Abstract
Background
Primary open angle glaucoma (POAG) is the most common form of glaucoma, with a multifactorial etiology that results in retinal ganglion cell death and loss of vision. In this study, we assessed the effects of myricetin on the trabecular meshwork cells in POAG.
Methods
In the in-vivo model, glaucoma was induced in Sprague-Dawley rats by injecting hyaluronic acid into the anterior chamber of the eye (every week for six-weeks). Treatment group rats were administered myricetin (25, 50 or 100 mg/ kg body weight via oral gavage) each day for of six weeks.
Results
POAG TM cells exposed to myricetin (25, 50 or 100 μM) exhibited significantly lowered reactive oxidative species (ROS) levels and lipid peroxidation products. The expressions of transforming growth factors (TGFβ1/β2), vascular endothelial growth factor, and senescence markers (senescence associated-β-galactosidase, cyclin-dependent kinase inhibitors-p16 and p21) were substantially down-regulated in POAG TM cells exposed to myricetin. Myricetin effectively prevented IOP elevation in glaucoma-induced rats and decreased inflammatory cytokines (IL-1α, IL-1β, IL-6, Il-8, TNF-α) in the aqueous humor and POAG TM cells of glaucoma-induced rats.
Conclusion
The observations of the study illustrate the protective effects of myricetin in glaucomatous TM cells.
Keywords: glaucoma, inflammatory mediators, myricetin, oxidative stress, senescence markers, trabecular meshwork cells
Introduction
Glaucoma constitutes a group of optic neuropathies that can cause loss of vision and gradual loss of retinal ganglion cells (RGC) [1]. Primary open angle glaucoma (POAG), one of the leading causes of visual impairment worldwide, is the most common form of glaucoma. POAG is a multifactorial disease with a complex, unknown etiology that causes irreversible damage to the optic nerve. The principal factor involved in the onset and progression of POAG is raised intraocular pressure (IOP) [2]. RGCs are highly vulnerable to damage caused by this abnormally raised IOP [3]. IOP is affected by the balance of aqueous humor (AH) secretion by the ciliary body, and outflow of AH into the venous circulation via the trabecular meshwork (TM), a specialised optic tissue [4, 5]. In POAG, the TM exhibits considerable abnormal changes including decreased cellularity, accumulation of extracellular matrix (ECM) components, and changes in the actin cytoskeleton [6, 7].
Reactive oxygen species (ROS)-induced oxidative stress has been reported to be critical in the pathology of increased IOP in POAG [8, 9, 10]. ROS including superoxide anions and H2O2 have been observed in AH. Izzotti et al. [11] reported that ROS increase outflow resistance in the anterior chamber [12]. Several other clinical studies have also described increased lipid peroxidation products in the TM cells of glaucomatous patients, suggesting high oxidative stress as a major factor in the pathophysiology of glaucoma [13, 14, 15, 16]. ROS are known to be associated with signalling pathways that influence the expressions of many cytokines and growth factors, such as transforming growth factor βs (TGFβs) [17]. Some pro-inflammatory and fibrogenic factors have also been detected in the aqueous humor, reflecting the ongoing inflammation associated with glaucoma [18, 19]. In glaucoma, pro-inflammatory cytokines secreted by macrophages include IL-1β, IL-6, and TNF-α. These cytokines cause further remodelling of the ECM that results in altered cytoskeletal interactions in TM cells [20].
In aging and glaucomatous TM cells, elevated cellular oxidative stress is found to activate senescence markers such as cyclin-dependent kinase (CDK) inhibitors p16 and p21 [21]. Several studies have reported the accumulation of senescence cells in POAG [22, 23] suggesting the role of oxidative stress in aging [24, 25]. The use of antioxidants is critical to combat the oxidative stressors caused by the production of ROS and for the maintenance of homeostasis. Under conditions of ROS overproduction, supplementation with compounds of a high antioxidant potential is immensely valuable. Studies have shown that antioxidants could offer protection against the ROS-induced pathogenesis seen in glaucoma [26]. Previous treatment with the antioxidant compound resveratrol was found to effectively reduce levels of ROS and inflammatory markers within the eye [23].
Flavonoids are naturally occurring polyphenolic compounds widely present in fruits and vegetables [27]. Flavonoids possess several bioactive properties including anti-oxidant, anti-inflammatory, and neuroprotective effects [28]. Studies have reported that flavonoids can reduce oxidative stress [28, 29] and improve ocular blood flow in POAG [30]. Myricetin, (3,5,7,3’,4’,5’-hexahydroxyflavone) is present in apples, oranges, berries, and vegetables. It has been found to possess antioxidant, anti-tumor, anti-inflammatory, neuroprotective [31, 32], and antibacterial properties [33, 34]. Myricetin treatment was also observed to inhibit hyperglycemia and decrease serum lipid levels in patients [31, 35]. In this study, we evaluated the effect of myricetin in glaucomatous TM cells.
Materials and methods
Chemicals and antibodies
Myricetin and buffers used in Western blotting analysis were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against TGF-β1, TGF-β2, TNF-α, IL-6, IL-1β, IL-8, VEGF, p16, and p21 were procured from Cell Signalling Technology (Danvers, MA, USA). Horseradish peroxidase-labelled IgG secondary antibodies and β-actin were obtained from Santa Cruz Biotechnology (Texas, USA). Other chemicals and reagents used for the experiment were from Sigma-Aldrich.
Human TM tissues
POAG TM tissues were isolated from patients and cultured using the protocol previously described by Stamer et al. [36]. Following approval of the study by the ethical committee of the Institute, normal non-glaucomatous TM tissues dissected from human eye donors were obtained from the eye banks of Qingdao Municipal Hospital (Qingdao, China). Prior to surgery the details of each patient were collected, including age, gender, and clinical data including the type of glaucoma, duration since diagnosis, IOP, and visual acuity. The isolated TM cells were maintained in a solution of Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing fetal bovine serum (10%), L-glutamine (0.292mg/mL), streptomycin (0.1mg/mL), and 100U/mL penicillin (Sigma-Aldrich). The medium was replenished every 48 to 72 hours and maintained at 37°C in 5% CO2. On reaching 70-80% confluence, the cells were trypsinized and subjected to experimentation. All the experiments were performed between 3-5 passages. During each passage, the cells were incubated with myricetin (25, 50 or 100μM in DMSO, from Sigma, Saint Louis, MO) or vehicle (1μl DMSO/mL culture media) every 72 h for 15 days.
Determination of intracellular ROS levels
Intracellular ROS levels were determined using the OxiSelect™ Intracellular ROS Assay Kit (Cell biolabs Inc, CA, USA). The protocol utilizes a 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) fluorogenic probe. DCFH-DA is deacetylated to non-fluorescent 2’,7’-dichlorodihydrofluorescein (DCFH) in the presence of cellular esterases, and then rapidly oxidized by intracellular ROS to 2’,7’-dichlorodihydrofluorescein (DCF). DCF is a highly fluorescent compound that accurately reflects the overall level of oxidative stress. Cells were incubated in Hank’s solution containing 10μM of DCFH-DA dye and the level of ROS, as reflected by the overall intracellular fluorescence, was detected (excitation at 485 nm and emission at 530 nm).
Determination of protein carbonylation content
The levels of protein carbonyl, the oxidative product of proteins, were measured in TM cells (The Protein Carbonyl Content Assay Kit, Sigma-Aldrich). The treatment of protein carbonyls with 2,4-dinitrophenylhydrazine (DNPH) results in stable dinitrophenyl (DNP) hydrazone adducts. The intensity of adducts read at 375nm reflects the overall protein carbonyl content.
Measurement of thiobarbituric acid reactive substance (TBARS)
TBARS is an oxidative stress marker that bonds to malondialdehyde. TBARS levels were determined with a TBARS Assay kit (Cayman Chemical) following the manufacturer’s protocol.
Detection of superoxide dismutase activity
The activity of superoxide dismutase (SOD), an antioxidant enzyme, was evaluated with a commercial assay kit (Cayman Chemical) as per the manufacturer’s instructions. The overall activity levels were measured as U/mg tissue protein.
Western blot analysis
TM cell lysates were prepared in cooled cell lysis buffer (buffer [50 mM Tris-HCl (pH7.6), 1% NP-40; sodium deoxycholate (0.5%), SDS (0.1%), PMSF, Aprotinin (1mg/L), Leupeptin (1mg/L)]. The whole protein concentration of the supernatant was assessed using a BCA assay kit (Bio-Rad, USA). Equivalent volumes of protein (60 μg) from different treatments were electrophoretically separated by SDS-PAGE (10-12%). Separated protein bands were transferred onto PVDF membrane (Invitrogen) and blocked for 60 min at 37℃ using TBST buffer (20 mM Tris -pH7.6; 137 mM NaCl; 0.1% Tween 20) containing 5% non-fat milk. Membranes were then incubated overnight at 4°C with specific primary antibodies (1:1000). Following this, membranes were washed three times with TBST and then incubated at 37℃ for 60 min with HRP-labelled secondary antibodies (1:2000). The positive bands were detected by an enhanced chemiluminescence method (Millipore, USA) and analyzed by the ChemiDoc XRS imaging system (Bio-Rad, USA). β-actin was used as the internal control by which the concentration of test proteins were normalized.
Assay of senescence-associated (SA)-β-galactosidase
An assay was performed to assess β-galactosidase activity in the TM cells. Activity levels were determined using a SA-β-galactosidase activity staining kit from Cell Signaling Technology (Danvers, MA, USA), based on the manufacturer’s instructions. TM cells cultured in monolayers were washed twice with PBS and incubated in fixative solution for 15 minutes. The cells were washed again with PBS and stained with the staining solution at 37 °C for 16 hours. Following incubation, the cells were washed with PBS for a final time and viewed under the microscope (Nikon ECLIPS Ti microscope, Nikon Corporation, Japan).
Quantitative real time PCR (RT-PCR)
Total RNA from the TM cells was isolated using the RNeasy kit (Qiagen Inc. Valencia, CA). Total RNA content was determined using RiboGreen fluorescent dye (Molecular Probes Inc. Eugene, OR). The initial strand of cDNA was created using a cDNA reverse transcription kit (Applied Biosystems, CA, USA). PCR was carried out using the 7300 Real-Time PCR System (Applied Biosystems) with SYBR green fluorescence. The primers used for amplification were as follows: IL-1α forward 5’-AAGTGTTGACAGGCCGTATG-3’; IL-1α reverse: 5’- TACCAGACTTCGCTCCCTCT-3’; IL-6 forward: 5’-GCTTCCAATCTGGGTTCAAT-3’, IL-6 reverse: 5’-CTAATCTGCACAGCCTCGAC-3’; GAPDH forward: 5’-CACCACCATGGAGAAGGC-3’, GAPDH reverse: 5’-CCATCCACAGTCTTCTGA-3’. Final PCR products were separated on 2% agarose gel and bands were stained with 0.05% ethidium bromide. Final intensities were analyzed using the Bio‑Gel imagery apparatus (Bio‑Rad, USA).
In-vivo studies
Animals
Sprague-Dawley rats (male; 250-280 g; n=60) were purchased from the study institute’s animal centre, housed in sterile cages (n=3/cage), and carefully monitored under a standard laboratory environment (12h:12h day/night cycle, 22-23°C, humidity 55-60%). The study was conducted in compliance with the International Guidelines for the Study of Laboratory Animals and the National Animal Welfare Law of China. The rats were acclimatized to the housing conditions for 5 days prior to the start of the study.
Study design
The rats were randomly separated into five groups (n=12/group). To induce glaucoma, rats were anesthetized with ketamine (100mg/mL) and xylazine (20mg/mL), and one drop of 0.5% proparacaine hydrochloride was applied to each eye. IOP was measured immediately following induction of anaesthesia. Following previous protocols described by Moreno et al., an intracameral injection of hyaluronic acid (HA; Sigma-Aldrich St. Louis, MO) was given once per week for 6 weeks to induce chronic IOP elevation [37]. Twenty-five μL of HA (10mg/mL freshly prepared each time in sterile saline solution) was injected, using a Hamilton syringe with a 30-gauge needle. One group of rats (groups III-V) received daily oral myricetin for 6 weeks at 25, 50 or 100mg/kg doses via gavage starting 24 hours after glaucoma induction. The glaucoma group (group II) received an equal volume of saline following glaucoma induction for 6 weeks. The control group (group I) had no hyaluronic acid injection; an equal volume of saline solution was injected into the right eye as an equivalent to hyaluronic acid.
IOP measurement
All IOP measurements were performed under anesthesia. A calibrated Tono-Pen-XL (Reichert, Inc., Depew, NY) tonometer was used. IOP was measured every week prior to, and immediately following, each glaucoma induction. With gentle manual restraint, IOP values were recorded in each eye using firm contact of the tonopen with the cornea. Measurements were recorded as IOP, as suggested by Moore et al. [38, 39].
ROS levels in AH
AH samples were carefully collected from the rats using a syringe. ROS levels were determined as mentioned in the in vitro experimentation methods.
Inflammatory markers
Levels of IL-1β and IL-6 (Biolegend, San Diego, CA, USA) were determined according to the manufacturer’s instructions. Cytokine levels were measured using the SpectraMax 190 automatic plate reader and analyzed by SoftMax pro software (Molecular Devices, Sunnyvale CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS software (version 21.0) (SPSS Inc., Chicago, USA). Multiple group comparisons were done by ANOVA (one-way analysis of variance) followed by post-hoc analysis using Duncan’s Multiple Range Test (DMRT). A value of p<0.05 was considered statistically significant.
Results
Myricetin reduced the ROS-induced oxidative stress in the POAG TM cells
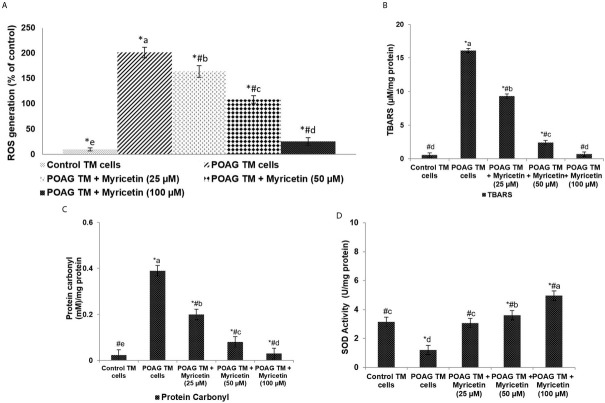
Oxidative stress has been well documented in the pathology of POAG. Our data showed a significant (p<0.05) rise in oxidative stress levels in the glaucomatous TM (GTM) cells, as reflected by the marked increase in ROS levels (Figure 1a). ROS generation was found to be significantly raised in POAG TM cells, as compared to the control TM cells. We also detected a significant (p<0.05) rise in TBARS and protein carbonyl content in GTM cells, along with a significant decrease in the activity levels of SOD (Figure 1b-d). Myricetin exposure significantly reduced the content of TBARS/MDA and protein carbonyl in GTM cells. In POAG TM cells, the production of intracellular ROS was reduced from 201.6% to 163.91%, 108.67%, and 25.81% upon exposure to 25, 50 and 100μM doses of myricetin, respectively. Myricetin treatment also significantly enhanced the levels of SOD. An amount of 100μM of myricetin increased SOD activity from 1.21U/mg protein to 4.97U/mg protein.
Figure 1.
Myricetin reduces oxidative stress in POAG. ROS levels (A), TBARS levels (B), Protein carbonyl levels (C) Superoxide dismutase Values are represented as mean±SD, n=6. * represents p<0.05 vs. control; # represents p<0.05 vs. POAG TM cells. a-e represent the mean values from different experimental groups that differ from each other
Myricetin down-regulates senescence markers in POAG TM cells
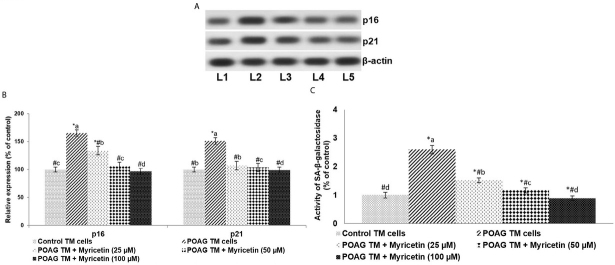
The effects of myricetin on levels of CDK inhibitors (p16 and p21) and SA-β-gal activity in the POAG TM cells were determined. p16 and p21 protein expressions were observed to be significantly (p<0.05) higher in the GTM cells vs. control (Figure 2 a and b). Further, a marked increase in SA-β-gal activity was found, as evidenced by staining intensity (Figure 2c). Myricetin was found to significantly (p<0.05) down-regulate p16 and p21 levels and reduce SA-β-galactosidase activity. 100μM of myricetin reduced the expression of p16 and p21 by approximately 1.7-fold and 1.52-fold, respectively.
Figure 2.
Myricetin down-regulates senescence markers.. Myricetin reduced the expression of CDK inhibitors p16 and p21 (A and B) and SA-β-galactosidase activity in POAG TM cells (C) “A” represents representative immunoblot. “B” indicates the relative expressions of proteins with control expression set at 100%. Values are represented as mean±SD, n=6. p<0.05 as determined by one-way ANOVA followed by DMRT analysis. * represents p<0.05 vs. control; # represents p<0.05 vs. POAG TM cells; a-d represent the mean values of different experimental groups that differ from each other at p<0.05 [L1-Control TM cells; L2-POAG TM cells; L3-POAG TM + Myricetin (25μM); L4-POAG TM + Myricetin (50μM); L5-POAG TM + Myricetin (100μM)]
Myricetin reduces inflammatory cytokines and growth factors in POAG TM cells
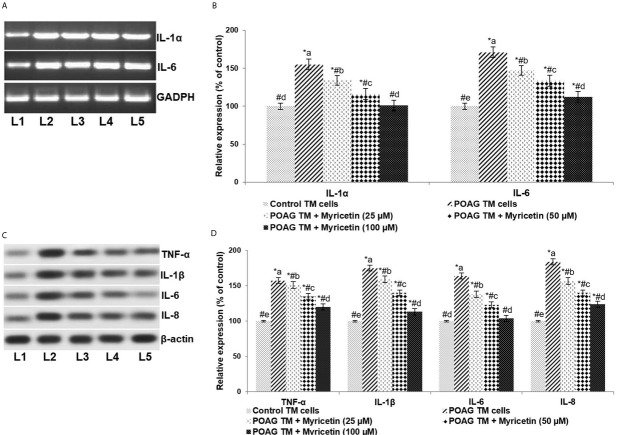
Inflammatory cytokines are known to be critically associated with glaucoma [20]. mRNA and protein expression analysis revealed significantly (p<0.05) elevated levels of pro-inflammatory cytokines (TNF-α, IL-1α, IL-1β, IL-6 and IL-8) in POAG TM cells as compared to normal TM cells (Figure 3). ROS have been known to induce cytokines [17]. The observed increase in the expression levels of inflammatory cytokines could be due to the increased ROS in POAG. Myricetin exposure resulted in a marked decrease in the expression of the inflammatory cytokines. Myricetin at 100μM was found to be more effective than the lower doses of 25 and 50μM.
Figure 3.
Myricetin down-regulates pro-inflammatory cytokines.. “A” represents the bands of RT-PCR products. “B” indicates the relative expressions of IL-1α and IL-6 mRNA levels with control expression set at 100%. “C” represents the immunoblot of pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8. “D” indicates the relative protein expressions with control expression set at 100%. Values are represented as mean±SD, n=6. * represents p< 0.05 vs.. control; # represents p<0.05 vs.. POAG TM cells. a-e represent the mean values from different experimental groups that differ from each other at p<0.05. [L1-Control TM cells; L2-POAG TM cells; L3-POAG TM + Myricetin (25μM); L4-POAG TM + Myricetin (50μM); L5-POAG TM + Myricetin (100μM)]
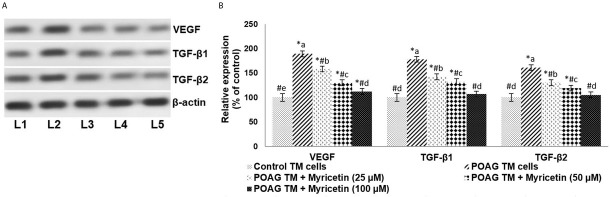
ROS have been known to influence the expression of growth factors such as VEGF and TGF-β. In the study, POAG TM cells exhibited significantly higher concentrations of VEGF, TGF-β1, and TGF-β2 compared to controls. 100 μM of myricetin was found to significantly down-regulate overall expression levels, respectively reducing VEGF, TGF-β1, and TGF-β2 by 1.6-fold, 1.7-fold, and 1.5-fold.
Myricetin effectively reduced IOP following glaucoma induction in rats
Raised intraocular pressure (IOP) is a major risk factor for POAG [40]. Following the experimental induction of glaucoma, a marked (p<0.05) increase in IOP was observed over 6 weeks (Figure 5). IOP in animals treated with oral myricetin (25, 50 or 100 mg) was found to be considerably lower than the IOP of glaucoma control rats over the period of study, although a noticeable increase was seen between the normal controls and myricetin-treated groups. IOP increased from 17mmHg in the 1st week to 35.5mmHg in the 6th week in the glaucoma group. IOP decreased from 35.5mmHg to 10mmHg on treatment with 100 mg myricetin. These results indicate the efficacy of myricetin in lowering IOP, which is of immense clinical value in the management of glaucoma.
Figure 5.
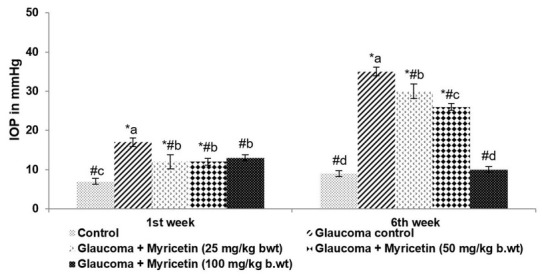
Myricetin reduced intraocular pressure in glaucoma-induced rats.. Values are represented as mean±SD, n=6. p<0.05 as determined by one-way ANOVA followed by DMRT analysis. * represents p<0.05 vs. control; # represents p<0.05 vs. Glaucoma control. a-d represent the mean values of different experimental groups that differ from each other at p<0.05.
Figure 4.
Myricetin down-regulates the expressions of VEGF and TGF-βs.. “A” represents the immunoblot. “B” indicates the protein expressions with control expression set at 100%. Values are represented as mean±SD, n=6. * represents p<0.05 vs. control; # represents p<0.05 vs. POAG TM cells. a-e represent the mean values from different experimental groups that differ from each other at p<0.05. [L1-Control TM cells; L2-POAG TM cells; L3-POAG TM + Myricetin (25μM); L4-POAG TM + Myricetin (50μM); L5-POAG TM + Myricetin (100μM)]
Effects of myricetin on ROS levels in the AH of hyaluronic acid-treated rats
ROS levels in the AH can affect TM cells in a way that can result in elevated IOP [41]. Significantly (p<0.05) elevated ROS levels were seen in hyaluronic acid-treated rats compared to normal controls (Figure 6). Increased ROS levels were seen with a concurrent rise in IOP. Myricetin at all three tested doses significantly decreased the level of ROS detected in the AH, with a maximal IOP lowering effect seen at the 100mg dose. ROS levels were reduced to 14.02% on treatment with 100 mg myricetin.
Figure 6.
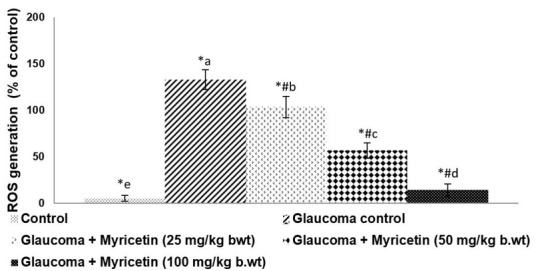
Myricetin reduces ROS levels in the aqueous humor of glaucoma-induced rats.. Values are represented as mean±SD, n=6. * represents p<0.05 vs. control; # p<0.05 vs. Glaucoma control. a-e represent the mean values from different experimental groups that differ from each other at p<0.05.
Myricetin reduced inflammatory cytokines in the AH of hyaluronic acid-treated rats
The levels of IL-1β and IL-6, key pro-inflammatory cytokines were determined in AH. Hyaluronic acid induction was found to cause a significant (p<0.05) increase in the levels of inflammatory cytokines as against normal control (Figure 7). Myricetin, remarkably (p<0.05) reduced the levels of IL-1β to 1.21, and IL-6 to 1.33. These observations demonstrate the potential of myricetin against glaucoma.
Figure 7.
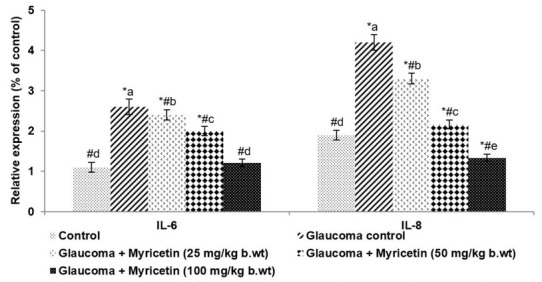
Myricetin down-regulates pro-inflammatory cytokines. Values are represented as mean±SD, n=6. p<0.05 as determined by one-way ANOVA followed by DMRT analysis. * represents p<0.05 vs. control; # represents p<0.05 vs. Glaucoma control. a-e represent the mean values of different experimental groups that differ from each other at p<0.05.
Discussion
Primary open angle glaucoma (POAG) is a common optic neuropathy characterized by visual impairment. The pathogenesis of POAG includes elevated IOP, oxidative stress, abnormal accumulation of ECM, cell senescence, inflammation, and loss of retinal ganglion cells [7, 42]. Currently therapies primarily lower IOP through the use of medications such as prostaglandin analogues, beta-blockers, alpha-agonists, and carbonic anhydrase inhibitors. While these drugs can effectively lower IOP, they do not offer complete protection. Some patients can also experience vision loss despite normal IOP [43]. As such, the need for the identification and development of novel drugs that can target mechanisms involved in the pathogenesis of POAG are highly valuable [44, 45].
It is well documented that oxidative stress damages biomolecules via peroxidation. Previous studies have shown that oxidative stress can elevate IOP, and is involved in the pathogenesis of glaucoma [8, 9, 46]. The use of antioxidant compounds in the treatment of glaucoma have been previously studied. Vitamin E, N-acetylcysteine and resveratrol have previously been shown to be beneficial in the management of POAG [10, 47, 48]. Furthermore, the consumption of flavonoid-containing foods has been found to reduce the overall risk of POAG and benefit long-term visual function [49, 50, 51, 52]. Our study found that the use of myricetin decreased IOP, and could therefore have a potential protective effect in POAG. Similar results reported by Hodges et al. [53] found that intravenously administration of 1mg/kg myricetin decreased IOP in normotensive rabbits. Here, we observed significantly increased ROS generation in the POAG TM cells and in the AH of glaucoma-induced rats. We also found elevated levels of oxidative stress markers TBARS/MDA and protein carbonyls in POAG TM cells. MDA is a by-product of lipid peroxidation and is a useful marker in assessing overall levels of oxidative stress [54]. Protein carbonyls are formed by ROS interacting with amino acids [55]. It has been reported that total protein carbonyl content increases with age, and the accumulation of these (carbonyls subsequently results in structural damage and dysfunction [56, 57]. Several studies have also reported significantly elevated protein carbonyl levels in glaucoma [58, 59, 60], and that an oxidant/antioxidant imbalance can increase the levels of ROS-induced TM damage [61, 62]. Our study found that glaucoma subjects had a 2-fold decrease in SOD activity (specify ‘the activity’). SOD is a chief antioxidant enzyme that protects against oxidative stress [15, 63]. The observed decrease in SOD activity could be due to overutilization under the conditions of high oxidative stress seen in POAG TM cells. Myricetin administration resulted in a significant increase in SOD activity, along with decreased levels of MDA and protein carbonyl content. The reduction in ROS levels illustrates the potent free-radical scavenging capacity of myricetin as an antioxidant. Increasing experimental data illustrate that the accumulation of peroxidation products in glaucomatous tissues results in oxidative stress–induced neurodegeneration [15, 64]. Animal studies of IOP lowering drops such as Latanoprost demonstrate that while these medications can lower IOP, they do not reverse or improve the oxidative stress or damage
caused by the glaucoma [65]. The observed results of the present study illustrate the efficacy of myricetin in reducing IOP and as well combating oxidative stress. Similar results were reported by Razali et al. [48] with the topical application of trans-resveratrol. Resveratrol concurrently reduced IOP and restored redox balance in rodents with steroid-induced glaucoma.
Inflammation is known to be increased in POAG [23]. Cytokines, including IL-1α, IL-1β, IL-6, and TNF-α have been shown to be associated in the pathogenesis of POAG [66]. Numerous studies have reported these cytokines in the AH and anterior chamber tissues of POAG patients [67, 68]. Glaucomatous TM cells have been found to constitutively express IL-1α that, in turn, up-regulates the expression of other inflammatory mediators [69, 70]. Luna et al. [23] reported raised levels of inflammatory mediators IL-1α, IL-6, and IL-8 in TM cells that were subjected to oxidative stress. In line with previous reports, we observed increased levels of TNF-α, IL-1α, IL-1β, IL-6, and IL-8 in POAG TM cells, and IL-1β and IL-6 in the AH of glaucoma-induced rats. These results reinforce the association between inflammation and glaucoma pathogenesis. Raised oxidative stress levels further induce an inflammatory response [17]. The inhibitory effect of myricetin on ROS levels may, in part, be responsible for the observed decrease in inflammatory mediators.
ROS-mediated oxidative stress induces abnormal cell signalling [10, 71]. ROS has been found to activate growth factors such as TGF-β that, in turn, can induce oxidative stress leading to further cellular damage [71, 72]. Raised VEGF levels have been found in glaucomatous TM cells [73]. Studies have shown that cultured human TM cells exposed to TGF-β1/2 exhibit phenotypic changes [6, 74] and upregulation of ECM proteins, such as α-SM-actin, which are associated with glaucoma pathogenesis [9, 75]. In keeping with previous studies, we found substantially elevated expressions of TGF-β1/2 and VEGF in the POAG group. These TM cell changes suggest that raised oxidative stress levels could be a contributing factor to the upregulation of these inflammatory markers. Previous in vitro studies have reported that myricetin exposure at various concentrations
between 10 and 100μM decrease the proliferation and migration of human retinal pigment epithelial (RPE) cells, and reduce VEGF secretion into the eye [76]. Our study demonstrated that myricetin administration at the tested doses markedly supressed VEGF expression.
Downregulation of TGF-β1/2 and VEGF upon myricetin exposure suggests that myricetin has a significant role in modulating the expression of major proteins within the ocular environment. These observations further illustrate the anti-oxidant and anti-inflammatory effects of myricetin. The reduction in TGF-β1/2 and VEGF levels could be due to the direct effects of myricetin, or due to myricetin-mediated decreases in ROS levels.
Along with the continued activation of inflammatory responses, TM cells exhibit decreased cellularity and upregulation of cellular senescence markers [22, 77]. Studies suggest that increased oxidative stress can result in decreased TM cellularity [77, 78] and activation of senescence markers such as β-galactosidase [8], p16, and p21 [21]. This implies that apoptosis and cellular senescence contribute to the loss of TM function in glaucoma. We noticed a near 2.5-fold enhancement of SA-β-galactosidase activity with elevated expression of p16 and p21 in the TM cells of POAG patients. As well, IL-6 and IL-8 have been found to be involved in the induction of senescence [79]. Exposure of TM cells to myricetin leads to a significant decrease in the expressions of p16 and p21 along with reduced SA-β-gal activity, suggesting a protective effect. Luna et al. [23] reported similar findings in POAG TM cells treated with resveratrol. We believe that treatment with myricetin can reduce IOP and restore redox balance within the eye by effectively modulating senescence markers and inflammatory mediators.
Conclusion
The results of our study suggest that myricetin is efficacious in preventing oxidative stress-induced senescence and inflammation in POAG TM cells. Myricetin could be further explored as a potential candidate for the management of glaucoma. Further studies are required to explore fully the mechanisms and molecular events involved in the protective effects of myricetin.
References
- [1].Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol. 2013;13:36. doi: 10.1016/j.coph.2012.09.003. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53:S3. doi: 10.1016/j.survophthal.2008.08.006. –. [DOI] [PubMed] [Google Scholar]
- [3].Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465. doi: 10.1016/s1350-9462(03)00026-0. –. [DOI] [PubMed] [Google Scholar]
- [4].Weinreb RN, Khaw PT.. Primary open-angle glaucoma. Lancet. 2004;363:1711. doi: 10.1016/S0140-6736(04)16257-0. –. [DOI] [PubMed] [Google Scholar]
- [5].Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007;52:S101. doi: 10.1016/j.survophthal.2007.08.002. –. [DOI] [PubMed] [Google Scholar]
- [6].Lütjen-Drecoll E.. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1. doi: 10.1016/j.exer.2005.02.008. –. [DOI] [PubMed] [Google Scholar]
- [7].Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769. doi: 10.1016/j.exer.2008.11.025. –. [DOI] [PubMed] [Google Scholar]
- [8].Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308:346. doi: 10.1016/s0006-291x(03)01385-8. –. [DOI] [PubMed] [Google Scholar]
- [9].Fuchshofer R, Yu AH, Welge-Lüssen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta 2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715. doi: 10.1167/iovs.06-0226. –. [DOI] [PubMed] [Google Scholar]
- [10].He Y, Leung KW, Zhang YH, Duan S, Zhong XF, Jiang RZ. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49:1447. doi: 10.1167/iovs.07-1361. et al. –. [DOI] [PubMed] [Google Scholar]
- [11].Izzotti A, Bagnis A. Saccà SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105. doi: 10.1016/j.mrrev.2005.11.001. –. [DOI] [PubMed] [Google Scholar]
- [12].Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9:963. doi: 10.3109/02713689009069932. –. [DOI] [PubMed] [Google Scholar]
- [13].Ohia SE, Opere CA, Leday AM. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res. 2005;579:22. doi: 10.1016/j.mrfmmm.2005.03.025. –. [DOI] [PubMed] [Google Scholar]
- [14].Baleriola J, García-Feijoo J, Martínez-de-la-Casa JM, Fernández-Cruz A, de la Rosa EJ. Fernández-Durango R. Apoptosis in the trabecular meshwork of glaucomatous patients. Mol Vis. 2008;14:1513. –. [PMC free article] [PubMed] [Google Scholar]
- [15].Sacca SC, Izzotti A, Rossi P, Traverso C.. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389. doi: 10.1016/j.exer.2006.10.008. –. [DOI] [PubMed] [Google Scholar]
- [16].Sacca SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385. doi: 10.1016/S0079-6123(08)01127-8. –. [DOI] [PubMed] [Google Scholar]
- [17].Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFβ2- induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226. doi: 10.1167/iovs.05-1060. –. [DOI] [PubMed] [Google Scholar]
- [18].Kokotas H, Kroupis C, Chiras D, Grigoriadou M, Lamnissou K, Petersen MB. Biomarkers in primary open angle glaucoma. Clin Chem Lab Med. 2012;50:2107. doi: 10.1515/cclm-2012-0048. et al. –. [DOI] [PubMed] [Google Scholar]
- [19].Huang W, Chen S, Gao X, Yang M, Zhang J, Li X. Inflammation-related cytokines of aqueous humor in acute primary angle-closure eyes. Invest Ophthalmol Vis Sci. 2014;55:1088. doi: 10.1167/iovs.13-13591. et al. –. [DOI] [PubMed] [Google Scholar]
- [20].Taurone S, Ripandelli G, Pacella E, Bianchi E, Plateroti AM, De Vito S. Potential regulatory molecules in the human trabecular meshwork of patients with glaucoma: immunohistochemical profile of a number of inflammatory cytokines. Mol Med Rep. 2015;11:1384. doi: 10.3892/mmr.2014.2772. et al. –. [DOI] [PubMed] [Google Scholar]
- [21].Fatma N, Kubo E, Toris CB, Stamer WD, Camras CB, Singh DP. PRDX6 attenuates oxidative stress- and TGFβ-induced abnormalities of human trabecular meshwork cells. Free Radic Res. 2009;43:783. doi: 10.1080/10715760903062887. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745. doi: 10.1016/j.exger.2005.06.005. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luna C, Li G, Liton PB, Qiu J, Epstein DL, Challa P. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47:198. doi: 10.1016/j.fct.2008.10.029. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Macip S, Igarashi M, Fang L, Chen A, Pan ZQ, Lee SW. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 2002;21:2180. doi: 10.1093/emboj/21.9.2180. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Petrova NV, Velichko AK, Razin SV, Kantidze OL.. Small molecule compounds that induce cellular senescence. Aging Cell. 2016;15:999. doi: 10.1111/acel.12518. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grover AK, Samson SE. Antioxidants and vision health: facts and fiction. Mol Cell Biochem. 2014;388:173. doi: 10.1007/s11010-013-1908-z. –. [DOI] [PubMed] [Google Scholar]
- [27].Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727. doi: 10.1093/ajcn/79.5.727. –. [DOI] [PubMed] [Google Scholar]
- [28].Milbury PE.. Flavonoid Intake and Eye Health. J Nutr Gerontol Geriatr. 2012;31:254. doi: 10.1080/21551197.2012.698221. –. [DOI] [PubMed] [Google Scholar]
- [29].Chu KO, Chan KP, Wang CC, Chu CY, Li WY, Choy KW. Green tea catechins and their oxidative protection in the rat eye. J Agric Food Chem. 2010;58:1523. doi: 10.1021/jf9032602. et al. –. [DOI] [PubMed] [Google Scholar]
- [30].Khoo NK, White CR, Pozzo-Miller L, Zhou F, Constance C, Inoue T. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic Biol Med. 2010;49:339. doi: 10.1016/j.freeradbiomed.2010.04.022. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Choi HN, Kang MJ, Lee SJ, Kim JI. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr Res Pract. 2014;8:544. doi: 10.4162/nrp.2014.8.5.544. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Semwal DK, Semwal RB, Combrinck S, Viljoen A. Myricetin: A dietary molecule with diverse biological activities. Nutrients. 2016;8:90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Xu HX, Lee SF. Activity of plant flavonoids against antibiotic-resistant bacteria. Phytother Res. 2001;15:39. doi: 10.1002/1099-1573(200102)15:1<39::aid-ptr684>3.0.co;2-r. –. [DOI] [PubMed] [Google Scholar]
- [34].Kang SS, Kim JG, Lee TH, Oh KB. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol Pharm Bull. 2006;29:1751. doi: 10.1248/bpb.29.1751. –. [DOI] [PubMed] [Google Scholar]
- [35].Chang CJ, Tzeng TF, Liou SS, Chang YS, Liu IM. Myricetin increases hepatic peroxisome proliferator-activated receptor - protein expression and decreases plasma lipids and adiposity in rats. Evid Based Complement Alternat Med. 2012;2012:787152. doi: 10.1155/2012/787152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000;20:347. –. [PubMed] [Google Scholar]
- [37].Moreno MC, Marcos HJ, Oscar Croxatto J, Sande PH, Campanelli J, Jaliffa CO. A new experimental model of glaucoma in rats through intracameral injections of hyaluronic acid. Exp Eye Res. 2005;81:71. doi: 10.1016/j.exer.2005.01.008. et al. –. [DOI] [PubMed] [Google Scholar]
- [38].Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34:363. –. [PubMed] [Google Scholar]
- [39].Moore CG, Epley D, Milne ST, Morrison JC. Long-term non-invasive measurement of intraocular pressure in the rat eye. Curr Eye Res. 1995;14:711. doi: 10.3109/02713689508998499. –. [DOI] [PubMed] [Google Scholar]
- [40].Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48. doi: 10.1001/archopht.121.1.48. –. [DOI] [PubMed] [Google Scholar]
- [41].Chhunchha B, Singh P, Stamer WD, Singh DP. Prdx6 retards senescence and restores trabecular meshwork cell health by regulating reactive oxygen species. Cell Death Discov. 2017;3:17060. doi: 10.1038/cddiscovery.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701. doi: 10.1001/archopht.120.6.701. et al. –. [DOI] [PubMed] [Google Scholar]
- [43].Beidoe G, Mousa SA. Current primary open-angle glaucoma treatments and future directions. Clinical Ophthalmology (Auckland, NZ) 2012;6:1699. doi: 10.2147/OPTH.S32933. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268. doi: 10.1001/archopht.120.10.1268. et al. –. [DOI] [PubMed] [Google Scholar]
- [45].Quigley HA. Glaucoma. Lancet. 2011;377:1367. doi: 10.1016/S0140-6736(10)61423-7. –. [DOI] [PubMed] [Google Scholar]
- [46].Sacca SC, Pulliero A, Izzotti A. The dysfunction of the trabecular meshwork during glaucoma course. J Cell Physiol. 2015;230:510. doi: 10.1002/jcp.24826. –. [DOI] [PubMed] [Google Scholar]
- [47].Ko ML, Peng PH, Hsu SY, Chen CF. Dietary deficiency of vitamin E aggravates retinal ganglion cell death in experimental glaucoma of rats. Curr Eye Res. 2010;35:842. doi: 10.3109/02713683.2010.489728. –. [DOI] [PubMed] [Google Scholar]
- [48].Razali N, Agarwal R, Agarwal P, Tripathy M, Kapitonova MY, Kutty MK. Topical trans-resveratrol ameliorates steroid-induced anterior and posterior segment changes in rats. Exp Eye Res. 2016;143:9. doi: 10.1016/j.exer.2015.09.014. et al. –. [DOI] [PubMed] [Google Scholar]
- [49].Ramdas WD, Wolfs RC, Kiefte-de Jong JC, Hofman A, de Jong PT, Vingerling JR. Nutrient intake and risk of open-angle glaucoma: the Rotterdam Study. Eur J Epidemiol. 2012;27:385. doi: 10.1007/s10654-012-9672-z. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Patel S, Mathan JJ, Vaghefi E, Braakhuis AJ. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841. doi: 10.1007/s00417-015-3168-y. –. [DOI] [PubMed] [Google Scholar]
- [51].Kang JH, Willett WC, Rosner B, Buys E, Wiggs JL, Pasquale LR. Association of dietary nitrate intake with primary open-angle glaucoma: a prospective analysis from the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016;134:294. doi: 10.1001/jamaophthalmol.2015.5601. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kang JH, Ivey KL, Boumenna T, Rosner B, Wiggs JL, Pasquale LR. Prospective study of flavonoid intake and risk of primary open-angle glaucoma. Acta Ophthalmol. 2018:14. doi: 10.1111/aos.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hodges LC, Kearse CE, Green K. Intraocular pressure-lowering activity of phenolic antioxidants in normotensive rabbits. Curr Eye Res. 1999;19:234. doi: 10.1076/ceyr.19.3.234.5320. –. [DOI] [PubMed] [Google Scholar]
- [54].Srour MA, Bilto YY, Juma M. Evaluation of different methods used to measure malonyldialdehyde in human erythrocytes. Clin Hemorheol Microcirc. 2000;23:23. –. [PubMed] [Google Scholar]
- [55].Stadtman ER, Oliver CN. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991;266:2005. –. [PubMed] [Google Scholar]
- [56].Stadtman ER.. Protein oxidation and aging. Science. 1992;257:1220. doi: 10.1126/science.1355616. –. [DOI] [PubMed] [Google Scholar]
- [57].Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313. doi: 10.1074/jbc.272.33.20313. –. [DOI] [PubMed] [Google Scholar]
- [58].Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3177. doi: 10.1167/iovs.05-0208. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490. doi: 10.1016/j.preteyeres.2006.07.003. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hondur G, Göktas E, Yang X, Al-Aswad L, Auran JD, Blumberg DM. Oxidative stress-related molecular biomarker candidates for glaucoma. Invest Ophthalmol Vis Sci. 2017;58:4078. doi: 10.1167/iovs.17-22242. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhao J, Wang S, Zhong W, Yang B, Sun L, Zheng Y. Oxidative stress in the trabecular meshwork (Review) Int J Mol Med. 2016;38:995. doi: 10.3892/ijmm.2016.2714. –. [DOI] [PubMed] [Google Scholar]
- [62].Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xu L, Chen JH, Li JJ, Luo L, Yang H, Zhang RX. The prevalence and its screening methods of primary open angle glaucoma in defined population-based study of rural and urban in Beijing. Zhonghua Yan Ke Za Zhi. 2004;40:726. et al. –. [PubMed] [Google Scholar]
- [64].Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458. doi: 10.1001/archopht.123.4.458. –. [DOI] [PubMed] [Google Scholar]
- [65].Fahmy HM, Saad EAES, Sabra NM, El-Gohary AA, Mohamed FF, Gaber MH. Treatment merits of Latanoprost/Thymoquinone - Encapsulated liposome for glaucomatus rabbits. Int J Pharm. 2018;548:597. doi: 10.1016/j.ijpharm.2018.07.012. –. [DOI] [PubMed] [Google Scholar]
- [66].Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A.. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152. doi: 10.1016/j.preteyeres.2011.11.002. –. [DOI] [PubMed] [Google Scholar]
- [67].Takai Y, Tanito M, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Invest Ophthalmol Vis Sci. 2012;53:241. doi: 10.1167/iovs.11-8434. –. [DOI] [PubMed] [Google Scholar]
- [68].Vohra R, Tsai JC, Kolko M. The role of inflammation in the pathogenesis of glaucoma. Surv Ophthalmol. 2013;58:311. doi: 10.1016/j.survophthal.2012.08.010. –. [DOI] [PubMed] [Google Scholar]
- [60].Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304. doi: 10.1038/85446. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang X, Schroeder A, Callahan EM, Coyle BM, Wang N, Erickson KA. Constitutive signalling pathway activity in trabecular meshwork cells from glaucomatous eyes. Exp Eye Res. 2006;82:968. doi: 10.1016/j.exer.2005.11.001. et al. –. [DOI] [PubMed] [Google Scholar]
- [71].Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/−mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFβ. Cell Death Differ. 2005;12:734. doi: 10.1038/sj.cdd.4401597. –. [DOI] [PubMed] [Google Scholar]
- [72].Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol. 2008;294:C842. doi: 10.1152/ajpcell.00540.2007. –. [DOI] [PubMed] [Google Scholar]
- [73].Micera A, Quaranta L, Esposito G, Floriani I, Pocobelli A, Saccà SC. Differential protein expression profiles in glaucomatous trabecular meshwork: An evaluation study on a small primary open angle glaucoma population. Adv Ther. 2016;33:252. doi: 10.1007/s12325-016-0285-x. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191. doi: 10.1167/iovs.06-0296. et al. –. [DOI] [PubMed] [Google Scholar]
- [75].Zhao X, Ramsey KE, Stephan DA, Russell P. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 2004;45:4023. doi: 10.1167/iovs.04-0535. –. [DOI] [PubMed] [Google Scholar]
- [76].Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol Vis. 2014;20:242. et al. –. [PMC free article] [PubMed] [Google Scholar]
- [77].Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and non-glaucomatous normals. Ophthalmology. 1984;91:564. doi: 10.1016/s0161-6420(84)34248-8. –. [DOI] [PubMed] [Google Scholar]
- [78].Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714. –. [PubMed] [Google Scholar]
- [79].Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019. doi: 10.1016/j.cell.2008.03.039. et al. –. [DOI] [PubMed] [Google Scholar]