Abstract
Background
Acute and subacute disturbances of wakefulness and cognitive function are common neurological manifestations in the hospital and in outpatient care. An important element of the differential diagnosis was described only a few years ago: autoimmune encephalitis, a condition whose diagnosis and treatment pose an interdisciplinary challenge.
Methods
This review is based on pertinent publications from the years 2005–2017 that were retrieved by a selective search in PubMed, and on the authors’ personal experience and case reports.
Results
The incidence of autoimmune encephalitis in Germany is estimated at 8–15 cases per million persons per year. In some patients with psychotic manifestations or impaired consciousness of acute or subacute onset, an autoimmune pathogenesis can be demonstrated by the laboratory detection of autoantibodies against neuronal target antigens (e.g., glutamate receptors). Testing of this type should be performed in patients with inflammatory changes in the cerebrospinal fluid or on magnetic resonance imaging (MRI), or those who have had an otherwise unexplained first epileptic seizure or status epilepticus. The cumulative sensitivity of testing for all potentially causative antineuronal antibodies in patients with clinically defined autoimmune encephalitis is estimated at 60–80 %. Figures on cumulative specificity are currently unavailable.
Conclusion
The detection of antineuronal antibodies in patients with the corresponding appropriate symptoms implies the diagnosis of autoimmune encephalitis. Observational studies have shown that rapidly initiated immunosuppressive treatment improves these patients’ outcomes. Further studies are needed to determine the positive predictive value of antineuronal antibody detection and to develop further treatment options under randomized and controlled conditions.
Acute or subacute disorders of wakefulness (quantitative impairment of consciousness, ICD-10 R40.-) and qualitative impairment of consciousness (confusion, impaired orientation, amnesia syndromes; ICD-10 R41.-) are frequently occurring causes of hospitalization. In a large neurological emergency department, quantitative disorders of consciousness were the cardinal symptom in every fifth patient seen (reduced vigilance 9%, epileptic seizures 11%) (1). Around 20 to 30% of all hospital inpatients, 50% of elderly patients, and up to 70% of intensive care patients suffer from delirium, i.e., acute deterioration of alertness, organized cognition, memory, attentiveness, and perception (2, e1– e3). Particularly with delirium syndromes the causes are unclear and the treatment principally comprises supportive measures, e.g., management of systemic infections or electrolyte shifts (3).
Recently an additional differential diagnosis has been described, namely a group of previously unknown immune-mediated forms of encephalitis with autoantibodies against neuronal antigens (4). These diseases are rare, but can be clearly delineated from noninflammatory causes with the aid of a rigorous diagnostic work-up for antibodies. Their detection and diagnosis is of great importance, as immune therapy is a causal, frequently successful form of treatment (5). Owing to the wide heterogeneity of immune-mediated forms of encephalitis, these diseases demand close interdisciplinary cooperation on the part of neurologists, intensive care specialists, oncologists, pediatricians, gynecologists, and psychiatrists (eBoxes 2 and 3).
eBOX 2. Case 1.
A 70-year-old woman was admitted to the internal medicine department because she was suspected to have dementia. There was a 4-month history of progressive short-term memory loss with personality changes and repeated erroneous actions. Clinical examination on admission found that the patient was disoriented in place and time, showed attention disorder, sometimes displayed diminished affect, and was intermittently indifferent. She had no focal neurological deficits. Clinical chemistry demonstrated hyponatremia of 125 mmol/L; the cerebrospinal fluid findings were normal. Neurocranial magnetic resonance tomography showed increased signal and bilateral thickening of the hippocampus. An autoimmune panel investigation for paraneoplastic antibodies, prompted by the patient’s weight loss, detected antibodies to LGI1 with a serum titer of 1:100. Each day during the patient’s stay in hospital there were several episodes, each lasting a few seconds, in which she grimaced and waved her arms around bizarrely. A neurologist later classified these events as faciobrachial dystonic seizures (FBDS). On the basis of the clinical and laboratory findings, LGI1-antibody-positive limbic encephalitis was diagnosed. The patient was treated with intravenous steroids (1 g methylprednisolone/day) for 3 days, followed by oral prednisolone for 5 months. Immune therapy rapidly resulted in remission of the FBDS and the patient’s orientation increasingly improved. With time, the symptoms resolved completely; however, there was retrograde amnesia for the time spent in the hospital.
eBOX 3. Case 2.
A 21-year-old woman was admitted to the hospital because of progressive personality change with repeated phases of transient mental distraction. The initial clinical examination found fluctuating disorientation but no focal neurological deficit. The patient subsequently developed a hallucinatory psychosis and both complex focal seizures and generalized seizures occurred. Neurocranial magnetic resonance imaging (MRI) showed no abnormalities. Notably, the patient had been admitted to the pediatric department at the age of 16 years and treated for several weeks due to subacute personality change with loss of motivation and weepy behavior. Clinical chemistry at that time had shown an inflammatory cerebrospinal fluid (CSF) syndrome with 47 leukocytes/µL and demonstrated a serum titer of 140 IU/mL for antibodies to thyroperoxidase (TPO). Cranial MRI, analysis of the antineuronal antibodies known in 2006, and a comprehensive survey for pathogens had revealed no abnormalities. Hashimoto encephalitis had been suspected, and treatment with glucocorticoids and neuroleptics had led to complete remission. A few weeks before this new admission, the patient had begun studying at the university. On admission, clinical chemistry again showed an inflammatory CSF syndrome, this time with 108 leukocytes/µL. Analysis for antineuronal antibodies, carried out in the form of an encephalitis profile, demonstrated the presence of antibodies to NMDA receptors in CSF and serum. Recurrence of anti-NMDA receptor encephalitis was diagnosed. Despite treatment with intravenous steroids, immunoadsorption, and intravenous immunoglobulins, the patient’s clinical condition deteriorated progressively and she experienced recurring complex focal seizures with some generalized seizures. A complex focal episode that necessitated midazolam anesthesia signaled the beginning of an 8-week period of intensive care. The immunosuppressive therapy was escalated by the addition of rituximab and cyclophosphamide, and a combination of levetiracetam, phenytoin, phenobarbital and lacosamide was introduced as anticonvulsive treatment. An extensive search, including explorative laparoscopy, revealed no teratoma. After 3 months the patient’s condition had become stable and she was transferred for rehabilitation. At a follow-up visit 9 months after admission there were minor residual deficits in verbal and nonverbal short-term memory and working memory. A year after falling ill, the patient was able to resume her studies.
The aim of this review is to impart basic familiarity with autoimmune encephalitis and the diagnostic options for this group of diseases. The following questions are answered:
What symptoms and findings may point to the presence of underlying autoimmune encephalitis?
In which patients is an antibody diagnostic work-up for autoimmune encephalitis appropriate and how should it be carried out?
What factors and sources of error must be considered in interpreting the results?
Methods
The PubMed database (www.pubmed.gov) was surveyed for relevant scientific research. The search strategy is described in eBox 1.
eBOX 1. Supplementary information on method.
The MEDLINE database (www.pubmed.gov) was searched for relevant publications using the terms “autoimmune encephalitis,” “autoimmune encephalopathy,” “NMDA receptor encephalitis,” and “LGI1 encephalitis” for the period from 2005 to 1 September 2017. Records were filtered for “clinical trials,” “reviews,” and “human species“ as well as for “NOT multiple sclerosis,” “NOT neuropathy,” “NOT lupus erythematosus,” and “NOT vasculitis”.
Articles without an abstract, articles in languages other than German or English, single case reports, case series with fewer than five patients, and reviews and original articles with a purely pathophysiological thrust were manually filtered out. The remaining 72 publications were screened and evaluated by the authors.
Results
Clinical symptoms
Many forms of autoimmune encephalitis manifest as “limbic encephalitis,” i.e., psychiatric symptoms or qualitative impairment of consciousness in combination with epileptic seizures and memory disorders (4). Because the seizures are often not generalized (6), but occur focally as psychomotor episodes with isolated impaired consciousness and disorientation (dyscognitive seizures) (7), these patients often receive an incorrect diagnosis of delirium, encephalopathy, or neurodegenerative dementia (5).
The year 2016 saw the publication of the first consensus criteria for the diagnosis of possible autoimmune encephalitis prior to knowledge of the antibody results (8). The principal warning signs are rapidly progressive (<3 months) qualitative or quantitative impairment of consciousness, lethargy, personality changes, and deterioration of short-term memory. Further diagnostic “red flags” are newly occurring epileptic seizures, psychiatric symptoms, changes in magnetic resonance tomography (MRI) findings in the temporal lobes (bilateral alterations of the mesial portions of the lobes on T2-weighted MRI), inflammatory changes of the cerebrospinal fluid (CSF), and alterations on electroencephalography (EEG) (epilepsy-typical potentials or regional decelerations) (8). In particular, an unprovoked first occurrence of status epilepticus should prompt suspicion of autoimmune encephalitis until another cause is demonstrated (9). In many patients, however, especially the elderly, the memory deterioration takes place over a period exceeding 3 to 6 months and the initially mostly focal epileptic seizures may be misconstrued by the patients and their relatives (10, 11, e4, e5).
Antineuronal antibodies
It has been known since the 1960s that limbic forms of encephalitis may occur as paraneoplastic neurological syndromes (e6). In these cases the patients are often found to have antibodies to neuronal intracellular antigens (onconeural antibodies) (e7), are mostly elderly, almost always have a malignant tumor, and respond only poorly to immunotherapy (table).
Table. Neuronal and glial autoantibodies in autoimmune encephalitis with acute or subacute impairment of consciousness.
| Antigen | Syndrome | Tumor association |
| Antibodies to neuronal intracellular antigens | ||
| Hu, CV2/CRMP5, amphiphysin | Limbic encephalitis | > 95 %; small-cell BC |
| Ma2 | Limbic encephalitis*1 | > 95 %; testicular tumors |
| GAD65 | Limbic encephalitis, temporal lobe epilepsy | Max. 25 % thymomas, small-cell BC |
| Antibodies to neuronal surface antigens | ||
| NMDA receptor (GluN1) | Anti-NMDA receptor encephalitis | 25–50 % ovarian teratomas in women aged 14 to 45 years |
| LGI1 | Limbic encephalitis, usually no inflammatory CSF, faciobrachial dystonic seizures | 5–10 % thymomas |
| CASPR2 | Limbic encephalitis, Morvan syndrome*2, neuropathic pain syndromes | 20–50 % thymomas(usually in Morvan syndrome) |
| GABA(B1) receptor | Limbic encephalitis | 50 % small-cell BC |
| AMPA receptor (GluR1/2) | Limbic encephalitis, refractory seizures | 65 % thymomas, small-cell BC |
| mGluR5 | Limbic encephalitis | 70 % Hodgkin lymphomas |
| GABA(A) receptor (α1/β3) | Limbic encephalitis, status epilepticus | < 5 % thymomas |
| DPPX | Limbic encephalitis, diarrhea, hyperekplexia, stiff-person spectrum | < 10 % lymphomas |
| Glycine receptor (αGlyR) | Stiff-person spectrum, progressive encephalomyelitis with rigidity and myoclonus (PERM) | < 5 % thymomas, lymphomas |
| Neurexin-3α | Encephalitis (similar to anti-NMDA receptor encephalitis) | None yet known |
| IgLON5 | Encephalitis with sleep disorders, stridor, chronic course | None yet known |
| Antibodies to glial antigens | ||
| MOG | ADEM *3, encephalopathy and focal seizures *4, neuromyelitis optica spectrum diseases *5 | None yet known |
In the column “Antigen”, in the case of multimeric receptors the principal antigenic subunits are given. *1 Often with distinct mesencephalic and hypothalamic involvement. *2 Morvan syndrome: combination of limbic encephalitis and neuromyotonia. *3 Acute, disseminated encephalomyelitis particularly in children. *4 Individual cases of focal cortical encephalitis with MOG antibodies have been described (e10, e11). *5 Generally without impairment of consciousness AMPA, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor; BC, bronchial carcinoma; CASPR2, contactin-associated protein 2; CRMP5, collapsin response-mediator protein 5; CSF, cerebrospinal fluid; DPPX, dipeptidyl-peptidase-like protein 6; GABA(A) receptor, ?-aminobutyric acid receptor A; GABA(B) receptor, ?-aminobutyric acid receptor B; GAD65, glutamate ?decarboxylase 65 kD; GFAP, glial fibrillary acidic protein; IgLON5, igLON family member 5; LGI1, leucine-rich glioma inactivated protein 1; mGluR5, metabotropic glutamate receptor 5; MOG, myelin oligodendrocyte glycoprotein; NMDA receptor, N-methyl-D-aspartate receptor
In 2005, previously unknown antineuronal antibodies were detected in patients with severe encephalitis and benign ovarian teratomas who responded well to immunotherapy. These antibodies were directed at structures located on the surface of axons and dendrites (e8). In time, the N-methyl-D-aspartate (NMDA) receptor was identified as the underlying target antigen (12). Thirteen further “neuronal surface antigens” were identified in the following few years, mostly receptors or synaptic scaffolding proteins (table) (13).
Incidence of autoimmune encephalitis
From what is known so far, autoimmune encephalitis is a rare disease. Its precise incidence in Germany has not yet been investigated. The German Network for Research on Autoimmune Encephalitis (GENERATE e. V.) estimates the incidence at 8 to 15 patients/1 000 000 inhabitants/year. Data from southern England suggest a similar figure (14). However, studies on the frequency of autoimmune encephalitis specifically in inpatients or outpatients with acutely or subacutely impaired consciousness are lacking as yet in Germany. Probably some members of this group of patients suffer from unrecognized autoimmune encephalitis. For example, an autoimmune or paraneoplastic etiology was found in 13 to 37% of patients with newly occurring, unprovoked, refractory status epilepticus (9, 15, 16). It can therefore be assumed that a meaningful number of undetected cases exist and that the incidence of autoimmune encephalitis is actually higher than previously thought.
Pathophysiology
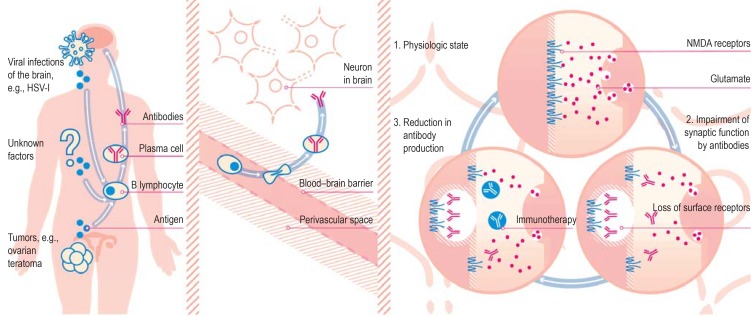
In some patients the primary trigger factor for autoimmune encephalitis is a (usually previously undetected) tumor that is expressing the target antigen, e.g., ovarian teratoma in the case of anti-NMDA receptor encephalitis (17, 18). The cause of cases of autoimmune encephalitis in which no tumor is demonstrated is unclear, although viral infection (e.g., after herpes [HSV-I] encephalitis) and genetic predisposition have been postulated (19– 21) (figure 1).
Figure 1.
Pathomechanism of synaptic encephalitis: the example of anti-NMDA receptor encephalitis Trigger factors: The misdirected autoimmune reaction may be triggered by the expression of N-methyl-D-aspartate (NMDA) receptors in ovarian teratomas or by the secretion of these receptors in the inflamed brain, e.g., following herpes simplex-I (HSV-I) encephalitis. In many cases, however, the trigger factor is unknown. Establishment of immune reaction: While the systemic immune reaction does not lead to sufficient antibody levels in the brain in the presence of an intact blood–brain barrier, the passage of antibody-producing plasma cells (e.g., triggered by infection) brings about high concentrations of antibodies in the cerebrospinal fluid. Antagonistic effect of antibodies on synapses: The presence of the autoantibodies causes internalization of the receptors and impairment of glutamate transfer. Immunotherapy and/or plasmapheresis lead to reduction of the antibody concentration in the synapse. This enables newly formed receptors to reach the surface of the neuron in sufficient numbers. (Reproduction by kind permission of Katja Duwe-Schrinner, Duwe.Design)
In a second step, probably unrelated events (e.g., systemic infections) (22) lead to migration of autoantibody-producing activated B lymphocytes across the blood–brain barrier into the brain (18). The antibodies that are then produced there exert a direct effect by binding to their target antigens (17, 23, 24). This effect is dose dependent and reversible, comparable with pharmacological inhibition (25, 26) (figure 1). Therefore, the symptoms of most forms of autoimmune encephalitis with synaptic antibodies can be reversed by early treatment (13).
Diagnosis
Cerebral imaging and the general CSF findings—leukocyte count, cytology, and analysis of autochthonous immunoglobulin synthesis—are important for the demonstration of autoimmune encephalitis (24). Both investigations serve above all to narrow down the differential diagnoses (box). The finding of bilateral temporomesial hyperintensities on T2/FLAIR MRI sequences may point to limbic encephalitis, but in most cases conventional imaging reveals only unspecific changes or a lack of abnormalities (27).
Differential diagnoses of autoimmune encephalitis.
-
Intoxication
Ethanol, opiates, benzodiazepines, tricyclic antidepressants, neuroleptics, carbon monoxide, etc.
-
Epileptic seizures
Other symptomatic forms of epilepsy, nonconvulsive status epilepticus
-
Infection
Herpes (HSV-I) encephalitis
Other viral encephalitides (varicella zoster virus [VZV], human herpes viruses [HHV6/7] especially in immunocompromised patients, tick-borne encephalitis [TBE], enteroviruses, West Nile virus, Japanese B-encephalitis)
Syphilis
Progressive multifocal leukocephalopathy (PML)
-
Other autoimmune diseases
Collagenoses (systemic lupus erythematosus, Sjögren syndrome)
Bickerstaff encephalitis/Miller–Fisher syndrome
-
Malignancies
Non-Hodgkin lymphoma, neoplastic meningitis
-
Cerebrovascular events
Vasculitis, septic focal encephalitis
-
Metabolic derangements
Wernicke encephalopathy, renal or hepatic encephalopathy
If the CSF shows signs of inflammation with pleocytosis or detection of isolated oligoclonal bands and infection has been excluded, the encephalitis may be of autoimmune origin (box). In autoimmune encephalitis the leukocyte count may be as high as 100/µl, or occasionally even 500/µl, and isolated oligoclonal bands are often present in the CSF (8, 28). In general, however, these inflammatory CSF changes are observed more frequently in acute cases (e.g., anti-NMDA receptor encephalitis) (28) and much less often in subacute/chronic autoimmune encephalitis (11). Therefore, while a constellation of CSF findings pointing to inflammation in a patient with impaired consciousness should always prompt consideration of underlying autoimmune encephalitis, the absence of such signs does not rule out encephalitis. Indeed, for some subforms, e.g., LGI1 encephalitis (LGI1: leucine-rich glioma inactivated protein 1), absence of these signs is typical (5, 11).
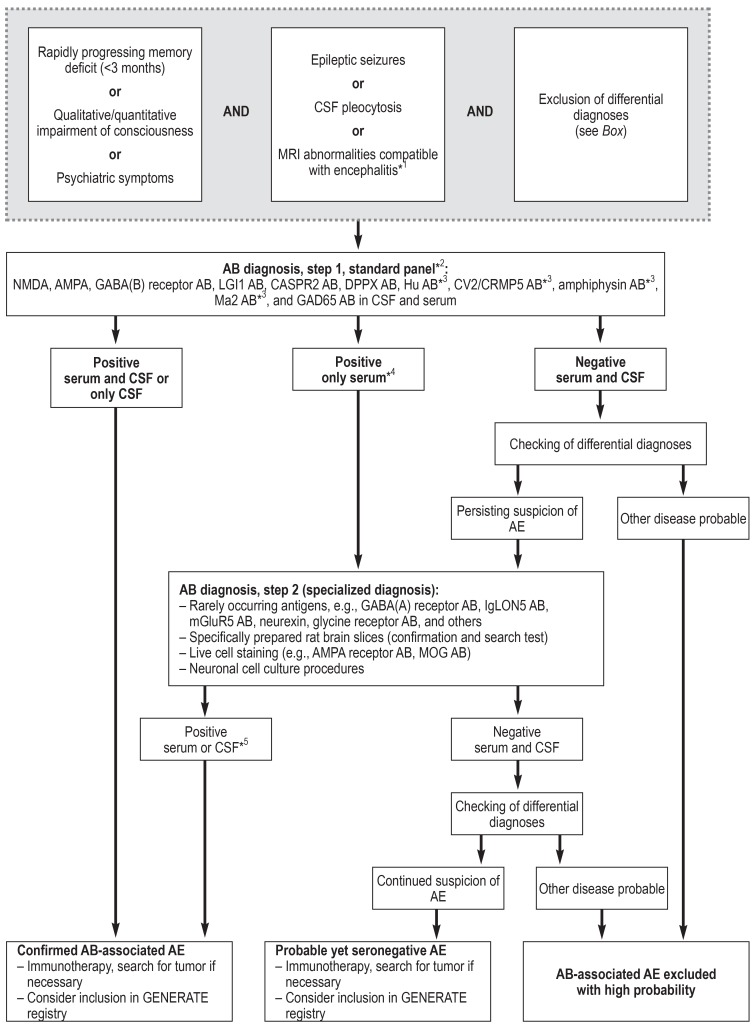
The international consensus, however, is that early diagnostic work-up for autoimmune encephalitis by means of antibody determination is required in all cases of acute or subacute qualitative or quantitative impairment of consciousness in which there is no convincing alternative etiology (figure 2) (8). This is particularly true in the presence of the following:
Figure 2.
Proposed diagnostic algorithm for investigation of autoimmune encephalitis (AE) (based on [8])
*1 A particularly strong sign of encephalitis on magnetic resonance imaging (MRI) is bilateral edema of the mesial temporal lobe. Unspecific changes are most common, however, and MRI serves especially to exclude differential diagnoses. *2 Standardized, commercially available test kits (immunofluorescence tests with tissue and fixed, transfected cells, ELISA, immunoblotting). *3 Serum testing is sufficient for these antibodies. *4 Immunofluorescence results with commercial cell-based assays with low titers (<1 : 40) but without corresponding CSF findings may be false positive. *5 For rarely occurring antibodies, individual decisions are necessary in the event of isolated serum positivity without corresponding CSF findings. AB, Antibodies; CSF, cerebrospinal fluid; GENERATE, German Network for Research on Autoimmune Encephalitis; for further abbreviations see foot of Table
An inflammatory CSF syndrome
Prominent epileptic seizures
MRI findings suggestive of unilateral or bilateral involvement of the limbic system (mesial temporal lobes) (8).
Antibody detection
As specific biomarkers, antineuronal antibodies in serum and CSF are of key importance (8). Proof of their presence enables both delineation from other differential diagnoses and also identification of the subform of autoimmune encephalitis involved. This differentiation is important for the prognosis and the recognition of a possible tumor association (table).
In some forms of autoimmune encephalitis, autoantibody testing possesses high diagnostic sensitivity and specificity (e.g., CSF sampling in anti-NMDA receptor encephalitis: specificity 100%, sensitivity 100% [98.5 to 100.0%]) (23). Predictive values cannot currently be ascertained owing to lack of data on prevalence of the antibodies in patients with impaired consciousness from other causes.
However, autoantibodies cannot always be demonstrated in cases where clinical findings indicate that autoimmune encephalitis is probable (8). For this reason the cumulative sensitivity of comprehensive antibody testing for all potential neuronal antibodies is lower (estimated at 60 to 80%) in clinically defined autoimmune encephalitis. No data are yet available on cumulative specificity.
The following aspects have to be taken into account when testing for autoantibodies:
• Standardized commercial test systems are now available for specific detection of the most frequently occurring antibodies (table). The examinations can thus be performed in any laboratory where the staff are familiar with autoimmune diagnostic methods (immunofluorescence, ELISA, immunoblotting). Because symptoms overlap among the various disease presentations, and owing to the multiplicity of newly identified autoantibodies, generation of an autoantibody profile (figure 2) with inclusion of neuronal tissue is superior to the testing of individual antibodies. In a retrospective study of 2716 requests for analysis (2608 results negative, 108 positive), around 30% of the positive tests identified antibodies other than those suspected (29).
• In certain subforms serum testing without accompanying CSF analysis is considerably less sensitive and specific (e.g., anti-NMDA receptor encephalitis: 16% false-negative results for serum testing alone) (23). For this reason it is recommended to test for antibodies in both CSF and serum in parallel.
• Our own observations, in agreement with published data (30), show that isolated analysis of serum for antibodies to neuronal surface antigens using transfected cells can result in false-positive rates of 1 to 2%. Therefore any low-titer detection of these antibodies in serum (<1:40) that is not backed up by corresponding findings in the CSF should be viewed skeptically and the findings should be double-checked by means of other techniques at a laboratory with special expertise in autoimmune encephalitis (figure 2) (17, 23).
• The autoantibodies associated with encephalitis belong, without exception, to immunoglobulin (Ig) class G (24). The diagnostic value of antineuronal antibodies of classes IgA and IgM is unclear. For example, IgA or IgM antibodies to NMDA receptors in serum are found in up to 10% of patients with various neuropsychiatric illnesses—and in the same proportion of healthy persons (30).
• In patients with anti-NMDA receptor encephalitis there is a correlation between disease activity and antibody titer in CSF but not in serum (23). However, the titer depends on the test system used, so this correlation may not be found in routine diagnostic practice. Moreover, persistence of the given antibodies has been described in the remission phase for patients with LGI1 encephalitis and anti-NMDA receptor encephalitis (11, 31).
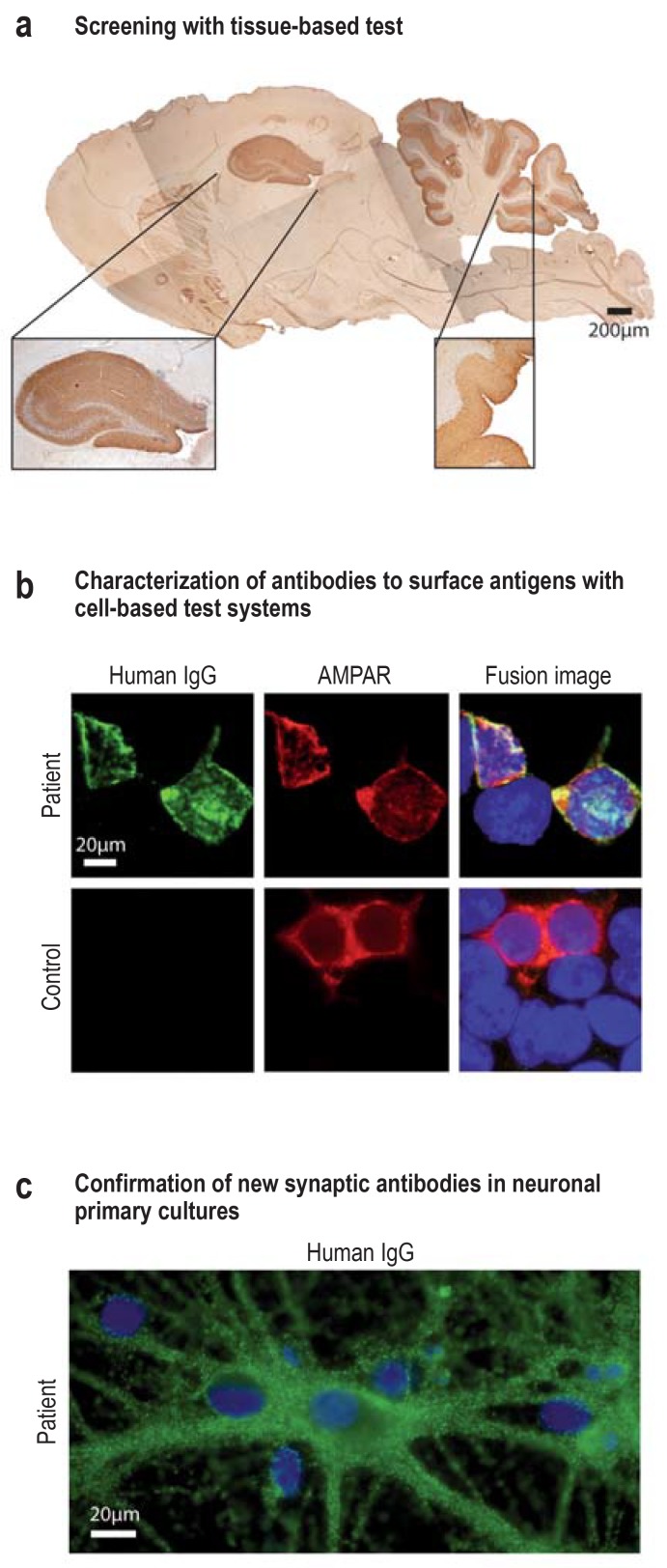
• The most frequently occurring antineuronal antibodies can be detected with the currently available test kits, but there are no validated systems for many less common target antigens. Furthermore, increasing numbers of new antigens are being identified (32– 34, e9). In patients with clinical suspicion of autoimmune encephalitis, this diagnostic gap can be bridged to some extent by means of special immunohistochemical methods, but these can usually only be carried out in specialized laboratories. These investigations, using specific tissue slices prepared in such a way as to preserve the integrity of membrane proteins, represent an expanded search system for new antibodies and simultaneously serve to verify and confirm known antibodies (Figure 2, eFigure) (13, 17). Therefore, clinically suspected autoimmune encephalitis can only be considered seronegative if this tissue-based screening test also yields no evidence of antineuronal antibody activity (8).
eFigure:
Experimental analyses
In the case of persistent clinical suspicion in the face of a negative result, or to check an implausible positive result, more detailed analysis of antineuronal antibodies can be carried out using experimental methods in specialized laboratories.
a) A tissue-based test using specially prepared sagittal slices of rat brain serves for both screening and confirmation. This panel shows the hippocampal and cerebellar staining after incubation of serum (1 : 200) from a patient suspected of having autoimmune encephalitis. The results point to the presence of a synaptic autoantibody with an unknown target antigen. Antihuman secondary antibody and DAB immunohistochemistry. Scale bar: 200 µm.
b) In contrast to commercial cell-based test kits, unfixed cell cultures could be used for antigen-specific testing in the specialized laboratory (live cell staining without potential fixation artifacts). This panel shows HEK293T cells (HEK, human embryonal kidney), which express AMPA receptors on their surface after transfection with specific cDNA and were stained with serum (green, 1 : 40) from a patient with anti-AMPA receptor encephalitis or a control patient. Incubation with a monoclonal antibody to AMPA receptors (red) is shown for comparison. The fusion images show that colocalization of both colors (yellow) occurs, which confirms the presence of AMPA receptor specific IgG antibodies in this patient’s serum. Scale bar: 20 µm.
c) Neuronal primary cell cultures are used to confirm a new neuronal surface antibody (e.g., from panel a). The example of an anti-IgLON5 antibody (in green) illustrates the binding of the autoantibody to the surface of the neurons and their dendrites. The nuclei of the neurons and the adjacent glial cells are stained blue (neuronal, embryonal, murine hippocampal primary culture DIV21; incubation with serum 1 : 200 in culture with unfixed, unpermeabilized neurons and staining with green-marked secondary antihuman IgG antibodies). Scale bar: 20 µm.
AMPAR, a-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; DAB, 3,3’-diaminobenzidine; IgG, immunoglobulin G; DIV21, 21 days in vitro
IgLON5, Iglon family member 5
• Not infrequently, other systemic (e.g., antinuclear antibodies, ANA) or organ-specific (e.g., anti-thyroperoxidase, TPO) autoantibodies are detected in patients with autoimmune encephalitis. Findings of this kind, which point to a general autoimmune diathesis, may give rise to incorrect diagnoses (such as neuropsychiatric lupus erythematosus or Hashimoto encephalitis) in the absence of knowledge of the specific antineuronal antibodies and disease pattern. These diseases should thus only be considered after comprehensive testing for antineuronal antibodies, including extended serological tests (8).
In summary, the following are required for rational antibody diagnosis in patients with qualitative or quantitative impairment of consciousness:
General familiarity with the clinical warning signs of underlying autoimmune encephalitis on the part of the treating physician
Consensus among hospital staff or provision by the laboratory of an antibody panel for standardized, syndrome-oriented antibody testing
Cooperation with a laboratory experienced in the diagnosis of autoimmune encephalitis
Close communication between treating physician and laboratory in the event of atypical clinical signs with a positive antibody constellation, or high clinical suspicion without antibody detection.
Treatment and prognosis
No randomized controlled trials on the topic of treatment for autoimmune encephalitis have been published. Encephalitis with antibodies to neuronal surface antigens generally have a good prognosis provided it is detected promptly and treated at an early stage (28). However, the prognosis in individual cases varies depending on the target antigen of the autoantibody, the presence or absence of associated tumors, the patient’s age, and the severity of the disease (10, 11, 28). For instance, 77 to 98% of patients with anti-NMDA receptor encephalitis are able to live independently by 2 years after diagnosis (multicenter observational study with 577 patients, evidence level III) (28). However, multimodal imaging and neuropsychological testing demonstrated long-term impairment of memory function (35, 36). In LGI1 encephalitis, structural damage to the hippocampus often leads to permanent cognitive deficits (37). These persisting deficits can, however, be prevented by early immunotherapy after the first epileptic seizures (retrospective cohort study, n = 80, 56% cognitive deficits without immunotherapy, 1.3% with immunotherapy, evidence level III) (6). In general, early and adequate immunotherapy seems to be one of the most important prognostic factors (11, 28).
While the swiftest possible initiation of treatment—usually intravenous steroid pulse therapy and plasma exchange—and resection of any underlying tumor are of primary importance, patients who do not respond positively should be given rituximab or cyclophosphamide as early as possible (8, 28). Those who remain refractory to this treatment have benefited from IL6 blockade (tocilizumab) or plasma cell-specific therapy (proteasome inhibitors) (38, 39). Nevertheless, it is not uncommon for a patient to need several months of convalescence with intensive rehabilitation (11, 28). Most cases of monophasic autoimmune encephalitis do not require immune therapy stretching over a period of years (evidence level V), but such measures can be considered in the event of repeated recurrence (evidence level V) (40). All the treatments described above are off label.
Despite all the laboratory tests that have recently become available, autoimmune encephalitis is still not infrequently seronegative (8). However, many of these patients benefit from immunotherapy. Therefore, all patients with high clinical suspicion of autoimmune encephalitis, even those who are seronegative, should be given immunotherapy (8). Clinical criteria for probabe seronegative autoimmune encephalitis have recently been published, but the diagnosis should be made only after exclusion of rarely occurring autoantibodies (figure 2) (8).
Crucial for the collection of data on the whole spectrum of autoimmune encephalitis are national and international networks and registers for this group of diseases (e.g., the German Network for Research on Autoimmune Encephalitis, GENERATE e. V.; www.generate-net.de [in German]).
Key Messages.
Autoimmune encephalitis is an important differential diagnosis in patients with acute and subacute impairment of consciousness; the warning signs are memory deficits, epileptic seizures (especially status epilepticus), inflammatory signs in the cerebrospinal fluid (CSF), and temporomesial abnormalities on magnetic resonance imaging.
In analogy to the observations in anti-NMDA receptor encephalitis, it can be assumed that also in other forms of antibody-mediated encephalitis the patient’s prognosis or disease pattern depends largely on early diagnosis and initiation of treatment (28).
The determination of antineuronal antibodies in serum and CSF defines subgroups of autoimmune encephalitis with different treatment needs and prognoses.
The antibody diagnostic work-up should include testing of samples from serum and CSF with a combination of various test systems in order to attain the best possible specificity and sensitivity.
Investigation should include a comprehensive profile of the frequently relevant antibodies. In the event of atypical antibody constellations (low titer only in serum, IgA/IgM isotypes) or an atypical clinical presentation, the findings should be verified in a specialized laboratory if at all possible. The same is true for seronegative findings in cases of high clinical suspicion of autoimmune encephalitis.
Acknowledgments
Translated from the original German by David Roseveare
Footnotes
Conflict of interest statement
Prof. Wandinger worked for Euroimmun up to December 2012. He has received payment for a lecture from the laboratory Dr. Fenner und Kollegen.
Dr. Leypoldt has received payments for lectures from Grifols, Teva, Roche, Biogen, Merck, and Fresenius.
All three authors—Prof. Wandinger, Dr. Leypoldt, and Prof. Junker—are employed at a university institute where the work includes investigation of antineuronal antibodies.
References
- 1.Royl G, Ploner CJ, Möckel M, Leithner C. [Neurological chief complaints in an emergency room] Nervenarzt. 2010;81:1226–1230. doi: 10.1007/s00115-010-3020-x. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DJ, O‘Regan NA, Caoimh RÓ, et al. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-001772. e001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94–114. doi: 10.1111/nyas.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escudero D, Guasp M, Ariño H, et al. Antibody-associated CNS syndromes without signs of inflammation in the elderly. Neurology. 2017;89:1471–1475. doi: 10.1212/WNL.0000000000004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson J, Bi M, Murchison AG, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. 2018;141:348–356. doi: 10.1093/brain/awx323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 8.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaspard N, Foreman BP, Alvarez V, et al. New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. 2015;85:1604–1613. doi: 10.1212/WNL.0000000000001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Sonderen A, Ariño H, Petit-Pedrol M, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87:521–528. doi: 10.1212/WNL.0000000000002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariño H, Armangue T, Petit-Pedrol M, et al. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. 2016;87:759–765. doi: 10.1212/WNL.0000000000003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalmau J. NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: the 2016 Cotzias Lecture. Neurology. 2016;87:2471–2482. doi: 10.1212/WNL.0000000000003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 15.Shin JW, Koo YS, Kim YS, et al. Clinical characterization of unknown/cryptogenic status epilepticus suspected as encephalitis: a multicenter cohort study. J Neuroimmunol. 2018;315:1–8. doi: 10.1016/j.jneuroim.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Atmaca MM, Tüzün E, Erdag E, Bebek N, Baykan B, Gurses C. Investigation of anti-neuronal antibodies in status epilepticus of unknown etiology: a prospective study. Acta Neurol Belg. 2017;117:841–884. doi: 10.1007/s13760-017-0796-5. [DOI] [PubMed] [Google Scholar]
- 17.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77:589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Sonderen A, Roelen DL, Stoop JA, et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann Neurol. 2017;81:193–198. doi: 10.1002/ana.24858. [DOI] [PubMed] [Google Scholar]
- 20.Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol. 2014;75:317–323. doi: 10.1002/ana.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller SH, Färber A, Prüss H, et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann Neurol. 2018;83:863–869. doi: 10.1002/ana.25216. [DOI] [PubMed] [Google Scholar]
- 22.Leypoldt F, Höftberger R, Titulaer MJ, et al. Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurol. 2015;72:180–186. doi: 10.1001/jamaneurol.2014.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;76:108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planaguma J, Leypoldt F, Mannara F, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis—relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. doi: 10.1016/j.neuroscience.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wandinger KP, Klingbeil C, Gneiss C, et al. New serological markers for the differential diagnosis of autoimmune limbic encephalitis. Journal of Laboratory Medicine. 2011;35:329–342. [Google Scholar]
- 30.Dahm L, Ott C, Steiner J, et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol. 2014;76:82–94. doi: 10.1002/ana.24189. [DOI] [PubMed] [Google Scholar]
- 31.Hansen HC, Klingbeil C, Dalmau J, Li W, Weißbrich B, Wandinger KP. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 2013;70:117–119. doi: 10.1001/jamaneurol.2013.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gresa-Arribas N, Planaguma J, Petit-Pedrol M, et al. Human neurexin-3a antibodies associate with encephalitis and alter synapse development. Neurology. 2016;86:2235–2242. doi: 10.1212/WNL.0000000000002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13:575–586. doi: 10.1016/S1474-4422(14)70051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the ophelia syndrome. Neurology. 2011;77:1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finke C, Kopp UA, Scheel M, et al. Functional and structural brain changes in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2013;74:284–296. doi: 10.1002/ana.23932. [DOI] [PubMed] [Google Scholar]
- 36.Finke C, Kopp UA, Pajkert A, et al. Structural hippocampal damage following anti-N-methyl-D-aspartate receptor encephalitis. Biol Psychiatry. 2016;79:727–734. doi: 10.1016/j.biopsych.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Finke C, Prüss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol. 2017;74:50–59. doi: 10.1001/jamaneurol.2016.4226. [DOI] [PubMed] [Google Scholar]
- 38.Lee WJ, Lee ST, Moon J, et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. 2016;13:824–832. doi: 10.1007/s13311-016-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheibe F, Prüss H, Mengel AM, et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology. 2017;88:366–370. doi: 10.1212/WNL.0000000000003536. [DOI] [PubMed] [Google Scholar]
- 40.Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378:840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- E1.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51:591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- E2.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Francis J. Delirium in older patients. J Am Geriatr Soc. 1992;40:829–838. doi: 10.1111/j.1532-5415.1992.tb01859.x. [DOI] [PubMed] [Google Scholar]
- E4.Bien CG, Mirzadjanova Z, Baumgartner C, et al. Anti-contactin-associated protein-2 encephalitis: relevance of antibody titres, presentation and outcome. Eur J Neurol. 2017;24:175–186. doi: 10.1111/ene.13180. [DOI] [PubMed] [Google Scholar]
- E5.Joubert B, Saint-Martin M, Noraz N, et al. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol. 2016;73:1115–1124. doi: 10.1001/jamaneurol.2016.1585. [DOI] [PubMed] [Google Scholar]
- E6.Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain. 1968;91:481–496. doi: 10.1093/brain/91.3.481. [DOI] [PubMed] [Google Scholar]
- E7.Darnell RB, Posner JB. Paraneoplastic syndromes affecting the nervous system. Semin Oncol. 2006;33:270–298. doi: 10.1053/j.seminoncol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- E8.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Jarius S, Wandinger KP, Horn S, Heuer H, Wildemann B. A new purkinje cell antibody (anti-Ca) associated with subacute cerebellar ataxia: immunological characterization. J Neuroinflammation. 2010;7 doi: 10.1186/1742-2094-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4 doi: 10.1212/NXI.0000000000000322. e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Hamid SHM, Whittam D, Saviour M, et al. Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol. 2018;75:65–71. doi: 10.1001/jamaneurol.2017.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]