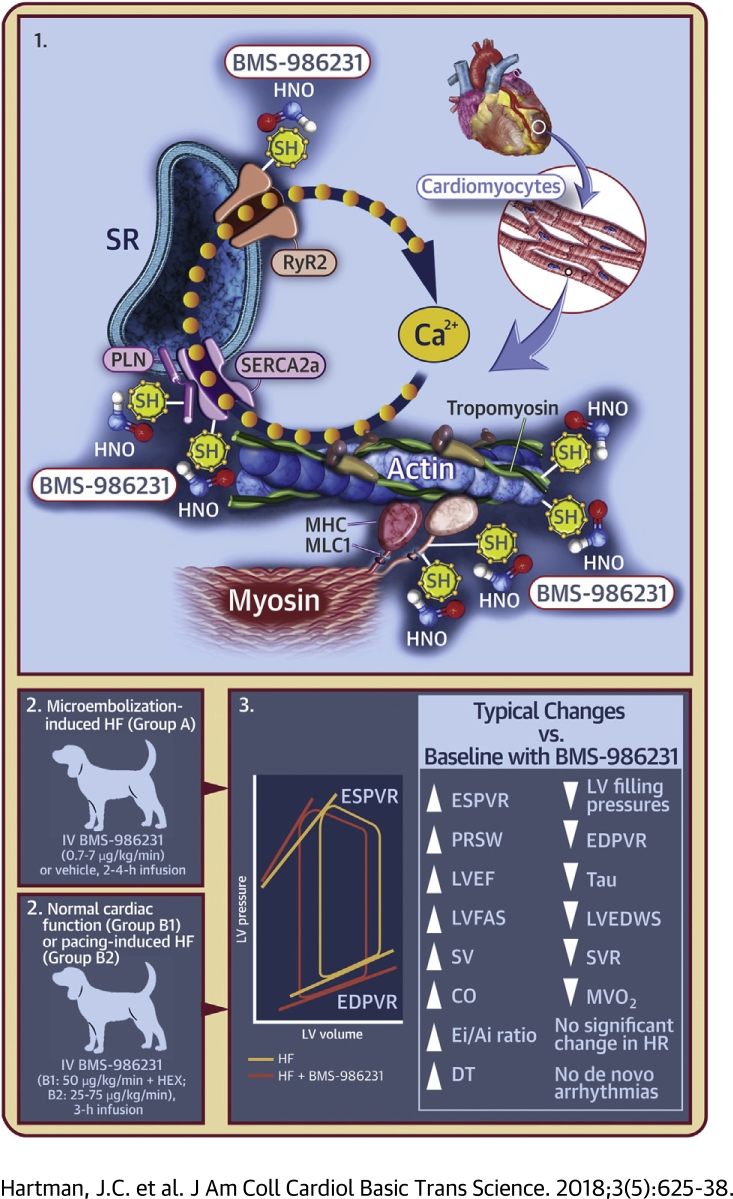
Visual Abstract
Key Words: canine, cardiomyopathies, heart failure, hemodynamics, nitroxyl
Abbreviations and Acronyms: DT, deceleration time of early mitral inflow velocity; EDPVR, end-diastolic pressure–volume relationship; Ei/Ai, the ratio of early-to-late filling time integrals; ESPVR, end-systolic pressure–volume relationship; HEX, Hextend (plasma volume-expanding solution); LVEDWS, left ventricular end-diastolic circumferential wall stress; LVEF, left ventricular ejection fraction; LVFAS, left ventricular fractional area shortening; MHC, myosin heavy chain; MLC1, myosin light chain 1; PRSW, pre-load-recruitable stroke work; RyR2, ryanodine receptor 2; SH, thiol group; SV, stroke volume; SVR, systemic vascular resistance; Tau, left ventricular relaxation time-constant
Highlights
-
•
Nitroxyl (HNO) enhances SR calcium uptake and release in cardiomyocytes, and improves myofilament calcium sensitivity without impacting L-type calcium channel or total SR calcium content.
-
•
The effects of the nitroxyl donor BMS-986231 on hemodynamics, left ventricular function, and pro-arrhythmic potential were assessed using canine models of HF.
-
•
BMS-986231 was associated with increased cardiac contractility and relaxation, as well as moderate vasodilatory effects; there was also a significant reduction in MVO2.
-
•
There were no clinically significant changes in HR, and no de novo arrhythmias were detected; BMS-986231 was also not associated with venotoxicity.
-
•
Thus, BMS-986231 has beneficial inotropic, lusitropic, and vasodilatory effects; clinical studies are ongoing.
Summary
The effects of the nitroxyl donor BMS-986231 on hemodynamics, left ventricular (LV) function, and pro-arrhythmic potential were assessed using canine heart failure models. BMS-986231 significantly (p < 0.05) increased LV end-systolic elastance, pre-load-recruitable stroke work, ejection fraction, stroke volume, cardiac output, ratio of early-to-late filling time integrals, and early mitral valve inflow velocity deceleration time. BMS-986231 significantly decreased LV filling pressures, end-diastolic stiffness, the time-constant of relaxation, end-diastolic wall stress, systemic vascular resistance, and myocardial oxygen consumption. BMS-986231 had little effect on heart rate and did not induce de novo arrhythmias. Thus, BMS-986231 has beneficial inotropic, lusitropic, and vasodilatory effects.
Heart failure (HF) affects approximately 26 million people worldwide and is the leading cause of hospitalizations in the developed world, comprising up to 4% of all admissions in the United States and Europe 1, 2. HF also accounts for 1% to 3% of total health care expenditures across the Americas and Western Europe (1). Acute decompensated HF is the most common reason for HF hospitalization (3). Of those hospitalized, 50% are readmitted within 6 months, and 17% to 45% die within 1 year of initial admission 1, 4. The median length of stay is 4 to 20 days, and the inpatient mortality rate is 4% to 30% (2).
There have been no major advances in therapies for acute HF (AHF) in recent decades, and current therapies have substantial limitations. Diuretics, although a cornerstone of AHF management, offer no direct myocardial effects and are known to cause electrolyte abnormalities and metabolic disturbances. Inotropes are associated with arrhythmias and increased mortality, and pure vasodilators can cause systemic hypotension 5, 6, 7, 8, 9. Thus, there is a need for new therapies that safely and effectively reduce cardiac load while enhancing cardiac output (CO). Accomplishing the latter safely has proven difficult because of the frequent reliance on therapies that are cyclic adenosine monophosphate (cAMP) stimulators, which are fraught with undesirable complications 5, 9, 10, 11.
Nitroxyl (HNO) donors are a class of molecules with unique biochemical and pharmacological properties that have been investigated over several years for their potential therapeutic applications. Although related to nitric oxide (NO), the pharmacological effects of HNO are different from NO; these effects produce arterial and venous dilation, as well as direct, beneficial, cAMP/protein kinase A-independent lusitropic and inotropic effects, in both the normal and failing myocardium 8, 10, 12, 13. HNO enhances sarcoplasmic reticular calcium uptake and release through modulation of sarcoplasmic reticulum calcium2+ adenosine triphosphatase (SERCA2a), phospholamban (PLN), and the ryanodine receptors. HNO donors also improve myofilament calcium sensitivity without affecting the L-type calcium channel or total sarcoplasmic reticular calcium content 8, 10, 14, 15, 16, 17.
Several exogenous HNO donors have been studied, but a clinically viable HNO therapy for AHF remains elusive. Angeli’s salt (Na2N2O3) decomposes rapidly to produce HNO and nitrite (which also has cardiovascular effects) under physiological conditions 12, 18, 19, 20. However, although it produces vasodilation and cAMP-independent inotropic and lusitropic effects in dogs, it is chemically unstable and not suitable for clinical use 18, 19, 21. CXL-1020 was a first-generation pure HNO donor (i.e., it did not generate nitrite in addition to HNO upon decomposition, but an inactive organic by-product, CXL-1051) and was shown to produce beneficial vasodilatory, inotropic, and lusitropic effects in normal and HF animal models; however, due to injection-site toxicity, its development was halted 8, 11. BMS-986231 (formerly CXL-1427) represents a second generation of HNO donor that is now in clinical development (22). BMS-986231 (C5H7NO4S) was originally developed by Cardioxyl Pharmaceuticals (Chapel Hill, North Carolina) and is a pH-sensitive molecule with a half-life of approximately 40 to 144 min in healthy humans (dose range 0.1 to 15 μg/kg/min) (23) and approximately 15 to 60 min in dogs (dose range 30 to 120 μg/kg/min) (Bristol-Myers Squibb, Princeton, New Jersey; data not shown). When administered intravenously, BMS-986231 nonenzymatically releases HNO and an inactive sulfinic acid by-product, BMT-284730.
Evaluation of this novel HNO donor molecule in established preclinical models of HF has not been described. The primary objective of these studies was to assess the effects of BMS-986231 on hemodynamics, left ventricular (LV) function, and pro-arrhythmic potential in 2 different canine models of HF, conducted at 2 separate U.S.-based study centers (see Methods).
Methods
The studies conformed to the Guidelines for Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (2011) (24) and were approved by the Institutional Animal Care and Use Committees of both the Henry Ford Health System (Detroit, Michigan) and QTest Labs, Inc. (Columbus, Ohio), which were the test sites.
Group A: Coronary microembolization-induced HF in dogs
This study was conducted in the Cardiovascular Research Laboratories at the Henry Ford Hospital (Detroit, Michigan). Seven male mongrel dogs underwent multiple sequential intracoronary microembolizations under general anesthesia to produce chronic LV dysfunction and HF. Microembolizations were discontinued when LV ejection fraction (LVEF), determined by ventriculography, was approximately 30%. This microembolization technique was previously validated (see Supplemental Material) 25, 26, 27.
While under general anesthesia, each animal received BMS-986231 (0.7, 2, and 7 μg/kg/min) and vehicle (15% Captisol [sulfobutylether-beta cyclodextrin; Ligand, Inc., San Diego, California] in sterile water), administered 1 week apart via continuous intravenous (IV) infusion over 4 h. The test compound was supplied by Cardioxyl Pharmaceuticals as a powder and formulated in 15% Captisol. Hemodynamic, ventriculographic, and echocardiographic measurements were recorded at baseline and at various time points until 5 h after infusion start (i.e., 4 h of infusion plus a 1-h washout period) (Supplemental Table 1). Peripheral venous blood samples were also collected.
All hemodynamic assessments, namely, arterial and LV pressures, peak rate of change of LV pressure during isovolumic contraction (LV peak +dP/dt) and relaxation (LV peak −dP/dt), LV end-diastolic pressure (LVEDP), CO, stroke volume (SV), and systemic vascular resistance (SVR), were made during left and right heart catheterizations. Ventriculographic measurements to determine LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), and LVEF were made during cardiac catheterization after completion of the hemodynamic measurements. Echocardiographic and Doppler studies were performed to determine LV fractional area shortening (LVFAS), LV end-diastolic circumferential wall-stress (LVEDWS), the ratio of early-to-late filling time integrals (Ei/Ai), and deceleration time (DT) of early mitral valve inflow velocity. With the 0.7- and 7-μg/kg/min infusions, myocardial oxygen consumption (MVO2) was assessed as described previously 8, 28, at baseline and again at 2 and 4 h (see Supplemental Material). Electrocardiography (ECG) was used to assess the QT interval throughout the study for determination of heart rate (HR).
As described previously (8), a separate cohort of 7 mongrel dogs with microembolization-induced HF was subjected to programmed ventricular stimulation (PVS) after a 2-h infusion of BMS-986231 7 μg/kg/min and vehicle control (see Supplemental Material). Each PVS session was terminated when it provoked ventricular fibrillation (VF) or a sustained monomorphic ventricular tachycardia (SVT) lasting >30 s. Threshold data for SVT or VF were quantified, in which progressively increasing scores indicated higher magnitudes of required stimulation (Supplemental Table 2). Hemodynamic and echocardiographic measurements were made at baseline, 2 h post-infusion before PVS protocol initiation, and again 15 min after protocol completion. Blinding was not used during the study.
Group B: Long-term instrumented dogs with normal cardiac function (B1) or pacing-induced cardiomyopathy (B2)
This study was conducted at QTest Labs, Inc. (Columbus, Ohio). All dogs were beagles and were surgically implanted with a radiotelemetry transmitter (TL11M3-D70-PCTP, Data Sciences Int., St. Paul, Minnesota) to provide systemic arterial blood and LV pressures, ECG, and body temperature. Each dog was also long-term instrumented with short-axis (endocardial) sono-micrometry crystals to allow hemodynamic load-independent, mechano-energetic assessments via pressure–volume relationships (PVRs) generated during brief and transient inferior vena cava occlusions. All dogs were fully conscious throughout the study.
Eight dogs with normal cardiac function (group B1) were administered BMS-986231 50 μg/kg/min IV over 3 h (as in group A, BMS-986231 was supplied in powder form and formulated in 15% Captisol). Due to the vasodilatory effects of HNO, this was followed by a 5 ml/kg IV bolus of plasma volume-expanding solution (Hextend [HEX], BioTime, Inc., Alameda, California) to restore cardiac preload and to confirm the load-independent effects of BMS-986231 on lusitropy and inotropy.
Six beagle dogs (group B2) were also subjected to an established overdrive cardiac pacing protocol to induce LV dysfunction and remodeling consistent with HF 8, 29. In short, the ventricles were asynchronously and continuously driven at 180 to 240 beats/min via an implanted pacemaker and ventricular lead. LV remodeling was confirmed by elevated N-terminal prohormone of brain natriuretic peptide levels after approximately 3 to 6 weeks of pacing. The dogs were then IV infused with 3 separate doses of BMS-986231 (25, 50, and 75 μg/kg/min) over 3 h.
Analog signals were digitally sampled (1,000 Hz) and recorded with a data acquisition system (IOX; EMKA Technologies, Falls Church, Virginia). ECG and pressure waveforms were continuously recorded, whereas HR, arterial pressure, and LV mechanical or geometric indexes were measured at baseline, and at 30, 60, 90, and 180 min after infusion start, with an additional measurement after the HEX bolus in normal dogs and at 1 h post-infusion in paced dogs. Blood samples were collected pre-dose and 3 h after infusion start.
The following indexes were recorded at the designated time points in both studies: 1) HR and systolic (SAP), diastolic (DAP), and mean (MAP) arterial pressures; and 2) LV mechanical and geometrical indexes from the LV pressure waveform as LV end-systolic pressure (LVESP), LVEDP, LV peak +dP/dt and −dP/dt, Tau, and cardiac volumes (LVESV, LVEDV, SV), as determined from integrated dimension signals measured from the myocardial crystals.
Furthermore, the following measurements were derived from LV PVRs generated during brief periods of preload reduction: 1) inotropic indexes (SV, pressure-volume area [PVA], and stroke work [SW]); 2) load-independent inotropy (preload-recruitable stroke work [PRSW], end-systolic elastance [ESPVR]); 3) estimated (linear) end-diastolic stiffness (EDPVR); and 4) LVESP and SV relationship (arterial elastance [Ea]). Blinding was not used during the study.
Statistical analyses
Within-group comparisons were made using repeated-measures analysis of variance. For group A, pairwise comparisons were made using the Student-Newman-Keuls test. For group B, post hoc comparisons against baseline were made using the Holm-Šidák method.
The T-statistic for 2 independent means was used to compare treatment effects (p < 0.05).
SVT and VF threshold scores with BMS-986231 7 μg/kg/min and vehicle were compared using the Student paired t-test (p < 0.05).
Results
Group A: Coronary microembolization-induced HF
All dogs met the LVEF entry criteria of approximately 30% (range 22% to 36%), and all dogs that entered into the 4-h study completed all 4 treatment arms. There were no adverse events (including venotoxicity) during or after drug or placebo administration.
BMS-986231 0.7- to 7-μg/kg/min infusion (4 h)
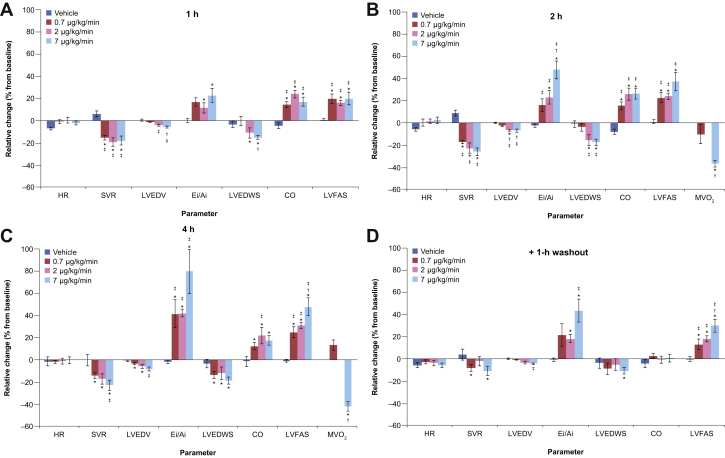
Parameter values at baseline and 4 h are summarized in Table 1; mean percentage changes in key parameters from baseline to the end of the washout period are shown in Figures 1A to 1D. With vehicle, all measured or calculated variables were stable from baseline through 4 h. Statistically, LVEDP was significantly reduced at the 7-μg/kg/min dose infusion (mean percentage change from baseline: −14%; p < 0.05). Compared with baseline, BMS-986231 was associated with significant dose-dependent decreases in LVESV over 4 h (−12% to −19%; p < 0.05) and with a small reduction in LVEDV (−4% to −9%), which were statistically significant for the 2 lower doses (Table 1, Figure 1C). In contrast, BMS-986231 was associated with relative increases versus baseline in LVEF (+18% to +30%) and LVFAS (+25% to +48%) over the same time period. Furthermore, BMS-986231 was associated with significant increases in SV, CO, and mean aortic flow compared with baseline (Table 1, Figures 1A to 1C). Effects were not dose-dependent. For some parameters (LVESV, LVFAS, and mean aortic flow), statistically significant increases versus baseline were still seen across all 3 doses 1 h after cessation of dosing; however, for other parameters, the statistical significance of changes versus baseline 1 h after dosing cessation was inconsistent across doses (Figure 1D). LV peak +dP/dt was not altered from baseline at any dose infusion level.
Table 1.
Parameters at Baseline and After 4 h for Vehicle and BMS-986231 0.7, 2, and 7 μg/kg/min
| Vehicle |
LV Function (n = 7) |
|||||||
|---|---|---|---|---|---|---|---|---|
| BMS-986231 (0.7 μg/kg/min) |
BMS-986231 (2 μg/kg/min) |
BMS-986231 (7 μg/kg/min) |
||||||
| Baseline | 4 h (%Δ From Baseline) | Baseline | 4 h (%Δ From Baseline) | Baseline | 4 h (%Δ From Baseline) | Baseline | 4 h (%Δ From Baseline) | |
| HR, beats/min | 83 ± 1 | 82 ± 3 (−2 ± 4) | 77 ± 1 | 76 ± 1 (−2 ± 1) | 87 ± 3 | 86 ± 4 (−1 ± 3) | 84 ± 2 | 84 ± 2 (−0.3 ± 3) |
| MAP, mm Hg | 76 ± 2 | 73 ± 2 (−4 ± 1) | 76 ± 2 | 72 ± 3 (−5 ± 2) | 78 ± 2 | 76 ± 2 (−1 ± 3) | 79 ± 3 | 70 ± 3∗† (−11 ± 3) |
| SAP, mm Hg | 89 ± 2 | 87 ± 2 (−2 ± 0.9) | 92 ± 2 | 89 ± 2 (−3 ± 2) | 90 ± 3 | 89 ± 2 (−1 ± 2) | 91 ± 3 | 86 ± 3 (−6 ± 3) |
| LVEDP, mm Hg | 14 ± 0.4 | 14 ± 0.6 (0 ± 3) | 13 ± 0.5 | 13 ± 0.6 (−3 ± 3) | 14 ± 0.4 | 13 ± 0.5 (−6 ± 4) | 14 ± 0.3 | 12 ± 0.8∗‡ (−14 ± 4) |
| LVEDV, ml | 85 ± 4 | 84 ± 4 (−1 ± 0.7) | 83 ± 5 | 80 ± 5∗ (−4 ± 1) | 86 ± 4 | 81 ± 4∗ (−6 ± 2) | 85 ± 4 | 78 ± 4‡ (−9 ± 2) |
| LVESV, ml | 61 ± 4 | 60 ± 4 (−1 ± 1) | 58 ± 5 | 51 ± 4∗‡ (−12 ± 0.9) | 61 ± 4 | 50 ± 2∗†‡ (−18 ± 2) | 60 ± 4 | 48 ± 3∗†‡ (−19 ± 2) |
| SV, ml | 24 ± 0.9 | 24 ± 0.8 (0.1 ± 1) | 25 ± 1 | 29 ± 2∗‡ (14 ± 3) | 25 ± 1 | 31 ± 2∗‡ (23 ± 7) | 25 ± 0.9 | 30 ± 1∗‡ (18 ± 2) |
| CO, l/min | 2.01 ± 0.06 | 1.97 ± 0.07 (−2 ± 5) | 1.94 ± 0.07 | 2.18 ± 0.13∗ (12 ± 3) | 2.20 ± 0.08 | 2.67 ± 0.16∗‡ (22 ± 8) | 2.11 ± 0.06 | 2.47 ± 0.11∗ (17 ± 5) |
| LVEF, % | 29 ± 1 | 29 ± 1 (2 ± 1) | 31 ± 2 | 36 ± 2∗‡ (18 ± 2) | 30 ± 1 | 38 ± 1∗†‡ (30 ± 6) | 30 ± 0.6 | 38 ± 0.7∗†‡ (30 ± 2) |
| LVFAS, % | 28 ± 2 | 27 ± 2 (−1 ± 2) | 27 ± 2 | 33 ± 2∗‡ (25 ± 5) | 26 ± 2 | 35 ± 2∗‡ (31 ± 3) | 26 ± 2 | 37 ± 2∗†‡ (48 ± 8) |
| Mean aortic flow, ml/s | 27 ± 2 | 27 ± 2 (−0.2 ± 2) | 27 ± 2 | 33 ± 2∗‡ (25 ± 6) | 27 ± 2 | 34 ± 3∗‡ (24 ± 4) | 27 ± 2 | 36 ± 3∗‡ (32 ± 5) |
| SVR (dynes·s·cm−5) | 3,060 ± 141 | 3,009 ± 120 (−0.5 ± 6) | 3,164 ± 170 | 2,706 ± 165∗ (−15 ± 3) | 2,847 ± 129 | 2,354 ± 189∗ (−17 ± 5) | 3,002 ± 114 | 2,298 ± 143∗‡ (−23 ± 5) |
| LV peak +dP/dt, mm Hg/s | 1,476 ± 85 | 1,595 ± 105 (10 ± 10) | 1,442 ± 102 | 1,517 ± 98 (6 ± 4) | 1,482 ± 110 | 1,560 ± 79 (7 ± 5) | 1,435 ± 75 | 1,458 ± 85 (3 ± 6) |
| Ei/Ai | 4.2 ± 0.4 | 4.1 ± 0.4 (−2 ± 2) | 4.1 ± 0.3 | 5.7 ± 0.6∗‡ (41 ± 13) | 4.1 ± 0.3 | 5.8 ± 0.5∗‡ (42 ± 4) | 3.6 ± 0.4 | 6.3 ± 0.7∗‡ (80 ± 20) |
| DT, ms | 77 ± 2 | 77 ± 2 (−0.3 ± 2) | 75 ± 2 | 86 ± 2∗‡ (14 ± 2) | 74 ± 3 | 87 ± 3∗‡ (17 ± 5) | 76 ± 2 | 89 ± 2∗‡ (16 ± 2) |
| LVEDWS, g/cm2 | 69 ± 6 | 67 ± 6 (−4 ± 4) | 65 ± 7 | 55 ± 6∗ (−14 ± 3) | 69 ± 8 | 58 ± 3∗ (−12 ± 6) | 70 ± 5 | 57 ± 4∗ (−19 ± 4) |
| MVO2, μmol/min | NT | NT | 52 ± 12 | 61 ± 13 (13 ± 5) | NT | NT | 170 ± 11 | 97 ± 7∗† (−42 ± 5) |
Values are mean ± SEM.
Δ = change; CO = cardiac output; DT = deceleration time of early mitral inflow velocity; Ei/Ai = ratio of Ei (time−velocity integral of the mitral inflow velocity waveform representing early filling) to Ai (time−velocity integral representing left atrial contraction); HR = heart rate; LV = left ventricular; LVEDP = LV end-diastolic pressure; LVEDV = LV end-diastolic volume; LVEDWS = LV end-diastolic circumferential wall stress; LVEF = LV ejection fraction; LVESV = LV end-systolic volume; LVFAS = LV fractional area shortening; LV peak +dP/dt = peak rate of change of LV pressure during isovolumic contraction; MAP = mean arterial pressure; MVO2 = myocardial oxygen consumption; NT = not tested; SAP = systolic arterial pressure; SV = stroke volume; SVR = systemic vascular resistance.
p < 0.05 vs. baseline.
p < 0.05 vs. 0.7-μg/kg/min dose infusion.
p < 0.05 vs. vehicle.
Figure 1.
Percentage Mean Changes in Parameters
(A to D) Percentage mean changes in parameters with BMS-986231 (0.7, 2, 7 μg/kg/min) and vehicle over time. All values shown are mean ± SEM. *p < 0.05 versus baseline; †p < 0.05 versus 0.7 μg/kg/min dose infusion; ‡p < 0.05 versus vehicle. CO = cardiac output; Ei/Ai = ratio of Ei (time-velocity integral of the mitral inflow velocity waveform representing early filling) to Ai (time-velocity integral representing left atrial contraction); HR = heart rate; LVEDV = left ventricular end-diastolic volume; LVEDWS = left ventricular end-diastolic circumferential wall stress; LVFAS = left ventricular fractional area shortening; MVO2 = myocardial oxygen consumption; SVR = systemic vascular resistance.
At 4 h, BMS-986231 was associated with significant increases of 41% to 80% in the Ei/Ai ratio from baseline; a significant increase in DT from baseline across all doses was also seen (Table 1, Figure 1C). The effects on Ei/Ai ratio (Figure 1D) and DT were sustained through the 1-h washout period. A significant decrease in LVEDWS versus baseline over 4 h was also observed (−12% to −19%) (Table 1, Figure 1C). There was no significant dose dependency for these endpoints. HR was not significantly affected by BMS-986231 infusion, regardless of dose (p = NS) (Table 1).
BMS-986231 resulted in a numerically but non-statistically significant reduction in SAP, and a drop in MAP that reached significance with the 7-μg/kg/min dose infusion (−11%) (Table 1). SVR was significantly reduced versus baseline across all doses at 4 h (−15% to −23%), with significant reductions also seen for all doses at the 1- and 2-h intervals (Table 1, Figures 1A to 1C). At both 2 and 4 h, MVO2 was significantly (p < 0.05) decreased with the highest BMS-986231 dose (Figures 1B and 1C).
No changes in QTc were observed with BMS-986231 for 4 h with 1 h of washout. No de novo ventricular or atrial arrhythmias were recorded during BMS-986231 or vehicle infusion.
For the 4-h study, blood levels of BMS-986231 (and its principal, inactive by-product BMT-284730) with the 0.7- and 2-μg/kg/min doses accumulated between 2 and 4 h (Table 2). With the 7-μg/kg/min dose infusion, 2- and 4-h levels of BMS-986231 were equivalent, whereas BMT-284730 levels continued to accumulate. At 1 h post-infusion, BMS-986231 levels were 70% to 80% lower across all doses than at 4 h; BMT-284730 levels were maintained at 1 h post-infusion (Table 2).
Table 2.
Plasma Concentrations of BMS-986231 and its Principal (Inactive) Metabolite, BMT-284730, in Groups A and B2 Animals at Specified Time Intervals After the Start of BMS-986231 Infusion
| BMS-986231 Infusion (μg/kg/min) | Plasma Concentration (ng/ml) |
||||
|---|---|---|---|---|---|
| +2 h | +3 h | +4 h | Washout (+1 h Post-Infusion) | ||
| Group A: Coronary microembolization-induced heart failure | |||||
| 0.7 | BMS-986231 | 59 ± 12 | N/A | 137 ± 20 | 42 ± 7 |
| BMT-284730 | 21 ± 3 | N/A | 48 ± 6 | 47 ± 7 | |
| 2 | BMS-986231 | 102 ± 15 | N/A | 207 ± 46 | 46 ± 8 |
| BMT-284730 | 58 ± 4 | N/A | 127 ± 11 | 119 ± 11 | |
| 7 | BMS-986231 | 489 ± 53 | N/A | 490 ± 41 | 101 ± 12 |
| BMT-284730 | 233 ± 15 | N/A | 466 ± 34 | 407 ± 28 | |
| Group B2: Pacing-induced cardiomyopathy | |||||
| 25 | BMS-986231 | N/A | 806 ± 80 | — | — |
| BMT-284730 | N/A | 1,200 ± 118 | — | — | |
| 50 | BMS-986231 | N/A | 1,272 ± 72 | — | — |
| BMT-284730 | N/A | 2,390 ± 314 | — | — | |
| 75 | BMS-986231 | N/A | 1,790 ± 159 | — | — |
| BMT-284730 | N/A | 3,868 ± 297 | — | — | |
NA = not available; — not applicable.
Values are mean ± SD. N = 6 plasma samples per dose infusion.
Dogs that underwent programmed ventricular stimulation after BMS-986231 7-μg/kg/min infusion (2 h)
Hemodynamic, echocardiographic, and ventriculographic findings with BMS-986231 7 μg/kg/min over 2 h in the 7 dogs that underwent PVS were broadly similar to those seen in the dose-escalation component of the study (Table 3). LVESV was significantly decreased, with little effect on LVEDV. Significant increases versus baseline were seen in LVEF, LVFAS, SV, and CO (Table 3). There was no significant change in HR with BMS-986231 versus baseline, and there was minimal effect on SAP and MAP. Vehicle administration was not associated with any effect on blood pressure or HR, and also had no effect on LV functional measures. During PVS, BMS-986231 increased the mean threshold score for SVT or VF 2-fold versus vehicle (18 vs. 9) and had no impact on subsequent cardioversion to restore sinus rhythm. Subsequently, all animals were successfully cardioverted.
Table 3.
Parameters at Baseline and 2 h in Animals That Underwent Programmed Ventricular Stimulation (vehicle and BMS-986231 7 μg/kg/min)
| Extrastimuli-Provoked VF or SVT >30 s (n = 7) |
||||
|---|---|---|---|---|
| Vehicle |
BMS-986231 (7 μg/kg/min) |
|||
| Baseline | 2 h (%Δ from baseline) | Baseline | 2 h (%Δ from baseline) | |
| HR, beats/min | 83 ± 3 | 81 ± 2 (−2 ± 4) | 83 ± 1 | 81 ± 2 (−2 ± 4) |
| MAP, mm Hg | 80 ± 2 | 80 ± 2 (0.9 ± 2) | 78 ± 2 | 81 ± 3 (3 ± 5) |
| SAP, mm Hg | 94 ± 2 | 93 ± 2 (−0.5 ± 2) | 90 ± 1 | 92 ± 3 (2 ± 5) |
| DAP, mm Hg | 69 ± 2 | 69 ± 2 (1 ± 3) | 69 ± 2 | 70 ± 3 (3 ± 6) |
| LVEDV, ml | 82 ± 3 | 82 ± 2 (−0.1 ± 0.6) | 82 ± 2 | 81 ± 2 (−2 ± 1) |
| LVESV, ml | 57 ± 2 | 57 ± 2 (−0.1 ± 1) | 57 ± 2 | 45 ± 2∗ (−20 ± 2†) |
| SV, ml | 26 ± 1 | 26 ± 1 (−0.1 ± 3) | 26 ± 1 | 35 ± 1∗ (37 ± 4†) |
| CO, l/min | 2.12 ± 0.11 | 2.08 ± 0.14 (−1.4 ± 6.8) | 2.13 ± 0.09 | 2.85 ± 0.09∗ (35.1 ± 6.7†) |
| LVEF, % | 31 ± 1 | 31 ± 1 (−0.4 ± 3) | 31 ± 1 | 44 ± 1∗ (42 ± 5†) |
| LVFAS, % | 32 ± 1 | 33 ± 1 (3 ± 2) | 33 ± 2 | 41 ± 2∗ (27 ± 5†) |
| Threshold score | — | 9 ± 0.7 | — | 18 ± 3∗ |
| Conversion by DC shock | — | 7/7 (100%) | — | 7/7 (100%) |
Values are mean ± SEM or n/N (%), unless otherwise indicated.
DAP = diastolic arterial pressure; DC = direct current; SVT = sustained monomorphic ventricular tachycardia; VF = ventricular fibrillation; other abbreviations as in Table 1.
p < 0.05 vs. baseline.
p < 0.05 vs. percentage change in vehicle.
Group B: Normal cardiac function (B1) and pacing-induced cardiomyopathy (B2)
To determine more directly whether BMS-986231 enhanced cardiac contractility in vivo, pressure-volume analysis were performed in conscious dogs before and during treatment in both control (i.e., healthy) conditions (B1) and after pacing-induced HF (B2).
Dogs with normal cardiac function (B1)
Hemodynamic, functional, and geometric parameters at baseline were within the normal physiological range (Table 4).
Table 4.
Parameters at Baseline and After 3 h for BMS-986231 50 μg/kg/min and BMS-986231 50 μg/kg/min + HEX, and at Baseline and After 3 h for BMS-986231 25, 50, and 75 μg/kg/min
| Animals With Normal Cardiac Function (n = 8) (%Δ From Baseline) |
Animals With Pacing-Induced Cardiomyopathy (n = 6) (%Δ From Respective Baselines) |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | 50 μg/kg/min (3 h) | 50 μg/kg/min (3 h + HEX) | Baseline (Dose, μg/kg/min) | 25 μg/kg/min (3 h) | 50 μg/kg/min (3 h) | 75 μg/kg/min (3 h) | |
| CO, l/min | 1.9 ± 0.1 | 1.9 ± 0.1 (2 ± 2) | 2.2 ± 0.2∗ (17 ± 3) | 1.4 ± 0.1 (25) 1.5 ± 0 (50) 1.5 ± 0 (75) |
1.5 ± 0.1∗ (14 ± 1) | 1.8 ± 0∗† (19 ± 2) | 1.8 ± 0.1∗† (20 ± 2) |
| SV, ml | 19 ± 1 | 20 ± 1 (5 ± 3) | 22 ± 0.7∗ (16 ± 4) | 14 ± 0.6 (25) 15 ± 0.6 (50) 14 ± 0.3 (75) |
16 ± 0.8∗ (18 ± 1) | 18 ± 0.5∗ (20 ± 3) | 18 ± 0.7∗ (28 ± 5) |
| HR, beats/min | 100 ± 8 | 96 ± 8 (−4 ± 3) | 101 ± 8 (1 ± 5) | 100 ± 5 (25) 103 ± 5 (50) 109 ± 4 (75) |
94 ± 5∗ (−5 ± 2) | 102 ± 5 (−1 ± 2) | 102 ± 5∗ (−6 ± 2) |
| SW, mm Hg • l | 2 ± 0.2 | 2 ± 0.2∗ (−11 ± 4) | 2 ± 0.2 (−5 ± 2) | 1 ± 0.1 (25) 1 ± 0.1 (50) 1 ± 0 (75) |
1 ± 0.1 (12 ± 4) | 1 ± 0 (5 ± 7) | 1 ± 0.1∗ (10 ± 5) |
| LVESP, mm Hg | 113 ± 4 | 93 ± 5∗ (−18 ± 2) | 93 ± 5∗ (−17 ± 2) | 98 ± 4 (25) 107 ± 5 (50) 108 ± 2 (75) |
90 ± 4∗ (−8 ± 2) | 92 ± 3∗ (−14 ± 3) | 90 ± 3∗ (−17 ± 1) |
| LVEDP, mm Hg | 7 ± 1 | 3 ± 1∗ (−63 ± 17) | 7 ± 1‡ (0 ± 19) | 23 ± 1 (25) 26 ± 2 (50) 25 ± 2 (75) |
20 ± 2∗ (−14 ± 3) | 21 ± 2∗ (−19 ± 4) | 18 ± 1∗ (−24 ± 6) |
| LV peak +dP/dt, mm Hg/s | 2,983 ± 167 | 3,412 ± 215∗ (14 ± 4) | 3,337 ± 177 (13 ± 5) | 1,789 ± 130 (25) 1,936 ± 217 (50) 1,777 ± 140 (75) |
1,945 ± 142 (9 ± 3) | 1,975 ± 182 (3 ± 2) | 1,805 ± 160 (1 ± 2) |
| LVEF, % | 65 ± 2 | 73 ± 2∗ (12 ± 1) | 73 ± 2∗ (12 ± 1) | 37 ± 0.9 (25) 38 ± 0.8 (50) 36 ± 0.6 (75) |
45 ± 2∗ (23 ± 2) | 48 ± 0.8∗ (26 ± 5) | 51 ± 2∗† (41 ± 6) |
| LVEDV, ml | 29 ± 2 | 27 ± 2∗ (−6 ± 2) | 30 ± 1 (3 ± 3) | 37 ± 0.9 (25) 39 ± 0.8 (50) 38 ± 0.5 (75) |
36 ± 1 (−4 ± 1) | 37 ± 1∗ (−5 ± 1) | 35 ± 0.8∗ (−9 ± 2) |
| Tau, ms | 17 ± 1 | 16 ± 0.8∗ (−6 ± 1) | 15 ± 0.8∗ (−10 ± 3) | 18 ± 0.4 (25) 17 ± 0.7 (50) 17 ± 1 (75) |
15 ± 0.9∗ (−18 ± 4) | 15 ± 0.9∗ (−14 ± 3) | 14 ± 0.8∗ (−16 ± 4) |
| PVA, mm Hg • l | 3 ± 0.3 | 3 ± 0.2∗ (−25 ± 4) | 3 ± 0.2∗ (−21 ± 3) | 6 ± 2 (25) 5 ± 2 (50) 4 ± 0.5 (75) |
3 ± 0.4∗ (−28 ± 10) | 3 ± 0.5∗ (−36 ± 6) | 2 ± 0.1∗ (−43 ± 6) |
| PRSW, mm Hg§ | 82 ± 6 | 92 ± 7∗ (12 ± 1) | 93 ± 7∗ (12 ± 1) | 51 ± 2 (25) 59 ± 6 (50) 50 ± 2 (75) |
58 ± 2∗ (10 ± 1) | 70 ± 7∗ (16 ± 1) | 61 ± 2∗ (22 ± 1) |
| MAP, mm Hg | 111 ± 4 | 96 ± 5∗ (−14 ± 1) | 98 ± 4 (−12 ± 2) | 100 ± 4 (25) 112 ± 5 (50) 108 ± 3 (75) |
93 ± 3∗ (−7 ± 2) | 97 ± 3∗ (−13 ± 2) | 92 ± 2∗ (−15 ± 1) |
| SAP, mm Hg | 129 ± 7 | 109 ± 6∗ (−15 ± 1) | 109 ± 5 (−15 ± 3) | 117 ± 4 (25) 126 ± 4 (50) 126 ± 4 (75) |
107 ± 4 (−8 ± 2) | 109 ± 4 (−14 ± 2) | 105 ± 3 (−16 ± 2) |
| DAP, mm Hg | 94 ± 4 | 85 ± 4∗ (−10 ± 2) | 87 ± 5 (−8 ± 3) | 87 ± 5 (25) 96 ± 5 (50) 92 ± 3 (75) |
82 ± 4 (−5 ± 2) | 86 ± 4 (−10 ± 2) | 79 ± 2 (−13 ± 1) |
| SVR, MAP/CO | 58 ± 5 | 49 ± 4∗ (−16 ± 2) | 42 ± 4∗‡ (−28 ± 3) | 56 ± 4 (25) 54 ± 4 (50) 56 ± 3 (75) |
46 ± 4∗ (−16 ± 3) | 38 ± 1∗ (−27 ± 3) | 40 ± 2∗ (−29 ± 2) |
| Ea, mm Hg/ml | 6 ± 0.3 | 5 ± 0.1∗ (−23 ± 3) | 4 ± 0.1∗ (−28 ± 4) | 7 ± 0.4 (25) 8 ± 0.4 (50) 8 ± 0.1 (75) |
6 ± 0.4∗ (−22 ± 1) | 5 ± 0.3∗ (−29 ± 2) | 5 ± 0.2∗ (−35 ± 2) |
| ESPVR, mm Hg/ml | 8 ± 0.9 | 9 ± 1∗ (19 ± 2) | 9 ± 1∗ (20 ± 1) | 5 ± 0.5 (25) 6 ± 0.5 (50) 5 ± 0.5 (75) |
6 ± 0.5∗ (11 ± 1) | 7 ± 0.7∗ (18 ± 1) | 7 ± 0.7∗ (29 ± 1) |
| EDPVR, mm Hg/ml | 1 ± 0.1 | 0.8 ± 0.1∗ (−28 ± 7) | 0.9 ± 0.2∗ (−18 ± 9) | 3 ± 0.4 (25) 3 ± 0.3 (50) 3 ± 0.1 (75) |
2 ± 0.4∗ (−17 ± 2) | 2 ± 0.3∗ (−22 ± 2) | 2 ± 0.2∗† (−39 ± 6) |
Values shown are mean ± SEM or mean ± SD (n).
Ea = arterial elastance; EDPVR = end-diastolic pressure-volume relationship; ESPVR = end-systolic pressure-volume relationship; HEX = Hextend (plasma volume-expanding solution); LVESP = LV end-systolic pressure; PRSW = preload-recruitable stroke work; PVA = pressure-volume area; SW = stroke work; Tau = LV relaxation time-constant; other abbreviations as in Tables 1 and 3.
p < 0.05 vs. respective baseline value.
p < 0.05 vs. 25 μg/kg/min dose.
p < 0.05 vs. 50 µg/kg/min (3 h).
mm Hg × ml/ml.
With BMS-986231 50 μg/kg/min, significant reductions in LVEDP and LVEDV (−63% and −6%, respectively) were seen after 3 h of infusion (Table 4). LVEF increased significantly and progressively during the infusion (+12% after 3 h), and this improvement was maintained with HEX (Table 4, Figure 2A). SV and CO were preserved after 3 h, although moderate but significant reductions in both parameters were seen 1 h into the infusion. However, when preload was restored with HEX, both SV and CO were significantly increased above baseline (Table 4, Figure 2A).
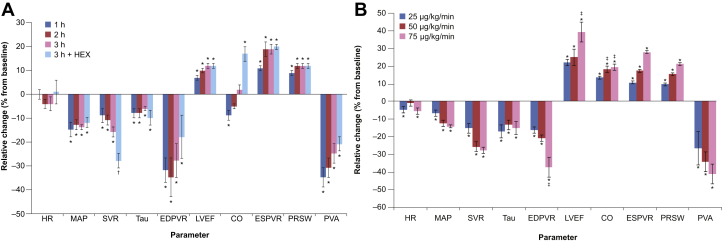
Figure 2.
Time Course and Dose Effects of BMS-986231
(A) Time course effects of BMS-986231 (50 μg/kg/min) in animals with normal cardiac function, and (B) dose effects of BMS-986231 (25, 50, and 75 μg/kg/min) after a 3-h infusion in animals with pacing-induced cardiomyopathy. All values shown are mean ± SEM. *p < 0.05 versus baseline; †p < 0.05 (3 h + HEX vs. 3 h); ‡p < 0.05 (vs. 25 μg/kg/min). EDPVR = end-diastolic pressure-volume relationship; ESPVR = end-systolic pressure-volume relationship; HEX = Hextend (plasma volume-expanding solution); LVEF = left ventricular ejection fraction; MAP = mean arterial pressure; PRSW = preload-recruitable stroke work; PVA = pressure-volume area; Tau = left-ventricular relaxation time-constant; other abbreviations as in Figure 1.
BMS-986231 50 μg/kg/min was associated with significant time-dependent enhancement in load-independent indexes of contractility (Figure 3A), which increased the slopes of both ESPVR (+19%) and PRSW (+12%) (Table 4, Figure 2A), even after preload was acutely restored (+HEX). Representative LV pressure–volume families also showed typical inotropic leftward shifts with BMS-986231 (Figure 3B). The load-dependent index, LV peak +dP/dt, was also significantly increased (+14%) after 3 h (Table 4).
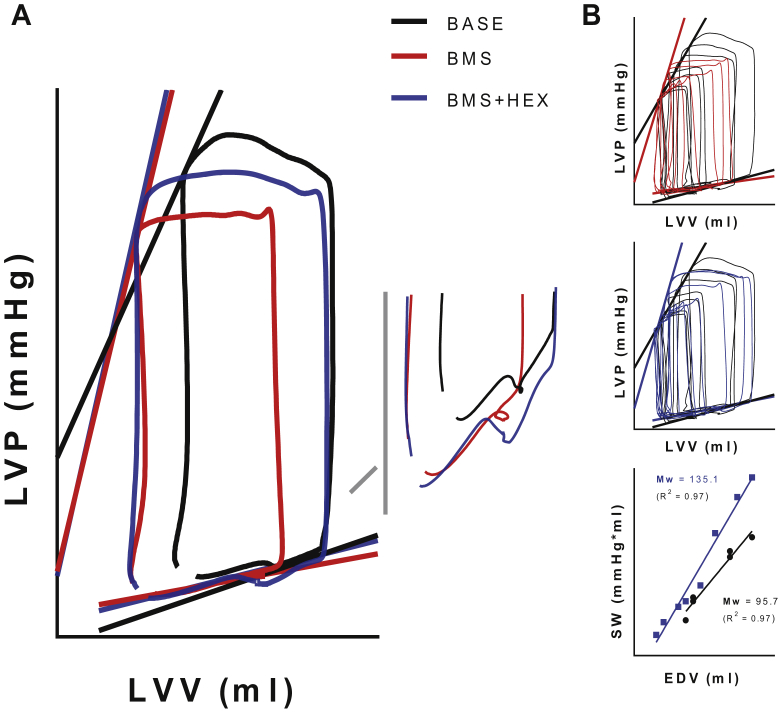
Figure 3.
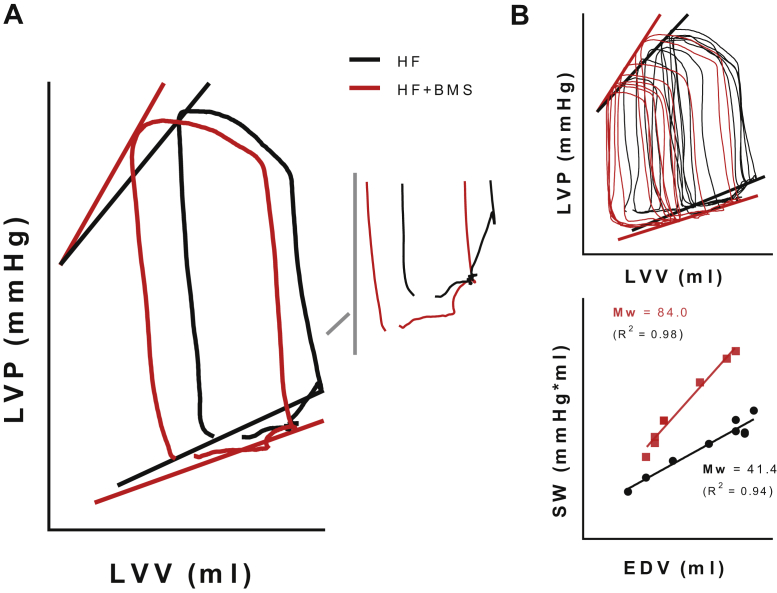
Representative Left Ventricular Pressure–Volume Relationships
Representative left ventricular pressure–volume relationships generated via acute preload reductions in conscious healthy dogs at baseline (BASE, black) and at 3 h following BMS-986231 (BMS) administration (50 μg/kg/min intravenously), both before (BMS, red) and after (BMS + HEX, blue) acute volume expansion. (A) Steady-state (pre-occlusion) pressure–volume curves of 1 cardiac cycle (inset, showing filling phase), demarcated by both the end-systolic (ESPVR) and by the estimated (linear) end-diastolic pressure–volume relationships (EDPVR). BMS-986231 decreased preload, and load-independently increased the end-systolic elastance (slope of the ESPVR). (B) Families used to generate the pressure–volume relationships (top and middle), as well as the stroke work (SW) to end-diastolic volume relationship (bottom), showing an increase in the slope of the PRSW (Mw) induced by BMS-986231. EDV = end-diastolic volume; HEX = Hextend (plasma volume-expanding solution); LVP = left ventricular pressure; LVV = left ventricular volume; PRSW = preload-recruitable stroke work.
Both Tau and the slope of the EDPVR were significantly reduced versus baseline with BMS-986231 50 μg/kg/min (−6% and −28%, respectively) (Table 4). A significant reduction in Tau was seen 30 min post-infusion start (data not shown), and was sustained throughout the infusion period.
With BMS-986231 50 μg/kg/min, there was a maximal but clinically insignificant reduction in HR of 4% during this period (Table 4, Figure 2A). MAP was reduced by 14%; this effect was both rapid and sustained (Figure 2A). After 3 h, both SVR and Ea were also significantly reduced versus baseline (−16% and −23%, respectively). HEX had negligible effects on PRSW, EDPVR, and HR.
BMS-986231 was also associated with significant reductions in LVSW and PVA after 3 h (−11% and −25% vs. baseline, respectively). The reduction in PVA was preserved with HEX (Figure 2A), suggesting a reduction in MVO2.
Dogs with pacing-induced cardiomyopathy (B2)
Pacing resulted in severely depressed LV function and ventricular modeling (Table 4). Mean LVEDP was elevated, and the LV peak +dP/dt was decreased; MAP and HR were within normal limits. Similarly, the Ea, LVEF, and slopes for PRSW and EDPVR post-pacing were consistent with HF-associated cardiac and vascular dysfunction.
In these paced dogs, BMS-986231 was associated with significant and dose-dependent decreases in filling and (albeit more moderate) systemic pressures. For instance, following 3 h of infusion, LVESP and LVEDP were significantly reduced (LVESP: −8 to −17%; LVEDP: −14 to −24%; all p < 0.05 vs. baseline) (Table 4).
BMS-986231 was also associated with significant reductions in LVEDV at 3 h (−4 to −9%) (Table 4). Compared with baseline, LVEF increased dose dependently by 23% to 41% (all p < 0.05) at 3 h. SV and CO also displayed dose-dependent increases versus baseline (Table 4, Figure 2B). These increases in LVEF, SV, and CO were sustained at 1 h post-infusion (data not shown).
All load-independent indexes of contractility also improved in the paced dogs during infusion. The slopes of the ESPVR and PRSW increased progressively and dose dependently over the 3-h infusion (e.g., ESPVR: +11% to +29%; PRSW: +10% to +22%; all p < 0.05 vs. baseline) (Table 4, Figure 2B, Figures 4A, and 4B). Increases in the slopes of both PRSW and ESPVR were sustained 1 h after infusion (data not shown).
Figure 4.
Representative Left Ventricular Pressure–Volume Relationships Generated Via Acute Preload Reductions in Conscious Dogs
Representative left-ventricular pressure–volume relationships generated via acute preload reductions in conscious dogs with tachypacing-induced heart failure (HF), taken both at baseline (HF, black) and at 3 h following BMS-986231 (BMS) administration (50 μg/kg/min intravenously, red). (A) Steady-state (pre-occlusion) pressure–volume curves of 1 cardiac cycle (inset, showing filling phase), demarcated by both the ESPVR as well as by the estimated (linear) EDPVRs. BMS-986231 decreased pre-load, and load-independently increased the end-systolic elastance (slope of the ESPVR). (B) Families used to generate the pressure–volume relationships (top), as well as the SW to end-diastolic volume relationship (bottom), showing a BMS-986231-induced increase in the slope of the PRSW (Mw). Abbreviations as in Figure 3.
After the 3-h infusion period, Tau was reduced by 18% (25 μg/kg/min), 14% (50 μg/kg/min), and 16% (75 μg/kg/min) (all p < 0.05) versus baseline. The estimated EDPVR was dose- dependently reduced by 17% to 39% (all p < 0.05) versus baseline (Table 4, Figure 2B). These diastolic improvements were partially sustained for both indexes at all doses 1 h after infusion (data not shown).
HR was marginally reduced versus baseline after 3 h, reaching statistical significance with the 25- and 75-μg/kg/min doses (−5% and −6%, respectively) (Table 4, Figure 2B). Ea and SVR were also significantly reduced after 3 h (Ea: −22% to −35%; SVR: −16% to −29%; all p < 0.05 vs. baseline) (Table 4, Figure 2B).
The PVA was significantly and dose-dependently reduced versus baseline at 3 h, with the 25-, 50-, and 75-μg/kg/min dose infusions leading to mean decreases of 28% to 43% (Table 4, Figure 2B). These reductions were partially preserved at 1 h post-infusion (data not shown).
Analysis of BMS-986231 plasma concentrations in dogs with pacing-induced cardiomyopathy after 3 h of infusion showed dose-dependent increases in BMS-986231 and BMT-284730 concentrations. BMT-284730 concentrations were consistently greater than BMS-986231 after 3 h (Table 2). Further analyses also indicated that BMS-986231 concentrations were significant linear predictors of change in both MAP and PRSW (Supplemental Figure 1).
Discussion
The results of these studies indicated that in dogs with experimentally induced HF, IV administration of BMS-986231 was associated with enhanced LV contractility and relaxation, and with a moderate degree of systemic vasodilation, all of which are desirable attributes for a pharmacological agent in patients with AHF.
BMS-986231 demonstrated significant beneficial inotropic effects, as evidenced by the increases in load-independent indexes, such as ESPVR (mean 11% to 29%) and PRSW (mean 10% to 22%) in group B animals with normal or failing hearts. LVESV was significantly reduced, whereas LVEF was significantly increased across both groups A and B, with relative increases in LVEF reaching beyond 40% versus baseline. Other measures of enhanced inotropy, including LVFAS (group A), SV, and CO, were also significantly increased. Mean aortic flow was increased by up to 32%, with the highest dose in group A. Many of these indexes were sustained at 1 h post-infusion. This enhanced systolic LV function was also evident with CXL-1020, which was associated with a significantly increased maximal LV power index and load-independent LV end-systolic elastance in dogs with experimentally-induced HF (8).
In addition to improving LV systolic function, BMS-986231 also improved LV diastolic function. In group A, the beneficial lusitropic effects were evident based on a significant increase in the Ei/Ai ratio after a 4-h infusion, as well as an increase in DT of early mitral inflow velocity and a reduction in LVEDWS. In group B2, the LV relaxation time-constant, Tau, was significantly reduced by a mean of up to 18%, and the EDPVR was reduced up to 39% after 3 h versus baseline in those animals with pacing-induced HF. BMS-986231 was also associated with significant reductions in pre-load measures of LVEDV and LVEDP across both group A and B cohorts. These beneficial findings on measures of cardiac relaxation are echoed in studies of CXL-1020 in dogs with experimentally-induced HF (8).
BMS-986231 was associated with vasodilatory effects in both group A and B, with significant reductions in SVR (up to a mean reduction of ∼30% in group B2) seen over their respective infusion periods. LVESP (as described in the group B study) was also reduced by approximately 17% with the 75-μg/kg/min dose infusion. Although a numerical reduction in SAP (and DAP for group B2) was seen in both models, MAP was significantly reduced by up to 15% versus baseline. These data support the theory that BMS-986231 exerts a systemic vasodilatory effect similar to that seen previously with IV administration of CXL-1020 (8).
No clinically meaningful impact of BMS-986231 on HR was observed, which was a finding consistent with that of CXL-1020 (8). MVO2, either directly measured (group A) or estimated from the PVA (group B), was significantly reduced with BMS-986231 in both models, showing relative decreases of >40% with the highest dose infusions tested (7 and 75 μg/kg/min). Again, reductions in MVO2 were reported with CXL-1020 (10 μg/kg/min over 4 h) in dogs with microembolization-induced HF (8); these findings reflect an improved mechanical efficiency of the failing heart. The reduction of MVO2 could be attributed to both direct reductions in the LV wall tension, preload, and afterload, or to increased calcium-handling and/or contractile efficiency, which together overcame the increase in MVO2 that was potentially elicited by an increase in contractility. HR, a major determinant of MVO2, was unchanged.
Similarly, in all of the present studies, BMS-986231 was not associated with the induction of the de novo arrhythmias that are commonly seen with legacy inotropes (11). Moreover, in group A, BMS-986231 increased the threshold for the induction of SVT and VF with ventricular stimulation. Together, these results suggested a negligible pro-arrhythmic potential; however, they require further investigation.
As expected, IV-infused BMS-986231 demonstrated dose- and time-dependent increases in blood concentration levels, indicating that BMS-986231 possesses a predictable pharmacokinetic profile. Furthermore, the fact that BMS-986231 plasma concentrations correlated strongly with relative changes in both MAP and PRSW over the same period in group B2 dogs provided further support for the beneficial effects BMS-986231 on cardiac function.
The findings of these 2 preclinical studies corroborated those of a placebo-controlled phase 2a study in hospitalized patients with advanced HF with reduced LVEF (22). In that study, BMS-986231, at 6-h dose infusions of up to 12 μg/kg/min, was well tolerated and was associated with rapid and sustained reductions in pulmonary capillary wedge pressure, as well as reductions in pulmonary arterial systolic and diastolic pressure, and right atrial pressure. It was also associated with increased CO, (noninvasively) measured as increases in the SV index and cardiac index. There was no meaningful change in HR with BMS-986231 and no evidence of arrhythmias detected on ECG. Although it was not possible to demonstrate conclusively with the techniques used in this phase 2a study, its findings were consistent with those of the 2 pre-clinical studies described here, in which invasive techniques were able to demonstrate changes in load-independent cardiac function (e.g., significantly increased ESPVR). Both the preclinical and clinical findings are encouraging, and further studies to assess the safety and efficacy of BMS-986231 in patients with AHF are needed. The phase 2b STANDUP AHF (Evaluate the Safety and Efficacy of 48-Hour Infusions of HNO [Nitroxyl] Donor in Hospitalized Patients With Heart Failure) study of BMS-986231 in hospitalized patients with HF and impaired systolic function (NCT03016325) is one such study.
Study limitations
In these studies, we described 2 distinct canine models of HF to comprehensively evaluate the physiological response to BMS-986231 and provide mutually supportive evidence of the inotropic, lusitropic, and moderately vasodilatory effects of the compound. Due to inherent differences between and the unique characteristics of each model, caution should be exercised when comparing parameters between the 2 models, both at baseline and at specific time intervals during the infusion. Apart from the method in which cardiomyopathy was induced (i.e., microembolism under anesthesia vs. pacing while fully conscious), the animals themselves were also different in terms of breed and size, with the mongrel dogs in group A being larger than the beagle dogs in group B. In addition, differences in the way specific parameters were assessed made meaningful comparison between the models difficult; for example, in the group A animals, SV was measured based on differences in LV volumes, whereas in the group B animals, it was measured from pressure-volume loops. As a result, the focus here was on responses to the drug within each model, relative to a respective baseline.
Regarding the difference in dose ranges used across the 2 models (0.7 to 7 μg/kg/min and 25 to 75 μg/kg/min), earlier tests (data not shown) showed BMS-986231 to be substantially more potent in the dogs with microembolism-induced HF (which were under general anesthesia) compared with the paced dogs (which were fully conscious). It is known that anesthesia leads to depressed cardiac function; therefore, it is possible that the activity of HNO observed in the microembolism-induced HF model might partly be due to reversal of the isoflurane-induced reduction in myofilament calcium sensitivity, in a manner similar to that previously described for another HNO donor (30).
The purpose of these studies was to assess the hemodynamic, rather than the biochemical, effects of BMS-985231 in different canine models; as a result, detailed biochemical analyses were not conducted as part of either study. One question that may arise is whether the effect of BMS-986231 may be mediated by NO, rather than HNO. Earlier in vitro nuclear magnetic resonance studies (data not shown) demonstrated that BMS-986231 in phosphate-buffered saline was converted stoichiometrically to HNO and its inactive by-product, BMT-284730; furthermore, when this conversion in phosphate-buffered saline took place in the presence of excess glutathione, stoichiometric trapping of HNO as a glutathione adduct occurred. In addition, a study of CXL-1020 in rats reported hemodynamic effects that were not only similar to those seen with BMS-986231 in the present studies with dogs, but were different to those of the NO donor sodium nitroprusside (13). Regarding the biochemical effects of BMS-986231 in humans, in the phase 2a study, BMS-986231 (3 to 12 μg/kg/min) was not associated with adverse changes in laboratory parameters, including brain natriuretic peptide, troponin I, serum creatinine, liver enzymes, hemoglobin, or platelet count (22).
Conclusions
In 2 experimental canine HF models, BMS-986231 was associated with outcomes indicative of beneficial inotropic, lusitropic, and vasodilatory effects, with no meaningful impact on HR and with a reduction in MVO2. BMS-986231 did not induce de novo ventricular arrhythmias, and significantly increased the threshold for triggering life-threatening SVT or VF episodes with PVS. These findings were closely aligned with those from studies of CXL-1020; unlike CXL-1020, however, there was no evidence of venotoxicity with BMS-986231 that led to termination of the clinical development of CXL-1020 (8). These findings corroborated those studies of BMS-986231 in the clinical setting, and together provided strong support for BMS-986231 as a suitable candidate for further clinical development for patients with AHF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In these 2 experimental studies, BMS-986231 demonstrated beneficial inotropic, lusitropic, and vasodilatory effects. It resulted in significant increases in measures of cardiac function (LV end-systolic elastance, and so forth) and significant decreases in other outcomes, including LV filling pressures, end-diastolic stiffness, systemic vascular resistance, and myocardial oxygen consumption. There were no clinically significant changes in HR. No de novo arrhythmias were detected. BMS-986231 was also not associated with venotoxicity.
TRANSLATIONAL OUTLOOK: Subsequent clinical research has provided support to the preclinical findings of increased inotropy and lusitropy and vasodilatory effects with BMS-986231, and that it is well tolerated. However, further studies are needed to fully assess its potential as a therapy for patients with AHF, and a phase 2b trial of BMS-986231 is currently underway in this patient population (NCT03016325).
Acknowledgments
The authors would like to thank the scientific staff at QTest Labs, Inc, for their technical assistance. Editorial support was provided by Laura Grace, PhD and Geraint Owens, PhD of Chameleon Communications International Ltd, with funding from Bristol-Myers Squibb.
Footnotes
This work was supported by Cardioxyl Pharmaceuticals (Chapel Hill, North Carolina) and Bristol-Myers Squibb (Princeton, New Jersey).
Drs. Hartman and Reardon were both employed by Cardioxyl Pharmaceuticals when these studies were being performed and the data summarized. Dr. del Rio has received research support from Cardioxyl Pharmaceuticals. Dr. Sabbah has received research grant support from and was a member of the scientific and clinical advisory boards of Cardioxyl Pharmaceuticals; and has received speaking honoraria from Bristol-Myers Squibb. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Peter Liu, MD, served as Guest Editor for this paper.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Ponikowski P., Anker S.D., AlHabib K.F. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosy A.P., Fonarow G.C., Butler J. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Desai A.S., Stevenson L.W. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg B. Acute decompensated heart failure - treatments and challenges. Circ J. 2012;76:532–543. doi: 10.1253/circj.cj-12-0130. [DOI] [PubMed] [Google Scholar]
- 6.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Chavey W.E., Hogikyan R.V., Van Harrison R., Nicklas J.M. Heart failure due to reduced ejection fraction: medical management. Am Fam Physician. 2017;95:13–20. [PubMed] [Google Scholar]
- 8.Sabbah H.N., Tocchetti C.G., Wang M. Nitroxyl (HNO): a novel approach for the acute treatment of heart failure. Circ Heart Fail. 2013;6:1250–1258. doi: 10.1161/CIRCHEARTFAILURE.113.000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 10.Tocchetti C.G., Wang W., Froehlich J.P. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arcaro A., Lembo G., Tocchetti C.G. Nitroxyl (HNO) for treatment of acute heart failure. Curr Heart Fail Rep. 2014;11:227–235. doi: 10.1007/s11897-014-0210-z. [DOI] [PubMed] [Google Scholar]
- 12.Flores-Santana W., Salmon D.J., Donzelli S. The specificity of nitroxyl chemistry is unique among nitrogen oxides in biological systems. Antioxid Redox Signal. 2011;14:1659–1674. doi: 10.1089/ars.2010.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roof S.R., Ueyama Y., Mazhari R. CXL-1020, a novel nitroxyl (HNO) prodrug, is more effective than milrinone in models of diastolic dysfunction-a cardiovascular therapeutic: an efficacy and safety study in the rat. Front Physiol. 2017;8:894. doi: 10.3389/fphys.2017.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancel S., Zhang J., Evangelista A. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froehlich J.P., Mahaney J.E., Keceli G. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 16.Sivakumaran V., Stanley B.A., Tocchetti C.G. HNO enhances SERCA2a activity and cardiomyocyte function by promoting redox-dependent phospholamban oligomerization. Antioxid Redox Signal. 2013;19:1185–1197. doi: 10.1089/ars.2012.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohr M.J., Kaludercic N., Tocchetti C.G. Nitroxyl enhances myocyte Ca2+ transients by exclusively targeting SR Ca2+-cycling. Front Biosci (Elite Ed) 2010;2:614–626. doi: 10.2741/e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Switzer C.H., Flores-Santana W., Mancardi D. The emergence of nitroxyl (HNO) as a pharmacological agent. Biochim Biophys Acta. 2009;1787:835–840. doi: 10.1016/j.bbabio.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paolocci N., Saavedra W.F., Miranda K.M. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg J.O., Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25:915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 21.Paolocci N., Katori T., Champion H.C. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling. Proc Natl Acad Sci U S A. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tita C., Gilbert E.M., Van Bakel A.B. A phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction. Eur J Heart Fail. 2017;19:1321–1332. doi: 10.1002/ejhf.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowart D., Venuti R., Guptill J., Noveck R., Foo S. A phase 1 study of the safety and pharmacokinetics of the intravenous nitroxyl prodrug, CXL-1427. J Am Coll Cardiol. 2015;65:A876. [Google Scholar]
- 24.National Research Council . 8 ed. National Academies Press; Washington D.C.: 2011. Guide for care and use of laboratory animals. [Google Scholar]
- 25.Sabbah H.N., Stein P.D., Kono T. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 26.Sabbah H.N., Goldberg A.D., Schoels W. Spontaneous and inducible ventricular arrhythmias in a canine model of chronic heart failure: relation to haemodynamics and sympathoadrenergic activation. Eur Heart J. 1992;13:1562–1572. doi: 10.1093/oxfordjournals.eurheartj.a060102. [DOI] [PubMed] [Google Scholar]
- 27.Sabbah H.N., Levine T.B., Gheorghiade M., Kono T., Goldstein S. Hemodynamic response of a canine model of chronic heart failure to intravenous dobutamine, nitroprusside, enalaprilat, and digoxin. Cardiovasc Drugs Ther. 1993;7:349–356. doi: 10.1007/BF00880158. [DOI] [PubMed] [Google Scholar]
- 28.Chandler M.P., Stanley W.C., Morita H. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- 29.Senzaki H., Isoda T., Paolocci N., Ekelund U., Hare J.M., Kass D.A. Improved mechanoenergetics and cardiac rest and reserve function of in vivo failing heart by calcium sensitizer EMD-57033. Circulation. 2000;101:1040–1048. doi: 10.1161/01.cir.101.9.1040. [DOI] [PubMed] [Google Scholar]
- 30.Ding W., Li Z., Shen X. Reversal of isoflurane-induced depression of myocardial contraction by nitroxyl via myofilament sensitization to Ca2+ J Pharmacol Exp Ther. 2011;339:825–831. doi: 10.1124/jpet.111.185272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.