Abstract
Background
Clinical guidelines recommend psychosocial care as an integral part of medical treatment, but access is often limited. Technology-based approaches provide an attractive opportunity to optimize health outcomes and quality of life in people with chronic somatic diseases e.g. by means of Internet- and mobile-based interventions (IMIs). The present article provides an overview on the basics of IMIs, applications and their evidence base for people living with chronic somatic diseases.
Methods
We conducted a selective literature search in the PubMed and Cochrane databases. Reviews which included randomized controlled trials investigating psychological IMIs were discussed pertaining to their relevance for the population described.
Results
IMIs lead to a change in unfavorable behavior connected to chronic somatic diseases. IMIs can foster protective factors like balanced physical activity or risk factors like smoking or alcohol consumption. However, studies reveal small effect sizes of d=0.25 for physical activity and an averaged effect size of d=0.20 for smoking and alcohol consumption. Additionally, IMIs can be used for the (co-)treatment of chronic somatic diseases, for instance to increase disease-specific self-efficacy in patients with diabetes (d=0.23). Studies included in meta-analyses are often highly heterogenous and are investigated in research contexts with limited health care services relevance.
Conclusion
IMIs are potentially effective when aiming at lifestyle changes and supporting medical treatment in people with chronic somatic diseases. However, results are still heterogenous and the evidence base is limited regarding specific settings, compounding the discussion of possible ways of implementing IMIs into our health-care systems.
On average, 70% of adults globally die from chronic somatic diseases and their complications (1) such as coronary artery disease, heart failure, cancer, asthma, chronic obstructive pulmonary disease (COPD), diabetes mellitus or human immunodeficiency virus (HIV). People with chronic somatic diseases might be confronted with a threat to their life, threats to independence and autonomy and threats to life goals, future plans, relationships and economic well-being (2). They often are confronted with a multitude of self-management challenges, including adhering to complex treatment plans, coping with the daily challenges due to their condition as well as dealing with mental health issues such as depression, anger or anxiety (2).
From health care services research it is well-known that adherence to treatment regimens often falls short (3). However, there are several barriers to psychosocial support like limited mobility, time constraints and stigmatization as well as limited availability of evidence-based interventions (4). Internet- and Mobile-Based Interventions (IMIs) might be an innovative and economically attractive way to lower these barriers and improve the health status and well-being of people with chronic somatic diseases (5).
The present review provides an overview on the evidence of psychosocial IMIs in people with chronic somatic diseases, as well as conclusions and future perspectives on applying IMIs to these patients.
Characterizing Internet- and Mobile-Based Interventions
IMIs are mostly standardized self-help programs, provided via a website (6). IMIs can be characterized with regard to
the extent of human support,
theoretical foundations,
technical implementation,
objectives and
areas of application (7).
They can be an integral part of the medical treatment or provided as stand-alone intervention. Areas of application comprise prevention, initiation of medical/psychological treatment, components of the medical/psychological treatment or follow-up as exemplified in the Box. Various evidence-based psychotherapeutic techniques can build the basis for IMIs aiming at behavior change, increasing health-related knowledge, awareness and understanding in users as well as fostering adherence to medical treatment (8), coping with the disease and tackling feelings of depression and anxiety (9, 10).
BOX. Area of application and phases of an Internet- and Mobile-Based Intervention (IMI).
-
Prevention
smoking cessation
alcohol consumption
healthy diet
sexual behavior
-
Initiation of Treatment
treatment expectations, treatment motivation
negative emotions
-
Component of Treatment
treatment/medication adherence
distress
depression
anxiety
-
Follow-Up
transfer to daily life
disease-management
stabilization of treatment effects
Pertaining to the extent of human support, IMIs can be classified by the degree of guidance (e.g. minimally, intensively, unguided) (6). For guided IMIs, e-coaches (e.g. psychologist/physician) guide participants through a modular program by sending short messages or feedbacks to the participant after each session. If the IMI is unguided, participants complete an instructive program by themselves.
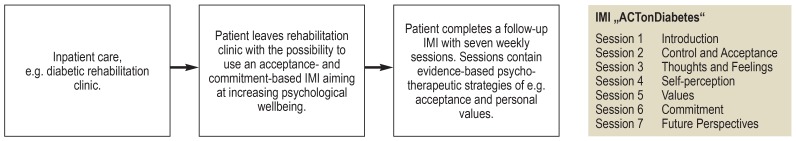
A wide range of studies across lifestyle factors (e.g. physical activity, smoking) and psycho-social (co-)treatment of medical conditions indicate the potential of IMIs for the prevention, treatment and follow-up in general (figure). IMIs designed to promote behavior change can be effectively used to influence various health-related behaviors, including physical activity, smoking, alcohol consumption, diet, sleep, or sexual behavior (11), all of which are key risk factors for chronic somatic diseases. Reviews and meta-analyses on IMIs aimed at changing lifestyle factors show small to medium effect sizes comparable to cognitive-behavioral face-to-face interventions (12).
Figure.
Example for the use of an Internet- and mobile-based Intervention (IMI) in the context of patient aftercare (e15).
Psychosocial IMIs for the (co-)treatment of chronic somatic diseases can be used for a large variety of problem areas. Psychological IMIs might aim at preoperative treatment expectations (13), coping skills, self-management, treatment/medication adherence and patient empowerment (10). Tackling problem areas of people with chronic somatic diseases might help to reduce limitations due to disability and to improve quality of life (10).
The following paragraphs describe the current evidence on IMIs for changing lifestyle factors and (co-)treating medical conditions in people living with chronic somatic disease.
Methods
Studies included in this selective review were identified by expert knowledge and a comprehensive literature search in the databases PubMed and Cochrane Library in November 2017 and April 2018 following the structure of a meta-review. The meta-review approach was selected given the broad scope of the present review.
Systematic reviews and meta-analyses of randomized controlled trials (RCTs) which compared Internet- and mobile-based psychosocial interventions to waitlist-control/minimal intervention/no intervention were selected by one of the authors (EB) and discussed in their relevance for people living with chronic somatic diseases. The search strategy was based on a combination of MeSH terms pertaining to IMIs for differenct chronic somatic diseases and different problem areas. The search strategy combined (“internet” OR “mobile-based” OR “web-based” OR “online-intervention” OR “e-health” OR m-health) AND (“chronic disease” OR “cardiovascular disease” OR “diabetes” OR “human immunodeficiency virus” OR “cancer” OR “respiratory disease”) AND (“self-management” OR “empowerment” OR “behavior change” OR “alcohol consumption” OR “smoking” OR “sleep” OR “physical activity” OR “adherence”) AND publication type (“meta-analysis” AND “systematic review”).
State of the evidence for Internet- and Mobile-Based Interventions (IMIs)
Changing lifestyle factors
Well-balanced physical activity can be considered as independent protective factor and cornerstone for all causes of burden and mortality (e.g. risk factors for obesity, type 2 diabetes, coronary artery disease) (14, 15). IMIs can reduce cardiovascular risk factors in high-at-risk adults by increasing physical activity (Standardized mean difference [SMD] = 0.25, 95% CI [0.10; 0.39]) (16). Foster et al. (17) reviewed IMIs versus control with low risk of bias at 12-month follow-up, finding significant effects on cardiovascular fitness (SMD=0.40, 95% CI [0.04; 0.76], n=2; high quality evidence) and on self-reported physical activity (SMD=0.20, 95%CI [0.11; 0.28]; moderate quality evidence, n=9). These effects might hold true for the population of people with chronic somatic diseases, e.g. people with diabetes (18), cancer (19) or COPD (20). However, the heterogeneity of studies within these populations is high and hampers the calculation of effect sizes. Additionally, limited information due to obstacles, moderators and users´ experience (21) highlight the need for a more differentiated view on how, when, why and for which population physical activity IMIs are indicated.
Smoking and drinking are core risk factors for developing a chronic somatic disease. IMIs can help to reduce tobacco use (21– 23) and alcohol consumption (23, 24). Meta-analyses indicate smoking cessation IMIs being superior control groups with no intervention, with a reported relative risk (RR) between 1.15 (95% CI: [1.02; 1.30]) and 2.16 (95% CI: [1.77; 2.62]) (21, 22, 25, 26).
For adult problem drinking, meta-analyses comparing IMIs to a passive control group revealed a small to medium effect size (g = 0.39, 95% CI: [0.23; 0.57]) in favor of the IMI to reduce alcohol consumption (27) (Hedges’g corresponds approximately Cohens’d; subsequent effects are interpreted based on (28) with g=±.2 indicating small, ±.5 medium, ±.8 large effects). Another review reveals that participants using IMIs drank approximately 23g pure alcohol less than participants of passive control groups or participants receiving minimal intervention (24). Additionally, one meta-analysis across alcohol and tobacco use revealed a weighted average effect size of d=0.20 (p<0.001) (23). Several studies included populations with HIV or other chronic somatic diseases (10). Thus, there is good reason to assume that IMIs aiming at smoking cessation and reduced alcohol consumption will work in people living with chronic somatic diseases as well.
Sleep disorders are associated with negative health outcomes and chronic somatic diseases (15). Individuals facing chronic somatic diseases are frequently affected by sleep disorders relative to the general population and simultaneously, insomnia is a risk factor for developing chronic somatic diseases (29, 30). Recent systematic reviews and meta-analyses revealed that in mixed populations, i.e. people with and without chronic somatic diseases, IMIs for insomnia have the potential to improve multiple indices of sleep with effect sizes comparable to cognitive-behavioral face-to-face therapy for insomnia, ranging from g=0.21 to g=1.09 (31). Sleep indices included the overall sleep duration, subjective sleep quality and the number of nightly awakenings. The effects were maintained at 4–48 week follow-up with g= 0.58 to g=0.68 (p<0.05, n=5) (31). In people who survived cancer, sleep-efficiency was improved due to cognitive-behavioral IMIs with a medium effect size of d=0.53 (95% CI: [0.39; 0.68]) in comparison to control (32). This effect persisted at a 6-month follow-up (small effect size d=0.33) (32). Thus, IMIs aiming at healthy sleep in people with chronic somatic diseases might bear the potential to substantially improve peoples’ well-being and physical health.
Similar results can be found for other risk and health behaviors such as sexual behavior (33) or a healthy diet in people with chronic somatic diseases (34). A statistically significant but not necessarily clinically meaningful (i.e. treatment effects that reach practically important impact on rates of behavioral risk factors) overall effect size (g=0.17, 95% CI: [0.14–0.19]) can be found across different behaviors (smoking cessation, physical activity, receiving regular mammography screening or eating a healthy diet) (35). Similarly, weight in people with chronic somatic diseases can be significantly reduced by IMIs compared to usual care or waitlist-control (weighted MD= –1.32 kg/m2, 95% CI: [–2.59;–0.06]) (36). Nevertheless, weight loss of one kg/m2 will hardly be regarded as clinically meaningful, irrespective of statistical significance (37). More information is needed on the differential effects of lifestyle IMIs and sustainability of effects in order to optimize their potential for people living with chronic somatic diseases.
The (co-)treatment of chronic somatic diseases
Self-management is a crucial factor for the adequate coping with chronic disease. Recent meta-analyses of self-management interventions for adults with metabolic diseases showed that IMIs aiming at improving self-management significantly improved adults’ HbA1c-levels (MD= –0.43%, 95%-CI: [–0.68; –0.19%], n=10), body weight and quality of life (38). The number of readmissions due to heart failure and emergency visits due to asthma might be reduced too (39) and asthma-control can be improved by IMIs (40). A recent review on internet-delivered self-management support for improving coronary heart disease (CHD) and self-management-related outcomes, revealed significant effects especially for the improvement of lifestyle-related behaviors (e1). However, data is too scarce or inconsistent to draw conclusions pertaining to clinical outcomes like morbidity or mortality in this population (e1, e2). However, other indices for improved self-management such as adherence to treatment and management of distress have been shown as well (e1).
Patient empowerment, that is, enhancing the involvement of patients in their own care, is another crucial factor in coping effectively with chronic disease. A systematic review which included various chronic somatic diseases (e.g. diabetes, heart failure, COPD) found, that IMIs significantly improved patient empowerment (SMD=0.61, 95% CI: [0.29; 0.94], n=2) and disease-specific self-efficacy (SMD=0.23, 95% CI: [0.12; 0.33], n=9) (e3).
Finally, IMIs might improve adherence to treatment or medication. Medication plans as well as treatment plans can often not develop their full potential, for approximately 50% of patients do not adhere to them as prescribed (3). A recent systematic review of IMIs on treatment adherence across different medical populations revealed significant improvements (p<0.05 to p<0.001) in 56% of the 27 included RCTs, while 37% revealed no significant results and 7% reported mixed results (8). These results demonstrate that the potential of IMIs to increase treatment adherence is high, but content and quality of investigated IMIs varies considerably between studies.
A systematic review and meta-analysis in persons with cardiovascular disease found significantly increased adherence to medical therapy (odds ratio [OR] 4.51, 95% CI: [2.38; 8.57], p<0.0001) as well as pharmacological and non-pharmacological therapy (OR 3.86, 95% CI: [1.35; 10.03], p<0.01) in the IMI group compared to usual care (e4). Besides promising results, its important to mention that data on plenty medical and psychosocial outcomes are missing for most somatic diseases. Nevertheless, the research landscape in this field is growing. Reported effect sizes are selectively recapped in Table 1 while Table 2 gives an overview of selected IMIs.
Table 1. Effectiveness of IMIs in high-at-risk groups and people with chronic somatic diseases based on selected meta-analyses.
| Target population | SMD | [95-%-KI] | N |
| Lifestyle Factors | |||
| Alcohol (23, 27, e16) | 0.39 | [0.23; 0.57] | 7 |
| 0.22 | [0.14; 0.29] | 28 | |
| 0.20 | [0.13; 0.27] | 16 | |
| Smoking (23) | 0.14 | [0.06; 0.23] | 13 |
| Physical activity (16) | 0.25 | [0.10; 0.39] | 14 |
| Overweight (36) | MD: 1.32 kg/m2 | [0.06; 2.59] | 5 |
| Diet (34) | 0.22 | [0.09; 0.34] | 25 |
| Sleep*1 (31) | 1.09 | [0.74; 1.45] | 8 |
| (Co-)treatment of chronic medical conditons | |||
| Diabetes (e3) | 0.61 | [0.29; 0.94] | 2 |
| Cardiovascular Disease (16, 40) | MD: 2.66 mm Hg | [1.52; 3.81] | 47 |
| Scoping Review*2 | 18 | ||
| Cancer (32) | 0.53 | [0.39; 0.68] | 8 |
| HIV (33) | Scoping Review*2 | 23 | |
| COPD (20) | Scoping Review*2 | 9 | |
| Asthma (40) | 0.25 | [0.12; 0.37] | 3 |
Estimates of SMD are based if available on systematic reviews and meta-analyses in the population of people with chronic somatic diseases or populations high at risk for developing a chronic disease.
*1 Several outcomes due to sleep indices were measured; selected outcome: insomnia severity
*2 Scoping review = Systematic reviews on IMIs for specific medical conditions with only partly meta-analytic data aggregation on plenty medical and psycho-social outcomes.
CI = Confidence interval; N = number of included RCTs in the meta-analysis; MD = mean difference;
SMD = standardized mean difference (Cohens’ d / Hedges’ g).
Table 2. Selected Internet- and Mobile-Based Interventions (IMIs).
| Name | Population | Goal | More information |
| Selected Tried and Tested Internet- and Mobile-Based Interventions. | |||
| Stepathlon | general | physical activity | www.stepathlon.com |
| SHUTi | general | insomnia | www.myshuti.com |
| QuitCoach | smokers | cessation | www.quitcoach.org.au |
| ACTonPain | chronic pain | psychological flexibility | www.geton-training.de/chronischeSchmerzen.php |
| Currently under investigation. | |||
| WIDeCAD / W-Decide | Coronary artery disease/Cancer | increasing psychological wellbeing | www.geton-training.de/WIDeCAD.php www.geton-training.de/W-Decide.php |
Conclusion
The present review provides evidence on the potential effectiveness of IMIs when targeting lifestyle factors and supporting medical treatment. Currently, the usage of IMIs in routine care increases globally, most often realized as (guided) stand-alone self-help interventions. Reported systematic reviews included RCTs that compared IMIs with at least one non-intervention control (31) as well as active control groups (e.g. minimal intervention, face-to-face intervention) (17, 21, 24).
Some systematic reviews did not find any evidence of publication bias (17, 32) while others tried to minimize publication bias by searching a wide range of databases as well as sources of unpublished literature (24) and adjusted for potential publication bias (31) or reported insufficient numbers of studies impeding the assessment of publication bias (21).
In summary, the evidence base and quality of studies in the population of people with chronic somatic diseases is often too scarce and heterogenous to calculate effect sizes (18, 19). Substantial heterogeneity emerged between included studies e.g. with regard to quality and scope of interventions, comparison conditions, participants characteristics, theoretical foundations and outcomes. It is important to mention, that systematic reviews and meta-analyses indicate that the positive effects of interventions on psycho-social outcomes are not easily translatable into physical outcomes (e.g. reduced HbA1c-levels in diabetes (e5), high-density lipidprotein or reduced colesterol in people with cardiovascular disease (e2). More research is needed on the question of to what extent IMIs can help to improve disease-related physical outcomes as well as clinical outcomes like hospitalization, morbidity and mortality-rates.
Furthermore, most of the included RCTs were carried out in selected research settings. Further research must show to what extent the results can be transferred into the specific health-care services context. Future RCTs should investigate if a similar picture can be drawn from research conducted in specific routine health-care settings. In this context, sustainability of intervention effects and cost-effectiveness analyses need to become more prominent (e6, e7). Similarly, negative effects of IMIs should be examined more carefully, investigating if patients might deteriorate or undergo adverse events (e8).
One systematic review on cognitive-behavioral IMIs for psychological distress in people living with chronic somatic disease highlighted that none of the 29 included studies reported a negative effect on any outcome measures (e9). Still, little is known about rare but serious adverse events that might be caused by largely automated interventions (e8).
Finally, some studies showed that the acceptance of IMIs in the respective target populations such as people with diabetes is relatively low (53.8% reported low acceptance) (e10, e11), questioning the current public health relevance of IMIs. The same might apply to health-care providers who are skeptical regarding the integration of IMIs into their everyday practice. However, targeted information can increase acceptance considerably (e10– e12) and acceptance is known to increase with IMIs becoming part of routine care (e12).
Thus, it might be safe to say that IMIs provide the possibility to further improve our health care systems, particularly if embedded in a systematic health care plan to support a digitally enabled and integrated patient-centered health-care like in Australia, where an established 10-year program aims at implementing statewide technology foundations for eHealth (e13). Thereby, IMIs could target lifestyle factors, the underlying or preventable medical conditions as well as mental comorbidities, a well studied topic (e14) beyond the scope of the present review.
Key Messages.
Chronic diseases are the leading cause of death and disability worldwide.
Research investigating innovative internet- and mobile-based interventions (IMIs) is growing rapidly.
Evidence-based IMIs might be an innovative way to improve the health-status of people living with chronic somatic diseases.
Practitioners should be informed about possibilities and limits of IMIs to make informed decisions on if, how when and why incorporating IMIs into their routine-care.
Footnotes
Conflict of interest statement
Prof. Andersson received honoraria for the authorship and co-authorship of a publication related to the topic of this review. (Author fee paid by Book Royalty).
Prof. Baumeister received third-party funds from DRV-Bund, Barmer GEK, and the SVLFG
Dr. Ebert holds shares in the company GET.ON Institut GmbH. He received honoraria for consultancy work from Techniker Krankenkasse, Sanofi, Novartis, SchönKliniken, Agaplesion Kliniken, Minddistrict GmbH, and Lantern Inc. He received third-party funds from BMBF, the EU, DRV, and Barmer GEK.
The other authors declare that no conflict of interest exists.
References
- 1.Forouzanfar MH, Afshin A, Alexander LT, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falvo DR, Holland BE. Falvo DR, Holland BE, editors. Psychosocial and funtional aspects of health conditions Medical and psychosocial aspects of chronic illness and disability. Burlington, Massachusetts: Jones & Bartlett Publishers. 2018:11–32. [Google Scholar]
- 3.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fradgley EA, Paul CL, Bryant J. A systematic review of barriers to optimal outpatient specialist services for individuals with prevalent chronic diseases: what are the unique and common barriers experienced by patients in high income countries? Int J Equity Health. 2015;14 doi: 10.1186/s12939-015-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers MA, Lemmen K, Kramer R, Mann J, Chopra V. Internet-delivered health interventions that work: systematic review of meta-analyses and evaluation of website availability. J Med Internet Res. 2017;19 doi: 10.2196/jmir.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barak A, Klein B, Proudfoot JG. Defining internet-supported therapeutic interventions. Ann Behav Med. 2009;38:4–17. doi: 10.1007/s12160-009-9130-7. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Ebert D, Lehr D, Berking M, Baumeister H. Internetbasierte kognitiv-behaviorale Behandlungsansätze: State of the Art und Einsatzmöglichkeiten in der Rehabilitation. Rehabilitation. 2013;52:155–163. doi: 10.1055/s-0033-1343491. [DOI] [PubMed] [Google Scholar]
- 8.Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17 doi: 10.2196/jmir.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charova E, Dorstyn D, Tully P, Mittag O. Web-based interventions for comorbid depression and chronic illness: a systematic review. J Telemed Telecare. 2015;21:189–201. doi: 10.1177/1357633X15571997. [DOI] [PubMed] [Google Scholar]
- 10.van Beugen S, Ferwerda M, Hoeve D, et al. Internet-based cognitive behavioral therapy for patients with chronic somatic conditions: a meta-analytic review. J Med Internet Res. 2014;16:88–93. doi: 10.2196/jmir.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12 doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther. 2018;47:1–18. doi: 10.1080/16506073.2017.1401115. [DOI] [PubMed] [Google Scholar]
- 13.Salzmann S, Euteneuer F, Laferton JAC, et al. Effects of preoperative psychological interventions on catecholamine and cortisol levels after surgery in coronary artery bypass graft patients. Psychosom Med. 2017;79:806–814. doi: 10.1097/PSY.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 15.Smith TW, Williams PG. Behavioral medicine and clinical health psychology In: Lambert MJ (ed.): Handbook of psychotherapy and behavior change. Hoboken: John Wiley & Sons. 2013:690–734. [Google Scholar]
- 16.Beishuizen CR, Stephan BC, van Gool WA, et al. Web-based interventions targeting cardiovascular risk factors in middle-aged and older people: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e55. doi: 10.2196/jmir.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster C, Richards J, Thorogood M, Hillsdon M. Remote and web 20 interventions for promoting physical activity. Cochrane Database Syst Rev. 2013,;9 doi: 10.1002/14651858.CD010393.pub2. CD010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotter AP, Durant N, Agne AA, Cherrington AL. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. J Diabetes Complications. 2014;28:243–251. doi: 10.1016/j.jdiacomp.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuijpers W, Groen WG, Aaronson NK, van Harten WH. A systematic review of web-based interventions for patient empowerment and physical activity in chronic diseases: relevance for cancer survivors. J Med Internet Res. 2013;15 doi: 10.2196/jmir.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundell S, Holmner Å, Rehn B, Nyberg A, Wadell K. Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir Med. 2015;109:11–26. doi: 10.1016/j.rmed.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Taylor GMJ, Dalili MN, Semwal M, Civljak M, Sheikh A, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD007078.pub5. CD007078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD006611.pub3. CD006611. [DOI] [PubMed] [Google Scholar]
- 23.Rooke S, Thorsteinsson E, Karpin A, Copeland J, Allsop D. Computer-delivered interventions for alcohol and tobacco use: a meta-analysis. Addiction. 2010;105:1381–1390. doi: 10.1111/j.1360-0443.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaner EF, Beyer FR, Garnett C, et al. Personalised digital interventions for reducing hazardous and harmful alcohol consumption in community-dwelling populations. Cochrane Database Syst Rev. 2017;9 doi: 10.1002/14651858.CD011479.pub2. CD011479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham A, Carpenter K, Cha S, et al. Systematic review and meta-analysis of internet interventions for smoking cessation among adults. Subst Abuse Rehabil. 2016;7 doi: 10.2147/SAR.S101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Medicine. 2013;10:1–45. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riper H, Spek V, Boon B, et al. Effectiveness of e-self-help interventions for curbing adult problem drinking: a meta-analysis. J Med Internet Res. 2011;13 doi: 10.2196/jmir.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen J. Taylor & Francis Inc. 2. London: 1988. Statistical power analysis for the behavioral sciences; pp. 20–27. [Google Scholar]
- 29.Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006;12:221–229. [PubMed] [Google Scholar]
- 30.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 31.Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. doi: 10.1016/j.smrv.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Muessig KE, Nekkanti M, Bauermeister J, Bull S, Hightow-Weidman LB. A systematic review of recent smartphone, internet and web 20 interventions to address the HIV continuum of care. Curr HIV/AIDS Rep. 2015;12:173–190. doi: 10.1007/s11904-014-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly JT, Reidlinger DP, Hoffmann TC, Campbell KL. Telehealth methods to deliver dietary interventions in adults with chronic disease: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1693–1702. doi: 10.3945/ajcn.116.136333. [DOI] [PubMed] [Google Scholar]
- 35.Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med (Baltim) 2010;51:214–221. doi: 10.1016/j.ypmed.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunenberg E, Lin J, Baumeister H. Wirksamkeit web-basierter psychologischer Interventionen zur Gewichtsreduktion - ein systematisches Review. Rehabilitation (Stuttg) 2013;52:182–187. doi: 10.1055/s-0033-1343489. [DOI] [PubMed] [Google Scholar]
- 37.Sorgente A, Pietrabissa G, Mauro Manzoni G, et al. Web-based interventions for weight loss or weight loss maintenance in overweight and obese people: a systematic review of systematic reviews. J Med Int. 2017;19:1–16. doi: 10.2196/jmir.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo CC, Su YJ, Lin CC. A systematic review and meta-analysis: effectiveness of internet empowerment-based self-management interventions on adults with metabolic diseases. J Adv Nurs. 2018 doi: 10.1111/jan.13574. doi: 10.1111/jan.13574. [DOI] [PubMed] [Google Scholar]
- 39.Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478–488. [PMC free article] [PubMed] [Google Scholar]
- 40.Hui CY, Walton R, McKinstry B, Jackson T, Parker R, Pinnock H. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc. 2017;24:619–632. doi: 10.1093/jamia/ocw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Palacios J, Lee GA, Duaso M, et al. Internet-delivered self-management support for improving coronary heart disease and self-management-related outcomes A systematic review. J Cardiovasc Nurs. 2017;32:E9–E23. doi: 10.1097/JCN.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Devi R, Singh SJ, Powell J, Fulton EA, Igbinedion E, Rees K. Rees K, editor. Internet-based interventions for the secondary prevention of coronary heart disease Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd 2015 Lt Pubmed: Cochrane Database Syst Rev. 2015;12 doi: 10.1002/14651858.CD009386.pub2. CD009386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Samoocha D, Bruinvels DJ, Elbers NA, Anema JR, van der Beek AJ. Effectiveness of web-based interventions on patient empowerment: a systematic review and meta-analysis. J Med Internet Res. 2010;12 doi: 10.2196/jmir.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Gandhi S, Chen S, Hong L, et al. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol. 2017;33:219–231. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- E5.Christensen J, Valentiner LS, Petersen RJ, Langberg H. The effect of game-based interventions in rehabilitation of diabetics: a systematic review and meta-analysis. Telemed e-Health. 2016;22:789–797. doi: 10.1089/tmj.2015.0165. [DOI] [PubMed] [Google Scholar]
- E6.Ebert DD, Nobis S, Lehr D, et al. The 6-month effectiveness of internet-based guided self-help for depression in adults with type 1 and 2 diabetes mellitus. Diabet Med. 2017;34:99–107. doi: 10.1111/dme.13173. [DOI] [PubMed] [Google Scholar]
- E7.Paganini S, Teigelkötter W, Buntrock C, Baumeister H. Economic evaluations of internet- and mobile-based interventions for the treatment and prevention of depression: a systematic review. J Affect Disord. 2018;225:733–755. doi: 10.1016/j.jad.2017.07.018. [DOI] [PubMed] [Google Scholar]
- E8.Rozental A, Andersson G, Boettcher J, et al. Consensus statement on defining and measuring negative effects of internet interventions. Internet Interv. 2014;1:12–19. [Google Scholar]
- E9.McCombie A, Gearry R, Andrews J, Mikocka-Walus A, Mulder R. Computerised cognitive behavioural therapy for psychological distress in patients with physical illnesses: a systematic review. J Clin Psychol Med Settings. 2015;22:20–44. doi: 10.1007/s10880-015-9420-0. [DOI] [PubMed] [Google Scholar]
- E10.Baumeister H, Seifferth H, Lin J, Nowoczin L, Lüking M, Ebert D. Impact of an acceptance facilitating intervention on patients’ acceptance of internet-based pain interventions. Clin J Pain. 2015;31:528–535. doi: 10.1097/AJP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- E11.Baumeister H, Nowoczin L, Lin J, et al. Impact of an acceptance facilitating intervention on diabetes patients’ acceptance of internet-based interventions for depression: a randomized controlled trial. Diabetes Res Clin Pract. 2014;105:30–39. doi: 10.1016/j.diabres.2014.04.031. [DOI] [PubMed] [Google Scholar]
- E12.Ebert DD, Berking M, Cuijpers P, Lehr D, Pörtner M, Baumeister H. Increasing the acceptance of internet-based mental health interventions in primary care patients with depressive symptoms A randomized controlled trial. J Affect Disord. 2015;176:9–17. doi: 10.1016/j.jad.2015.01.056. [DOI] [PubMed] [Google Scholar]
- E13.Ministry of NSW Health—eHealth strategy for NSW Health. A digitally enabled and integrated health system delivering patient centred health experiences and quality health outcomes [Internet]. 2016: 1-35. www.health.nsw.gov.au/ehealth/documents/ehealth-strategy-for-nsw-health-2016-2026.pdf (last accessed on 16 May 2918) [Google Scholar]
- E14.Ebert DD, van Daele T, Nordgreen T, et al. Internet- and mobile-based psychological interventions: applications, efficacy, and potential for improving mental health. Eur Psychol. 2018;23:167–187. [Google Scholar]
- E15.Bendig E, Bauereiss N, Fluhr L, Baumeister H. German Clinical Trial Register: ACTonDiabetes—a randomised-controlled feasibility trial of an acceptance and commitment therapy-based internet intervention for people with diabetes mellitus Available from. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00013193. 2017. p. Identifier: DRKS00013193 (last accessed on 16 May 2918) [Google Scholar]
- E16.Riper H, Blankers M, Hadiwijaya H, et al. Effectiveness of guided and unguided low-intensity internet interventions for adult alcohol misuse: a meta-analysis. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0099912. [DOI] [PMC free article] [PubMed] [Google Scholar]