Abstract
Global developmental delay (GDD) and intellectual disability (ID) are common concerns in the paediatric setting. Etiologies of both conditions are highly heterogeneous. The American Academy of Pediatrics, the American Academy of Neurology and the British Columbia-based Treatable Intellectual Disability Endeavor (TIDE) protocol have each proposed multitiered investigations of GDD/ID to guide physicians toward an understanding of etiology that optimizes therapeutic yield. This statement provides a framework for the clinical investigation of GDD/ID in children, along with an updated protocol for Canadian physicians to follow in the etiological investigation of GDD/ID. The revised protocol is based on current knowledge and existing guidelines. Key elements of investigation include formal vision and hearing testing, chromosomal microarray, Fragile-X DNA testing and first-tier testing for treatable inborn errors of metabolism. Brain imaging is recommended in the presence of specific neurological findings.
Keywords: Chromosome microarray, Global developmental delay, Intellectual disability, IEM
Global developmental delay (GDD) and intellectual disability (ID) affect up to three per cent of the paediatric population (1,2). The diagnosis of GDD is limited to children younger than 5 years old, but these children often evolve to meet diagnostic criteria for ID and probably represent the same population (Table 1). Because the etiological diagnoses of GDD and ID overlap, it is natural that investigations in pursuit of a definitive diagnosis for either disorder are similar. Early detection is crucial for initiating rehabilitation services and treatment as soon as possible. The etiology of GDD/ID can be identified in many cases (40% to 80%) (3). Therefore, it is essential that general paediatricians in Canada coordinate the etiological evaluation of this patient population with subspecialists, using an integrative approach.
Table 1.
Diagnostic criteria
| Global developmental delay |
|---|
| • Significant delay (at least 2 SDs below the mean with standardized tests) in at least two developmental domains from the following: |
| Gross or fine motor |
| Speech/language |
| Cognition |
| Social/personal |
| Activities of daily living |
| Reserved for children <5 years old |
| Intellectual disability (intellectual developmental disorder)*: |
| The following three criteria must be met: |
| 1. Deficits in intellectual functions, such as reasoning, problem-solving, planning, abstract thinking, judgment, academic learning and learning from experience, confirmed by both clinical assessment and individualized, standardized intelligence testing. |
| 2. Deficits in adaptive functioning that result in failure to meet developmental and socio-cultural standards for personal independence and social responsibility. Without ongoing support, the adaptive deficits limit functioning in one or more activities of daily life, such as communication, social participation and independent living, across multiple environments, such as home, school, work and community. |
| 3. Onset of intellectual and adaptive deficits during the developmental period. |
A diagnosis is critical because it allows for (2):
Timely initiation of causal treatment or supportive management,
Prevention of complications,
Improved prognostication,
Accurate genetic counselling regarding recurrence risk and prenatal/preimplantation genetic diagnosis, when indicated,
Better access to services in the community, and
Resolution of a diagnostic odyssey or (better still) avoidance of inappropriate, costly and traumatizing tests.
The goal of this statement is to provide a framework for etiological investigation of GDD/ID in children that helps clinicians to implement evidence-based guidelines. We also propose a stepwise approach suited for clinical practice in Canada, always understanding that it must be tailored to the specific clinical context and availability of local resources.
ETIOLOGY OF GDD AND ID
The probability of finding an etiological diagnosis varies in different studies and according to the kind of investigation and the severity of GDD/ID. In severe ID (as defined in DSM-5), an identifiable cause was detected in up to 80% of cases (4,5). The yield appears to be lower in mild ID, with a cause identified in approximately 24% of cases (6). The categories of etiological diagnosis and proportion of diagnostic yield for the most common diagnoses are presented in Table 2.
Table 2.
Causes of global developmental delay/intellectual disability
| Broad category | Possible causes | Proportion of diagnostic yield* |
|---|---|---|
| Prenatal intrinsic | Genetic | Up to 47% |
| Central nervous system malformations | Up to 28% | |
| Metabolic | ||
| Prenatal extrinsic | Teratogens/toxins (drugs of abuse, medications, etc.) | Up to 21% |
| Infections | ||
| Perinatal | Asphyxia | Up to 55% |
| Prematurity | ||
| Neonatal complications | ||
| Postnatal | Neglect/psychosocial environment | Up to 11% |
| Infections | ||
| Trauma | ||
| Toxins |
Data taken from ref. (3).
*Percentage of total cases of GDD/ID with an identified etiologic diagnosis who fall into this specific category.
Etiological investigation
Algorithms recommended by the American Academy of Pediatrics (AAP) (2), the American Academy of Neurology (AAN) (4) and the Treatable Intellectual Disability Endeavour (TIDE) protocol (5) are intended to simplify investigation of GDD/ID by limiting tests that are time-consuming or not clinically relevant and to promote efficient use of limited health care resources.
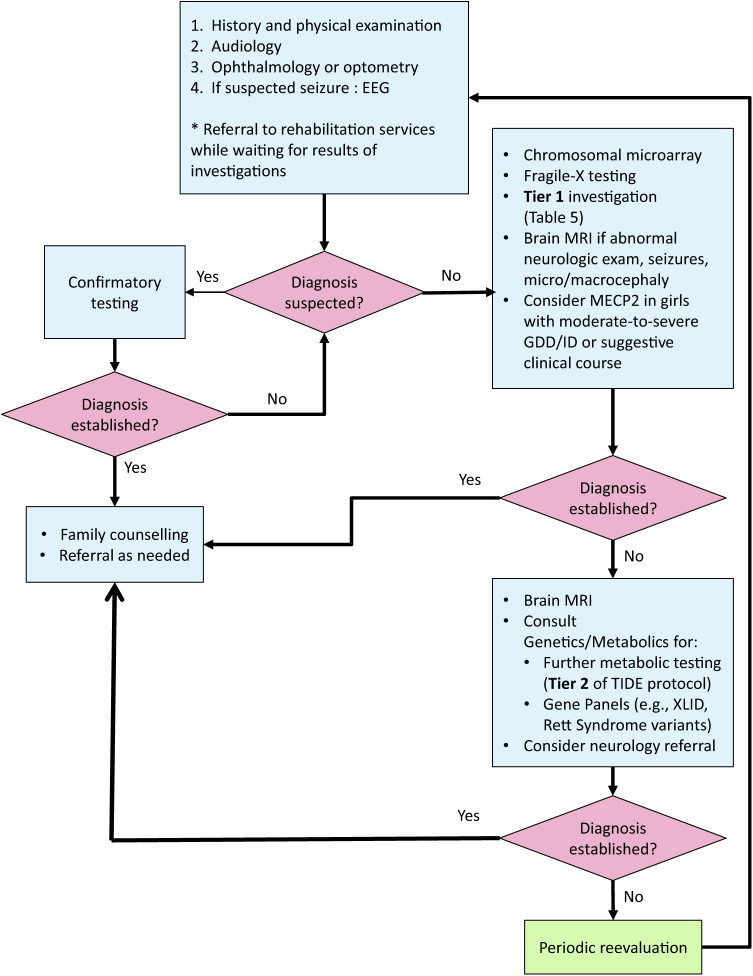
Each algorithm was developed to screen for the most common or treatable etiologies first. By contrast, other pathways propose an approach based on checklists and likelihood ratio models, stopping investigation when the clinician feels that it would not alter outcome, even without a diagnosis (3). One important ‘clinical pearl’ is to look for clinical characteristics pointing toward a specific etiology and order testing for that diagnosis first. When no apparent cause can be identified, a stepwise approach—conducted in collaboration with a geneticist—is recommended, with paediatricians leading the investigation whenever possible. See Figure 1 for a suggested approach to testing.
Figure 1.
Algorithm for investigating global developmental delay or intellectual disability. Figure available in colour online. EEG Electroencephalogram; GDD Global developmental delay; ID Intellectual disability; MRI magnetic resonance imaging; XLID X-linked intellectual disability
History and physical examination
In one recent review, an etiological diagnosis based on history and physical examination was found in 12.5% to 38.6% of cases (3), confirming that these steps mark the most important phase of investigation (2,3,7,8). A three-generation family history, a psychosocial history, detailed prenatal and birth histories and the timing of major milestones should be recorded as accurately as possible (Table 3). A neurodevelopmental assessment, including current developmental level and a systematic physical examination (Table 3) can either point toward a specific diagnosis or guide laboratory testing. When a specific etiology is suspected at that point or when a family history of disorder associated with GDD/ID has been established, specific testing for this disorder should be ordered first (Figure 1).
Table 3.
History and physical and neurodevelopmental exams
| History | Physical and neurodevelopmental exams |
|---|---|
| Family history | Physical exam |
| Three-generations review, looking for: | • Growth parameters |
| • Recurrent miscarriages | • Head shape |
| • Birth defects | • Fontanelle |
| • Infant deaths | • Cutaneous stigmata |
| • GDD/ID | • Spine |
| • Neurologic conditions | • Heart abnormalities |
| • Genetic conditions | • Abdomen check for organomegaly |
| • Ethnic background | • Limb abnormalities |
| • Consanguinity | • Genital abnormalities |
| Psychosocial history | Neurodevelopmental exam |
| • Parent language, education, employment | • Neurological exam |
| • Parental drug/alcohol abuse | • Congenital abnormalities |
| • Child care arrangements | • Dysmorphic features |
| • History of abuse or neglect and involvement of child protective services | • Current developmental level |
| Prenatal history | |
| • Prenatal ultrasound | |
| • Screening for fetal aneuploidy | |
| • Maternal diabetes or hypertension | |
| • Infections | |
| • Exposure to medications or toxins | |
| Birth history | |
| • Weight and height | |
| • Head circumference | |
| • Apgar score | |
| • Length of hospitalization | |
| Red flags suggestive of inborn errors of metabolism | |
| • Table 4 | |
| Developmental milestones | |
| • Regression or lack of milestones | |
| • Timing of parents’ first concern |
GDD Global developmental delay; ID Intellectual disability
Sensory evaluation
According to the AAN (4) and other reviews (5,7,9), children with GDD/ID should be referred for a formal assessment of their vision (optometry or ophthalmology) and hearing. Identifying a sight or hearing deficit can alter management course and guide further investigation.
Genetic testing
Chromosome microarray
The use of chromosome microarray (CMA, also referred to as comparative genomic hybridization or CGH) as a first-line investigation in children with GDD/ID, is endorsed by the AAP, the AAN, the International Standard Cytogenetic Array and the American College of Medical Genetics (2,4,9,10). It is the single test with the best diagnostic yield (7,8) (at 8% to 20%), exceeded in efficacy only by clinical evaluation from an experienced clinician specializing in GDD/ID (2,4,11). The variation in yield reported in different studies can be explained by the absence of stratification for severity and the presence of other anomalies. Therefore, it remains uncertain whether CMA is useful in mild (according to DSM-5) familial ID. Those patients could simply represent the lower percentiles of the IQ Gauss curve and the etiologies are often multifactorial. When multiple congenital anomalies are present, the American College of Medical Genetics still recommends CMA as a first-line investigation, unless a specific diagnosis is being considered (10).
Karyotype
The use of standard karyotyping is not recommended as a first-line test, because its sensitivity is less than one-half that of CMA in children diagnosed with GDD/ID. The resolution of conventional chromosomal analysis is 5 Mb to 10 Mb compared with 0.05 Mb to 0.1 Mb with CMA. However, karyotyping is recommended instead of CMA for clinically suspected aneuploidy (e.g., Turner syndrome, trisomy 21) or a family history of chromosomal rearrangements or multiple spontaneous abortions (4,12). For the latter scenario, parental chromosome karyotyping should be ordered first.
Fragile X DNA testing
For children with ID, Fragile X is the most common genetic cause, representing 2% to 6% of affected boys and 1% to 4% of affected girls. Because the clinical phenotype is often nonspecific in infants and young children with Fragile X, AAP and AAN guidelines both recommend that Fragile X DNA (FMR1) testing be considered as part of first-line investigation for boys and girls with GDD/ID as defined in the DSM-5 (1,2,4,9,12,13). Panels for X-linked ID exist but should only be considered for families with two or more affected males. They should be guided by a geneticist (2).
Rett syndrome testing
Rett syndrome is found in 1.5% of girls with moderate-to-severe ID (2). According to the AAP and the AAN, MECP2 molecular analysis should be ordered when characteristic symptomatology is present (i.e., initially normal development followed by loss of speech and purposeful hand use, stereotypical hand movement, gait abnormalities) or for moderately-to-severely affected girls (2,4).
Whole-exome or -genome sequencing
Whole-exome sequencing permits analysis of coding regions for known genes and the identification of causal mutations in up to 40% of patients with severe ID (14). This relatively new technique is becoming clinically accessible at lower cost in some regions of Canada. Variations of unknown significance are still a challenge and need to be interpreted with caution. Given these limitations, exome or genome sequencing is not actually recommended for primary care physicians but may become a first-line investigation in the near future. Use of this test by medical geneticists in moderate-to-severe ID or in syndromic cases is endorsed by the Canadian College of Medical Geneticists (15).
Metabolic workup
Red flags suggestive of an inborn error of metabolism (IEM) are listed in Table 4. Even if these findings, when present, raise the diagnostic yield of a metabolic workup, some IEMs present in a more subtle manner (5). In 2011, the AAN recommended that metabolic testing be performed only in the presence of strong clinical suspicion, in the absence of neonatal screening or after genetic testing and neuroimaging have not been diagnostic (4). As Canada does not have a universal newborn screening panel for hereditary disorders, neonatal screening programs vary among provinces/territories. Even with an effective screening program, some IEMs are easy to miss (2,5).
Table 4.
Red flags suggestive of inborn errors of metabolism
| • Family history of IEM or developmental disorder or unexplained neonatal or sudden infant death |
| • Consanguinity |
| • Intrauterine growth retardation |
| • Failure to thrive |
| • Head circumference or stature growth abnormality (>2 SD above or under the mean) |
| • Recurrent episodes of vomiting, ataxia, seizures, lethargy, coma |
| • History of being severely symptomatic and needing longer to recover with benign illnesses (e.g., upper respiratory tract infection) |
| • Unusual dietary preferences (e.g., protein or carbohydrate aversion) |
| • Regression in developmental milestones |
| • Behavioural or psychiatric problems (e.g., psychosis at a young age) |
| • Movement disorder (e.g., dystonia) |
| • Facial dysmorphism (e.g., coarse facial features) |
| • Organomegaly |
| • Severe hypotonia |
| • Congenital nonfacial anomalies |
| • Sensory deficits, especially if progressive (e.g., cataracts, retinopathy) |
| • Noncongenital progressive spine deformities |
| • Neuro-imaging abnormalities |
Data taken from ref. (5).
IEM Inborn error of metabolism; SD Standard deviation.
While rapid access to a clinical geneticist or metabolic specialist for an evaluation identifying the most probable IEM would be ideal, it is not a reality in most of Canada. Also striking is that as much as two-thirds of children with GDD/ID have no recognizable pattern of symptoms pointing toward a specific diagnosis. Nonspecificity often precludes the timely identification of a potentially treatable disorder, especially in late-onset disorders or in milder cases, where complete symptomatology has not developed. The typical metabolic workup (lactate, ammonia, chromatography of plasma amino acids and urinary organic acids) has a diagnostic yield of less than 1% to 5% (7), therefore supporting testing only when clinical red flags are present. However, previous studies were designed to identify an etiological yield and ignored the ‘therapeutic yield’ (i.e., the identification of a treatable disorder). Series with a more extended metabolic workup revealed a yield of more than 5% (5). It is also known that many treatable causes of GDD/ID do not present with developmental regression (5). One Canadian initiative from the B.C. Children’s Hospital (5), based on a review of the literature (16,17) identifying 89 IEMs amenable to treatment (17), aims to identify diseases before they become severe or irremediable complications develop. This protocol proposes a two-tiered algorithm. Tier 1 comprises a group of tests capable of identifying at least three IEMs, along with being readily accessible, minimally invasive and economical (in Vancouver, the whole test group costs about $528).
First-tier tests can identify 60% of currently known treatable IEMs causing ID. TreatableID.org is a web app (www.treatable-id.org) containing an algorithm that is regularly updated and describes 81 treatable IDs by their biochemical defects, diagnostic tests, clinical features and treatment modalities (17). The algorithm developers recommend tier-1 testing before genetic testing and neuroimaging, emphasizing the treatable nature of the disorders included and the relative urgency to identify them. The AAP recommends considering a metabolic workup at the same time or soon after CMA and Fragile X DNA testing (2). Tier-1 content is the same for both groups, except for the addition of copper and ceruloplasmin in the TIDE protocol. The AAN adds basic metabolic tests that can guide further testing: blood sugar, blood gas, lactate and creatine kinase. Table 5 and Figure 1 summarize first-tier laboratory investigations that should be ordered for all patients whose GDD/ID presents without a recognizable constellation of symptoms. An evaluation or a discussion with a metabolics specialist should be considered in the presence of red flags to tailor the laboratory investigation to that specific patient.
Table 5.
Tier-1 laboratory investigations for unexplained GDD/ID
| Blood* | Urine* |
|---|---|
| • Complete blood count | • Organic acids |
| • Glucose | • Creatine metabolites |
| • Blood gas | • Purines, pyrimidines |
| • Urea, creatinine | • Glycosaminoglycans |
| • Electrolytes (to calculate anion gap) | |
| • AST, ALT | |
| • TSH | |
| • Creatine kinase | |
| • Ammonia | |
| • Lactate | |
| • Amino acids | |
| • Acylcarnitine profile, carnitine (free and total) | |
| • Homocysteine | |
| • Copper, ceruloplasmin** | |
| • Biotinidase*** | |
| • Ferritin, vitamin B12 when dietary restriction or pica are present | |
| • Lead level when risk factors for exposure are present |
ALT Alanine aminotransferase; AST Aspartate aminotransferase; GDD Global developmental delay; ID Intellectual disability; TSH Thyroid-stimulating hormone.
*Perform testing after 4 h to 8 h of fasting. **Recommended tier-1 test in the TIDE protocol, but not by AAP, AAN. Consider as a first-line investigation when hepatomegaly, dystonia, abnormal liver function findings are present. ***Clinical expert recommendation only. Consider biotinidase testing when severe hypotonia, seizures are present.
ADDITIONAL INVESTIGATIONS
Thyroid testing
Hypothyroidism is a common, reversible cause of GDD/ID, with an incidence of approximately 1 out of 3,500 live births. Many study authors recommend screening for thyroid function (9), but the AAN states that the test does not need to be repeated when newborn screening is present (4). It is included in tier 1 (Table 5) whether or not newborn screening is performed, such that acquired cases and hypothyroidism cases of hypothalamic or pituitary origin are not missed.
Iron, vitamin B12
An Australian group (9) recommends including complete blood count, ferritin and vitamin B12 in the initial workup of children with GDD/ID, especially when there is a history of pica or feeding restrictions. Iron deficiency anemia is an easily identifiable and treatable cause of altered development.
Lead
Lead poisoning can affect mental and physical development severely, especially in children younger than 5 years of age, leading to conditions such as autism spectrum disorder, loss of milestones (particularly related to language) and encephalopathy (18). The AAN is the only association to recommend lead level dosing in children with risk factors for exposure.
Testing for congenital infections
One study (9) suggests evaluating for congenital infections (TORCH: toxoplasmosis, others, rubella, CMV, herpes) when neurological anomalies, microcephaly, hearing and/or vision loss are present. Consider consulting with infectious disease specialists whenever a congenital infection is suspected.
Neuroimaging
Neuroimaging studies, including computed tomography or magnetic resonance imaging (MRI) reveal nonspecific abnormalities in approximately 30% of children with GDD/ID ([6], anywhere between 2% and 80%, depending on the study), but neuroimaging contributes to understanding the etiology underlying GDD/ID in only 0.2% to 2.2% of cases (2). The diagnostic yield for neuroimaging improves when an abnormal neurological examination, seizures or macro- or microcephaly are present. MRI is preferred to computed tomography because it is more sensitive for identifying clinically significant structural abnormalities and anomalies related to myelination and neuronal migration (2,4,9). Because sedation is often required to perform an MRI and finding an abnormality rarely leads to an etiological diagnosis, the AAP does not recommend neuroimaging as a routine investigation for children with GDD/ID. While the AAN recommends performing an MRI on all patients when chromosomal microarray, Fragile X testing and MECP2 (if indicated) have been inconclusive (4), others recommend this test only when neurological findings are present (9). According to expert opinion, a brain MRI with spectroscopy is indicated in all cases of intractable epilepsy or developmental regression.
Electroencephalogram
Uncontrolled epilepsy or epileptic syndromes, such as Landau-Kleffner syndrome, can be associated with developmental delays or regression. Seizures are a common symptom of IEMs. An electroencephalogram is justified when there is clinical suspicion of seizures, speech regression or neurodegenerative disorder (9).
A testing algorithm
A stepwise approach, based on the AAP’s 2014 policy statement, AAN’s 2011 guidelines and the TIDE protocol, with some modifications arising from the literature and expert consensus, is outlined above (Figure 1).
SUMMARY
GDD and ID are common disorders in children, and paediatricians are often involved in the etiological workup needed for diagnosis and next steps. Even if early identification and stimulation are of paramount importance, establishing an etiological diagnosis can help relieve family stress, limit invasive and inappropriate testing, guide prognosis and, in some cases, alter management and treatment and prevent complications. ‘Therapeutic yield’ is gaining on pure diagnostics as grounds for testing in this rapidly evolving field, and children with suspected GDD/ID are sure to benefit from the newer approaches described here.
RECOMMENDATIONS
The following recommendations are based on evidence-based clinical practice guidelines and expert opinion.
History and physical examination are still the best first steps for establishing a diagnosis and should be systematically conducted for each child with suspected global developmental delay (GDD) and intellectual disability (ID). When a more specific diagnosis is suspected following clinical evaluation, investigation to confirm that etiology should be ordered first.
When a specific diagnosis is not suspected following clinical evaluation, consider a stepwise approach to investigation. The scope of investigation will depend on paediatric experience, the accessibility of subspecialists and the availability of resources.
To promote an evidence-based approach to evaluating children with GDD/ID, coordinating physician efforts with testing at provincial/territorial or regional referring centres is essential.
Formal vision and hearing testing is critical for all patients with suspected GDD/ID.
When no etiological diagnosis has been identified following history and physical examination, Fragile X, chromosomal microarray, Tier-1 metabolic testing, +/- brain imaging is recommended. If the diagnosis is not established, consider consultation with genetics/metabolic specialist.
Chromosomal microarray and Fragile X DNA testing are first-line investigations for children with unexplained GDD/ID.
Evidence supports Tier-1 (Table 5) testing for treatable inborn errors of metabolism (IEMs) in children with unexplained GDD/ID, even when clinical red flags are absent and a normal newborn screen has been obtained.
Brain imaging is recommended as a first-line investigation for patients with microcephaly, macrocephaly, seizures or abnormal neurological findings. For others, imaging may be postponed until first-line genetic and metabolic investigations have been performed. Consider the risks and benefits of sedation in each case. Magnetic resonance imaging (MRI) is the modality of choice.
Order lead level and iron studies for children at risk.
Whole-exome or -genome sequencing may be indicated in the clinical setting in future, when these tests are more readily available
Acknowledgements
This position statement has been reviewed by the Community Paediatrics Committee and the Early Years Task Force of the Canadian Paediatric Society.
All Canadian Paediatric Society position statements and practice points are reviewed regularly and revised as needed. Consult the Position Statements section of the CPS website www.cps.ca/en/documents for the most current version. Retired statements are removed from the website.
CANADIAN PAEDIATRIC SOCIETY MENTAL HEALTH AND DEVELOPMENTAL DISABILITIES COMMITTEE
Members: Debra Andrews MD (Chair), Stacey A. Bélanger MD (past Chair), Alice Charach MD, Brenda Clark MD (past member), Mark Feldman MD (Board Representative), Benjamin Klein MD, Daphne Korczak MD (past member), Oliva Ortiz-Alvarez MD
Liaisons: Sophia Hrycko MD, Canadian Academy of Child and Adolescent Psychiatry; Angie Ip MD, CPS Developmental Paediatrics Section; Aven Poynter MD, CPS Mental Health Section
Principal authors: Stacey A. Bélanger MD PhD, Joannie Caron MD
References
- 1. American Psychiatric Association. Intellectual disabilities. In: Diagnostic and Statistical Manual of Mental Disorders, 5th edn Washington, D.C: American Psychiatric Publishing, 2013. [Google Scholar]
- 2. Moeschler JB, Shevell M; Committee on Genetics Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics 2014;134(3):e903–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jimenez-Gomez A, Standridge SM. A refined approach to evaluating global developmental delay for the international medical community. Pediatr Neurol 2014;51(2):198–206. [DOI] [PubMed] [Google Scholar]
- 4. American Academy of Neurology. Evaluation of the Child with Global Developmental Delay 2011. www.aan.com/guidelines (Accessed March 17, 2017).
- 5. van Karnebeek CD, Stockler-Ipsiroglu S. Early identification of treatable inborn errors of metabolism in children with intellectual disability: The Treatable Intellectual Disability Endeavor protocol in British Columbia. Paediatr Child Health 2014;19(9):469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaffer LG; American College of Medical Genetics Professional Practice and Guidelines Committee American College of Medical Genetics guideline on the cytogenetic evaluation of the individual with developmental delay or mental retardation. Genet Med 2005;7(9):650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tirosh E, Jaffe M. Global developmental delay and mental retardation–A pediatric perspective. Dev Disabil Res Rev 2011;17(2):85–92. [DOI] [PubMed] [Google Scholar]
- 8. Wong VC, Chung B. Value of clinical assessment in the diagnostic evaluation of global developmental delay (GDD) using a likelihood ratio model. Brain Dev 2011;33(7):548–57. [DOI] [PubMed] [Google Scholar]
- 9. Silove N, Collins F, Ellaway C. Update on the investigation of children with delayed development. J Paediatr Child Health 2013;49(7):519–25. [DOI] [PubMed] [Google Scholar]
- 10. Manning M, Hudgins L; Professional Practice and Guidelines Committee, American College of Medical Genetics Array-based Technology and Recommendations for Utilization in Medical Genetics Practice for Detection of Chromosomal Abnormalities 2010. www.acmg.net/StaticContent/PPG/Array_based_technology_and_recommendations_for.13.pdf (Accessed March 17, 2017).
- 11. Sherr EH, Michelson DJ, Shevell MI, Moeschler JB, Gropman AL, Ashwal S. Neurodevelopmental disorders and genetic testing: current approaches and future advances. Ann Neurol 2013;74(2):164–70. [DOI] [PubMed] [Google Scholar]
- 12. Flore LA, Milunsky JM. Updates in the genetic evaluation of the child with global developmental delay or intellectual disability. Semin Pediatr Neurol 2012;19(4):173–80. [DOI] [PubMed] [Google Scholar]
- 13. Hersh JH, Saul RA; Committee on Genetics Health supervision for children with Fragile X syndrome. Pediatrics 2011;127(5):994–1006. [DOI] [PubMed] [Google Scholar]
- 14. Wright CF, McRae JF, Clayton S, et al. Making new genetic diagnoses with old data: iterative reanalysis and reporting from genome-wide data in 1,133 families with developmental disorders. Genet Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boycott K, Hartley T, Adam S, et al. ; Canadian College of Medical Geneticists. The clinical application of genome-wide sequencing for monogenic diseases in Canada: Position statement of the Canadian College of Medical Geneticists. J Med Genet 2015;52(7):431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Karnebeek CD, Stockler S. Treatable inborn errors of metabolism causing intellectual disability: A systematic literature review. Mol Genet Metab 2012;105(3):368–81. [DOI] [PubMed] [Google Scholar]
- 17. van Karnebeek CD, Shevell M, Zschocke J, Moeschler JB, Stockler S. The metabolic evaluation of the child with an intellectual developmental disorder: Diagnostic algorithm for identification of treatable causes and new digital resource. Mol Genet Metab 2014;111(4):428–38. [DOI] [PubMed] [Google Scholar]
- 18. Lowry JA. Childhood Lead Poisoning: Clinical Manifestations and Diagnosis 2016. www.uptodate.com/contents/childhood-lead-poisoning-clinical-manifestations- and-diagnosis?source=search_result&search=Childhood+lead+poisoning&selectedTitle=1%7E150 (Accessed March 17, 2017).