Abstract
Aim:
SSAT-1 is an enzyme that plays a critical role in cell growth. Amantadine, a FDA-approved antiviral drug, is a substrate for SSAT-1. The utility of amantadine as an agent to demonstrate elevated SSAT-1 activity linked to cancer was conducted.
Results:
High levels of SSAT-1 expression were measured in tumor human cell lines, and in breast, prostate and lung tumor tissue. An increase in the urinary levels of acetylated amantadine in cancer patients was observed.
Conclusion:
Increases in SSAT-1 contents in tumor tissue could be of value in targeting cancers with high SSAT-1 expression for confirmation/quantification. The high levels of acetylated amantadine could be used as a simple and useful screening test for the presence of cancer.
Keywords: : acetylation, amantadine, biomarkers, cancer diagnostics, cancer screening, spermidine/spermine N1-acetyltransferase-1
Lay abstract
In response to cancer, cells tend to overproduce specific enzymes as a self-defense mechanism. By using a safe and reliable method to capture and measure the excess enzyme spermidine/spermine N1-acetyltransferase-1, the presence of cancer can be established. This study describes a novel approach of detecting and screening cancer noninvasively in the urine of cancer patients using a safe and approved drug called amantadine that acts as a smart-tracking agent. Higher levels of the acetylated form of amantadine are detectable in the urine of cancer patients, which may serve as a detection tool. In addition, increases in the amount of spermidine/spermine N1-acetyltransferase-1 in tumor tissue may provide a tool for determining the presence of cancer during pathology assessment.
According to the American Cancer Society [1], one in seven deaths worldwide is due to cancer. Cancer is the second leading cause of death in high-income countries (following cardiovascular diseases) and the third leading cause of death in low- and middle-income countries (following cardiovascular diseases, and infectious and parasitic diseases). Excluding non-melanoma skin cancer cases, the International Agency for Research on Cancer has estimated that there were 14.1 million new cases of cancer in the world in 2012, of which 8 million occurred in developed nations. The corresponding estimates for total cancer deaths in 2012 were 8.2 million (about 22,000/day). By 2030, the global burden of cancer is expected to be 21.7 million new cases and 13 million cancer deaths.
Lung cancer is by far the leading cause of cancer death among both men and women, accounting for a 25% of cancer deaths. Each year, more people die of lung cancer than of colon, breast and prostate cancers combined. According to the Canadian Cancer Society [2], cancer is the leading cause of death in Canada and is responsible for 30% of all deaths, with an estimated 202,400 new cases and 78,800 deaths for the current year. In 2015, it was estimated that >20,000 Canadians died from lung cancer. Prognosis and survival depend on many factors; however, early and accurate diagnosis/prognosis plays a critical role in the treatment outcome and quality of life.
Several factors discourage the implementation of cancer screening for detection of early-stage cancer for the population in general. Two principal barriers are access to screening and its cost. These barriers could be overcome with a simple, accurate, reproducible and inexpensive test on a yearly basis as a general screening tool.
SSAT-1 is involved in the homeostasis of the polycationic aliphatic amines spermine and spermidine. These polyamines have multiple functions in eukaryotic cells, such as maintaining the membrane potential, and controlling intracellular pH and cell volume. Both polyamines regulate inflammatory processes, lipid metabolism, cell growth, proliferation and death [3,4]. The upregulation of SSAT-1 in different types of cancer is well documented [5–7]. Our laboratory discovered that the antiviral agent, amantadine, is a specific substrate for acetylation by SSAT-1 [8,9]. Thus, amantadine can be used to determine SSAT-1 cellular activity by measuring excretion of N-acetylamantadine (AA), which may indicate the presence of cancer.
SSAT-1 appears to be ubiquitous in mammalian tissues. While it is present in very small amounts in normal healthy cells, SSAT-1 can be induced by a number of factors, including toxic agents, hormones, drugs and growth factors [4,10–12]. Indeed, in human prostate cancer, there is increased expression of SSAT-1 to prevent polyamine concentrations from reaching levels that would be toxic to the cell [13]. The increased production of polyamines in cancer results in increased levels of polyamines and N1-acetylspermidine, reflecting increased SSAT-1 activity [14,15]. Indeed, an increase in monoacetylated polyamines has been detected in human and animal tumor cells/tissue [16–19].
In this study, the SSAT-1 expression levels in human normal and tumor cell lines as well as in primary patient-derived tumor tissues were assessed. Results demonstrated that high SSAT-1 expressions are present in specific cancers and the elevated SSAT-1 activity measured as the excretion of AA in urine could serve as a diagnostic test for cancer in humans.
Materials & methods
Cell culture
Normal human bronchial epithelial cells (ATCC® PCS-300-010™, VA, USA), prostate epithelial cells (ATCC PCS-440-010™), mammary epithelial cells (ATCC PCS-600-010™), A549 (human lung tumor cells, ATCC CCL-185™), LNCaP (human prostate adenocarcinoma cells, ATCC CRL-1740™) and T-47D (human breast tumor cells, ATCC HTB133™) were used. Cells were cultured in appropriate cell growth kit, supplemented with additional growth factors as provided by ATCC, and maintained in a 5% CO2 humidified incubator at 37°C.
Transcript analysis by qRT-PCR
Research and ethics approval was obtained from the University of Manitoba Research Ethics Board (Ethics File # HS 15822 [H2012:334]) prior to the study. Total RNA was extracted from tumor tissue (obtained from the Manitoba Tumor Bank, CancerCare Manitoba, Winnipeg, Canada) or from human cancer cell lines using Qiagen QIA Shredder Kit and RNeasy Mini Kit (Qiagen, ON, Canada). The RNA concentration in each sample was confirmed by nanodrop measurement. The RNA integrity was evaluated by measurement of the RNA integrity number (absorbance ratio at 260/280 nm of around 2.0). The SSAT-1 expression was determined by qRT-PCR using a cDNA probe specific for the gene (forward: 5′-TCATCACGAAGAAGTCCTCAAG-3′ and reverse: 5′- AGCACCCCTTTTACCACTG-3′, Integrated DNA Technologies, IA, USA) using Qiagen QuaniTect SYBR Green RT-PCR kit (Qiagen). The mRNA expression levels of the human housekeeping gene GAPDH (and hPRT1 in the experiments with human cancer cell lines) was measured in parallel using the corresponding PCR primers for this gene. The SSAT-1 expression levels were normalized with GAPDH and/or hPRT1 as the internal reference. Normalized SSAT-1 expression was further analyzed by the ΔΔct method.
SSAT-1 immunoblotting
Crude homogenate proteins (20–40 μg protein/sample) from four different primary tissues for each lung, prostate and breast cancers were resolved on SDS-PAGE (15%, polyacrylamide) and transferred onto a polyvinylidene fluoride membrane (Pall Canada, ON, Canada). After blocking with 3% skimmed milk in phosphate-buffered saline (PBS), the membrane was probed with 1:2000 primary monoclonal antibody against SSAT-1 (OriGene Technologies, MD, USA). After washing with PBS-Tween 0.05% ×3, polyvinylidene fluoride membranes were incubated with horseradish peroxidase-conjugated goat antimouse secondary antibody for 1 h at room temperature, following washing with PBS-Tween ×3. Protein bands were visualized by enhanced chemiluminescence (NEN Life Sciences, MA, USA). Mouse antitubulin (1:4000; Cedarlane, ON, Canada) was used as a control and the data were expressed as a ratio to the housekeeping protein.
Regulatory & institutional review board approvals
Ethics approval was obtained from the University of Manitoba Research Ethics Board (ethics file #: B2003:089) prior to study implementation. The in vivo human study protocol was reviewed and approved by Health Canada (file # 9427-U0304-22C); notice of authorization dated 3 June 2003) and was also listed on the NIH Clinicaltrials.gov website (identifier: NCT00755898). Clinical studies were completed under GCP and GLP conditions in accordance with the standards established by the Canadian Tri-Council Policies, following approval by the University of Manitoba Research Ethics Board and Health Canada.
Experimental subjects
In the first study, 99 patients from the CancerCare Manitoba outpatient clinics at various stages of treatment and 51 healthy adult controls provided a signed informed consent for participation. Volunteers aged >18 years were included in the study. Exclusion criteria were declared as follows: alcohol consumption within 5 days of amantadine ingestion, previous adverse reaction to amantadine and currently pregnant or lactating. On the day of the study, participants were requested to orally ingest 200 mg amantadine capsules (mylan–amantadine, amantadine hydrochloride, United States Pharmacopeia) 2 h after supper and to collect urine for 12-h post amantadine ingestion for AA analysis.
Analytical procedures
Urine was analyzed for AA by established and validated GLP-compliant HPLC methods using d3-acetylamantadine as the internal standard for quantitation at Biopharmaceuticals Research Inc. (Vancouver, BC, Canada). Health Canada authorized Biomark AA assay standard under application number: 229838 on 7 October 2014 (investigational testing authorization).
During the development of the LC/MS/MS assay for the quantitation of AA and amantadine in human urine, calibration standards were prepared over the concentration range from 0.1 to 100 ng/ml for AA and 0.08 to 24 μg/ml for amantadine plus blank controls, based on a volume of 1 ml human urine. Quality control samples in human urine were prepared at 0.4, 4, 20, and 80 ng/ml for AA and 0.32, 3.2, and 16 μg/ml concentration levels for amantadine. All calibration standards, quality control samples and test samples were spiked with the internal standard (IS), N-acetyl-d3-amantadine, and processed by liquid–liquid extraction. Samples were analyzed using HPLC on a Synergi Hydro-RP 80Å 4 μm (50 × 2.0 mm, id; Phenomenex, CA, USA) column, with tandem MS/MS detection using an electrospray ionization triple-quadruple mass analyzer (Agilent 1100, Agilent Technologies, CA, USA). Positively charged matrix factors and IS ions were monitored using the multiple reaction monitoring mode. Quantitation of ex vivo spiked AA and amantadine in human urine was performed based on the peak area response ratio of AA or amantadine to the IS added to all samples. This LC/MS/MS assay method was successfully implemented for the measurement of AA in human urine collected from the present clinical study.
Urine creatinine was used as an estimate of completeness of the sample, in other words, normal creatinine levels indicated that the test sample is complete and appropriate for AA testing. Urine creatinine was measured by the accredited Health Sciences Centre Clinical Biochemistry Department (Winnipeg, MB, Canada).
Data cross validation
Cross validation of findings was performed with HPLC of urine samples for the presence of AA [20,21]. Samples were coded by the study staff and the technician analyzing the biological samples did not have access to any patient information other than the code on the label.
Statistical analysis
The concentration, total amount of AA and its excretion rate were compared using Minitab version 15.1.0.0. The data were categorized by sex, age and stage of cancer. The results were unblinded for statistical analyses. Microcal Origin version 7.5 (Origin Lab Corp., MA, USA) was used for some of the statistical analyses of the data. Some of the gene expression values as well as human volunteer data are expressed as mean ± standard error of the mean. The differences among all groups were evaluated by one-way ANOVA followed by Student's t-test for comparisons between two groups with Bonferroni correction for multiple analyses. A probability of p < 0.05 was considered significant.
Results
SSAT-1 gene expression & protein contents in tumor samples
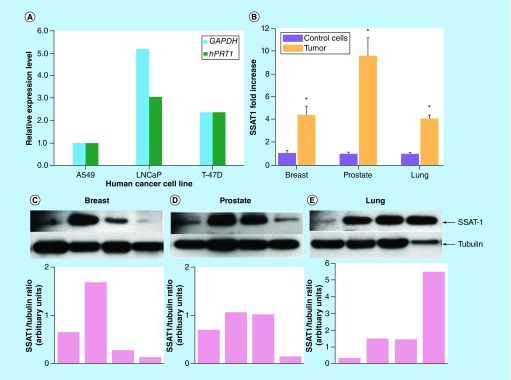
SSAT-1 gene and protein was detectable in all tumor tissue examined. Analysis of the SSAT-1 gene expression revealed detectable levels of SSAT-1 gene in A549 (human lung tumor cells) LNCaP (human prostate adenocarcinoma cells) and T-47D (human breast tumor cells) (Figure 1A). Furthermore, analysis of patient-derived breast, prostate and lung primary tumors revealed approximately a five- to ten-fold increase in SSAT-1 mRNA levels, as compared with respective noncancerous normal human bronchial epithelial cells, prostate epithelial cells and mammary epithelial cells that were used as proxy controls (Figure 1B). Immunoblotting also revealed increased SSAT-1 protein contents in the corresponding tumor tissue (Figure 1C–E). It should be noted that, in view of the very low levels of SSAT-1 in normal cells, we were unable to detect SSAT-1 protein in the noncancerous human control cells. Accordingly, these western blot images have not been presented. Table 1 shows the demographics (patient age and sex, tumor laterality/site, morphology and staging) of the tumor tissue used for the gene and protein expression studies.
Figure 1. . Relative SSAT-1 gene expression in human tumor cells and human primary tumor and SSAT-1 protein contents in human tumor tissue.
The relative SSAT-1 expression levels in (A) cancer cell lines using GAPDH and hPRT1 as housekeeping genes and (B) primary human breast, prostate and lung tumor tissue normalized with GAPDH as measured by qRT-PCR. Data for tissue expression levels are shown as the mean ± SEM of 6–7 different primary tumor tissues. Experiments were performed in triplicate. Representative immunoblots showing 27 kDa SSAT-1 protein levels were quantified by densitometry using tubulin from four different patients for (C) breast tumor tissue, (D) prostate tumor tissue and (E) lung tumor tissue. Normal human bronchial epithelial cells, prostate epithelial cells and mammary epithelial cells were used as controls as described in the methods.
SEM: Standard error of the mean.
Table 1. . Demographic information of primary tumor tissue used for gene and protein expression studies.
| Tumor tissue | Age (y) | Sex | Laterality/site | Morphology | Stage |
|---|---|---|---|---|---|
| Breast | |||||
| 1† | 61 | F | Left | Infilt. Ductal & lobular mixed carcinoma | TxN0M0 |
| 2† | 55 | F | Right | Infilt. Ductal & lobular mixed carcinoma | T2N1aM0 |
| 3 | 80 | F | Right | Infilt. Ductal carcinoma | T2N1aM0 |
| 4 | 44 | F | Right | Infilt. Ductal carcinoma | T2N0M0 |
| 5 | 43 | F | Left | Infilt. Ductal & lobular mixed carcinoma | T2N2M0 |
| 6† | 40 | F | Right | Infilt. Ductal & lobular mixed carcinoma | T1cN1M0 |
| 7† | 87 | F | Right | Infilt. Ductal & lobular mixed carcinoma | T4bN3aM0 |
| Prostate | |||||
| 1 | 61 | M | – | Adenocarcinoma | N/A |
| 2† | 65 | M | – | Adenocarcinoma | T3bN1M0 |
| 3† | 62 | M | – | Adenocarcinoma | T3bN1M0 |
| 4 | 66 | M | – | Adenocarcinoma | T3bN1M0 |
| 5† | 50 | M | – | Adenocarcinoma | T2cN0M0 |
| 6† | 64 | M | – | Adenocarcinoma | T2cN0M0 |
| Lung | |||||
| 1† | 52 | M | RML | Adenocarcinoma with acinar, solid pattern | T2N0M0 |
| 2† | 75 | F | RUL | Adenocarcinoma | T2N0M0 |
| 3 | 68 | F | RUL | Adenocarcinoma | T2N0M0 |
| 4† | 80 | F | LUL | Adenocarcinoma | T2bN0M0 |
| 5† | 69 | F | LUL | Adenocarcinoma | T2aN0M0 |
| 6 | 55 | F | LLL | Adenocarcinoma | T3N1M0 |
| 7 | 71 | F | LUL | Adenocarcinoma | T2aN2M1b |
†Samples used for immunoblotting.
Infilt.: Infiltrating; LLL: Left lower lobe; LUL: Left upper lobe; M: Metastasis; N: Lymph nodes; RML: Right middle lobe; RUL: Right upper lobe; T: Staging, tumor.
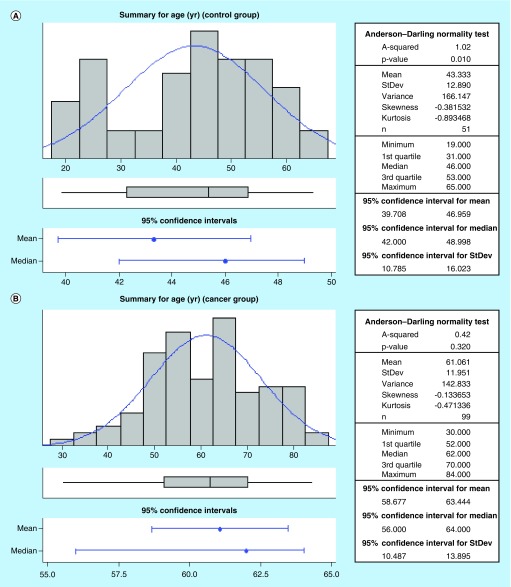
Healthy control & cancer patient characteristics
The characteristics of the participants in this study are shown in Table 2. There were no statistical differences between the age of the female (43 ± 2 years) and male (44 ± 3 years) participants in the healthy control group. Similarly, there were no statistical differences between the age of the female (62 ± 3 years) and male (61 ± 2 years) participants in the cancer group. Of note, the participants in the cancer group were older than the healthy control volunteers (Figure 2A & B). No significant differences in body weight and serum creatinine levels in the health volunteers and cancer patients were observed. Table 3 shows the different cancer types groupings as well as some staging information with a vast majority (56/99) of patients with lung cancer. Available staging information revealed 15 lung cancer patients at stage 3 and 12 patients with stage 4 disease (Table 3).
Table 2. . Participant demographics.
| Parameter | Healthy volunteers | Cancer patients |
|---|---|---|
| Age (y) | 43 (31–53) | 62 (53–70) |
| Males/females | 23/28 | 58/41 |
| Body weight (kg) | 75 (64–89) | 77.8 (63.5–89.8) |
| Urine creatinine (μmol/l) | 79 (67–84) | 74 (61–91) |
Data presented as mean and range in parentheses.
Figure 2. . Age distribution in healthy control and cancer patients.
Table 3. . Cancer type and staging of patients.
| Cancer† | Patients (n) | Stage | ||
|---|---|---|---|---|
| II | III | IV | ||
| Lung | 56 | 1 | 15 | 12 |
| GI | 16 | 1 | 1 | 2 |
| Breast | 4 | 1 | – | – |
| Prostate | 1 | – | – | – |
| Oral/naso | 11 | 1 | – | 1 |
| Other | 11 | – | 2 | – |
| Total | 99 | 4 | 18 | 15 |
†Lung cancer includes all patients with nonsmall cell, adenocarcinoma, squamous, small cell and mesothelioma. GI cancer is all patients with colon, rectal, cecal, eosophagus and pancreatic cancers. For others, cancer type includes angiosarcoma met, brain met, non-Hodgkins lymphoma, myosarcoma met, follicular lymphoma, parotid gland, B-cell lymphoma, multiple myeloma and lymphoma. Staging information was not available for all samples.
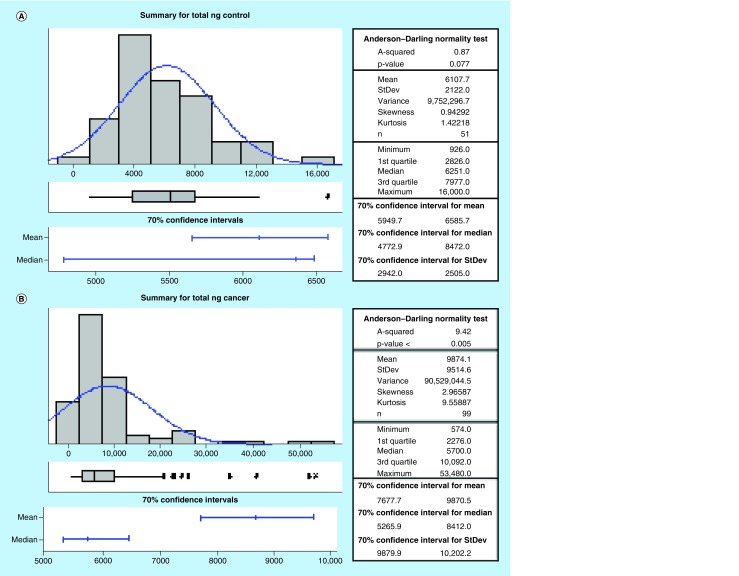
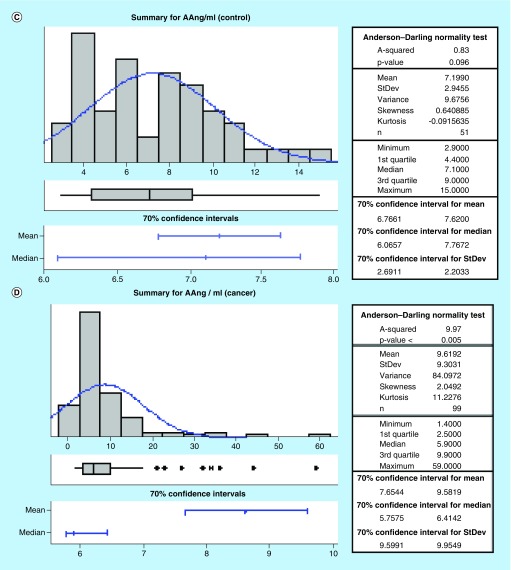
Urinary acetylamantadine levels in healthy & cancer patients
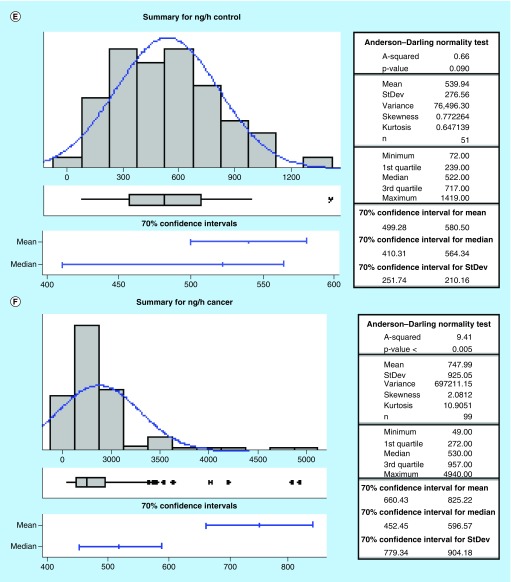
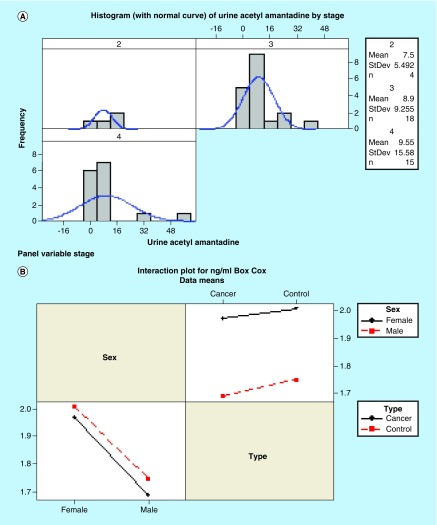
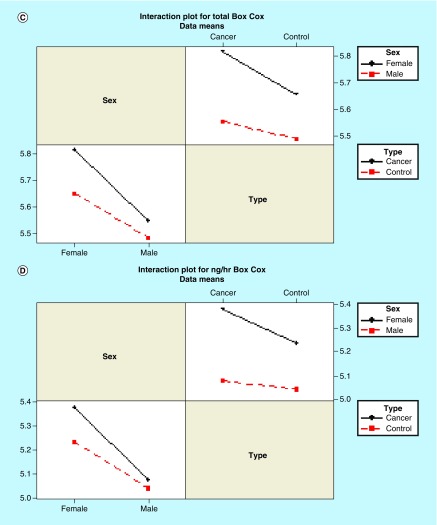
All participants had detectable AA in their urine. The data revealed that the control group has a lower amount of total urinary AA as compared with the cancer group by parametric analysis (Figure 3A & B). Furthermore, cancer patients excreted AA at higher concentration and rate compared with healthy controls (Figure 3C–F). Interestingly, a gradual increase in the urinary AA concentration was measured with progressing cancer severity, in other words, stage II to stage IV (Figure 4A). Also, the SSAT-1 response was higher in females than in males. In this regard, females were observed to exhibit a greater difference in the total AA amount and rate of excretion (Figure 4B & C) as compared with male counterparts. These data suggest that females may have a higher sensitivity (cancer vs control) of detection as compared with males.
Figure 3. . Total concentration and excretion rates for urinary acetylamantadine as determined by LC/MS/MS technique.
A parametric method was used to analyze the data for total AA (in ng amount) in (A) healthy controls and (B) cancer patients as well as for the AA concentration in (C) healthy controls and (D) cancer patients. Values are expressed as ng/ml. The excretion rate was calculated by total ng amount of AA divided by 12 and is expressed as ng/h and is shown for (E) healthy controls and (F) cancer patients. Urine was collected over a 12-h period after the ingestion of amantadine.
AA: Acetylamantadine.
Figure 4. . Acetylamantadine concentration in the urine of cancer patients according to stage of disease and sensitivity of acetylamantadine detection in male and female healthy control participants and cancer patients.
Mean values for AA concentration in (A) cancer patients are expressed in ng/ml for all cancers stage 2 (n = 4), stage 3 (n = 18) and stage 4 (n = 15), whereas the sensitivity data is shown for (B) AA concentration (ng/ml), (C) total AA (ng) and (D) rate of excretion (ng/h).
AA: Acetylamantadine.
Discussion
We, as well as others, have shown that SSAT-1 signals can be a useful marker for assessing the presence and potentially the stage/aggressiveness of cancer [8,9,14–19]. The combined approach revalidates the linear correlation between SSAT-1 gene expression, protein level and activity.
In this study, an LC/MS/MS assay method was developed for the quantitation of AA and amantadine in human urine based on a quadratic calibration regression function. The performance of the assay for AA and amantadine in human urine was successfully qualified based on the assessment of assay specificity, selectively, linearity, accuracy, precision, quantitation range, recovery and LLOQ against predetermined assay acceptance criteria. The detection of AA by the developed method is reliable, accurate, highly sensitive and reproducible.
Results of this study suggest that using amantadine as a proxy to quantify indirectly the increased SSAT-1 acetylation activity in cancer have clinical utility. The growth of transplantable tumors is associated with an intensification of sulfadimidine acetylation. Treatment of the tumors caused a decrease in acetylation rate and when tumor suppression failed, the rate of sulfadimidine acetylation did not diminish [22]. Consistent with this finding, the level of N-acetylation of sulfadimidine in cancer patients is higher in both fast and slow acetylator phenotypes, compared with corresponding controls [23,24]. Comparison of phenotyped malignant and benign breast tissue showed increased acetylation of both p-aminobenzoic acid and sulfamethazine in human malignant tissue. Enzyme activity was twice as great for prototypical N-acetyl transferase 2 (NAT2) compared with NAT1 acetyltransferase substrates in malignant tissue [25]. Effective treatment of malignant lymphoma caused a decrease in sulfadimidine acetylation [24]. It has been assumed that the increased acetylation activity could be attributed to the acetyltransferases NAT1 and NAT2. Although it is generally accepted that NATs are constitutive enzymes and are not inducible, induction of NAT2 has been demonstrated in mouse kidney under the influence of glucocorticoid or androgen [26]. The increases in acetylation described in malignancy for NAT2-selective substrates in humans may also be explained by increased SSAT-1 activity. We have previously reported that amantadine is a specific substrate of SSAT-1 and can differentiate between acetylation by SSAT-1 and NATs, since amantadine is not a substrate for acetylation by either NAT1 or NAT2 [8,9].
Accordingly, we hypothesized that amantadine acetylation would serve as a biomarker for malignancy since it occurs only by SSAT-1, an enzyme upregulated in tumor tissue. Our hypothesis was supported by the total AA and AA/h response and suggests that amantadine acetylation has potential as a diagnostic test for cancer. The data from our Phase II proof of concept study provided the basis for designing a Phase III study that will determine the effectiveness of this biomarker in a large, untreated, newly diagnosed patient population after optimization of the urine collection time and dosage in order to understand the pharmacokinetics and pharmacodynamics of AA.
We have identified SSAT-1, an enzyme in a key metabolic polyamine pathway that is overexpressed showing high activity in cancer patients compared with healthy controls. Interestingly, SSAT-1 has a unique feature that binds very specifically to amantadine, an approved US FDA drug. Then, shortly upon ingestion of the drug, it enters the cells in the body, where it is acetylated by SSAT-1. The catalytic activity of SSAT-1 on amantadine released the product AA, which passes through the urine (noninvasive) and, is easily detected. Because the assay detects a final byproduct (AA), which is not further metabolized, the signal is higher compared with traditional cancer biomarkers. In this regard, the already known blood-based protein biomarkers such as [27–34] carcinoembryonic antigen, carbohydrate antigen 19-9 l and α-fetoprotein alone are unreliable as well as lack the sensitivity and specificity needed for early diagnosis of cancer [27–34], which underscores the importance of identifying and validating more sensitive and accurate markers [27]. It should be noted that side effects from amantadine ingestion are highly unlikely since only single doses of amantadine are administered. Any side effects, if they occur, may include insomnia, jitteriness, difficulty in concentrating and mental depression. However, these are associated with long-term ingestion of amantadine and are highly unlikely to occur with ingestion of a single dose [35,36].
It should be mentioned that the data distribution of the cancer group presented in the current study was non parametric with a very wide spread of values suggesting the presence of interfering factors. These uncontrolled confounding factors may include any ongoing treatment, other co-morbidities and the fact that all cancer patient data were pooled. On the other hand, the data distribution for the control group was parametric, suggesting a minimized contribution of any possible confounding factors. In view of the higher AA concentrations, total AA amount, AA excretion rates and the gradual increase in AA concentration with higher cancer disease severity, classification stage suggests that a strong correlation exits between advancing disease, stage of detection and SSAT-1 activity level. It is also important to note that the detection of SSAT-1 transcripts and protein in primary human tumor tissue could also serve as a valuable prognostic and diagnostic assessment tool for testing for the presence of cancer at biopsy. Interestingly, an additional hypothesis is borne out from the present study that links increased SSAT1 levels with epigenetics, particularly DNA hypomethylation. In this regard, secondary to increased putrescine levels, as a consequence of increased SSAT1 activity, as well as with stabilization of adenomethionine decarboxylase, S-adenosylmethionine is directed for the biosynthesis of polyamines. However, with this increased consumption of S-adenosylmethionine in the polyamine pathway, there could be a reduction in the availability of methyl groups, resulting in DNA hypomethylation. DNA hypomethylation, which is a hallmark of the early phase of certain cancers [37] and nonneoplastic diseases [38], may be linked to elevated SSAT-1. This hypothesis is therefore an additional consideration for the importance of SSAT-1 in human cancer.
Although considerable advances in cancer diagnostics have been developed in the last 30 years, the overall mortality rate for most cancers has shown little improvement. Currently, the industry is dominated by imaging modalities (computed tomography, MRI and mammograms) followed by molecular and biomarker diagnostic assays. Imaging modalities are expensive, often inaccessible with the consequences of exposure to ionizing radiation. On the other hand, biopsies for molecular and biomarker diagnostics are invasive and uncomfortable. They may or may not result in the detection of the carcinoma. Limitations of these techniques have sequestered their use to post-symptomatic rather than pre-symptomatic detection.
With the recent emergence of metabolomics and the identification of specific enzymes being overregulated or overexpressed in cancer cells [39–42], it is our viewpoint that the enzyme is not the biomarker, but that the biomarker is the product (metabolite) of the enzymatic reaction. An overexpressed enzyme related to cancer will produce many times more metabolite, thereby exponentially increasing the signal-to-background noise ratio. The result is an amplified biomarker that acts like beacon for early detection that no traditional biomarker can match; however, while this remains to be proven, our findings demonstrate that the product of elevated SSAT-1 and the generation of AA as a product of its enzymatic activity are quantifiable and clinically relevant.
Conclusion
Increases in SSAT-1 protein contents and gene expression in primary tumors is linked to a concomitant higher level of AA in urine of cancer patients upon ingestion of amantadine.
Future perspective
While a larger study is necessary, our initial findings support further investigations to determine the utility of our amantadine test as a simple screening test for the presence of cancer as well as a surveillance test in populations at high risk for developing cancer. The elevated SSAT-1 expression could be utilized for confirmation/quantification during pathology assessment. The multiple approaches, undertaken in the present study to quantify SSAT-1 could also serve as prognostic (staging) and diagnostic tools for different types of cancer.
Summary points.
SSAT-1 (gene and protein) is upregulated in human cancer.
Amantadine is a specific substrate for acetylation by SSAT-1.
A higher urinary level of acetylamantadine is detected in patients with different types of cancer that may also be linked to staging.
SSAT-1 response appeared to be higher in females than in males.
The amantadine test may represent a novel simple screening and surveillance tool for different types of cancer.
Acknowledgements
We thank the volunteers, research nurses D Hewitt and E Friesen, for their expert assistance. Biopharmaceutical Research Inc., completed the LC/MS/MS analyses. Infrastructural support was provided by the St. Boniface Hospital Foundation and the University of Manitoba.
Footnotes
Financial & competing interests disclosure
This study was supported by the Manitoba Tumor Bank, Winnipeg, Manitoba and a member of the Canadian Tumor Repository Network and funded in part by Cancer Care Manitoba and the Canadian Institutes of Health Research and the Maunders-McNeil Foundation. The study was also funded by the University of Manitoba and Biomark Diagnostics Inc. None of the authors has conflict of interest to disclose. R Ahmed is the president and CEO and B Cheng is the acting CTO of BioMark Diagnostics Inc. (Richmond, BC, Canada), which has partially sponsored this study. AW Maksymiuk, DS Sitar, H Bach, PS Tappia and B Ramjiawan are minor shareholders of BioMark Diagnostics Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approvals and have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, informed consent has been obtained from the participants involved.
Author contributions
DS Sitar, AW Maksymiuk and R Ahmed designed the study and were involved in data analysis, and contributed to writing the paper and had primary responsibility for final content as well as procuring financial support for the experimental and clinical studies. DS Sitar and AW Maksymiuk were also responsible for the development of overall research plan, as well as for enrollment and informed consent. AW Maksymiuk provided medical oversight for the clinical study. B Cheng and DS Sitar were involved in the development of the LC/MS/MS assay for detection of acetylated amantadine. B Cheng also analyzed the data and performed the statistical analysis. H Bach was responsible for the cell SSAT-1 gene expression studies and participated in the data analysis and writing of the manuscript. PS Tappia, RA Bagchi and N Aroutiounova were responsible for the tumor tissue SSAT-1 gene expression and immunoblotting experiments. PS Tappia also wrote the initial draft of the paper. B Ramjiawan provided the expertise for regulatory and institutional review board approvals and contributed to the design of the study. All authors approved the manuscript.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Global cancer facts & figures. 2018. www.cancer.org/research/cancer-facts-statistics/global.html
- 2.Canadian Cancer Society. Canadian cancer statistics publication. 2018. http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/?region=on
- 3.Pegg AE. Functions of polyamines in mammals. J. Biol. Chem. 2016;291(29):14904–14912. doi: 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008;294(6):E995–E1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]; •• An excellent and comprehensive review of the cellular function of spermidine/spermine N1-acetyl transferase (SSAT).
- 5.Babbar N, Hacker A, Huang Y, Casero RA. Tumor necrosis factor α induces spermidine/spermine N1-acetyltransferase through nuclear factor κB in non-small-cell lung cancer cells. J. Biol. Chem. 2006;281(34):24182–24192. doi: 10.1074/jbc.M601871200. [DOI] [PubMed] [Google Scholar]; •• An important discovery demonstrating that a common mediator of inflammation can lead to the induction of SSAT expression by activating the NF-κB signaling pathway in non-small-cell lung cancer cells.
- 6.Gabrielson E, Tully E, Hacker A, et al. Induction of spermidine/spermine N1-acetyltransferase in breast cancer tissues treated with the polyamine analogue N1,N11-diethylnorspermine. Cancer Chemother. Pharmacol. 2004;54(2):122–126. doi: 10.1007/s00280-004-0786-1. [DOI] [PubMed] [Google Scholar]
- 7.Huang W, Eickhoff JC, Mehraein-Ghomi F, et al. Expression of spermidine/spermine N1-acetyl transferase (SSAT) in human prostate tissues is related to prostate cancer progression and metastasis. Prostate. 2015;75(11):1150–1159. doi: 10.1002/pros.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bras AP, Hoff HR, Aoki FY, Sitar DS. Amantadine acetylation may be effected by acetyltransferases other than NAT1 or NAT2. Can. J. Physiol. Pharmacol. 1998;76(7–8):701–706. doi: 10.1139/cjpp-76-7-8-701. [DOI] [PubMed] [Google Scholar]; •• First demonstration that amantadine is acetylated in humans by another acetyltransferase enzyme that is neither linked to N-acetyl transferase 1 nor to N-acetyl transferase 2.
- 9.Bras APM, Jänne J, Porter CW, Sitar DS. Spermidine/spermine N1-acetyltransferase catalyzes amantadine acetylation. Drug Metab. Dispos. 2001;29(5):676–680. [PubMed] [Google Scholar]; •• Provides evidence that amantadine can be acetylated by SSAT and that it may be a specific drug substrate for this enzyme.
- 10.Matsui I, Wiegand L, Pegg AE. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J. Biol. Chem. 1981;256(5):2454–2459. [PubMed] [Google Scholar]
- 11.Pegg AE, Seely JE, Pösö H, della Ragione F, Zagon IA. Polyamine biosynthesis and interconversion in rodent tissues. Fed. Proc. 1982;41(14):3065–3072. [PubMed] [Google Scholar]
- 12.Seiler N. Functions of polyamine acetylation. Can. J. Physiol. Pharmacol. 1987;65(10):2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- 13.Bettuzzi S, Davalli P, Astancolle S, et al. Tumor progression is accompanied by significant changes in the levels of expression of polyamine metabolism regulatory genes and clusterin (sulfated glycoprotein 2) in human prostate cancer specimens. Cancer Res. 2000;60(1):28–34. [PubMed] [Google Scholar]
- 14.Russell DH. Increased polyamine concentrations in the urine of human cancer patients. Nature New Biol. 1971;233(39):144–145. doi: 10.1038/newbio233144a0. [DOI] [PubMed] [Google Scholar]
- 15.Suh JW, Lee SH, Chung BC, Park J. Urinary polyamine evaluation for effective diagnosis of various cancers. J. Chromatogr. B Biomed. Sci. Appl. 1997;688(2):179–186. doi: 10.1016/s0378-4347(96)00266-6. [DOI] [PubMed] [Google Scholar]
- 16.Takenoshita S, Matsuzaki S, Nakano G, et al. Selective elevation of the N1-acetylspermidine level in human colorectal adenocarcinomas. Cancer Res. 1984;44(2):845–847. [PubMed] [Google Scholar]
- 17.Kingsnorth AN, Wallace HM. Elevation of monoacetylated polyamines in human breast cancers. Eur. J. Cancer Clin. Oncol. 1985;21(9):1057–1062. doi: 10.1016/0277-5379(85)90291-3. [DOI] [PubMed] [Google Scholar]
- 18.Pine M, Huben R, Pegg A. Production of N1-acetyl spermidine by renal cell tumors. J. Urol. 1989;141(3):651–655. doi: 10.1016/s0022-5347(17)40925-6. [DOI] [PubMed] [Google Scholar]
- 19.Sessa A, Perin A. Increased synthesis of N1-acetylspermidine in hepatic preneoplastic nodules and hepatomas. Cancer Lett. 1991;56(2):159–163. doi: 10.1016/0304-3835(91)90091-u. [DOI] [PubMed] [Google Scholar]
- 20.Hyvönen T, Keinänen TA, Khomutov AR, Khomutov RM, Eloranta TO. Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J. Chromatogr. 1992;574(1):17–21. doi: 10.1016/0378-4347(92)80093-6. [DOI] [PubMed] [Google Scholar]
- 21.Lou G, Zhang M, Minuk GY. Effects of acute ethanol exposure on polyamine and gamma-aminobutyric acid metabolism in the regenerating liver. Alcohol. 1999;19(3):219–227. doi: 10.1016/s0741-8329(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 22.Dilman VM, Anisimov VN, Kolosov AI, Bulovskaya LN. On the relationship between the activity of acetylation, growth of experimental tumors and efficacy of their suppression by hydrazine sulphate. Oncology. 1976;33(5–6):219–221. doi: 10.1159/000225149. [DOI] [PubMed] [Google Scholar]
- 23.Bulovskaya LN, Krupkin RG, Bochina TA, Shipkova AA, Pavlova MV. Acetylator phenotype in patients with breast cancer. Oncology. 1978;35(4):185–188. doi: 10.1159/000225282. [DOI] [PubMed] [Google Scholar]
- 24.Chekharina Y, Bulovskaya LN, Pavlova MV, Krupkin RG. Activity of N-acetyltransferase in patients with malignant lymphomas. Neoplasma. 1978;25(4):471–475. [PubMed] [Google Scholar]
- 25.Geylan YS, Dizbay S, Güray T. Arylamine N-acetyltransferase activities in human breast cancer tissues. Neoplasma. 2001;48(2):108–111. [PubMed] [Google Scholar]
- 26.Estrada-Rodgers L, Levy GN, Weber WW. Characterization of a hormone response element in the mouse N-acetyltransferase 2 (Nat2*) promoter. Gene Expr. 1998;7(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Bhardwaj M, Erben V, Schrotz-King P, Brenner H. Cell line secretome and tumor proteome markers for early detection of colorectal cancer: a systematic review. Cancers. 2017;9(11):156. doi: 10.3390/cancers9110156. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this review the results of studies evaluating markers derived from the secretome and tumor proteome for blood-based early detection of colorectal cancer are summarized.
- 28.Moertel CG, Fleming TR, Macdonald JS, et al. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA. 1993;270(8):943–947. [PubMed] [Google Scholar]
- 29.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23(4):338–351. doi: 10.1081/cnv-58878. [DOI] [PubMed] [Google Scholar]
- 30.Mroczko B, Groblewska M, Wereszczynska-Siemiatkowska U, et al. The diagnostic value of G-CSF measurement in the sera of colorectal cancer and adenoma patients. Clin. Chim. Acta. 2006;371(1–2):143–147. doi: 10.1016/j.cca.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Pons M, Cruz-Correa M. Colorectal cancer biomarkers: where are we now. Biomed. Res. Int. 2015;2015:149014. doi: 10.1155/2015/149014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang SY, Lin M, Zhang HB. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19–9 for colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015;8(8):9404–9409. [PMC free article] [PubMed] [Google Scholar]
- 33.Cartlidge CR, MRA U, Alkhatib AMA, Taylor-Robinson SD. The utility of biomarkers in hepatocellular carcinoma: review of urine-based 1H-NMR studies-what the clinician needs to know. Int. J. Gen. Med. 2017;10:431–442. doi: 10.2147/IJGM.S150312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of α-fetoprotein in hepatocellular carcinoma: both or neither? Am. J. Gastroenterol. 2006;101(3):524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 35.Aoki FY, Sitar DS. Clinical pharmacokinetics of amantadine hydrochloride. Clin. Pharmacokinet. 1988;14(1):35–51. doi: 10.2165/00003088-198814010-00003. [DOI] [PubMed] [Google Scholar]; • A comprehensive account on the pharmacology and pharmacokinetics of amantadine.
- 36.Gaudry SE, Sitar DS, Smyth DD, McKenzie JK, Aoki FY. Gender and age as factors in the inhibition of renal clearance of amantadine by quinine and quinidine. Clin. Pharmacol. Ther. 1993;54(1):23–27. doi: 10.1038/clpt.1993.104. [DOI] [PubMed] [Google Scholar]; • Interesting study examining the influence of sex and age on the renal clearance of amantadine.
- 37.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neidhart M. DNA Methylation and Complex Human Disease. Academic Press; Cambridge, MA, USA: 2015. DNA methylation and epigenetic biomarkers in non-neoplastic diseases; pp. 29–43. [Google Scholar]
- 39.Kang YP, Ward NP, DeNicola GM. Recent advances in cancer metabolism: a technological perspective. Exp. Mol. Med. 2018;50(4):31. doi: 10.1038/s12276-018-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín-Martín N, Carracedo A, Torrano V. Metabolism and transcription in cancer: merging two classic tales. Front. Cell. Dev. Biol. 2017;5:119. doi: 10.3389/fcell.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh D, Arora R, Kaur P, et al. Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell Biosci. 2017;7:62. doi: 10.1186/s13578-017-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel S, Ahmed S. Emerging field of metabolomics: big promise for cancer biomarker identification and drug discovery. J. Pharm. Biomed. Anal. 2015;107:63–74. doi: 10.1016/j.jpba.2014.12.020. [DOI] [PubMed] [Google Scholar]; •• An excellent review that discusses the efficacy of metabolomics in identifying biomarkers associated with diagnosis, prognosis and treatment of cancer.