Summary
Chemical modifications to nucleosomal DNA and histone tails greatly influence transcription of adjacent and distant genes, a mode of gene regulation referred to as epigenetic control. Here, the authors summarize recent findings that have illustrated crucial roles for epigenetic regulatory enzymes and reader proteins in the control of cardiac fibrosis. Particular emphasis is placed on epigenetic regulation of stress-induced inflammation and fibroblast activation in the heart. The potential of developing innovative small molecule “epigenetic therapies” to combat cardiac fibrosis is highlighted.
Key Words: epigenetics, fibroblast, fibrosis, inflammation
Abbreviations and Acronyms: Ang II, angiotensin II; BET, bromodomain and extraterminal protein; DNMT, DNA methyltransferase; ECM, extracellular matrix; HAT, histone acetyltransferase; HDAC, histone deacetylase; IL, interleukin; KDM, lysine demethylase; KMT, lysine methyltransferase; LPS, lipopolysaccharide; MI, myocardial infarction; NF-κB, nuclear factor-κB; SASP, senescent-associated secretory phenotype; SE, super-enhancer; SMA, smooth muscle actin; TET, ten-eleven translocation; TNF, tumor necrosis factor; Treg, regulatory T cell; TSA, trichostatin A; VPA, valproic acid
Central Illustration
Cardiac fibrosis may be beneficial, replacing regions of myocyte loss with a structural scar following myocardial infarction (MI), or maladaptive, involving excessive extracellular matrix (ECM) deposition in response to long-standing stress (1). Unrestrained cardiac fibrosis can elicit various deleterious effects. For example, interstitial fibrosis increases the passive stiffness of the myocardium, contributing to diastolic dysfunction 2, 3, and disrupts electrical conduction in the heart, causing arrhythmias and sudden cardiac death (4). Unfortunately, despite the widely accepted roles of fibrosis in cardiac dysfunction, no targeted antifibrotic drugs for the heart currently exist.
A growing body of evidence suggests that “epigenetic therapies” have great potential to combat organ fibrosis and could provide an innovative approach to treat heart disease. In cell nuclei, chromosomal DNA is packaged together with histone proteins to form chromatin. The basic unit of chromatin is the nucleosome, which encompasses ∼150 base pairs of DNA wrapped around a histone octamer. Chemical modifications to nucleosomal DNA and histone tails can profoundly influence transcription of neighboring or distant genes, and this mode of gene regulation is referred to as epigenetics. Here, we discuss histone acetylation, histone methylation, and DNA methylation as epigenetic events that contribute to the pathogenesis of cardiac fibrosis and heart failure.
Vincent Allfrey’s discovery that histones can be post-translationally acetylated, altering RNA synthesis in vitro, laid the foundation for our current understanding of the role of this modification in the control of transcription 5, 6. Acetylation of histones at gene promoters and enhancers can alter gene expression, positively or negatively, by modifying the electrostatic charge of chromatin and creating a combinatorial histone code that governs recruitment of “reader” proteins and chromatin remodeling factors that dictate expression of downstream target genes (7).
Histone acetyltransferases (HATs) catalyze the addition of acetyl groups to lysine residues in histone tails. HATs are divided into 2 typical families, Gcn5 and MYST, named for their founding members (8). Other proteins, such as p300/CBP, Taf1, and nuclear receptor coactivators also have catalytic acetyltransferase activities; however, they do not contain true consensus HAT domains and are categorized as an orphan class. The enzymes that remove acetyl groups from histone tails are histone deacetylases (HDACs). To date, the identified mammalian HDACs are classified into 4 classes: Class I (HDAC1, HDAC2, HDAC3, and HDAC8), Class II (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10), Class III (SIRT1–7), and Class IV (HDAC11) (9). Class I, II, and IV HDACs are zinc-dependent, whereas Class III HDACs (also known as sirtuins) use NAD+ as a cofactor for catalytic activity.
Acetylation of histones that mark active transcription start sites and enhancers can create docking sites for acetyl-readers, such as members of the bromodomain and extraterminal domain (BET) family of proteins (BRD2, BRD3, BRD4, and BRDT). BRD4 has the capacity to activate RNA Polymerase II (Pol II) by recruiting a kinase component (CDK9) of the P-TEFb complex, leading to transcription elongation 10, 11, 12. An emerging role for BRD4 is in the formation of large, dynamic, cell state–specific enhancers, referred to as super-enhancers (SEs) 13, 14. As discussed in the following text, BRD4 plays a central role in the control of cardiac fibrosis, in part through its ability to enhance the formation of SEs (15). Additional reader domains for acetyl-histone (e.g., YEATs), as well as readers of other histone marks such as methylation, have been described (16). However, nothing is known about the functions of these other factors in the context of cardiac disease.
Methylation is another crucial histone modification, and the addition of this mark is mediated by lysine methyltransferases (KMTs). In general, methylation of histones H3K4 and H3K36 by KMT2, KMT3, and KMT7 family members results in stimulation of gene expression, whereas methylation of H3K9, H4K20, and H3K27 by KMT1, KMT5, KMT6, and KMT8 enzymes results in gene repression (17). In contrast to KMTs, lysine demethylases (KDMs) remove methyl groups from histones, with KDM1, KDM2, KDM5, and KDM6 family members demethylating H3K4, and KDM3 and KDM7 enzymes demethylating H3K9 (18). The repressive methyl marks on H3K36 are removed by KDM2 (monomethylation and dimethylation) and KDM4 (dimethylation and trimethylation) family members. H3K27 di- and trimethylation are removed by KDM6; KDM7 also targets dimethylated H3K27, and can remove monomethyl from this site as well (18).
Nucleosomal DNA can be methylated by DNA methyltransferases (DNMTs), which are divided into 4 families, DNMT1, DNMT2, DNMT3, and Chromomethylase (19). DNA methylation occurs in intragenic, intergenic, and CpG islands in promoter regions and is associated with gene repression. Several mechanisms appear to explain how DNA methylation leads to transcriptional repression, one of which is to create docking sites for methyl-DNA reader proteins that function to inhibit downstream target gene expression 20, 21. Recently, a novel family of Fe2+- and 2-oxoglutarate–dependent dioxygenases known as ten-eleven translocation (TET) proteins was identified, and includes TET1, TET2, and TET3. These proteins were found to mediate DNA demethylation by oxidizing 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxylcytosine 22, 23, 24.
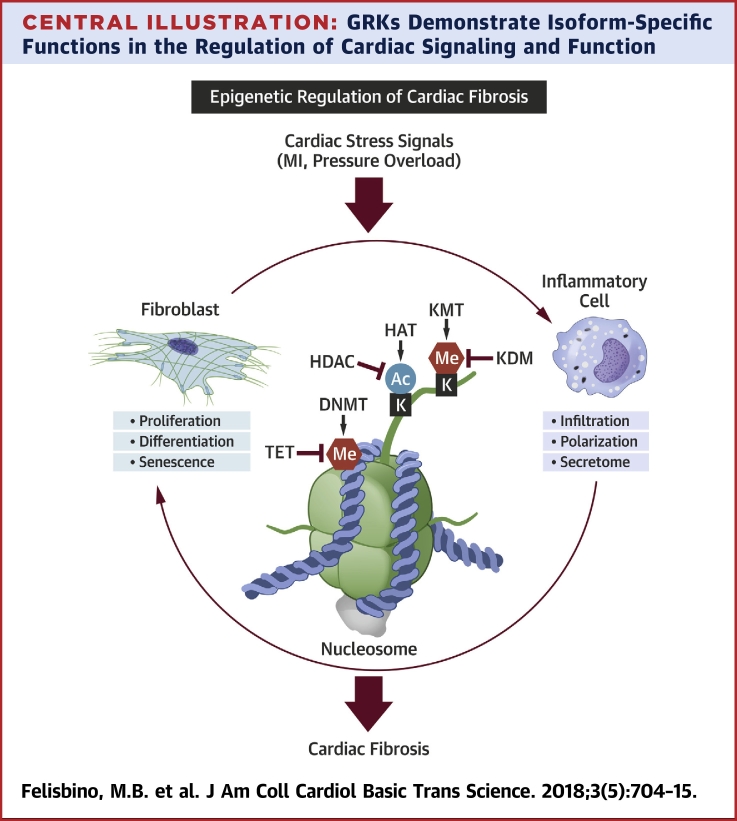
Cardiac fibrosis is a complex, multifactorial process 25, 26. For the purposes of this review, rather than simply listing epigenetic events that contribute to fibrosis of the heart, we describe roles of epigenetic regulatory effectors within the framework of distinct, but overlapping, phases of the fibrotic response: inflammation and fibroblast activation (Central Illustration).
Central Illustration.
Central Roles for Epigenetic Regulators in Mediating the Fibroblast Activation and Inflammation That Culminates in Cardiac Fibrosis
In response to pathological stresses such as myocardial infarction (MI) or pressure overload, epigenetic machinery is activated to promote cardiac inflammation and cardiac fibroblast proliferation, leading to cardiac fibrosis. Complex interplay between histone deacetylase (HDAC), histone acetyltransferase (HAT), lysine methyltransferase (KMT), lysine demethylase (KDM), DNA methyltransferase (DNMT), and ten-eleven translocation (TET) enzymes governs acetylation (Ac) of histones and methylation (Me) of histones and DNA that make up nucleosomes.
Epigenetics in Inflammation
Epigenetic control of inflammation
Inflammation has long been recognized as an important component of heart failure pathogenesis (27). It is thought that cardiac stress (e.g., MI) leads to nuclear factor-κB (NF-κB)–mediated stimulation of cytokine/chemokine gene expression in resident cardiac cells 28, 29, 30, 31. The release of proinflammatory mediators, as well as damage-associated molecular patterns (DAMPs), induces recruitment of inflammatory cells such as neutrophils and monocytes into the myocardium, promoting phagocytic clearance of debris 32, 33, 34. Although initially beneficial, failure to resolve this inflammatory cell extravasation leads to insidious cardiac inflammation, resulting in long-term fibroblast activation and subsequent fibrosis 35, 36, 37.
CD4+ T lymphocytes and FoxP3+ regulatory T cells (Tregs) have been shown to aid in the repair of the injured heart following MI (38). Furthermore, adoptive transfer of Tregs, which are generally anti-inflammatory, was demonstrated to decrease infiltration of neutrophils, monocytes, and lymphocytes into the heart post-MI, and blunted cardiac fibrosis in response to transverse aortic constriction, long-term angiotensin II (Ang II) infusion, or coxsackievirus infection 39, 40, 41, 42. However, it was recently shown that T cells can also promote cardiac fibrosis in the setting of long-term pressure overload, in which Th1 effector T cells were found to directly stimulate fibroblast-to-myofibroblast differentiation and promote fibrotic remodeling of the heart (43).
Epigenetic regulators clearly serve immunomodulatory roles in the heart 44, 45. For example, treatment of spontaneously hypertensive rats for 20 weeks with valproic acid (VPA), a weak HDAC inhibitor, led to a reduction in interleukin (IL)-1β and tumor necrosis factor (TNF)-alpha expression in the left ventricle, which correlated with attenuated cardiac hypertrophy and fibrosis, and improved cardiac function (46). The pan-HDAC inhibitor, SAHA, which is Food and Drug Administration approved for the treatment of cancer, reduced IL-1α, IL2, and TNF-α expression, and decreased perivascular and interstitial cardiac fibrosis in a model of hypertension-induced cardiac remodeling (47).
In addition to influencing proinflammatory gene expression via alterations in nucleosomal tail acetylation, HDAC-mediated deacetylation of nonhistone targets also regulates inflammatory signaling (48). For example, treatment of macrophages with the pan-HDAC inhibitor trichostatin A (TSA) led to enhanced acetylation of MAPK phosphatase-1, thereby promoting its ability to suppress proinflammatory p38 kinase signaling (49). Furthermore, HDAC-mediated deacetylation of interferon regulatory factor-7 was shown to be required for efficient DNA binding of this proinflammatory transcription factor (50). Finally, the p65 subunit of NF-κB can be deacetylated by HDAC3, promoting its nuclear exclusion via association with IκBα (51). Whether these nongenomic roles for HDACs regulate cardiac inflammation remains to be determined.
BET epigenetic reader proteins are crucial positive regulators of NF-κB–dependent proinflammatory gene expression (52). Utilizing the transverse aortic constriction and MI-induced mouse models of heart failure, as well as cultured human induced pluripotent stem cell–derived cardiomyocytes, small molecule BET bromodomain inhibition was shown to potently suppress a network of cardiac NF-κB–responsive genes that control the innate immune response (53). Mechanistically, the BET family member BRD4 directly interacts with NF-κB through acetylated Lys310 of the p65 subunit, an interaction that is required for NF-κB transactivation (Figure 1A) (54). In a genome-wide study employing TNF-α–stimulated endothelial cells, acetylated p65 was also shown to establish new SEs proximal to genes governing the canonical inflammatory response, and BET inhibition blocked NF-κB–directed SE reorganization (55). Future studies will determine whether a similar BRD4-dependent mechanism controls cardiac inflammation and fibrosis.
Figure 1.
Three Mechanisms by Which Epigenetic Regulators Can Control Inflammation
(A) Binding of BRD4 to acetylated p65 subunit of NF-κB leads to enhanced cyclin-dependent kinase 9 (CDK9)-mediated phosphorylation of RNA polymerase II (Pol II) and increased transcription of downstream proinflammatory genes. This provides a general mechanism by which BRD4 promotes inflammatory signaling in diverse cell types. (B) The lysine demethylases JMJD3 and UTX remove repressive H3K27 trimethylation marks at regulatory sites for proinflammatory genes in macrophages, thereby stimulating downstream target gene expression. (C) Acetylation of lysine residues in FoxP3 promotes its DNA binding and transcriptional activity, thereby leading to enhanced regulatory T cell (Treg) differentiation and anti-inflammatory function.
Epigenetic control of macrophages and Tregs
To our knowledge, no studies have addressed epigenetic mechanisms in specific inflammatory cell types in the context of cardiac disease, highlighting an area of emphasis for future investigation. Nonetheless, important epigenetic regulatory mechanisms have been detailed in inflammatory cells in other organ systems, and will likely apply to the heart as well. For this review, we focused on macrophages and T cells.
It is well established that both resident macrophages and those derived from monocyte recruitment play fundamental roles in the control of cardiac inflammation 56, 57. Macrophages are commonly categorized as M1 or M2, although we acknowledge that this is an oversimplification 58, 59. M1 macrophages secrete proinflammatory cytokines and chemokines, induce phagocytosis, and promote oxidative-dependent killing mechanisms. By contrast, M2 macrophages trigger resolution of inflammation and enhance wound healing.
Histone and DNA methylation have been linked to the establishment of macrophage phenotype. Association of the transcription factor interferon regulatory factor (IRF)-5 with SETDB1, a KMT1 family member, represses proinflammatory genes associated with the M1 phenotype by promoting the formation of H3K9me3 marks on regulatory elements (60). Likewise, the KMT3 family member Smyd2 negatively regulates the proinflammatory M1 phenotype by increasing repressive H3K36 dimethylation at promoter regions of proinflammatory genes (61). Conversely, KDM6 family members JMJD3 and UTX have been reported to promote the M1 macrophage phenotype by removing repressive H3K27 trimethylation marks at regulatory sites for proinflammatory genes (62) (Figure 1B). Regarding DNA methylation, in the setting of obesity, DNMT1 was recently shown to promote the M1 macrophage phenotype by hypermethylating and thereby reducing expression of the pro-M2 transcription factor PPARγ1 (63). Furthermore, in a mouse model of diabetes, DNMT1 was shown to hypermethylate regulatory elements for the genes Notch1, PU.1, and Klf4, thereby skewing macrophage polarization toward an M1 phenotype (64).
The initial link between HDACs and macrophage polarization was first suggested by the demonstration that VPA treatment suppresses the M1 phenotype of cultured macrophages (65). Moreover, the pan-HDAC inhibitor scriptaid was subsequently shown to promote the M2 anti-inflammatory phenotype of macrophages in a mouse model of brain injury (66). It has been shown that HDAC3 induces macrophages to produce IFN-γ in response to lipopolysaccharide (LPS), and the proinflammatory effect of this Class I HDAC appears to be related to its ability to bind to PU.1 and deacetylate H3K9 in M2 signature genes 67, 68. Class IIa HDAC7 was found to negatively regulate differentiation of pre-B cells into macrophages and to block macrophage function, whereas a splice variant of HDAC7 lacking amino-terminal residues promoted inflammatory gene expression in macrophages 69, 70. The related Class IIa HDAC, HDAC9, has also been implicated as a positive regulator of the M1 proinflammatory phenotype (71). Regarding Class III HDACs, extensive studies suggest that both SIRT1 and SIRT2 negatively regulate proinflammatory gene expression and prevent the M1 phenotype through deacetylation of the p65 subunit of NF-κB 72, 73.
BET reader proteins also play fundamental roles in regulating macrophage inflammatory phenotypes. This was exemplified by the recent demonstrations that NF-κB-dependent proinflammatory gene expression was reduced in Brd4-null bone marrow–derived macrophages (BMDMs), and that crosstalk between glucocorticoid receptors and BRD4 controlled LPS-induced proinflammatory gene expression in macrophages 74, 75. Furthermore, small-molecule BET protein inhibition has been shown to block NF-κB–directed SE formation on proinflammatory genes in macrophages, and macrophages derived from Brd2 hypomorphic mice exhibit diminished proinflammatory cytokine expression in response to LPS 55, 76.
The development and function of anti-inflammatory Tregs is governed by the FoxP3 transcription factor, which is a nongenomic target of HDAC and HAT enzymes (77) (Figure 1C). Acetylation of lysine residues in FoxP3 promotes its DNA binding activity and transcriptional transactivation capacity, thereby leading to enhanced expression of anti-inflammatory factors (e.g., IL-10) from Tregs. Multiple HDACs have been implicated in the control of FoxP3 acetylation and as negative regulators of Treg function, including SIRT1 78, 79, 80, HDAC6 81, 82, HDAC9 81, 83, 84, 85, and HDAC11 (86). Conversely, HDAC3 appears to enhance Treg function (87).
Coadministration of TSA with subtherapeutic doses of rapamycin for 14 days after transplant boosted Treg function, induced allograft tolerance, and dramatically improved survival in mouse cardiac and pancreatic islet allograft models (85). Consistent with these findings, suberoylanilide hydroxamic acid (SAHA) acted synergistically with tacrolimus (FK506) to prevent murine cardiac allograft rejection and enhance the proportion of Tregs by inducing T effector cell apoptosis (88). Furthermore, the efficacy of VPA in mouse models of collagen-induced arthritis, and TSA and SAHA in mouse colitis models, was also shown to correlate with induction of Treg action 84, 89. It is likely that HDAC inhibition blunts cardiac inflammation and fibrosis, at least in part, by stimulating Treg function in the heart.
Epigenetics in Fibroblast Activation
Epigenetic control of cardiac fibroblast proliferation
Cardiac fibroblasts are the key cellular mediators that drive the fibrotic response of the heart (25). In uninjured hearts, a stable matrix network protects quiescent fibroblasts from mechanical stimuli. Conversely, injury and the resulting inflammation disrupt the structural integrity of the matrix and expose these cells to mechanical stress as well as stimulation by growth factors. DAMPs, fibroblast growth factor-2, Ang II, platelet-derived growth factor, and the mast cell-derived proteases tryptase and chymase are potentially important activators of the proliferative response in fibroblasts 1, 34. Recent advances in the development of more specific fibroblast lineage tags have permitted a better understanding of the source of cardiac fibroblasts 90, 91, 92. Past conclusions regarding the contributions of bone marrow–derived cells and endothelial cells to the activated fibroblast population following injury appear to have been overestimated, and it is now widely acknowledged that resident cardiac fibroblasts represent the major cellular origin 93, 94, 95, 96.
In mouse models of pressure overload, MI, and isoproterenol-induced cardiac injury, fibroblast proliferation spiked within the first week following induction of cardiac remodeling, and thereafter, it returned to basal levels in a manner that correlated with reduced inflammation (97). Class I HDAC inhibitors such as mocetinostat (MGCD0103) have been demonstrated to potently suppress cardiac fibroblast proliferation and mitigate fibrotic remodeling in response to cardiac injury 98, 99, 100, 101. Thus, HDAC inhibitor–mediated suppression of cardiac fibrosis is likely due, at least in part, to the ability of these compounds to squelch expansion of the ECM-producing fibroblast pool in the heart. The mechanism by which HDAC inhibitors block cardiac fibroblast proliferation involves induction of genes encoding the cyclin-dependent kinase inhibitors p15 and p57, which leads to reduced retinoblastoma (Rb) protein phosphorylation and blockade of downstream target genes that promote the G1-to-S transition 100, 101 (Figure 2A). MGCD0103 treatment of atrial CD90+ cells was also shown to stimulate the p53/p21 axis, inducing cell cycle arrest and apoptosis (99).
Figure 2.
Three Mechanisms by Which Epigenetic Regulators Can Control Cardiac Fibroblast Proliferation and Myofibroblast Differentiation
(A) HDACs repress expression of genes encoding the cyclin-dependent kinase inhibitors, p15 and p57, which leads to enhanced retinoblastoma (Rb) protein phosphorylation and stimulation of downstream target genes that promote cardiac fibroblast proliferation. (B) Binding of BRD4 to acetyl-histone H3K27 helps create super-enhancers (SEs), which drive expression of target genes that promote extracellular matrix (ECM) production and myofibroblast differentiation. (C) DNA methyltransferase (DNMT)-mediated methylation of DNA leads to suppression of antiproliferative genes and genes that normally repress myofibroblast differentiation.
The potential role of BET proteins in the regulation of cardiac fibroblast proliferation has yet to be addressed. However, histone and DNA methylation have been implicated in the epigenetic control of cardiac fibroblast cell cycle control (102). Furthermore, inhibition of the p300 HAT was recently shown to block the proliferative response of cardiac fibroblasts and suppress Ang II–mediated cardiac fibrosis (103). Additional investigation is warranted to explain the seemingly paradoxical findings that increasing histone acetylation using an HDAC inhibitor or reducing histone acetylation with an HAT inhibitor can both attenuate cardiac fibroblast proliferation.
Epigenetic regulation of myofibroblast activation
Conversion of fibroblasts into myofibroblasts occurs late in the proliferative phase. This process is characterized by expression and incorporation of contractile proteins such as α-smooth muscle actin (α-SMA) into the cytoskeleton (104), and the synthesis and deposition of structural and matricellular ECM proteins (105). Several members of the matricellular family, including thrombospondin-1 and -2, osteopontin, SPARC, periostin, tenascin-C, and CCN2, are important modulators of the myofibroblast phenotype and play essential roles in regulating growth factor signaling (1). However, it is important to note that only a subset of fibroblasts that expand following cardiac stress will express α-SMA, suggesting that not all “activated fibroblasts” become prototypical myofibroblasts (97). Future studies are needed to further define these α-SMA–negative cells and determine their potential contributions to cardiac fibrosis.
Formation of a collagen-based matrix marks the end of the proliferative phase and the beginning of the maturation of the scar, a poorly understood process that is characterized by matrix crosslinking (1). The dense, crosslinked collagen matrix provides a strong structural scar to protect the heart following an MI, but can also enhance tissue stiffness, contributing to diastolic dysfunction and creating a substrate for arrhythmias (106). In the mature scar, deprivation of growth factors and removal of matricellular proteins may promote myofibroblast apoptosis or quiescence (105). However, myofibroblasts can exhibit persistent activation due to increased hemodynamic load, leading to long-term fibrotic changes and heart failure (25). Therapies that promote myofibroblast apoptosis or cellular senescence (further discussed later in the text) might be beneficial in this context.
Studies focused on epigenetic modulation of myofibroblast differentiation have mostly concentrated on the regulation of α-SMA gene expression. TSA was shown to block α-SMA expression in lung fibroblasts in association with reduced activation of AKT (107), and MGCD0103 was demonstrated to reverse α-SMA expression in CD90+/cKit− cardiac fibroblasts (99). Furthermore, the balance of HAT and HDAC expression was found to influence TGF-β–induced transcription of SM22α, another myofibroblast marker, demonstrating that histone hyperacetylation of the SM22α promoter is a target of TGF-β signaling (108).
Our preliminary studies suggest that BRD4 functions downstream of TGF-β within resident cardiac fibroblasts to form SEs that positively control genes that are essential for myofibroblast differentiation and production of ECM (M.S. Stratton, S.M. Haldar, and T.A. McKinsey, unpublished data, June 2018) (Figure 2B). Consistent with this possibility, BET inhibitors have been shown to block conversion of fibroblasts from other tissues, including liver, pancreas, and skin, into α-SMA+ myofibroblasts 15, 109, 110, 111.
BRD4 also likely regulates profibrotic crosstalk between cardiomyocytes and cardiac fibroblasts. Whole-genome chromatin immunoprecipitation sequencing (ChIP-seq) studies of BRD4 dynamics in cardiomyocytes revealed that, in response to stress signaling, this chromatin reader accumulates on SEs for genes encoding secreted profibrotic factors such as TGF-β2 (112). Thus, BRD4 appears to control genes that control paracrine signaling events leading to fibroblast activation in the heart.
The H3K9 demethylase, JMJD1A, was also shown to be important for myofibroblast differentiation of hepatic stellate cells, because knockdown of JMJD1A led to increased expression of α-SMA (113). Conversely, the H3K4 methyltransferase, KMT2H (ASH1), was found to directly bind regulatory elements of profibrotic genes, including those encoding α-SMA, and its depletion resulted in broad suppression of fibrogenic gene expression (114).
DNMTs are also linked to cardiac myofibroblast differentiation (Figure 2C). Human cardiac fibroblasts exposed to hypoxic conditions exhibited increased DNA methylation in association with elevated expression of DNMT1 and DNMT3B, and knockdown of DNMT3B or treatment of the cells with the DNMT inhibitor 5-aza-2'-deoxycytidine was shown to block α-SMA expression (115). In the failing human heart, fibrosis correlated with enhanced DNA methylation of promoter sequences regulating the gene encoding RASAL1, a RAS-GTPase–activating protein that suppresses RAS activity, and reduced RASAL1 expression was linked to enhanced endothelial-to-mesenchymal transition (116). Whole-genome epigenomics analyses are needed to address the potential crosstalk between DNA and histone modifications in the control of cardiac myofibroblast conversion.
Epigenetic regulation of cardiac fibroblast senescence
Cellular senescence is an irreversible form of cell cycle arrest initially described as a consequence of replicative exhaustion and telomere erosion; however, it is now known that it can be triggered by various cellular stresses, including DNA damage, oncogene activation, and reactive oxygen species 117, 118. A recent study demonstrated that, in response to pressure overload, a burst of mouse cardiac fibroblast proliferation is followed by the accumulation of senescent fibroblasts, the majority of which are α-SMA+ myofibroblasts (119). Furthermore, cardioprotection in aged osteopontin-deficient mice was linked to enhanced cardiac fibroblast senescence and reduced fibrosis in the heart (120). It is believed that fibroblast senescence provides a means to limit organ fibrosis by reducing the number of ECM producing cells and triggering the release of matrix degrading factors from the senescent cells (see later in the text) (121), although others have proposed that senescence promotes cardiac fibrosis post-MI (122).
Senescent cells can be distinguished from most quiescent cells by up-regulation of p16INK4a, p21CIP1/WAF1, and senescence-associated β-galactosidase activity (123). Furthermore, senescent cells are characterized by the senescent-associated secretory phenotype (SASP) (124). Among the components of this secretome are ECM-degrading enzymes (MMP-1, -3, -8, -10, -11, -12, and -13), suggesting that the SASP could promote reversal of tissue fibrosis. Consistent with this notion, ectopic expression of the senescence-inducing integrin-binding protein CCN1 in the mouse heart suppressed fibrosis and improved cardiac function, whereas inhibition of senescence by compound deletion of the Trp53 and Cdkn2a genes enhanced cardiac fibrosis (119). Thus, there is interest in developing senescence-inducing therapies for the treatment of pathological fibrosis. Nonetheless, it should be noted that long-term accumulation of senescent cells in tissues has been linked to age-associated organ dysfunction (125).
HDAC inhibitors have long been recognized to trigger a senescence phenotype in association with accumulation of hypophosphorylated Rb, which is antiproliferative (126). Additionally, HDAC inhibitors have been shown to stimulate expression of components of the SASP 127, 128. Whether HDAC inhibitors trigger senescence of cardiac fibroblasts has not been determined. However, we recently demonstrated that MGCD0103 and MS-275, but not structurally distinct HDAC inhibitors, potently stimulate expression of the SASP components PAI-1 and MMP-13 in cardiac fibroblasts (100). Further studies are required to determine whether these Class I HDAC inhibitors are bona fide inducers of cardiac fibroblast replicative senescence, and whether other epigenetic regulators, such as BET proteins, KMTs, and DNMTs, influence the cardiac SASP.
Conclusions
A proposal to treat cardiac fibrosis and heart failure with small-molecule inhibitors of epigenetic regulators is often met with skepticism because the regulators to be targeted are ubiquitously expressed and govern fundamental transcriptional mechanisms in a multitude of cell types. Nonetheless, there are 4 Food and Drug Administration–approved HDAC inhibitors, 2 approved DNMT inhibitors, and a multitude of other epigenetic modifying therapies in clinical development for oncology and non-oncology indications (129). Thus, the use of epigenetic therapies to treat human diseases has been validated.
The clinical experiences with HDAC inhibitors should serve as a useful guide for development of drugs targeting epigenetic regulators for the treatment of cardiac fibrosis. In cancer patients, who typically receive maximum tolerated doses of HDAC inhibitors, gastrointestinal disturbance, fatigue, and hematological toxicity have been reported (130). However, on the basis of our unpublished preclinical studies of cardiac fibrosis, the antifibrotic activity of HDAC inhibitors is observed at doses far lower than the maximum tolerated dose. Furthermore, we recently showed that givinostat, an HDAC inhibitor in phase 3 clinical testing for Duchenne muscular dystrophy, prevented diastolic dysfunction in rodents at concentrations that failed to elicit signs of hematological toxicity (131). Importantly, in the phase 2 study of givinostat in Duchenne muscular dystrophy patients, the HDAC inhibitor was found to be safe, well tolerated, and to reduce the amount of fibrotic tissue in skeletal muscle of afflicted adolescent boys (132). These findings establish the feasibility of employing an HDAC inhibitor to treat striated muscle fibrosis in humans, and justify the advancement of givinostat or other epigenetic targeted therapies into clinical trials to assess efficacy in the heart.
Despite significant advancements in our understanding of the transcriptional control of cardiac fibrosis, there are still major gaps in information related to the role of epigenetics in this process. This is exemplified by the remarkable fact that, to our knowledge, there are no published reports of whole-genome analyses of epigenetic modifications in cardiac fibroblasts. Blending studies of purified cardiac fibroblasts with state-of-the-art epigenomic technologies (e.g., high-resolution ChIP-seq) and pharmacological inhibitors of epigenetic regulators is a crucial next step toward uncovering novel mechanisms of epigenetic control of cardiac fibroblast proliferation, myofibroblast differentiation and senescence.
As detailed in this review, a significant body of published data supports the notion that epigenetic regulators are critical mediators of cardiac fibrosis (Table 1). Nonetheless, much of this work has been based on the use of small molecule inhibitors. An advantage of this approach is that preclinical findings made with pharmacological inhibitors can, in theory, be rapidly translated into human clinical trials. Conversely, a weakness of this avenue of investigation is that the inhibitors are typically systemically exposed, thus hindering evaluation of cell-autonomous roles for epigenetic regulators in the control of fibroblast activation and cardiac fibrosis. As such, future studies should address the consequences of fibroblast-specific deletion of genes encoding epigenetic factors on cardiac ECM deposition and remodeling. Emerging mouse lines, such as periostin-Cre and Tcf21-Cre, could be employed for these experiments 94, 133, 134.
Table 1.
Epigenetic Control of Inflammation and Fibroblast Activation
| Phase of Fibrosis Development | Epigenetic Modification | Evidence | Ref. # |
|---|---|---|---|
| Inflammation | |||
| Histone acetylation | VPA treatment leads to a reduction in IL-1β and TNF-α expression in the left ventricle. | (46) | |
| SAHA reduces IL-1α, IL2, and TNF-α expression in DOCA-salt hypertensive rats. | (47) | ||
| VPA treatment suppresses the M1 phenotype of cultured macrophages. | (65) | ||
| Class I HDAC inhibitors promote the M2 anti-inflammatory phenotype of macrophages. | 66, 67, 68 | ||
| Class IIa HDACs are positive regulators of the M1 proinflammatory phenotype. | 69, 70, 71 | ||
| BET reader | BET inhibition potently suppresses a network of cardiac NF-κB responsive genes that control the innate immune response. | 53, 54, 55 | |
| BET reader proteins play fundamental roles in regulating macrophage inflammatory phenotypes. | 74, 75, 76 | ||
| Histone methylation | KMT1 represses proinflammatory genes associated with the M1 phenotype by promoting the formation of H3K9me3 marks on regulatory elements. | (60) | |
| KMT3 negatively regulates the proinflammatory M1 phenotype by increasing repressive H3K36 dimethylation at promoter regions of proinflammatory genes. | (61) | ||
| KDM6 promotes the M1 macrophage phenotype by removing repressive H3K27 trimethylation marks at regulatory sites for proinflammatory genes. | (62) | ||
| DNA methylation | In the setting of obesity and diabetes, DNMT1 promotes the M1 macrophage phenotype by hypermethylating of the pro-M2 transcription factor, PPARγ1. | 63, 64 | |
| Fibroblast proliferation/activation | |||
| Histone acetylation | MGCD0103 potently suppresses cardiac fibroblast proliferation and mitigates fibrotic remodeling in response to cardiac injury. | 98, 99, 100, 101 | |
| Inhibition of the p300 HAT blocks the proliferative response of cardiac fibroblasts and suppresses Ang II–mediated cardiac fibrosis. | (103) | ||
| TSA blocks α-SMA expression in lung fibroblasts in association with reduced activation of AKT. | (107) | ||
| The balance of HAT and HDAC expression influences TGF-β–induced transcription of SM22α. | (108) | ||
| BET reader | BET inhibitors block conversion of liver, pancreas and skin fibroblasts into α-SMA+ myofibroblasts. | 109, 110, 111 | |
| In response to stress signaling, BRD4 accumulates on SEs for genes encoding secreted profibrotic factors such as TGF-β2. | (112) | ||
| Knockdown of JMJD1A increases expression of α-SMA. | (113) | ||
| KMT2H directly binds regulatory elements of profibrotic genes, including those encoding α-SMA. | (114) | ||
| DNA methylation | Knockdown of DNMT3B or treatment with the DNMT inhibitor blocks α-SMA expression. | (115) | |
| Cardiac fibrosis correlates with enhanced DNA methylation of promoter sequences regulating the gene encoding RASAL1, a RAS-GTPase–activating protein. | (116) |
BET = bromodomain and extraterminal protein; DNMT = DNA methyltransferase; HDAC = histone deacetylase; SAHA = suberoylanilide hydroxamic acid; SE = superenhancer; TSA = trichostatin A; VPA = valproic acid.
It is important to note that most of the epigenetic regulators described in this review also have nonhistone targets. The first “acetylome” was reported 9 years ago (135), and combined with subsequent studies, thousands of acetylation sites on thousands of proteins have been identified in mammalian cells (136), underscoring the importance of acetylation in biological control beyond epigenetics. In the heart, many proteins involved in metabolism, intra- and extracellular signaling, gene regulation, cell–cell communication and contraction have been shown to be acetylated, although little is known regarding if or how this post-translational modification alters the function of the target proteins 137, 138, 139. Consistent with this, nongenomic, non-nuclear roles for canonical epigenetic regulators such as HDAC1 and HDAC2 in the heart are beginning to emerge 131, 140.
In summary, this is an exquisitely exciting time for our field. Advances in the basic science of epigenetics, nonhistone epigenetic marks, and cardiac fibrosis promise to be rapidly forthcoming and to facilitate the clinical translation of innovative “epigenetic therapies” for patients who have heart disease.
Acknowledgment
The authors thank Joshua Travers for critical reading of the manuscript.
Footnotes
Dr. McKinsey was supported by the National Institutes of Health (HL116848 and HL127240) and the American Heart Association (16SFRN31400013). Both authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Shinde A.V., Frangogiannis N.G. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diez J., Querejeta R., Lopez B., Gonzalez A., Larman M., Martinez Ubago J.L. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 3.Mohammed S.F., Hussain S., Mirzoyev S.A., Edwards W.D., Maleszewski J.J., Redfield M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis Stuart S.D., De Jesus N.M., Lindsey M.L., Ripplinger C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J Mol Cell Cardiol. 2016;91:114–122. doi: 10.1016/j.yjmcc.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allfrey V.G., Faulkner R., Mirsky A.E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdin E., Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16:258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 7.Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 8.Lee K.K., Workman J.L. Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 9.Gregoretti I.V., Lee Y.M., Goodson H.V. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Bisgrove D.A., Mahmoudi T., Henklein P., Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc Natl Acad Sci U S A. 2007;104:13690–13695. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang M.K., Mochizuki K., Zhou M., Jeong H.S., Brady J.N., Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005;19:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z., Yik J.H., Chen R. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Amaral P.P., Bannister A.J. Re-place your BETs: the dynamics of super enhancers. Mol Cell. 2014;56:187–189. doi: 10.1016/j.molcel.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Loven J., Hoke H.A., Lin C.Y. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratton M.S., Haldar S.M., McKinsey T.A. BRD4 inhibition for the treatment of pathological organ fibrosis. F1000Res. 2017;6 doi: 10.12688/f1000research.11339.1. F1000 Faculty Rev-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews F.H., Strahl B.D., Kutateladze T.G. Insights into newly discovered marks and readers of epigenetic information. Nat Chem Biol. 2016;12:662–668. doi: 10.1038/nchembio.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Wen H., Shi X. Lysine methylation: beyond histones. Acta Biochim Biophys Sin (Shanghai) 2012;44:14–27. doi: 10.1093/abbs/gmr100. [DOI] [PubMed] [Google Scholar]
- 18.Nowak R.P., Tumber A., Johansson C. Advances and challenges in understanding histone demethylase biology. Curr Opin Chem Biol. 2016;33:151–159. doi: 10.1016/j.cbpa.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Goll M.G., Bestor T.H. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 20.Collings C.K., Waddell P.J., Anderson J.N. Effects of DNA methylation on nucleosome stability. Nucleic Acids Res. 2013;41:2918–2931. doi: 10.1093/nar/gks893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrich B., Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–6547. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer L.M., Tahiliani M., Rao A., Aravind L. Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids. Cell Cycle. 2009;8:1698–1710. doi: 10.4161/cc.8.11.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastor W.A., Aravind L., Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14:341–356. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tahiliani M., Koh K.P., Shen Y. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travers J.G., Kamal F.A., Robbins J., Yutzey K.E., Blaxall B.C. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seta Y., Shan K., Bozkurt B., Oral H., Mann D.L. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2:243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]
- 28.Frangogiannis N.G. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshminarayanan V., Lewallen M., Frangogiannis N.G. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol. 2001;159:1301–1311. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L., Chen S.S., Zhang J.Q., Ramires F.J., Sun Y. Activation of nuclear factor-kappaB and its proinflammatory mediator cascade in the infarcted rat heart. Biochem Biophys Res Commun. 2004;321:879–885. doi: 10.1016/j.bbrc.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 31.Morishita R., Sugimoto T., Aoki M. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 32.Frangogiannis N.G., Smith C.W., Entman M.L. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 33.Nahrendorf M., Swirski F.K., Aikawa E. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner N.A. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs) J Mol Cell Cardiol. 2016;94:189–200. doi: 10.1016/j.yjmcc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Hartupee J., Mann D.L. Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol. 2016;93:143–148. doi: 10.1016/j.yjmcc.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann D.L. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Bauersachs J., Langer H.F. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–1389. doi: 10.1002/ejhf.942. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann U., Beyersdorf N., Weirather J. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y., Xu W., Xiong S. Adoptive transfer of regulatory T cells protects against Coxsackievirus B3-induced cardiac fibrosis. PLoS One. 2013;8:e74955. doi: 10.1371/journal.pone.0074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanellakis P., Dinh T.N., Agrotis A., Bobik A. CD4(+)CD25(+)Foxp3(+) regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J Hypertens. 2011;29:1820–1828. doi: 10.1097/HJH.0b013e328349c62d. [DOI] [PubMed] [Google Scholar]
- 41.Kvakan H., Kleinewietfeld M., Qadri F. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 42.Tang T.T., Yuan J., Zhu Z.F. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 43.Nevers T., Salvador A.M., Velazquez F. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. 2017;214:3311–3329. doi: 10.1084/jem.20161791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKinsey T.A. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol Med. 2011;17:434–441. doi: 10.2119/molmed.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright L.H., Menick D.R. A class of their own: exploring the nondeacetylase roles of class IIa HDACs in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2016;311:H199–H206. doi: 10.1152/ajpheart.00271.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardinale J.P., Sriramula S., Pariaut R. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iyer A., Fenning A., Lim J. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br J Pharmacol. 2010;159:1408–1417. doi: 10.1111/j.1476-5381.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shakespear M.R., Halili M.A., Irvine K.M., Fairlie D.P., Sweet M.J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32:335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Cao W., Bao C., Padalko E., Lowenstein C.J. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caillaud A., Prakash A., Smith E. Acetylation of interferon regulatory factor-7 by p300/CREB-binding protein (CBP)-associated factor (PCAF) impairs its DNA binding. J Biol Chem. 2002;277:49417–49421. doi: 10.1074/jbc.M207484200. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Fischle W., Verdin E., Greene W.C. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 52.Xu Y., Vakoc C.R. Brd4 is on the move during inflammation. Trends Cell Biol. 2014;24:615–616. doi: 10.1016/j.tcb.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan Q., McMahon S., Anand P. BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aah5084. eaah5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang B., Yang X.D., Zhou M.M., Ozato K., Chen L.F. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol Cell Biol. 2009;29:1375–1387. doi: 10.1128/MCB.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown J.D., Lin C.Y., Duan Q. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epelman S., Lavine K.J., Beaudin A.E. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lavine K.J., Epelman S., Uchida K. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ginhoux F., Schultze J.L., Murray P.J., Ochando J., Biswas S.K. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 59.Murray P.J., Allen J.E., Biswas S.K. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eames H.L., Saliba D.G., Krausgruber T., Lanfrancotti A., Ryzhakov G., Udalova I.A. KAP1/TRIM28: an inhibitor of IRF5 function in inflammatory macrophages. Immunobiology. 2012;217:1315–1324. doi: 10.1016/j.imbio.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 61.Xu G., Liu G., Xiong S., Liu H., Chen X., Zheng B. The histone methyltransferase Smyd2 is a negative regulator of macrophage activation by suppressing interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) production. J Biol Chem. 2015;290:5414–5423. doi: 10.1074/jbc.M114.610345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruidenier L., Chung C.W., Cheng Z. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X., Cao Q., Yu L., Shi H., Xue B., Shi H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight. 2016;1:e87748. doi: 10.1172/jci.insight.87748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan J., Tie G., Wang S. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat Commun. 2018;9:33. doi: 10.1038/s41467-017-02425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu C., Li A., Leng Y., Li Y., Kang J. Histone deacetylase inhibition by sodium valproate regulates polarization of macrophage subsets. DNA Cell Biol. 2012;31:592–599. doi: 10.1089/dna.2011.1401. [DOI] [PubMed] [Google Scholar]
- 66.Wang G., Shi Y., Jiang X. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X., Barozzi I., Termanini A. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc Natl Acad Sci U S A. 2012;109:E2865–E2874. doi: 10.1073/pnas.1121131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mullican S.E., Gaddis C.A., Alenghat T. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barneda-Zahonero B., Roman-Gonzalez L., Collazo O. HDAC7 is a repressor of myeloid genes whose downregulation is required for transdifferentiation of pre-B cells into macrophages. PLoS Genet. 2013;9:e1003503. doi: 10.1371/journal.pgen.1003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shakespear M.R., Hohenhaus D.M., Kelly G.M. Histone deacetylase 7 promotes Toll-like receptor 4-dependent proinflammatory gene expression in macrophages. J Biol Chem. 2013;288:25362–25374. doi: 10.1074/jbc.M113.496281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Q., Rong S., Repa J.J., St Clair R., Parks J.S., Mishra N. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2014;34:1871–1879. doi: 10.1161/ATVBAHA.114.303393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 73.Lo Sasso G., Menzies K.J., Mottis A. SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS One. 2014;9:e103573. doi: 10.1371/journal.pone.0103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bao Y., Wu X., Chen J. Brd4 modulates the innate immune response through Mnk2-eIF4E pathway-dependent translational control of IkappaBalpha. Proc Natl Acad Sci U S A. 2017;114:E3993–E4001. doi: 10.1073/pnas.1700109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sacta M.A., Tharmalingam B., Coppo M. Gene-specific mechanisms direct glucocorticoid-receptor-driven repression of inflammatory response genes in macrophages. Elife. 2018;7:e34864. doi: 10.7554/eLife.34864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belkina A.C., Nikolajczyk B.S., Denis G.V. BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. J Immunol. 2013;190:3670–3678. doi: 10.4049/jimmunol.1202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Loosdregt J., Coffer P.J. Post-translational modification networks regulating FOXP3 function. Trends Immunol. 2014;35:368–378. doi: 10.1016/j.it.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Beier U.H., Wang L., Bhatti T.R. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. 2011;31:1022–1029. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Loosdregt J., Brunen D., Fleskens V., Pals C.E., Lam E.W., Coffer P.J. Rapid temporal control of Foxp3 protein degradation by sirtuin-1. PLoS One. 2011;6:e19047. doi: 10.1371/journal.pone.0019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Loosdregt J., Vercoulen Y., Guichelaar T. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 81.Beier U.H., Wang L., Han R., Akimova T., Liu Y., Hancock W.W. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Zoeten E.F., Wang L., Butler K. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol. 2011;31:2066–2078. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beier U.H., Angelin A., Akimova T. Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Zoeten E.F., Wang L., Sai H., Dillmann W.H., Hancock W.W. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138:583–594. doi: 10.1053/j.gastro.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tao R., de Zoeten E.F., Ozkaynak E. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 86.Huang J., Wang L., Dahiya S. Histone/protein deacetylase 11 targeting promotes Foxp3+ Treg function. Sci Rep. 2017;7:8626. doi: 10.1038/s41598-017-09211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L., Liu Y., Han R. FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest. 2015;125:1111–1123. doi: 10.1172/JCI77088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X., Han S., Kang Y. SAHA, an HDAC inhibitor, synergizes with tacrolimus to prevent murine cardiac allograft rejection. Cell Mol Immunol. 2012;9:390–398. doi: 10.1038/cmi.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saouaf S.J., Li B., Zhang G. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol. 2009;87:99–104. doi: 10.1016/j.yexmp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ivey M.J., Tallquist M.D. Defining the cardiac fibroblast. Circ J. 2016;80:2269–2276. doi: 10.1253/circj.CJ-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moore-Morris T., Cattaneo P., Puceat M., Evans S.M. Origins of cardiac fibroblasts. J Mol Cell Cardiol. 2016;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tallquist M.D., Molkentin J.D. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol. 2017;14:484–491. doi: 10.1038/nrcardio.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ali S.R., Ranjbarvaziri S., Talkhabi M. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115:625–635. doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 94.Kanisicak O., Khalil H., Ivey M.J. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moore-Morris T., Cattaneo P., Guimaraes-Camboa N. Infarct fibroblasts do not derive from bone marrow lineages. Circ Res. 2018;122:583–590. doi: 10.1161/CIRCRESAHA.117.311490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moore-Morris T., Guimaraes-Camboa N., Banerjee I. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ivey M.J., Kuwabara J.T., Pai J.T., Moore R.E., Sun Z., Tallquist M.D. Resident fibroblast expansion during cardiac growth and remodeling. J Mol Cell Cardiol. 2018;114:161–174. doi: 10.1016/j.yjmcc.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nural-Guvener H., Zakharova L., Feehery L., Sljukic S., Gaballa M. Anti-fibrotic effects of class I HDAC inhibitor, mocetinostat is associated with IL-6/Stat3 signaling in ischemic heart failure. Int J Mol Sci. 2015;16:11482–11499. doi: 10.3390/ijms160511482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nural-Guvener H.F., Zakharova L., Nimlos J., Popovic S., Mastroeni D., Gaballa M.A. HDAC class I inhibitor, Mocetinostat, reverses cardiac fibrosis in heart failure and diminishes CD90+ cardiac myofibroblast activation. Fibrogenesis Tissue Repair. 2014;7:10. doi: 10.1186/1755-1536-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schuetze K.B., Stratton M.S., Blakeslee W.W. Overlapping and divergent actions of structurally distinct histone deacetylase inhibitors in cardiac fibroblasts. J Pharmacol Exp Ther. 2017;361:140–150. doi: 10.1124/jpet.116.237701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams S.M., Golden-Mason L., Ferguson B.S. Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol. 2014;67:112–125. doi: 10.1016/j.yjmcc.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghosh A.K., Rai R., Flevaris P., Vaughan D.E. Epigenetics in reactive and reparative cardiac fibrogenesis: the promise of epigenetic therapy. J Cell Physiol. 2017;232:1941–1956. doi: 10.1002/jcp.25699. [DOI] [PubMed] [Google Scholar]
- 103.Rai R., Verma S.K., Kim D. A novel acetyltransferase p300 inhibitor ameliorates hypertension-associated cardio-renal fibrosis. Epigenetics. 2017;12:1004–1013. doi: 10.1080/15592294.2017.1370173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Willems I.E., Havenith M.G., De Mey J.G., Daemen M.J. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 105.Chen W., Frangogiannis N.G. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–953. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holmes J.W., Borg T.K., Covell J.W. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 107.Guo W., Shan B., Klingsberg R.C., Qin X., Lasky J.A. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–L870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qiu P., Ritchie R.P., Gong X.Q., Hamamori Y., Li L. Dynamic changes in chromatin acetylation and the expression of histone acetyltransferases and histone deacetylases regulate the SM22alpha transcription in response to Smad3-mediated TGFbeta1 signaling. Biochem Biophys Res Commun. 2006;348:351–358. doi: 10.1016/j.bbrc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 109.Ding N., Hah N., Yu R.T. BRD4 is a novel therapeutic target for liver fibrosis. Proc Natl Acad Sci U S A. 2015;112:15713–15718. doi: 10.1073/pnas.1522163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ijaz T., Jamaluddin M., Zhao Y. Coordinate activities of BRD4 and CDK9 in the transcriptional elongation complex are required for TGFbeta-induced Nox4 expression and myofibroblast transdifferentiation. Cell Death Dis. 2017;8:e2606. doi: 10.1038/cddis.2016.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumar K., DeCant B.T., Grippo P.J. BET inhibitors block pancreatic stellate cell collagen I production and attenuate fibrosis in vivo. JCI Insight. 2017;2:e88032. doi: 10.1172/jci.insight.88032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stratton M.S., Lin C.Y., Anand P. Signal-dependent recruitment of BRD4 to cardiomyocyte super-enhancers is suppressed by a microRNA. Cell Rep. 2016;16:1366–1378. doi: 10.1016/j.celrep.2016.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang Y., Wang S., Zhao Y. Histone H3K9 demethylase JMJD1A modulates hepatic stellate cells activation and liver fibrosis by epigenetically regulating peroxisome proliferator-activated receptor gamma. FASEB J. 2015;29:1830–1841. doi: 10.1096/fj.14-251751. [DOI] [PubMed] [Google Scholar]
- 114.Perugorria M.J., Wilson C.L., Zeybel M. Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast transdifferentiation. Hepatology. 2012;56:1129–1139. doi: 10.1002/hep.25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watson C.J., Collier P., Tea I. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum Mol Genet. 2014;23:2176–2188. doi: 10.1093/hmg/ddt614. [DOI] [PubMed] [Google Scholar]
- 116.Xu X., Tan X., Tampe B. Epigenetic balance of aberrant Rasal1 promoter methylation and hydroxymethylation regulates cardiac fibrosis. Cardiovasc Res. 2015;105:279–291. doi: 10.1093/cvr/cvv015. [DOI] [PubMed] [Google Scholar]
- 117.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 119.Meyer K., Hodwin B., Ramanujam D., Engelhardt S., Sarikas A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol. 2016;67:2018–2028. doi: 10.1016/j.jacc.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 120.Sawaki D., Czibik G., Pini M. Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation. 2018 Mar 2 doi: 10.1161/CIRCULATIONAHA.117.031358. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 121.Jun J.I., Lau L.F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu F., Li Y., Zhang J. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One. 2013;8:e74535. doi: 10.1371/journal.pone.0074535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dimri G.P., Lee X., Basile G. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Young A.R., Narita M. SASP reflects senescence. EMBO Rep. 2009;10:228–230. doi: 10.1038/embor.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mavrogonatou E., Pratsinis H., Papadopoulou A., Karamanos N.K., Kletsas D. Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol. 2017 Oct 21 doi: 10.1016/j.matbio.2017.10.004. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 126.Ogryzko V.V., Hirai T.H., Russanova V.R., Barbie D.A., Howard B.H. Human fibroblast commitment to a senescence-like state in response to histone deacetylase inhibitors is cell cycle dependent. Mol Cell Biol. 1996;16:5210–5218. doi: 10.1128/mcb.16.9.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Orjalo A.V., Bhaumik D., Gengler B.K., Scott G.K., Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pazolli E., Alspach E., Milczarek A., Prior J., Piwnica-Worms D., Stewart S.A. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 2012;72:2251–2261. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shortt J., Ott C.J., Johnstone R.W., Bradner J.E. A chemical probe toolbox for dissecting the cancer epigenome. Nat Rev Cancer. 2017;17:268. doi: 10.1038/nrc.2017.26. [DOI] [PubMed] [Google Scholar]
- 130.Marks P.A. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Investig Drugs. 2010;19:1049–1066. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jeong M.Y., Lin Y.H., Wennersten S.A. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aao0144. eaao0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bettica P., Petrini S., D'Oria V. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:643–649. doi: 10.1016/j.nmd.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 133.Acharya A., Baek S.T., Banfi S., Eskiocak B., Tallquist M.D. Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis. 2011;49:870–877. doi: 10.1002/dvg.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swonger J.M., Liu J.S., Ivey M.J., Tallquist M.D. Genetic tools for identifying and manipulating fibroblasts in the mouse. Differentiation. 2016;92:66–83. doi: 10.1016/j.diff.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choudhary C., Kumar C., Gnad F. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 136.Choudhary C., Weinert B.T., Nishida Y., Verdin E., Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- 137.Foster D.B., Liu T., Rucker J. The cardiac acetyl-lysine proteome. PLoS One. 2013;8:e67513. doi: 10.1371/journal.pone.0067513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Horton J.L., Martin O.J., Lai L. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2:e84897. doi: 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lundby A., Lage K., Weinert B.T. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herr D.J., Baarine M., Aune S.E. HDAC1 localizes to the mitochondria of cardiac myocytes and contributes to early cardiac reperfusion injury. J Mol Cell Cardiol. 2018;114:309–319. doi: 10.1016/j.yjmcc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]