-2):241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henninger J, Santoso B, Hans S, et al. . Clonal fate mapping quantifies the number of haematopoietic stem cells that arise during development. Nat Cell Biol. 2017;19(1):17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murayama E, Kissa K, Zapata A, et al. . Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25(6):963-975. [DOI] [PubMed] [Google Scholar]
- 21.Mahony CB, Pasche C, Bertrand JY. Oncostatin M and Kit-ligand control hematopoietic stem cell fate during zebrafish embryogenesis. Stem Cell Reports. 2018;10(6):1920-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue Y, Lv J, Zhang C, Wang L, Ma D, Liu F.. The vascular niche regulates hematopoietic stem and progenitor cell lodgment and expansion via klf6a-ccl25b. Dev Cell. 2017;42(4):349-362. [DOI] [PubMed] [Google Scholar]
- 23.Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108(4):581-594. [DOI] [PubMed] [Google Scholar]
- 24.Drummond IA, Majumdar A, Hentschel H, et al. . Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125(23):4655-4667. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24(3-4):220-229. [DOI] [PubMed] [Google Scholar]
- 26.Pham VN, Roman BL, Weinstein BM. Isolation and expression analysis of three zebrafish angiopoietin genes. Dev Dyn. 2001;221(4):470-474. [DOI] [PubMed] [Google Scholar]
- 27.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires nephrin, podocin and the FERM domain protein mosaic eyes. Dev Biol. 2005;285(2):316-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapp FG, Perlin JR, Hagedorn EJ, et al. . Protection from UV light is an evolutionarily conserved feature of the haematopoietic niche. Nature. 2018;558(7710):445-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4(12):1238-1246. [DOI] [PubMed] [Google Scholar]
- 30.Campbell C, Su T, Lau RP, et al. . Zebrafish embryonic stromal trunk (ZEST) cells support hematopoietic stem and progenitor cell (HSPC) proliferation, survival, and differentiation. Exp Hematol. 2015;43(12):1047-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stachura DL, Reyes JR, Bartunek P, Paw BH, Zon LI, Traver D. Zebrafish kidney stromal cell lines support multilineage hematopoiesis. Blood. 2009;114(2):279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf A, Aggio J, Campbell C, et al. . Zebrafish caudal haematopoietic embryonic stromal tissue (CHEST) cells support haematopoiesis. Sci Rep. 2017;7(1):44644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19(19):2331-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474(7350):220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa M, Ichikawa M, Kumano K, et al. . AML1/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood. 2006;108(10):3329-3334. [DOI] [PubMed] [Google Scholar]
- 36.Konantz M, Alghisi E, Müller JS, et al. . Evi1 regulates Notch activation to induce zebrafish hematopoietic stem cell emergence. EMBO J. 2016;35(21):2315-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gore AV, Monzo K, Cha YR, Pan W, Weinstein BM. Vascular development in the zebrafish. Cold Spring Harb Perspect Med. 2012;2(5):a006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen PD, Hollway GE, Sonntag C, et al. . Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature. 2014;512(7514):314-318. [DOI] [PubMed] [Google Scholar]
- 39.Mahony CB, Fish RJ, Pasche C, Bertrand JY. tfec controls the hematopoietic stem cell vascular niche during zebrafish embryogenesis. Blood. 2016;128(10):1336-1345. [DOI] [PubMed] [Google Scholar]
- 40.Blaser BW, Moore JL, Hagedorn EJ, et al. . CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J Exp Med. 2017;214(4):1011-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glass TJ, Lund TC, Patrinostro X, et al. . Stromal cell-derived factor-1 and hematopoietic cell homing in an adult zebrafish model of hematopoietic cell transplantation. Blood. 2011;118(3):766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977-988. [DOI] [PubMed] [Google Scholar]
- 43.Murayama E, Sarris M, Redd M, et al. . NACA deficiency reveals the crucial role of somite-derived stromal cells in haematopoietic niche formation. Nat Commun. 2015;6(1):8375. [DOI] [PubMed] [Google Scholar]
- 44.Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117(4):e49-e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiau CE, Kaufman Z, Meireles AM, Talbot WS. Differential requirement for irf8 in formation of embryonic and adult macrophages in zebrafish. PLoS One. 2015;10(1):e0117513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Travnickova J, Tran Chau V, Julien E, et al. . Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat Commun. 2015;6(1):6227. [DOI] [PubMed] [Google Scholar]
- 47.Espín-Palazón R, Stachura DL, Campbell CA, et al. . Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159(5):1070-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Esain V, Teng L, et al. . Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 2014;28(23):2597-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theodore LN, Hagedorn EJ, Cortes M, et al. . Distinct roles for matrix metalloproteinases 2 and 9 in embryonic hematopoietic stem cell emergence, migration, and niche colonization. Stem Cell Reports. 2017;8(5):1226-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153(6):1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heissig B, Hattori K, Dias S, et al. . Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heissig B, Werb Z, Rafii S, Hattori K. Role of c-kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003;90(4):570-576. [DOI] [PubMed] [Google Scholar]
- 53.Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91(12):4523-4530. [PubMed] [Google Scholar]
- 54.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116-120. [DOI] [PubMed] [Google Scholar]
- 56.Katayama Y, Battista M, Kao WM, et al. . Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407-421. [DOI] [PubMed] [Google Scholar]
- 57.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. . Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crisan M, Kartalaei PS, Vink CS, et al. . BMP signalling differentially regulates distinct haematopoietic stem cell types [published correction appears in Nat Commun. 2015;6:8793]. Nat Commun. 2015;6(1):8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crisan M, Solaimani Kartalaei P, Neagu A, et al. . BMP and hedgehog regulate distinct AGM hematopoietic stem cells ex vivo. Stem Cell Reports. 2016;6(3):383-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinho S, Marchand T, Yang E, Wei Q, Nerlov C, Frenette PS. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell. 2018;44(5):634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan YA, Freundlich T, Weissman TA, et al. . Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development. 2013;140(13):2835-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Livet J, Weissman TA, Kang H, et al. . Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450(7166):56-62. [DOI] [PubMed] [Google Scholar]
- 63.Mosimann C, Kaufman CK, Li P, Pugach EK, Tamplin OJ, Zon LI. Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development. 2011;138(1):169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quillien A, Abdalla M, Yu J, Ou J, Zhu LJ, Lawson ND. Robust Identification of Developmentally Active Endothelial Enhancers in Zebrafish Using FANS-Assisted ATAC-Seq. Cell Reports. 2017;20(3):709-720. [DOI] [PMC free article] [PubMed] [Google Scholar]