Abstract
Human reproduction is an inefficient process. There are several drivers of complications along the path to and during pregnancy, one of which is inflammation. Treatments to mitigate the deleterious effects of aberrant inflammation with something inexpensive and widely available like aspirin could have dramatic global impact. The Effects of Aspirin in Gestation and Reproduction (EAGeR) trial enrolled women aged 18 to 40 years with one to two prior pregnancy losses and no diagnosis of infertility. Patients were randomized to either low-dose aspirin or placebo. Here, we review the collective findings of the EAGeR trial to date and discuss several important lessons learned from the unique data resulting from this groundbreaking trial. Findings reported from this trial provide significant advances in the understanding of aspirin’s potential mechanisms in modulating reproductive processes and the role of inflammation in these processes. This review describes the collective findings of the EAGeR trial in the context of the existing literature regarding aspirin and inflammation in reproduction to inform relevant next steps in fertility and obstetric research, as well as potential implications for clinical care.
Keywords: aspirin, inflammation, implantation
Human reproduction is an inefficient process with the chance of conception per menstrual cycle thought to be approximately 25%1–3 and almost 30% of clinically recognized pregnancies ending in a loss.4 Furthermore, the riskof a spontaneous pregnancy loss increases with the number of prior pregnancy losses.5 Loss from aneuploidy is the most common cause of early loss (accounting for 50%).6 Other causes of loss include uterine anomalies,7 antiphospholipid antibody syndrome,8 and poorly controlled endocrine disorders such as overt hypothyroidism and diabetes.9,10 However, the cause of pregnancy loss in euploid pregnancies is unknown in the majority of cases. Abnormally decreased blood flow to the reproductive tract and aberrant inflammation may play a role in the pathologic process.11
Furthermore, while a balance of pro- and anti-inflammatory factors is involved in implantation12 and parturition,13 overactive inflammation has been shown to contribute to preterm birth,13 spontaneous early pregnancy loss,14 gestational diabetes,15 and preeclampsia.16 Excess inflammation has also been implicated in many causes of infertility including poor oocyte quality,17 pelvic inflammatory disease,18 polycystic ovarian syndrome,19 and endometriosis.20 Through modulation of excess inflammation, aspirin therapy has the potential to reduce or correct poor obstetric outcomes. Indeed, aspirin has the ability to increase blood flow and decrease inflammationinreproductiveorgans.21Further, aspirin is very low cost, has relatively few side effects, and is widely available.
Studies of aspirin and reproduction have generated mixed results and recent evidence indicates timing of initiating therapy may be critical to its effectiveness. When initiated at the start of the cycle for in vitro fertilization (IVF), aspirin improved endometrial growth and vascularization22 and when initiated at stimulation a recent meta-analysis showed an improvement in clinical pregnancy rate, though not live birth.23 A clinical trial randomizing women with two or more pregnancy losses to low-dose aspirin (LDA), heparin and LDA, or placebo starting preconception or before 6 weeks’ gestation showed no benefit to LDA or heparin for increasing livebirth.24 Postconception LDA has been studied extensively in recurrent pregnancy loss patients without benefit shown.25,26 Collectively, these findings suggest that LDA initiated preconceptionally may have beneficial effects on downstream events. However, the use of LDA preconceptionally has not been extensively studied outside of infertility treatment.
Given the suggestive evidence of benefit from preconception aspirin treatment on improving ART outcomes, perhaps through improvements in endometrial vascularization and implantation, in addition to a biological rationale for a potential benefit on preventing pregnancy loss and other adverse outcomes, the recently completed Effects of Aspirin in Gestation and Reproduction (EAGeR) trial investigated the impact of preconception-initiated LDA treatment on live birth in 1,228 women recruited from four U.S. university medical centers who were attempting spontaneous conception after experiencing one or two prior pregnancy losses. Findings reported from this trial provide significant advances in the understanding of LDA’s potential mechanisms in modulating reproductive processes and the role of inflammation in these processes. This review describes the collective findings of the EAGeR trial in the context of the existing literature regarding aspirin and inflammation in reproduction to inform relevant next steps in fertility and obstetric research, as well as potential implications for clinical care.
The EAGeR Trial: Lessons from Preconception Aspirin Therapy
As described earlier, prior trials of LDA treated women who were either already pregnant24,26–30 or undergoingIVF.22,31–33 Furthermore, LDA is currently recommended to begin at 12 weeks’ gestation for women who are high risk for preeclampsia.34 Thus, the EAGeR trial findings produced novel information regarding the impacts of aspirin and the role of inflammation in the preconception period for women attempting spontaneous conception. Women included in EAGeR were aged 18 to 40 years with up to two prior live births. Women with one prior loss less than 20 weeks of gestation within the preceding 12 months with no infertility diagnosis were enrolled under the “original” eligibility stratum. Additionally, women with one or two prior losses at any gestational age and at any time in the past were enrolled into the “expanded” eligibility stratum, more closely representing a general obstetric population with a history of pregnancy loss. Patients were followed up for six menstrual cycles plus the duration of pregnancy if applicable.
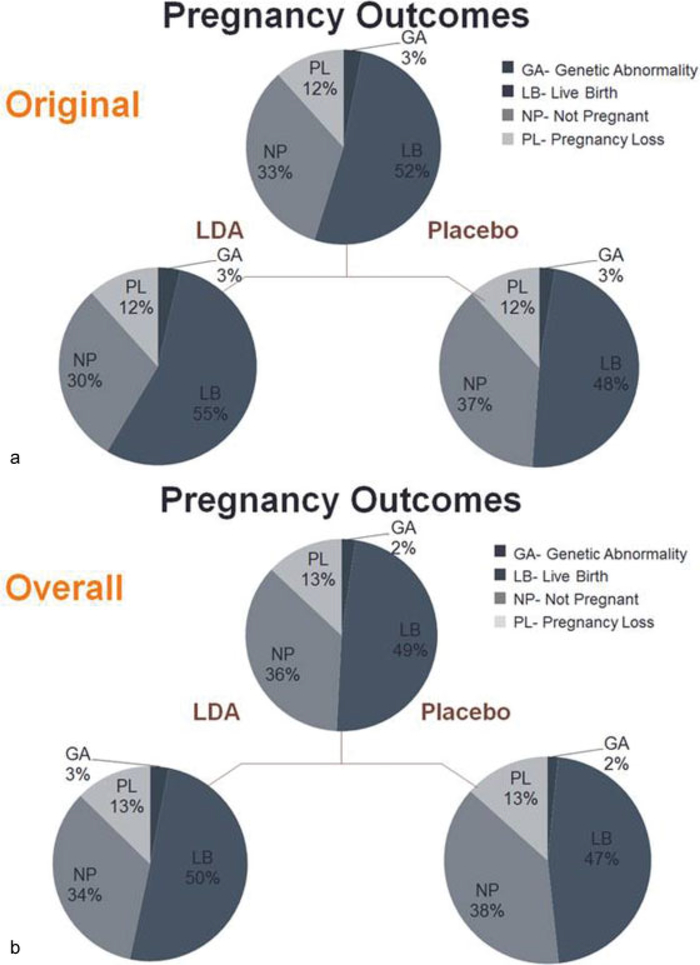
First, the findings regarding the primary outcome of live birth in the overall trial and by eligibility stratum indicated some beneficial impact of aspirin, but only among certain women. Among all participants, there was no statistically significant improvement in live birth attributable to LDA compared with placebo (relative risk [RR]: 1.10, 95% confidence interval [CI]: 0.98, 1.22); however, there was a statistically significant difference in live birth between the LDA and placebo group within women with a single recent loss that made up original stratum (62 vs. 53%, p = 0.045).35 In the expanded stratum alone, there was no difference between treatment groups (54 vs. 52%, p = 0.74). Interestingly, there were significantly more positive pregnancy tests and ultrasound-confirmed pregnancies in the LDA versus placebo group, accounting for the increased live birth rate observed in the original stratum and the marginally increased livebirths in the trial overall (►Fig. 1a, b). Concordantly, despite a hypothesis that aspirin may decrease pregnancy loss through a potential improvement in endometrial blood flow and reduced inflammation,21 there was no effect of LDA on pregnancy loss at any stage of pregnancy, either in the overall trial cohort or within eligibility strata.35 Moreover, to further investigate aspirin’s potential effect on supporting existing pregnancy, the type of loss (i.e., implantation failure, early pregnancy loss, or stillbirth) was examined in detail, and LDA was not found to have any effect on any particular subtype of loss. Notably, an analysis of chromosomal abnormalities determined from products of conception collected from early pregnancy losses further demonstrated that LDA did not decrease the rate of euploid pregnancy loss.36 Collectively, these primary trial findings suggested that initiating LDA therapy prior to conception may have important impacts in successful establishment, as opposed to maintenance, of pregnancy. Of note, such effects could not be observed in previous trial initiating LDA therapy after pregnancy confirmation.
Fig. 1.
(a) Overall cohort by pregnancy outcome. There is a slight nonstatistically significant increase in live births with low-dose aspirin (LDA) compared with placebo, with no difference in pregnancy loss. (b). Original stratum by pregnancy outcome. There is a statistically significant increase in live births with LDA compared with placebo, with no difference in pregnancy loss. (Adapted from Schisterman et al.35)
Indeed, a specific analysis of LDA’s effect on time to pregnancy in the overall cohort reflected thefindings of pregnancy and live birth rates in that LDA increased fecundability by 28% in the LDA compared with placebo group within the original stratum. This was true for either a human chorionic gonadotropin–positive pregnancy or an ultrasound-confirmed pregnancy. This would support the hypothesis that aspirin’s effect is not on supporting an established pregnancy but rather exerting its effects on earlier events, such as ovulation or embryo survival and successful implantation.37 Though direct assessment of each of these underlying mechanisms is impossible in humans, an examination of the effect of LDA on anovulation was recently reported.
Anovulation
In eumenorrheic women, reports of the per-cycle chance of sporadic anovulation have ranged from 0 to 11%.38–42 Prior evidence for an effect of LDA on ovulation is indirect and mixed.22,43,44 To better answer whether LDA impacts ovulatory function and to better elucidate the mechanism of increased fecundability observed among certain EAGeR participants, a secondary analysis of EAGeR trial data was performed to evaluate the impact of LDA compared with placebo on the occurrence of sporadic anovulation.
For the purposes of this analysis in EAGeR, a cycle was considered ovulatory if the urinary luteinizing hormone was 2.5-fold greater than the mean of the previous 5 days.40 Anovulation occurred in 12.2% of all cycles and LDA did not have an effect on the rate of anovulation overall (13.4% LDA vs 11.1% placebo; RR: 1.16, 95% CI: 0.88, 1.52) or among the original stratum (11.1% LDA vs. 10.1% placebo, RR: 1.06, 95% CI: 0.68, 1.54).45 These data suggest that the increase in fecundability seen in the LDA group previously37 was not mediated by decreasing risk of having an anovulatory cycle, but rather some other mechanism.
Inflammation and Implantation
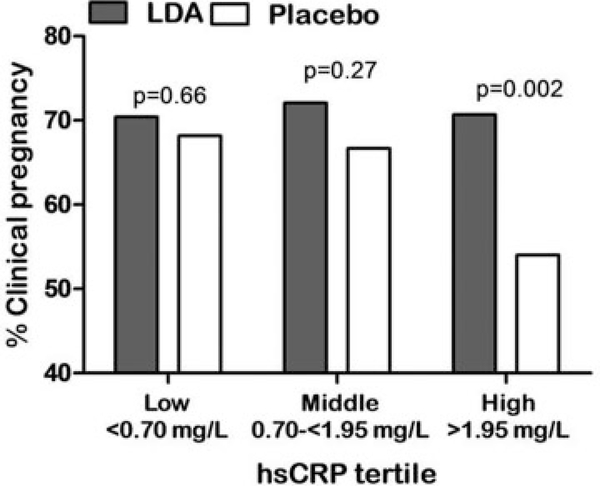
Given the potential role of aspirin to affect endometrial receptivity and implantation through modulating inflammation, a secondary analysis stratifying by tertile of high-sensitivity C-reactive protein (hsCRP) at study entry, as a marker of chronic low-grade inflammation, was next undertaken. Indeed, among women in the highest tertile (hsCRP:1.95–9.99mg/L, excluding women with hsCRP ≥10 mg/L) of preconception hsCRP, LDA significantly increased clinicallyconfirmedpregnanciesby31% and increased live births by 35% compared with the placebo group. In contrast, among women in the middle and lower tertiles of hsCRP, LDA did not affect clinically confirmed pregnancies or live birth (►Fig. 2).46 Interestingly, the chance of clinically confirmed pregnancy and live birth decreased with increasing level of hsCRP, and among the highest tertile of hsCRP, LDA appeared to restore the pregnancy and live birth rates to similar rates as women with lower hsCRP levels (►Fig. 2). Also, since obesity is known to be an inflammatory state which may be contributing to otherwise healthy women having low-grade inflammation,47 women with hsCRP in the top tertile (hsCRP ≥1.95 mg/L) were further stratified by adiposity status, considering both waist:hip ratio, a marker of central obesity, and body mass index (BMI) as an overall measure of adiposity. Interestingly, LDA showed a significant positive effect on pregnancy and live birth among leaner women only, including those below the cohort median waist: hip ratio and also among those with normal BMI (<25 kg/m2) (RR: 1.60 95% CI: 1.11, 2.30). In contrast, women above the cohort median waist:hip ratio, or with BMI ≥25 kg/m2, exhibited an attenuated LDA effect (RR: 1.18 95% CI: 0.89, 1.56).46 Thus, inflammation level at study entry appeared to significantly modify the effect of LDA on pregnancy chances, with hsCRP acting as a useful biomarker to identify women most likely to benefit from LDA therapy, similar to its application in cardiovascular medicine research. While potentially important for future clinical practice, these findings may also help shed light on the reproductive stage or mechanism through which LDA may impact reproduction. Importantly, it is thought that inflammatory mediators exchanged between the embryo and endometrial surface are an important component of the cross talk which drives the endometrium’s function as a sensor of the preimplantation embryo’s viability, with such communication enabling or disrupting successful embryonic implantation.48
Fig. 2.
Clinical pregnancy based on tertile of high-sensitivity C-reactive protein (hsCRP). In the highest tertile, clinical pregnancy was restored in the low-dose aspirin (LDA) group versus the placebo group. In the lower two tertiles, there was no difference between LDA and placebo. (Reprinted with permission from Sjaarda et al.46)
Indeed, a crucial purpose of the decidua’s sensor function is to recognize chromosomally abnormal embryos by their increased metabolic activity and prevent their implantation with a proteotoxic stress response.48 Thus, it was hypothesized that this appropriate inflammatory response may become pathological in the presence of an underlying disordered maternal inflammatory milieu, resulting in rejection of chromosomally normal embryos. Moreover, male embryos may be more vulnerable to rejection by an overactive maternal inflammatory environment, as preimplantation male embryos have demonstrated greater metabolic activity than female embryos.49,50 Further, in mice and bovine models, preimplantation embryos have been shown to exhibit a sexually dimorphic response to maternal inflammation, with male embryos exhibiting greater vulnerability.51,52 As such, the resulting sex ratio of women treated with LDA could lend a clue to whether the key effects of an anti-inflammatory agent such as aspirin occur at the time of implantation when this delicate embryo–endometrium crosstalk is occurring. Examining impacts of exogenous factors on sex ratio is not new, as the ratio of male-to-female newborn infants has trended downward globally over the past few decades.53–57 Parental exposure to smoking,58 dioxin,59 lead,60 methylmercury,61 pesticides,62 and earthquakes63,64 have all been linked to reduced male-to-female sex ratio.
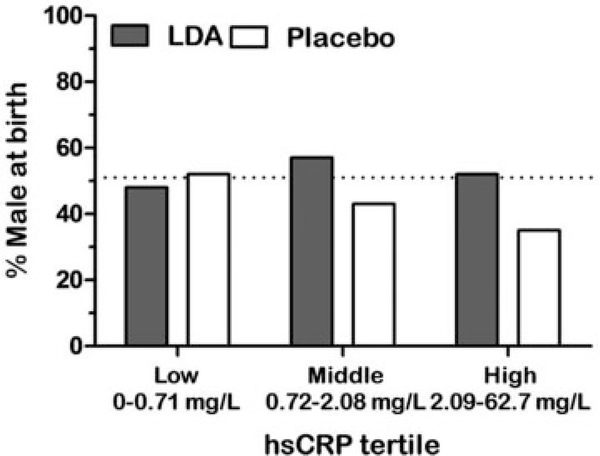
Thus, in a prespecified secondary analysis of the EAGeR trial65 to investigate the impacts of LDA therapy on offspring sex ratio, LDA was shown to increase male live births overall (RR: 1.31, 95% CI: 1.07, 1.59) and had no effect on female live birth. A further analysis found a similar positive effect of LDA on pregnancy with male offspring by including the outcome of pregnancy with a male embryo, whether the pregnancy ended in live birth or loss. Karyotype or microarray determined the offspring sex from pregnancy losses.65 To further evaluate the hypotheses that inflammation was hazardous to male embryos, and that LDA was subsequently protective, the analysis was stratified by hsCRP level at study entry. Congruent with the findings of the stratified analysis of LDA and live birth, the percentage male at birth decreased with increasing tertile of hsCRP among the placebo group, but the proportion of males remained at the expected level among the LDA-treated group (►Fig. 3). Thus, the lower pregnancy rates observed in women with higher inflammation appeared to be driven by a discordant lack of male pregnancies in women with chronic inflammation, and this discordance was restored with preconception LDA therapy.
Fig. 3.
Percentage of male live births by high-sensitivity C-reactive protein (hsCRP) tertile. In the highest tertile, the placebo group was less likely to have a male live birth than the low-dose aspirin (LDA) group. (Adapted from Radin et al.45)
Collectively, these data support and help elucidate LDA’s effect on implantation, a key regulatory step tied to delicate inflammatory mediators, in successful establishment of a healthy, ongoing pregnancy. There is good biologic plausibility to these findings given the intricately involved mechanisms of inflammatory mediators and endometrial receptivity and implantation.48 LDA’s inhibition of COX-2 function may lead to downstream inhibition of chronic inflammatory pathways.66 It has been well documented that patients with central obesity have higher levels of endogenous inflammation compared with individuals with normal BMI.17 However, the impact of LDA on obesity-driven inflammation is still unclear, as a minimal effect of LDA was observed in patients with higher levels of hsCRP who were overweight/obese. It may be that LDA, at 81 mg, is too low a dose to overcome the overall greater level of inflammation in women with excess adiposity, or that obesity-driven inflammation represents a different inflammatory milieu that may require treatments impacting different pathways than those inhibited by aspirin. As the percentage of overweight and obese females of reproductive age is almost 60% in the United States,67 further work to elucidate the complex interplay of adiposity, inflammation, and the role of anti-inflammatory treatments to improve reproduction in this population is needed.
Adverse Pregnancy Outcomes
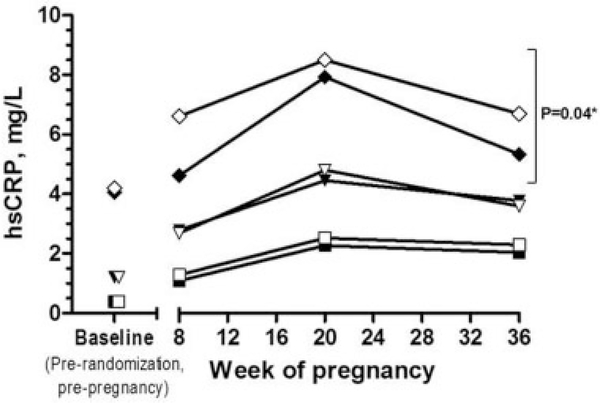
In addition to the effect of LDA on pregnancy and live birth rates, stratified by level of inflammation, the effect of LDA on hsCRP concentrations through pregnancy was also completed. Indeed, inflammation has been implicated in many adverse pregnancy outcomes, including preeclampsia and gestational diabetes.15,16,68 Examining the effect of LDA on hsCRP at 8, 20, and 36 weeks of gestation revealed that women in the highest tertile of hsCRP at study entry treated with LDA demonstrated a significant reduction in hsCRP’s elevation throughout pregnancy, whereas LDA had no effect on hsCRP concentrations through pregnancy in women beginning the study in the lower tertiles (►Fig. 4).46 Given this potential role for LDA to lower excessive elevation of hsCRP concentrations during pregnancy, an obvious potential application of LDA therapy may be to prevent adverse pregnancy outcomes such as preterm birth, which is a major cause of neonatal mortalityand morbidity.69,70 Furthermore, LDA is currently recommended for women at high risk for preeclampsia but not until 12 weeks’ gestation.34 A large systematic review of LDA initiated during pregnancy among more than 30,000 women noted that the relative risk of delivery before 37 weeks of gestation was 0.92 (95% CI: 0.88, 0.97) compared with no LDA.71 Aspirin therapy was mostly started after the first trimester in these studies, however. Within this large prior analysis, the decrease in preterm birth is thought to be from a decrease in medically indicated births, though this is not specified. Thus, given these prior findings and the observation of a reduction in excess inflammation during pregnancy in women in the third hsCRP tertile in EAGeR, an important step was also to examine the impact of LDA on adverse pregnancy outcome.
Fig. 4.
Difference in high-sensitivity C-reactive protein (hsCRP) concentrations in low-dose aspirin (LDA) versus placebo groups during pregnancy. Symbols indicate geometric means of log-transformed high-sensitivity C-reactive protein (hsCRP) baseline (pre-randomization) and throughout pregnancy. Squares indicate the lower hsCRP tertile (closed, LDA; open, placebo), triangles indicate middle hsCRP tertile (closed, LDA; open, placebo), and diamonds indicate higher hsCRP tertile (closed, LDA; open, placebo). Notation of significance indicates treatment group (LDA vs. placebo) difference across pregnancy (excluding baseline) from generalized estimating equations (GEE) models within each hsCRP tertile (assigned at baseline). (Reprinted with permission from Sjaarda et al.46)
In this secondary analysis in EAGeR participants,72 no significant reduction in the preterm birth rate was observed among the overall study population (RR: 0.72; 95% CI: 0.42, 1.23). A separate analysis of medically indicated and spontaneous preterm births demonstrated a nonsignificant trend toward lower risk in the LDA group for spontaneous preterm birth. However, when restricting to only patients achieving a clinically confirmed pregnancy, a reduction in preterm birth in the original stratum (RR: 0.39; 95% CI: 0.16, 0.94) but not in either the expanded cohort or in the overall population was observed. Interestingly, these lower rates of preterm birth tended to be more from spontaneous births than medically indicated births, which is consistent with a recent metaanalysis.73 Originally, it was postulated that the main mechanism for this reduction in preterm birth is aspirin’s effects on reducing the rate of preeclampsia and intrauterine growth restriction.71 Thus, these data from the EAGeR trial are of particular interest, as they clearly demonstrate that preconception LDA may reduce spontaneous preterm birth more so than medically indicated preterm birth, and also demonstrate no reduction in preeclampsia, though cases of preeclampsia in EAGeR were few (33 LDA vs. 22 placebo, p = 0.17). The inflammatory nature of labor13 lends biologic plausibility to a greater impact on spontaneous preterm birth. Parturition involves moving from a uterine quiescent state to a proinflammatory state which then promotes uterine contraction, cervical dilation, and rupture of membranes.13 Aspirin’s ability to inhibit cyclooxygenase decreases prostaglandin formation, which is known to play a role in both normal and abnormal labor.74 Furthermore, cyclooxygenase inhibitors are used as tocolytics75 and regular-dose aspirin (325 mg) is associated with a delay in the onset of labor.76 It is important to note that the EAGeR trial was not powered to detect an effect of LDA on preterm birth, however, and overall rates of pregnancy complications were low, as this population was selected to be without chronic medical conditions. As such, an examination of the impact of LDA on preterm birth and other adverse pregnancy outcomes stratified by hsCRP was not possible and remains an important area of future study, given the findings of LDA’s effect on lowering excess hsCRP throughout pregnancy in women beginning the study with low-grade inflammation.
Finally, as aspirin is inexpensive and widely available, its potential to decrease spontaneous preterm birth, a leading cause of neonatal morbidity and mortality, would have dramatic global implications. With this in mind, the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparous (ASPIRIN) trial (NCT02409680) currently conducted by the NICHD Global Network for Women’s and Children’s Health is open and actively enrolling patients to better elucidate the effect of aspirin on preterm birth.
Safety
While there is much yet to discover for the best clinical role for aspirin in fertility and obstetric care, a discussion of its role would be incomplete without a careful examination of risks of aspirin during pregnancy. Aspirin has repeatedly been shown to be of low risk to both mother and fetus77 and its use during pregnancy has been found to reduce the risk of preeclampsia and is recommended for women at high risk for developing preeclampsia.34 Based on these data, it is currently recommended that daily LDA therapy be initiated after the 12th week of pregnancy, as is recommended for preeclampsia prevention.78 This recommendation comes from several randomized controlled trials starting aspirin after the first trimester.34 The EAGeR trial afforded the opportunity to assess the effects of preconception aspirin use and its safety during early pregnancy as well.79 With the evidence of aspirin’s effects on fecundity discussed here and potential effects on other reproductive outcomes, there may be an increase in its preconception use.
Overall there was no difference in symptoms related to aspirin use (i.e., gastrointestinal discomfort, nausea or vomiting, allergic reaction, and difficulty breathing) in the LDA versus placebo groups. In terms of maternal complications, vaginal bleeding and subchorionic hematoma (26 vs. 20%, p = 0.01) occurred more often in the LDA group than in the placebo group. There was no difference between treatment groups in regard to subchorionic hemorrhage, epistaxis, placental abruption, and postpartum hemorrhage. It is important to note that there was no difference in pregnancy loss between the two groups despite higher rates of vaginal bleeding in the LDA group.35 Very few fetal or neonatal complications occurred in the trial overall and there were no differences in neonatal deaths, fetal deaths, or congenital anomalies by treatment group observed.79
These data are important in that aspirin was well tolerated while trying to conceive and then after conception was tolerated by the fetus and neonate. These findings are consistent with previous pooled data on aspirin started after the first trimester.71,77 Our noted increase in vaginal bleeding is in agreement with Truong et al, who found that LDA use increased the risk of subchorionic hematoma.80 This retrospective analysis did not report on birth outcomes, whereas the EAGeR trial prospectively followed up the pregnancies and showed no increase in pregnancy loss in the aspirin group.35 Importantly, neonatal and fetal complications were similar in each group. This was of particular importance in that the embryo is exposed to aspirin during organogenesis. This study had insufficient sample size to detect a difference in birth defects, but the data are reassuring and are in agreement with other large study findings.81,82 Overall, these data should reassure providers using LDA in the preconception period.
Conclusion
The EAGeR trial set out to examine the effects of LDA initiated prior to conception on live birth among women attempting spontaneous conception with a history of pregnancy loss, but no history of infertility. The unfolding story of discovery of LDA’s impact on the trial’s primary and secondary outcomes (live birth, time to pregnancy, preterm birth, sex ratio, and safety) combined with secondary analyses focused on the modifying effect of low-grade inflammation has led to significant advances in knowledge of aspirin, inflammation, and reproduction. Specifically, four important lessons can be gleaned from this evolving body of work. First, the timing of medication likely plays a very important role. This trial is unique in that LDA was started preconceptionally. Most trials, aside from ones involving IVF, started LDA after the establishment of pregnancy; given the data reviewed, beginning LDA after pregnancy confirmation may be of no benefit as the increase in live birth observed was from establishment of pregnancy and not loss prevention. Second, not all patients benefitted in the same way, and the use of a biomarker such as hsCRP may provide an avenue for personalized medicine to identify women most likely to benefit from LDA therapy. Moreover, participants with higher hsCRP and low BMI received the greatest benefit from preconception LDA, leading to new hypotheses regarding the interplay of adiposity, inflammation, and reproduction in the majority of reproductive age women who suffer from excess body weight. Third, because LDA is inexpensive, widely available, and generally regarded as safe, its expanded use in the field of reproductive care could have dramatic global impact. Fourth, inflammation and the modulation of inflammation play a key role in female reproduction with higher inflammation contributing to lower pregnancy rates, particularly pregnancies with male embryos, and excess hsCRP elevation throughout gestation. In conclusion, the findings from EAGeR trial to date have initiated many new paths of clinical discovery in women’s reproductive health that have the potential to improve the lives of women and children globally.
Acknowledgments
We express our deepest thanks to the EAGeR participants for their extraordinary commitment to the study, all of the EAGeR investigators and staff who devoted their time and energy to the success of the trial, and the Data Safety and Monitoring Board members for ongoing oversight, constant support, and advice throughout the trial.
Funding
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (contract nos. HHSN267200603423, HHSN267200603424, and HHSN 267200603426) and by the National Institutes of Health (NIH) Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and alumni of student research programs and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Footnotes
Trial Registration
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- 1.Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril 1996; 65(03):503–509 [PubMed] [Google Scholar]
- 2.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med 1988;319(04):189–194 [DOI] [PubMed] [Google Scholar]
- 3.Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956;161(09):855–860 [DOI] [PubMed] [Google Scholar]
- 4.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340(23): 1796–1799 [DOI] [PubMed] [Google Scholar]
- 5.Risch HA, Weiss NS, Clarke EA, Miller AB. Risk factors for spontaneous abortion and its recurrence. Am J Epidemiol 1988;128(02): 420–430 [DOI] [PubMed] [Google Scholar]
- 6.Eiben B, Bartels I, Bähr-Porsch S, et al. Cytogenetic analysis of 750 spontaneous abortions with the direct-preparation method of chorionic villi and its implications for studying genetic causes of pregnancy wastage. Am J Hum Genet 1990;47(04):656–663 [PMC free article] [PubMed] [Google Scholar]
- 7.Acién P Reproductive performance of women with uterine malformations. Hum Reprod 1993;8(01):122–126 [DOI] [PubMed] [Google Scholar]
- 8.Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Anti-phospholipid syndrome. Obstet Gynecol 2012;120(06):1514–152123168789 [Google Scholar]
- 9.Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid 2002;12(01):63–68 [DOI] [PubMed] [Google Scholar]
- 10.Greene MF. Spontaneous abortions and major malformations in women with diabetes mellitus. Semin Reprod Endocrinol 1999;17 (02):127–136 [DOI] [PubMed] [Google Scholar]
- 11.Silver RM, Branch DW. Sporadic and recurrent pregnancy loss In: Reece EA, Hobbins JC, eds. Medicine of the Fetus and Mother. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1999:195–216 [Google Scholar]
- 12.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011;1221:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345(6198):760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalagiri RR, Carder T, Choudhury S, et al. Inflammation in complicated pregnancy and its outcome. Am J Perinatol 2016; 33(14):1337–1356 [DOI] [PubMed] [Google Scholar]
- 15.Bossick AS, Peters RM, Burmeister C, Kakumanu N, Shill JE, Cassidy-Bushrow AE. Antenatal inflammation and gestational diabetes mellitus risk among pregnant African-American women. J Reprod Immunol 2016;115:1–5 [DOI] [PubMed] [Google Scholar]
- 16.Thilaganathan B, Wormald B, Zanardini C, Sheldon J, Ralph E, Papageorghiou AT. Early-pregnancy multiple serum markers and second-trimester uterine artery Doppler in predicting preeclampsia. Obstet Gynecol 2010;115(06):1233–1238 [DOI] [PubMed] [Google Scholar]
- 17.Boots CE, Jungheim ES. Inflammation and human ovarian follicular dynamics. Semin Reprod Med 2015;33(04):270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reljic M, Gorisek B. C-reactive protein and the treatment of pelvic inflammatory disease. Int J Gynaecol Obstet 1998;60(02): 143–150 [DOI] [PubMed] [Google Scholar]
- 19.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril 2011;95(03):1048–58.e1, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest 2006;62(03):139–147 [DOI] [PubMed] [Google Scholar]
- 21.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 2003;110(5–6):255–258 [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein M, Marazzi A, Polak de Fried E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo-controlled assay. Fertil Steril 1999; 71(05):825–829 [DOI] [PubMed] [Google Scholar]
- 23.Dentali F, Ageno W, Rezoagli E, et al. Low-dose aspirin for in vitro fertilization or intracytoplasmic sperm injection: a systematic review and a meta-analysis of the literature. J Thromb Haemost 2012;10(10):2075–2085 [DOI] [PubMed] [Google Scholar]
- 24.Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med 2010;362(17):1586–1596 [DOI] [PubMed] [Google Scholar]
- 25.Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin or anticoagulants for treating recurrent miscarriage in women without antiphospholipid syndrome. Cochrane Database Syst Rev 2009;(01):CD004734. [DOI] [PubMed] [Google Scholar]
- 26.Tulppala M, Marttunen M, Söderstrom-Anttila V, et al. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Hum Reprod 1997; 12(07):1567–1572 [DOI] [PubMed] [Google Scholar]
- 27.Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, Abdelall I. Low-molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. J Obstet Gynaecol 2008; 28(03):280–284 [DOI] [PubMed] [Google Scholar]
- 28.Clark P, Walker ID, Langhorne P, et al. ; Scottish Pregnancy Intervention Study (SPIN) collaborators. SPIN (Scottish Pregnancy Intervention) study: a multicenter, randomized controlled trial of low-molecular-weight heparin and low-dose aspirin in women with recurrent miscarriage. Blood 2010;115(21):4162–4167 [DOI] [PubMed] [Google Scholar]
- 29.Dolitzky M, Inbal A, Segal Y, Weiss A, Brenner B, Carp H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil Steril 2006;86(02):362–366 [DOI] [PubMed] [Google Scholar]
- 30.Visser J, Ulander VM, Helmerhorst FM, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia. HABENOX: a randomised multicentre trial. Thromb Haemost 2011;105(02):295–301 [DOI] [PubMed] [Google Scholar]
- 31.Dirckx K, Cabri P, Merien A, et al. Does low-dose aspirin improve pregnancy rate in IVF/ICSI? A randomized double-blind placebo controlled trial. Hum Reprod 2009;24(04):856–860 [DOI] [PubMed] [Google Scholar]
- 32.Duvan CI, Ozmen B, Satiroglu H, Atabekoglu CS, Berker B. Does addition of low-dose aspirin and/or steroid as a standard treatment in nonselected intracytoplasmic sperm injection cycles improve in vitro fertilization success? A randomized, prospective, placebocontrolled study. J Assist Reprod Genet 2006;23(01):15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambers MJ, Hoozemans DA, Schats R, Homburg R, Lambalk CB, Hompes PG. Low-dose aspirin in non-tubal IVF patients with previous failed conception: a prospective randomized doubleblind placebo-controlled trial. Fertil Steril 2009;92(03):923–929 [DOI] [PubMed] [Google Scholar]
- 34.LeFevre ML; U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161(11):819–826 [DOI] [PubMed] [Google Scholar]
- 35.Schisterman EF, Silver RM, Lesher LL, et al. Preconception lowdose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014;384(9937):29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mumford SL, Silver RM, Sjaarda LA, et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod 2016;31(03):657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schisterman EF, Mumford SL, Schliep KC, et al. Preconception low dose aspirin and time to pregnancy: findings from the effects of aspirin in gestation and reproduction randomized trial. J Clin Endocrinol Metab 2015;100(05):1785–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haiman CA, Pike MC, Bernstein L, et al. Ethnic differences in ovulatory function in nulliparous women. Br J Cancer 2002; 86(03):367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol 2003;102(02):317–318 [DOI] [PubMed] [Google Scholar]
- 40.Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril 2007;88(03):684–690 [DOI] [PubMed] [Google Scholar]
- 41.Gaskins AJ, Mumford SL, Zhang C, et al. ; BioCycle Study Group. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr 2009;90(04):1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh EE, Shaw ND, Klingman KM, et al. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab 2011; 96(10):3199–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matyas RA,Mumford SL, Schliep KC, et al. Effects of over-the-counter analgesic use on reproductive hormones and ovulation in healthy, premenopausal women. Hum Reprod 2015;30(07):1714–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nargund G, Wei CC. Successful planned delay of ovulation for one weekwithindomethacin.JAssistReprodGenet 1996;13(08):683–684 [DOI] [PubMed] [Google Scholar]
- 45.Radin RG, Sjaarda LA, Perkins NJ, et al. Low-dose aspirin and sporadic anovulation in the EAGeR randomized trial. J Clin Endocrinol Metab 2017;102(01):86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sjaarda LA, Radin RG, Silver RM, et al. Preconception low-dose aspirin restores diminished pregnancy and live birth rates in women with low grade inflammation: a secondary analysis of a randomized trial. J Clin Endocrinol Metab 2017;102(05):1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obesity Wolowczuk I. – an inflammatory state. Acta Vet Scand 2015;57(Suppl 1):K5–K5 [Google Scholar]
- 48.Macklon NS, Brosens JJ. The human endometrium as a sensor of embryo quality. Biol Reprod 2014;91(04):98. [DOI] [PubMed] [Google Scholar]
- 49.Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci U S A 2010;107(08):3394–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardner DK, Larman MG, Thouas GA. Sex-related physiology of the preimplantation embryo. Mol Hum Reprod 2010;16(08):539–547 [DOI] [PubMed] [Google Scholar]
- 51.Pérez-Crespo M, Ramírez MA, Fernández-González R, et al. Differential sensitivity of male and female mouse embryos to oxidative induced heat-stress is mediated by glucose-6-phosphate dehydrogenase gene expression. Mol Reprod Dev 2005; 72(04):502–510 [DOI] [PubMed] [Google Scholar]
- 52.Dobbs KB, Gagné D, Fournier E, et al. Sexual dimorphism in developmental programming of the bovine preimplantation embryo caused by colony-stimulating factor 2. Biol Reprod 2014;91(03):80. [DOI] [PubMed] [Google Scholar]
- 53.Allan BB, Brant R, Seidel JE, Jarrell JF. Declining sex ratios in Canada. CMAJ 1997;156(01):37–41 [PMC free article] [PubMed] [Google Scholar]
- 54.Mathews TJ, Hamilton BE. Trend analysis of the sex ratio at birth in the United States. Natl Vital Stat Rep 2005;53(20):1–17 [PubMed] [Google Scholar]
- 55.Davis DL, Gottlieb MB, Stampnitzky JR. Reduced ratio of male to female births in several industrial countries: a sentinel health indicator? JAMA 1998;279(13):1018–1023 [DOI] [PubMed] [Google Scholar]
- 56.Møller H Change in male:female ratio among newborn infants in Denmark. Lancet 1996;348(9030):828–829 [DOI] [PubMed] [Google Scholar]
- 57.Parazzini F, La Vecchia C, Levi F, Franceschi S. Trends in male: female ratio among newborn infants in 29 countries from five continents. Hum Reprod 1998;13(05):1394–1396 [DOI] [PubMed] [Google Scholar]
- 58.Fukuda M, Fukuda K, Shimizu T, Andersen CY, Byskov AG. Parental periconceptional smoking and male: female ratio of newborn infants. Lancet 2002;359(9315):1407–1408 [DOI] [PubMed] [Google Scholar]
- 59.Mocarelli P, Gerthoux PM, Ferrari E, et al. Paternal concentrations of dioxin and sex ratio of offspring. Lancet 2000;355(9218): 1858–1863 [DOI] [PubMed] [Google Scholar]
- 60.Hertz-Picciotto I The evidence that lead increases the risk for spontaneous abortion. Am J Ind Med 2000;38(03):300–309 [DOI] [PubMed] [Google Scholar]
- 61.Aschengrau A, Zierler S, Cohen A. Quality of community drinking water and the occurrence of spontaneous abortion. Arch Environ Health 1989;44(05):283–290 [DOI] [PubMed] [Google Scholar]
- 62.Garry VF, Harkins M, Lyubimov A, Erickson L, Long L. Reproductive outcomes in the women of the Red River Valley of the north. I. The spouses of pesticide applicators: pregnancy loss, age at menarche, and exposures to pesticides. J Toxicol Environ Health A 2002; 65(11):769–786 [DOI] [PubMed] [Google Scholar]
- 63.Fukuda M, Fukuda K, Shimizu T, Møller H. Decline in sex ratio at birth after Kobe earthquake. Hum Reprod 1998;13(08): 2321–2322 [DOI] [PubMed] [Google Scholar]
- 64.Saadat M Decline in sex ratio at birth after Bam (Kerman Province, Southern Iran) earthquake. J Biosoc Sci 2008;40(06):935–937 [DOI] [PubMed] [Google Scholar]
- 65.Radin RG, Mumford SL, Silver RM, et al. Sex ratio following preconception low-dose aspirin in women with prior pregnancy loss. J Clin Invest 2015;125(09):3619–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest 1996;97(11):2672–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.ACOG Practice Bulletin No 156: Obesity in pregnancy. Obstet Gynecol 2015;126(06):e112–e126 [DOI] [PubMed] [Google Scholar]
- 68.Gandevani SB, Banaem LM, Mohamadi B, Moghadam NA, Asghari M. Association of high-sensitivity C-reactive protein serum levels in early pregnancy with the severity of preeclampsia and fetal birth weight. J Perinat Med 2012;40(06):601–605 [DOI] [PubMed] [Google Scholar]
- 69.Mathews TJ, Menacker F, MacDorman MF; Centers for Disease Control and Prevention, National Center for Health Statistics. Infant mortality statistics from the 2002 period: linked birth/ infant death data set. Natl Vital Stat Rep 2004;53(10):1–29 [PubMed] [Google Scholar]
- 70.Anderson RN, Smith BL. Deaths: leading causes for 2001. Natl Vital Stat Rep 2003;52(09):1–85 [PubMed] [Google Scholar]
- 71.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2007;(02):CD004659. [DOI] [PubMed] [Google Scholar]
- 72.Silver RM, Ahrens K, Wong LF, et al. Low-dose aspirin and preterm birth: a randomized controlled trial. Obstet Gynecol 2015; 125(04):876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Vliet EO, Askie LA, Mol BW, Oudijk MA. Antiplatelet agents and the prevention of spontaneous preterm birth: a systematic review and meta-analysis. Obstet Gynecol 2017;129(02):327–336 [DOI] [PubMed] [Google Scholar]
- 74.MacIntyre DA, Sykes L, Teoh TG, Bennett PR. Prevention of preterm labour via the modulation of inflammatory pathways. J Matern Fetal Neonatal Med 2012;25(Suppl 1):17–20 [DOI] [PubMed] [Google Scholar]
- 75.Abramovici A, Cantu J, Jenkins SM. Tocolytic therapy for acute preterm labor. Obstet Gynecol Clin North Am 2012;39(01):77–87 [DOI] [PubMed] [Google Scholar]
- 76.Lewis RB, Schulman JD. Influence of acetylsalicylic acid, an inhibitor of prostaglandin synthesis, on the duration of human gestation and labour. Lancet 1973;2(7839):1159–1161 [DOI] [PubMed] [Google Scholar]
- 77.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160 (10):695–703 [DOI] [PubMed] [Google Scholar]
- 78.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122(05):1122–1131 [DOI] [PubMed] [Google Scholar]
- 79.Ahrens KA, Silver RM, Mumford SL, et al. Complications and safety of preconception low-dose aspirin among women with prior pregnancy losses. Obstet Gynecol 2016;127(04): 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Truong A, Sayago MM, Kutteh WH, Ke RW. Subchorionic hematomas are increased in early pregnancy in women taking lowdose aspirin. Fertil Steril 2016;105(05):1241–1246 [DOI] [PubMed] [Google Scholar]
- 81.Nørgård B, Puhó E, Czeizel AE, Skriver MV, Sørensen HT. Aspirin use during early pregnancy and the risk of congenital abnormalities: a population-based case-control study. Am J Obstet Gynecol 2005;192(03):922–923 [DOI] [PubMed] [Google Scholar]
- 82.Kozer E, Nikfar S, Costei A, Boskovic R, Nulman I, Koren G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol 2002; 187(06):1623–1630 [DOI] [PubMed] [Google Scholar]