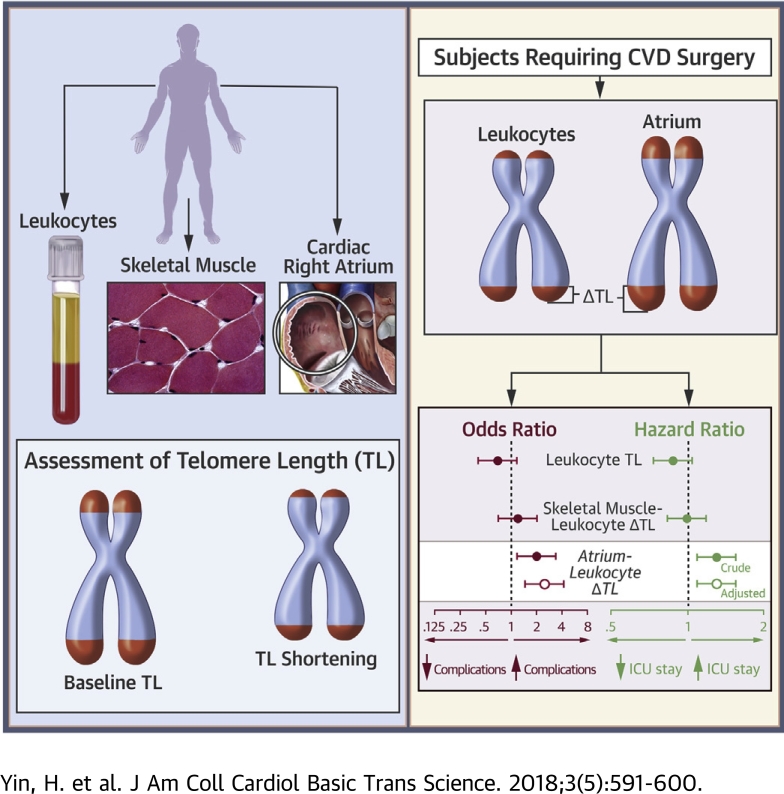
Visual Abstract
Key Words: atrium, risk, surgery, telomere shortening
Abbreviations and Acronyms: bp, base pair; CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; OR, odds ratio; PCR, polymerase chain reaction; TL, telomere length; ΔTLRA-L, right atrium-leukocyte TL difference
Highlights
-
•
Short leukocyte telomeres have been associated with adverse cardiovascular outcomes in population studies, but this relationship has not translated to patient care. The authors report a telomere length autologous referencing strategy that has the potential to mark biological aging and to identify high-risk individuals.
-
•
Among 163 patients who underwent cardiovascular surgery, telomeres in leukocytes and skeletal muscle displayed age-related shortening, whereas the telomere length in the cardiac right atrium was stable during 6 decades of life.
-
•
The magnitude of the telomere length gap between cardiac atrial tissue and leukocytes was associated with post-operative complications and length of stay in the intensive care unit.
-
•
This study provided proof of concept that a single-time, internally referenced assessment of leukocyte telomere shortening behavior could inform acute risks in patients with cardiovascular disease.
Summary
Leukocyte telomere shortening reflects stress burdens and has been associated with cardiac events. However, the patient-specific clinical value of telomere assessment remains unknown. Moreover, telomere shortening cannot be inferred from a single telomere length assessment. The authors investigated and developed a novel strategy for gauging leukocyte telomere shortening using autologous cardiac atrial referencing. Using multitissue assessments from 163 patients who underwent cardiovascular surgery, we determined that the cardiac atrium-leukocyte telomere length difference predicted post-operative complexity. This constituted the first evidence that a single-time assessment of telomere dynamics might be salient to acute cardiac care.
Leukocyte telomere length (TL) and leukocyte telomere shortening are 2 genomic DNA attributes that have generated considerable interest because of their relationships with health risks. Leukocyte TL is measured from a single blood sample and has received particular attention in human studies. Several large cross-sectional studies have revealed that adults with short leukocyte telomeres are at increased risk of aging-associated diseases, including coronary artery disease 1, 2, 3, 4, heart failure (5), and stroke 2, 3, 4. However, despite these associations, the value of knowing an individual’s leukocyte TL is unclear, and whether telomere assessment is useful for clinical decision-making is uncertain 6, 7.
Leukocyte telomere shortening is an alternative telomere assessment that, in theory, might provide meaningful information for patient care. Leukocyte telomeres shorten in response to replicative and oxidative stresses, and the extent of shortening has been considered to serve as an aggregate indicator of these biological stresses 8, 9, 10. Although less studied in humans than leukocyte TL, leukocyte telomere shortening has also been associated with cardiovascular outcomes 11, 12. However, leukocyte telomere shortening cannot be inferred from a single leukocyte TL measurement. This is because the major determinant of leukocyte TL is inheritance, which accounts for up to 80% of the TL variation in the population 13, 14, 15. In addition, serially measuring TL to ascertain the shortening rate has limited value in the clinical setting. The relatively low leukocyte telomere attrition rate (∼20 to 40 base pairs [bps] per year) 2, 13, 16, 17 means that typically 5 to 10 years of follow-up are required to discriminate a change in TL 12, 18.
Recently, an alternative approach to assessing leukocyte telomere attrition was proposed based on referencing leukocyte TL to that of a nonreplicating tissue in the same individual (19). A quasi-longitudinal assessment of telomere shortening might thus be made. In the dog, skeletal muscle TL was found to be stable over time, and the difference in TL between skeletal muscle and leukocytes correlated with age better than leukocyte TL alone did (19). Comparing leukocyte and skeletal muscle TL has been termed the “blood-and-muscle model” and has yielded insights into human telomere length dynamics 15, 20, 21.
Although conceptually interesting, whether an internal TL referencing approach has the power to inform clinical decision-making is unknown. At a more fundamental level, it is also unclear what the most suitable reference tissue for this purpose might be. Ideally, this would be tissue in which the TL is stable over the course of adulthood. However, there are limited human data on telomere dynamics in tissues other than leukocytes. In addition, although some reports have suggested that human skeletal muscle TL is invariant over time 22, 23, recent larger studies have indicated that skeletal muscle telomeres shorten in adults 20, 21.
The purpose of this study was to determine if there was a muscle-based strategy for gauging leukocyte TL shortening that was biologically rational and clinically meaningful for individuals with advanced cardiovascular disease. We studied 163 patients who underwent cardiac surgery and measured TL in their leukocytes, skeletal muscle, and cardiac right atrium. We found that, although there was evidence for telomere shortening in adult skeletal muscle, shortening was not evident for telomeres in the right atrium during 6 decades of life. Furthermore, a wide intraindividual difference between right atrium TL and leukocyte TL was associated with a more complicated post-operative course following surgery. The findings raised prospects for a single-time assessment of leukocyte telomere dynamics that could have patient-specific clinical usefulness.
Methods
Study population and sample preparation
Patients who underwent coronary artery bypass grafting, cardiac valve replacement or repair, ascending aortic replacement, or a combination of these procedures were enrolled in the study in accordance with protocols approved by the Institutional Review Board/Research Ethics Committee at The University of Western Ontario. Participants gave written informed consent. Samples from participants enrolled between March 2011 and August 2014 were studied. The samples studied were consecutive, excluding those in which the DNA integrity was insufficient for analysis (identified in 2.5% of subjects). Whole blood samples were obtained pre-operatively, and leukocytes were harvested using Histopaque-1077 (Sigma, Oakville, Ontario, Canada) for immediate isolation of genomic DNA. Tissue samples were harvested intraoperatively from trimmed and otherwise discarded fragments of skeletal muscle from the anterior chest wall (suprasternal, supraclavicular, and pectoralis major territories), as well as the right atrial appendage. Tissues were cleaned of adherent adipose tissue and stored at −80°C, within 45 min of surgical resection. A single thaw was undertaken for DNA extraction.
Genomic DNA preparation
Genomic DNA was isolated from peripheral blood leukocytes and muscle tissues using the QIAamp DNA mini kit (QIAGEN, Toronto, Ontario, Canada). The integrity of genomic DNA was ascertained by agarose gel electrophoresis. Samples were excluded if there was evidence for loss of integrity (smear >10% of total DNA signal), which was a rare finding that, when present, was exclusively in the muscle tissue. Concentration was determined in a 2-stage procedure, starting with ultraviolet spectrophotometry (NanoDrop Products, Wilmington, Delaware) and then with ultrasensitive fluorescent DNA staining (Quanti-iT PicoGreen dsDNA Assay Kit, Life Technologies, Burlington, Ontario, Canada).
TL measurement using quantitative polymerase chain reaction
TL was measured using quantitative polymerase chain reaction (PCR), based on described methods 24, 25, 26. A telomere repeat signal (T) was determined relative to that of the single copy gene, 36B4 (acidic ribosomal phosphoprotein P0, S). PCR was performed in a 10-μl reaction volume containing 5-ng genomic DNA and RT2 SYBR Green ROX Mastermix (QIAGEN), using an ABI 7500 Real-time PCR system or ViiA 7 Real-time PCR system (ThermoFisher Scientific, Burlington, Ontario, Canada). We used the following primers: GGTTTTT(GAGGGT)5 (T-forward), TCCCGACTA(TCCCTA)5 (T-reverse), CAGCAAGTGGGAAGGTGTAATCC (S-forward), and CCCATTCTATCATCAACGGGTACAA (S-reverse). A standard curve was generated for each run using DNA harvested from a single preparation of HepG2 cells, serially diluted by a factor of 1.68 (0.52 to 19.7 ng/μl).
To control for run-to-run variability, control genomic DNA samples from 7 different cell lines were included in each PCR run (25). Specifically, a single preparation from each of HEK293, HeLa, HT1080, THP1, U87, HITC6 vascular smooth muscle cells that stably expressed hTERT 27, 28 and human umbilical vein endothelial cells were used. The respective T/S ratios were divided by the average T/S ratio of that same DNA from 20 PCR runs to yield a normalizing factor. The average normalizing factor from all 7 control DNA preparations was used to correct the patient sample T/S ratios. Patient sample T/S ratios were measured in triplicate in 2 runs, or 3 runs if the first 2 values differed by >15%. The interassay coefficient of variation was 6.3%. Conversion of T/S ratios to absolute length (kilobase pair) was undertaken using DNA oligomer standards for both telomere repeats and 36B4, as described previously (26).
Adverse post-operative outcomes
Post-operative complications were ascribed if the patient developed ≥1 of the following: in-hospital mortality, cardiac arrest, life-threatening arrhythmia, need for an intra-aortic balloon pump, myocardial infarction, stroke, delirium, bleeding that necessitated reoperation, new renal failure that required dialysis, respiratory failure, septicemia, or mediastinitis; operational definitions were used for each endpoint as previously reported 29, 30. All of these are documented hazards in the post-operative period and were included as an aggregate endpoint (29). The length of stay in the intensive care unit (ICU) was recorded in days.
Statistical analysis
The target sample size for this study was based on a post-operative event rate of 15% 29, 31 and an assumption that the effect size for telomere shortening on post-operative events would be similar to that of leukocyte TL on mortality 32, 33. This yielded a sample size of 119 to detect an odds ratio (OR) of 1.4 per 1 SD change in TL, with a power of 85% and α of 0.05. Descriptive data were expressed as mean ± SD. Mean tissue TLs were compared using 1-way analysis of variance with the Bonferroni post hoc test. The lengths of telomeres in different tissues within an individual were compared using Pearson's correlation analysis. Relationships between TL and age were evaluated by linear regression analysis. Relationships between TL or ΔTL and sustaining a post-operative complication were evaluated using binary logistic regression analysis. A multivariate logistic regression model was used for non-telomere covariate adjustment (Table 1). Because of the sample size and predicted event rate, 1 covariate was used for multivariate modeling, selected based on the results of univariate analysis. Relationships with lengths of stay in the ICU, excluding 3 patients who died in hospital, were assessed using Cox proportional hazards regression analysis. Risks were adjusted using multivariate proportional hazards regression, and the covariate was selected as per the previously described strategy. The proportional hazard assumption was tested using Schoenfeld residuals, and no violations were observed. Multiple comparisons of different telomere indexes with outcomes were adjusted using the Bonferroni procedure to control the family-wise error rate at 0.05. ICU duration was further assessed in age-stratified tertiles of TL and ΔTL by chi-square analysis. Five age strata were used (<50, 50 to 59, 60 to 69, 70 to 79, and ≥80 years). Statistical analyses were performed using SPSS 19 (IBM Corp., Armonk, New York). All tests were 2-sided.
Table 1.
Demographic, Clinical, and Operative Characteristics of the Study Population
| All (N = 163, 100%) | Men (n = 130, 80%) | Women (n = 33, 20%) | |
|---|---|---|---|
| Demographic and clinical features | |||
| Age, yrs (range) | 62 ± 15 (30–89) | 62 ± 15 (30–89) | 64 ± 16 (30–85) |
| Caucasian | 144 (88) | 114 (88) | 30 (91) |
| Obesity (BMI ≥30 kg/m2) | 57 (35) | 42 (32) | 15 (46) |
| Smoking | 79 (49) | 66 (51) | 13 (39) |
| Atrial fibrillation | 42 (26) | 34 (26) | 8 (24) |
| Diabetes | 36 (22) | 31 (24) | 5 (15) |
| Comorbidities other than diabetes | 68 (42) | 56 (43) | 12 (36) |
| Recent (30 days) myocardial infarction | 8 (5) | 7 (6) | 1 (3) |
| Cerebrovascular disease | 16 (10) | 14 (11) | 2 (6) |
| Congestive heart failure | 14 (9) | 12 (9) | 2 (6) |
| Peripheral vascular disease | 26 (16) | 22 (17) | 4 (12) |
| Chronic obstructive pulmonary disease | 23 (14) | 20 (15) | 3 (9) |
| Renal failure requiring dialysis | 3 (2) | 2 (2) | 1 (3) |
| Re-operation | 4 (2) | 3 (2) | 1 (3) |
| Operative features | |||
| Surgical procedure | |||
| CABG | 68 (42) | 57 (44) | 11 (33) |
| Valve | 25 (15) | 17 (13) | 8 (24) |
| CABG and valve | 6 (4) | 4 (3) | 2 (6) |
| Aortic | 64 (39) | 52 (40) | 12 (36) |
| Operating room time, h (interquartile range) | 4.2 (3.3–6.1) | 4.4 (3.3–6.1) | 4.1 (3.1–5.8) |
| Urgent | 28 (17) | 21 (16) | 7 (21) |
Values are n, mean ± SD (range), or n (%).
BMI = body mass index; CABG = coronary artery bypass grafting.
Results
Telomeres in skeletal muscle and right atrium are longer than those in circulating leukocytes
A total of 163 patients who underwent coronary artery bypass graft, cardiac valve surgery, ascending aortic replacement, or a combination of any of these procedures were enrolled in the study. Demographic, clinical, and operative data are listed in Table 1. No sex differences were identified. The average TLs of peripheral blood leukocytes, chest wall skeletal muscle, and the right atrium are shown in Table 2. Telomeres in skeletal muscle were an average of 77% longer than leukocyte telomeres (p < 0.001). Telomeres in the right atrium were 85% longer than leukocyte telomeres (p < 0.001).
Table 2.
Telomere Length Measurements of Study Population
| All (N = 163, 100%) | Men (n = 130, 80%) | Women (n = 33, 20%) | |
|---|---|---|---|
| Age, yrs | 62 ± 15 (30–89) | 62 ± 15 (30–89) | 64 ± 16 (30–85) |
| TL, kb | |||
| Leukocyte | 3.58 ± 0.86 (162) | 3.61 ± 0.83 (129) | 3.46 ± 0.98 (33) |
| Skeletal muscle | 6.35 ± 1.78 (154) | 6.35 ± 1.82 (121) | 6.37 ± 1.64 (33) |
| Right atrium | 6.62 ± 1.76 (129) | 6.56 ± 1.84 (103) | 6.86 ± 1.42 (26) |
| ΔTL, kb | |||
| Skeletal muscle — leukocyte | 2.77 ± 1.62 (153) | 2.73 ± 1.66 (120) | 2.92 ± 1.47 (33) |
| Right atrium — leukocyte | 3.04 ± 1.59 (129) | 2.94 ± 1.69 (103) | 3.41 ± 1.07 (26) |
Values are mean ± SD (range) or mean ± SD (n).
kb = kilobase; TL = telomere length; ΔTL = change in TL.
Biased synchrony in TLs between leukocyte and muscle tissues
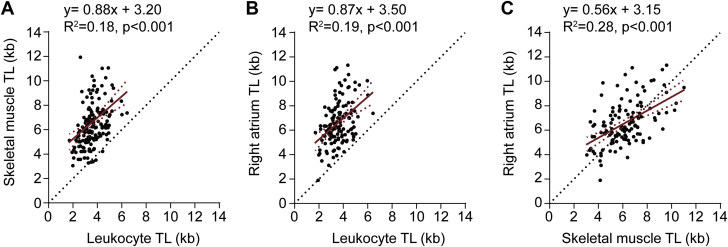
There was wide interindividual variation in leukocyte TL, with a coefficient of variation of 24%. The coefficients of variation for skeletal muscle TL and atrial TL were also wide, at 28% and 27%, respectively (Table 2). However, correlation analysis revealed synchrony in TL among tissues from an individual, that is, an individual with relatively long leukocyte telomeres also had relatively long skeletal muscle telomeres and relatively long atrial telomeres, and vice versa (Figures 1A to 1C) (p < 0.001). However, there was a systemic deviation from the unity line such that, on an individual basis, leukocyte telomeres were consistently shorter than those in either skeletal muscle (148 of 153 individuals) or the right atrium (128 of 129 individuals). Thus, the differences in TL between the 2 muscle-rich tissues and leukocytes were not simply a feature of the aggregate data but reflected a consistent within-subject phenomenon.
Figure 1.
TL Synchrony Among Leukocytes, Skeletal Muscle, and Right Atrium in Individuals Who Underwent Cardiovascular Surgery
Plots showing the intraindividual relationships between (A) skeletal muscle telomere length (TL) and leukocyte TL, (B) right atrium TL and leukocyte TL, and (C) right atrium TL and skeletal muscle TL in patients who underwent cardiovascular surgery.
Age-dependency of leukocyte and skeletal muscle TL but not atrial TL
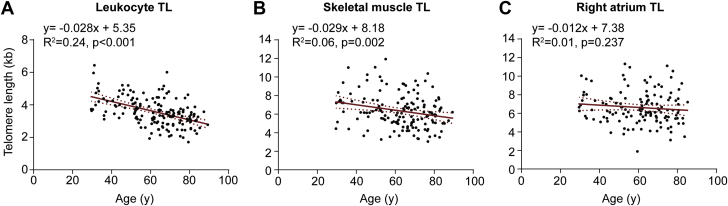
To gauge telomere shortening in the respective tissues, we examined the relationship between TL and age. Figure 2A shows that leukocyte TL was inversely associated with patient age (p < 0.001). Regression analysis revealed that age explained 24% of the variation in leukocyte TL. Interestingly, skeletal muscle TL was also inversely related to age (p < 0.001), with age explaining 6% of the variability in skeletal muscle TL (Figure 2B). The derived telomere attrition rates were similar for both tissues (28 and 29 bp/year, respectively). In contrast, there was no detectable association between cardiac atrial TL and patient age (p = 0.24) (Figure 2C). These findings revealed differences in telomere dynamics among different muscle-rich tissues, with relative stability of TL in the right atrium.
Figure 2.
TL Dynamics in Leukocytes, Skeletal Muscle, and the Right Atrium
Plots showing a relationship between (A) leukocyte TL and age and (B) skeletal muscle TL and age, (C) but not the right atrium TL and age. Abbreviation as in Figure 1.
Right atrium-leukocyte TL difference is associated with the risk of post-operative events
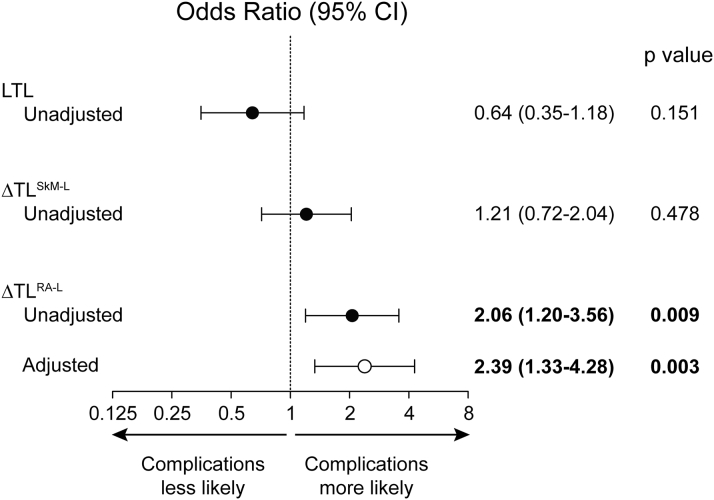
Because atrial tissue had long telomeres, with little to no evidence for age-related shortening at a tissue level, atrial cell TL might serve as an autologous reference for gauging leukocyte telomere shortening. This, in turn, might be a risk biomarker. Therefore, we next determined whether the right atrium-leukocyte TL difference (ΔTLRA-L) was associated with post-operative outcomes. There were 123 patients in whom TL measurements were obtained from all 3 compartments (Table 3). Leukocyte TL was not found to confer risk for post-operative complications, nor did the skeletal muscle-leukocyte TL difference (Figure 3). Likewise, skeletal muscle TL and right atrial muscle TL did not confer risk for complications (p = 0.890 and p = 0.090, respectively). However, ΔTLRA-L was significantly associated with the occurrence of a post-operative complication. For each SD increment in ΔTLRA-L, the risk of sustaining ≥1 post-operative complication increased by 106% (OR: 2.06; 95% confidence interval [CI]: 1.20 to 3.56; p = 0.009) (Figure 3). The association between ΔTLRA-L and adverse post-operative events persisted after adjusting for diabetes (OR: 2.39; 95% CI: 1.33 to 4.28; p = 0.003, with a threshold p = 0.010) (Figure 3), which was the covariate selected for multivariate analysis based on its association with complications (p = 0.014) on univariate analysis.
Table 3.
Adverse Post-Operative Outcomes and Length of Stay in the ICU
| All (N = 123, 100%) | Men (n = 97, 79%) | Women (n = 26, 21%) | |
|---|---|---|---|
| Post-operative complications | 15 (12) | 11 (11) | 4 (15) |
| In-hospital mortality | 3 (2) | 1 (1) | 2 (8) |
| Cardiac arrest/life-threatening arrhythmia | 4 (3) | 2 (2) | 2 (8) |
| Intraaortic balloon pump use | 1 (1) | 1 (1) | 0 (0) |
| Myocardial infarction | 1 (1) | 1 (1) | 0 (0) |
| Stroke and/or delirium | 10 (8) | 8 (8) | 2 (8) |
| Reoperation for bleeding | 4 (3) | 3 (3) | 1 (4) |
| New renal failure requiring dialysis | 1 (1) | 1 (1) | 0 (0) |
| Respiratory failure | 5 (4) | 2 (2) | 3 (12) |
| Septicemia | 0 (0) | 0 (0) | 0 (0) |
| Mediastinitis | 2 (2) | 1 (1) | 1 (4) |
| Length of stay in ICU, days, median, (interquartile range, min-max) | 1 (1–2, 1–32) | 1 (1–2, 1–32) | 2 (1–3, 1–19) |
Values are n (%), unless otherwise noted.
ICU = intensive care unit.
Figure 3.
Risk of Post-Operative Complications Following Cardiovascular Surgery According to TL and Intraindividual Telomere Length Differences
Odds ratios for developing a post-operative complication per SD in each of leukocyte TL, the difference between skeletal muscle and leukocyte TL (ΔTLSkM-L), and the difference between right atrium and leukocyte TL (ΔTLRA-L). Solid circles = unadjusted data. Open circles = data adjusted for diabetes. The threshold p value was 0.010. CI = confidence interval; LTL = leukocyte TL; other abbreviation as in Figure 1.
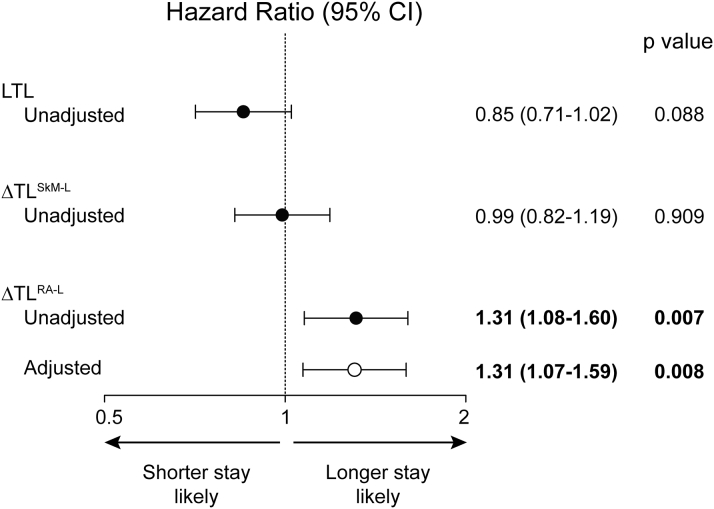
Right atrium-leukocyte TL difference is associated with a prolonged ICU stay
The length of stay in the ICU following cardiac surgery is a powerful determinant of long-term survival and functional capacity (34). We therefore analyzed the potential risk conferred by TL indexes on the length of ICU stay using a Cox proportional hazard model. This revealed that neither leukocyte TL or skeletal muscle-leukocyte TL difference conferred a detectable hazard (Figure 4). Skeletal muscle TL and right atrial muscle TL also did not confer hazard (p = 0.348 and 0.133, respectively). However, for each SD increase in ΔTLRA-L, the risk of remaining in the ICU increased by 31% (95% CI: 1.08 to 1.60; p = 0.007). A heightened risk persisted after adjusting for operating room time (hazard ratio: 1.31; 95% CI: 1.07 to 1.59, p = 0.008). The latter was selected as covariate for multivariate analysis because it was associated with length of stay in ICU on univariate analysis (p = 0.018). Consistent with these findings, among subjects in the upper tertile of ΔTLRA-L, 24% required at least 5 days of ICU care, whereas only 3% and 2% of patients in each of the middle and lower tertiles required this amount of ICU care, respectively (p < 0.001). There were no differences in ICU length of stay among tertiles of leukocyte TL (p = 0.795).
Figure 4.
Risk of Remaining in the ICU Following Cardiovascular Surgery According to TL and Intraindividual TL Differences
Hazard ratios for remaining in the intensive care unit (ICU) for each SD increment in TL or TL (ΔTL). Solid circles = unadjusted data. Open circles = data adjusted for operating room time. The threshold p value was 0.010. Abbreviations as in Figures 1 and 3.
Discussion
We quantified TL in leukocytes, skeletal muscle, and cardiac atrial tissue in patients who underwent cardiovascular surgery. We determined that: 1) within an individual, telomeres in skeletal muscle and cardiac atrial cells were substantially longer than those in leukocytes; 2) an inverse relationship between TL and age was evident in leukocytes and skeletal muscle, but right atrium TL was stable; and 3) patients with a relatively wide difference between their atrium TL and leukocyte TL were more likely to have a complex post-operative course. These findings provide evidence that an innately referenced, single time-point assessment of leukocyte telomere shortening might be relevant in the acute clinical care setting.
The leukocyte telomere attrition rate of ∼30 bp/year that we established is in keeping with several large studies 2, 13, 16. Our finding of similar shortening in skeletal muscle was noteworthy because it supported an emerging reconsideration of telomere dynamics in this “post-mitotic” tissue. Notwithstanding the longer average TL of skeletal muscle, which was likely attributable to developmental program differences with bone marrow cells, the telomere shortening pointed to ongoing skeletal muscle turnover and progenitor cell activity in this adult tissue (20).
In contrast to skeletal muscle, we did not find evidence for age-associated telomere shortening in the cardiac atrium. The explanation for this difference was speculative but might reflect lower progenitor cell activity in adult atria. Recent carbon-14 dating studies showed that cardiomyocyte turnover was substantially less than that of skeletal muscle cells 35, 36. It was also possible that emergence of noncardiomyocyte cells with inherently longer telomeres could counterbalance telomere shortening from cell turnover. In this regard, it is noteworthy that 26% of subjects had a history of atrial fibrillation, a condition associated with atrial fibrosis. Although the TL of atrial fibroblasts versus cardiomyocytes is unknown, fibroblast-like cells in liver were found to have longer telomeres than parenchymal cells (37). As well, a recent study of patients with atrial fibrillation did not find an association between atrial TL and age (38). Regardless of the exact atrial dynamics, our findings empirically established that the net effect of the cellular events within the adult human atria was a stable TL during 6 decades of life. The present data suggested that this stability, in turn, could provide a framework for internally referencing leukocyte TL, so that that the interindividual variability in TL due to inheritance is partially accounted for.
Recent data from Sabharwal et al. (15) indicated that a difference between skeletal muscle TL and leukocyte TL arises developmentally and in early life (15). A similar situation might exist for cardiac muscle. Accordingly, in the present cohort of patients with cardiovascular disease, the ΔTLRA-L might reflect a TL gap in early life plus widening of this gap by ongoing leukocyte TL shortening from replicative and oxidative stresses imposed during adulthood. Thus, assessing the ΔTLRA-L might be analogous, albeit not quantitatively identical, to measuring leukocyte telomere shortening from serial TL assessments, a measurement that has been associated with cardiovascular events and mortality 11, 12. Importantly, the autologous comparison approach tested here obviated the need for extended-term serial measurements and, as such, might be more salient to clinical care.
Individuals with a wide ΔTLRA-L were more likely to have complications following open-heart surgery. This finding constituted the first evidence that a telomere-based measurement was associated with the early response to an intervention. It was noteworthy that adverse post-operative events were not associated with leukocyte TL, which suggested that information conveyed by the atrial-referenced leukocyte TL was different from that conveyed by leukocyte TL itself. An association between the atrial-leukocyte TL difference and clinical care outcomes was further supported by the relationship with length of stay in the ICU. Complications leading to a prolonged stay in the ICU could effectively abrogate the benefits of cardiac surgery, and the ICU length of stay was strongly related to reduced survival and long-term functional decline 34, 39. Notably, only the atrium-leukocyte TL difference and the duration of the surgical procedure independently predicted this key endpoint. The latter is a well-recognized predictor of ICU duration and is related to the technically imposed stresses of the prolonged procedures. The telomere shortening index, in contrast, is a measure of previous biologically imposed stresses and thereby captures a different risk dimension.
Our report had several strengths. We undertook multitissue TL measurements and were the first to compare TL among different muscle-rich tissues in an individual. The telomere measurement strategy used had a small interassay coefficient of variation, and we used a multiple cell line design that controlled for run-to-run variability. These features enabled reliable TL comparisons between tissues and across the study population. As well, this was the first report to investigate a linkage between telomere dynamics and acute outcomes in a high-risk population. In this regard, ΔTLRA-L might carry conceptually unique risk factor information. There are established predictors of outcomes following cardiovascular surgery, including chronological age, diabetes, and smoking. Although ΔTLRA-L did not delineate a specific risk factor, it did report the impact of risk factors, particularly those that imposed replicative and oxidative burdens. In other words, the telomere shortening index afforded by ΔTLRA-L might serve as an innate, patient-specific readout of the consequences of risk factor loading, and thus the ability of an individual to withstand interventions or other additional stresses. That is, ΔTLRA-L might serve as an indicator of biological aging and thus patient resilience to new insults.
Study limitations
Our study was a single-site study. Although there are several large biobanks of leukocyte DNA from clinically followed individuals, this is not the case for skeletal muscle or cardiac DNA. Accordingly, this was a proof-of-concept study. It will be important to assess the value of atrial TL referencing in other cohorts and in larger samples with more post-operative events, to ascertain whether it might be a predictive tool with incremental value over current predictive strategies. It is also possible that a larger cohort may uncover some age-associated atrial telomere attrition. However, the fact that telomere attrition was readily identified in skeletal muscle, but not in the atria from the same individuals, points to the relative stability of atrial TL.
Although we did not identify an association between leukocyte TL and post-operative events, this study did not negate the potential value of leukocyte TL as a risk biomarker or as a potentially causal determinant of cardiovascular disease 14, 40, 41. In this regard, a recent study identified short telomeres, but not telomere attrition, as being associated with carotid atherosclerosis (42). Thus, there may be distinctions between leukocyte TL and telomere attrition with respect to clinical associations. Finally, we recognize that pre-emptively harvesting cardiac tissue is not feasible in most instances. It is nonetheless possible that longer term management of patients who have undergone cardiac surgery may be informed by this index. In this regard, the present cohort will be followed for an extended period. Importantly, the present findings also open the potential for assessing other approaches, such as right ventricular endomyocardial biopsy, which might increase opportunities for clinical usefulness.
Conclusions
We found that relating leukocyte TL to cardiac atrial TL constituted a biologically rational strategy for gauging leukocyte telomere shortening. The findings raise the possibility of using a personalized index of telomere dynamics to inform care.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Ascertaining biological age is important to cardiac risk assessment. An autologous referencing approach to gauging leukocyte telomere shortening may have an important advantage over assessing only leukocyte TL because it is not confounded by the inherited TL. Moreover, it obviates the need for serial TL measurement, which requires several years. Our study is the first to report that a single-time, referenced assessment of telomere dynamics may be salient to acute care of individuals with advanced cardiovascular disease.
TRANSLATIONAL OUTLOOK: Strategies to evaluate leukocyte telomere attrition by internal referencing may identify high-risk individuals. If similar strategies can be adapted pre-operatively, the approach may allow for identification of risk subgroups to guide cardiac surgical decisions. This proof-of-concept study also sets the stage for ascertaining if long-term adverse effects are predicted by the cardiac leukocyte TL difference.
Footnotes
Dr. Pickering was supported by grants from the Canadian Institutes of Health Research (CIHR FRN-11715, FDN-143326), the Heart and Stroke Foundation of Canada (T7081), and the Lawson Health Research Institute (IRF-013-09). Dr. Yin was supported by a CIHR Fellowship.
Dr. Chu has received speaker honoraria from Medtronic, Livanoa, Abbott Vascular, Boston Scientific, and Cryolife. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Brouilette S.W., Moore J.S., McMahon A.D. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick A.L., Kronmal R.A., Gardner J.P. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 3.D'Mello M.J., Ross S.A., Anand S.S. Telomere length and risk of myocardial infarction in a multiethnic population: the INTERHEART study. J Am Coll Cardiol. 2016;67:1863–1865. doi: 10.1016/j.jacc.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 4.D'Mello M.J., Ross S.A., Briel M., Anand S.S., Gerstein H., Pare G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 5.van der Harst P., van der Steege G., de Boer R.A. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Glei D.A., Goldman N., Risques R.A. Predicting survival from telomere length versus conventional predictors: a multinational population-based cohort study. PloS One. 2016;11:e0152486. doi: 10.1371/journal.pone.0152486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Alvira J.M., Fuster V., Dorado B. Short telomere load, telomere length, and subclinical atherosclerosis: the PESA study. J Am Coll Cardiol. 2016;67:2467–2476. doi: 10.1016/j.jacc.2016.03.530. [DOI] [PubMed] [Google Scholar]
- 8.Epel E.S., Blackburn E.H., Lin J. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 10.Sack M.N., Fyhrquist F.Y., Saijonmaa O.J., Fuster V., Kovacic J.C. Basic biology of oxidative stress and the cardiovascular system: part 1 of a 3-part series. J Am Coll Cardiol. 2017;70:196–211. doi: 10.1016/j.jacc.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epel E.S., Merkin S.S., Cawthon R. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baragetti A., Palmen J., Garlaschelli K. Telomere shortening over 6 years is associated with increased subclinical carotid vascular damage and worse cardiovascular prognosis in the general population. J Intern Med. 2015;277:478–487. doi: 10.1111/joim.12282. [DOI] [PubMed] [Google Scholar]
- 13.Slagboom P.E., Droog S., Boomsma D.I. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 14.Codd V., Nelson C.P., Albrecht E. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. doi: 10.1038/ng.2528. 427e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabharwal S., Verhulst S., Guirguis G. Telomere length dynamics in early life: the blood-and-muscle model. FASEB J. 2018;32:529–534. doi: 10.1096/fj.201700630R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rufer N., Brummendorf T.H., Kolvraa S. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frenck R.W., Jr., Blackburn E.H., Shannon K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masi S., D'Aiuto F., Martin-Ruiz C. Rate of telomere shortening and cardiovascular damage: a longitudinal study in the 1946 British Birth Cohort. Eur Heart J. 2014;35:3296–3303. doi: 10.1093/eurheartj/ehu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benetos A., Kimura M., Labat C. A model of canine leukocyte telomere dynamics. Aging Cell. 2011;10:991–995. doi: 10.1111/j.1474-9726.2011.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniali L., Benetos A., Susser E. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benetos A., Toupance S., Gautier S. Short leukocyte telomere length precedes clinical expression of atherosclerosis: blood-and-muscle model. Circ Res. 2018;122:616–623. doi: 10.1161/CIRCRESAHA.117.311751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponsot E., Lexell J., Kadi F. Skeletal muscle telomere length is not impaired in healthy physically active old women and men. Muscle Nerve. 2008;37:467–472. doi: 10.1002/mus.20964. [DOI] [PubMed] [Google Scholar]
- 23.Decary S., Mouly V., Hamida C.B., Sautet A., Barbet J.P., Butler-Browne G.S. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- 24.Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aviv A., Hunt S.C., Lin J., Cao X., Kimura M., Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Callaghan N.J., Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S., Fan Y.S., Chow L.H. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ Res. 2001;89:517–525. doi: 10.1161/hh1801.097165. [DOI] [PubMed] [Google Scholar]
- 28.Li S., Sims S., Jiao Y., Chow L.H., Pickering J.G. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ Res. 1999;85:338–348. doi: 10.1161/01.res.85.4.338. [DOI] [PubMed] [Google Scholar]
- 29.Chu M.W., Stitt L.W., Fox S.A. Prospective evaluation of consultant surgeon sleep deprivation and outcomes in more than 4000 consecutive cardiac surgical procedures. Arch Surg. 2011;146:1080–1085. doi: 10.1001/archsurg.2011.121. [DOI] [PubMed] [Google Scholar]
- 30.Novick R.J., Fox S.A., Stitt L.W. Cumulative sum failure analysis of a policy change from on-pump to off-pump coronary artery bypass grafting. Ann Thorac Surg. 2001;72:S1016–S1021. doi: 10.1016/s0003-4975(01)02949-6. [DOI] [PubMed] [Google Scholar]
- 31.Novick R.J., Fox S.A., Stitt L.W. Impact of the opening of a specialized cardiac surgery recovery unit on postoperative outcomes in an academic health sciences centre. Can J Anaesth. 2007;54:737–743. doi: 10.1007/BF03026870. [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick A.L., Kronmal R.A., Kimura M. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66:421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deelen J., Beekman M., Codd V. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. Int J Epidemiol. 2014;43:878–886. doi: 10.1093/ije/dyt267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bashour C.A., Yared J.P., Ryan T.A. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit Care Med. 2000;28:3847–3853. doi: 10.1097/00003246-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann O., Bhardwaj R.D., Bernard S. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spalding K.L., Bhardwaj R.D., Buchholz B.A., Druid H., Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 37.Wiemann S.U., Satyanarayana A., Tsahuridu M. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 38.Roberts J.D., Dewland T.A., Longoria J. Telomere length and the risk of atrial fibrillation: insights into the role of biological versus chronological aging. Circ Arrhythm Electrophysiol. 2014;7:1026–1032. doi: 10.1161/CIRCEP.114.001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moitra V.K., Guerra C., Linde-Zwirble W.T., Wunsch H. Relationship between ICU length of stay and long-term mortality for elderly ICU survivors. Crit Care Med. 2016;44:655–662. doi: 10.1097/CCM.0000000000001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan Y., Karlsson I.K., Karlsson R. Exploring the causal pathway from telomere length to coronary heart disease: a network mendelian randomization study. Circ Res. 2017;121:214–219. doi: 10.1161/CIRCRESAHA.116.310517. [DOI] [PubMed] [Google Scholar]
- 41.Telomeres Mendelian Randomization C. Haycock P.C., Burgess S. Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol. 2017;3:636–651. doi: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toupance S., Labat C., Temmar M. Short telomeres, but not telomere attrition rates, are associated with carotid atherosclerosis. Hypertension. 2017;70:420–425. doi: 10.1161/HYPERTENSIONAHA.117.09354. [DOI] [PMC free article] [PubMed] [Google Scholar]