Abstract
Mesoporous silica with hexagonal type structure containing amine functional group was introduced. Firstly, aminopropyl hexagonal mesoporous silica was synthetized in a co-condensation process, via templating route of n-dodecylamine. Then synthesized mesoporous material were characterized, and FT-IR spectrum confirmed the presence of amine group and CHN analysis determined the amount of organic layer. The high surface area (750 m2/g) was determined by applying nitrogen adsorption-desorption technique. The morphology was examined by scanning electron microscopy which proved hexagonal structure. The crystallinity of mesoporous material was observed in XRD pattern of this material. According to previous background of such material in adsorbing drug, herein, the efficiency of this material in adsorbing of 5-fluorouracil was evaluated through solid phase extraction method in aqueous and plasma media with high performance liquid chromatography. The extraction efficiency was evaluated for drug concentrations of 500–2000 ng/ml by means of 5–20 mg/ml hexagonal mesoporous silica in both media. The results showed good to excellent recovery rate of in both aqueous and plasma medium which confirmed that the aminopropyl functionalized hexagonal mesoporous silica could be considered as promising device for drug bioanalysis.
Keywords: Pharmaceutical science, Materials chemistry, Pharmaceutical chemistry
1. Introduction
The pyrimidine analogue, 5-fluorouracil (5-FU), has found a wide range of therapeutic applications, in various malignant tumors, including colorectal, breast, head and neck, ovarian and pancreatic cancers [1]. This chemotherapeutic agent is the drug of choice in treatment, for example, colorectal carcinoma, which have to be administrated in high dose and continuously, to provide desired level of drug concentration in target sites [2]. The high and repeated dose of IV infusion of this antineoplastic agent increases the risk of toxicity, mostly without any evidence of toxicities [3]. However, bone marrow depression to the point of leukopenia and critical thrombocytopenia, hemorrhagic tendency, intractable vomiting and ulcerations in the gastrointestinal tracts are acute and chronic symptoms of toxicity that probably occur in such treatment processes [4]. Accordingly, developing a sensitive, selective and rapid method for proper monitoring and pharmacokinetics determination of 5-FU is essential in proper dosage adjustment [5]. To determine the precise concentration of 5-FU in a biological medium some techniques have been developed, including high performance liquid chromatography (HPLC) [6], electrochemical assay [7], chromatography [8], electrophoresis and solid-phase extraction method [9]. Solid-phase extraction (SPE) has greatly considered in analyte extraction from complex matrices, recently. This technique finds increasing applications in the area of food, environmental and pharmaceutical analysis [10, 11]. SPE offers various advantages such as simplicity of sample preparation, minimizing organic solvent consumption, high selectivity and sensitivity [12], and can be coupled with different analytical techniques, including gas chromatography (GC) [13, 14, 15], HPLC [16, 17], liquid or gas chromatography with mass spectrometry [18, 19] and capillary electrophoresis (CE) [20, 21]. In recent years nanomaterials have been intended as an interesting device in isolation and extraction techniques due to their high surface area [22] which provided sufficient capacity for the adsorption of analytes. According to this, meso structures have attracted significant attention; and one of the well-known such materials is mesoporous silica (MS) which are typically synthesized by utilizing templating precursor mostly a surfactant-type agent and alkoxysilane precursors [23, 24, 25]. The inorganic framework would form around organic template which will be removed after silanization. By altering template structure different kinds of MS materials would be obtained like; SBA-15, HMS and MCM-41 while all types have high surfaces area [25]. Hexagonal mesoporous silica (HMS), are prepared through soft templating route by n-dodecyl amine as neutral long chain template which offer ease of removing [26, 27]. Long chain template resulted in the formation of long range hexagonal structure. Diverse applications of these materials in different fields include; separations and purification technology [28], catalysis [29], advanced engineered materials [30], selective adsorption as well as being the host for guest molecules [31, 32, 33]. Moreover, high chemical/thermal stability, well defined and manageable morphology, and the capability of intimate functionalization even through co-condensation step or after purification made them as potential candidates for biological applications [32, 34].
As it was described in our previous study, such materials play an impressive role in biological applications including drug adsorbing and delivery [31]. The aim of present study is to apply hexagonal modified mesoporous silica (HMS) as a solid sorbent of 5-FU through SPE method and evaluate the extraction performance in aqueous and human plasma media. Fig. 1 is an illustration of the whole processes.
Fig. 1.
An illustration of HMS application in solid-phase extraction of 5-Fu and determination of the extracted amount by HPLC.
2. Materials and methods
2.1. General remarks
5-FU was purchased from Ebewe Pharma (in concentration 50 mg/ml, Astria). All other chemicals were purchased from Merck and Sigma-Aldrich Company and used without further treatment. Aanalytical techniques including, FT-IR, CHN, XRD, were utilized for characterizing HMS-NH2 functionality and structure. N2 adsorption-desorption technique were applied to assess the specific surface area of the samples using the Brunauer–Emmet–Teller (BET) method. Particle size analysis with DLS, scanning electron microscopy (SEM) and atomic force microscopy (AFM) were performed to determine the sizes and the particle sizes distribution. 5-FU assay was performed by high performance liquid chromatography (HPLC). The instruments’ applied for characterizations are listed here. Fourier transform infrared spectroscopy (FT-IR)/Shimadzu FTIR-8300 (Japan), X-ray diffraction with XRD MPD 3000, SEM with Philips (Germany)/XL-30 FEG, surface area by Quantachrome Chem BET-3000, AFM with JPK nanowizard II and CHN analysis was performed using TermoFinningan, FIASHEA IIIZ, Germany for CHN analysis were applied for material characterization. HPLC was performed by Knauer, Wellchrom®/model k-1001.
2.2. Synthesis of HMS-NH2
Aminopropyl hexagonal mesoporous silica (HMS-NH2) was prepared according to previously reported procedure [27]. Briefly, tetraethyl orthosilicate (0.045 mol) and (3-aminopropyl) trimethoxysilane (0.02 mol) were admixed in a solution of absolute ethanol/deionized water (23 ml/27ml) containing n-dodecylamine (0.013 mol) at 20 °C for 18 hours. After that, the resulting white solid was recovered by sinter glass filtration and washing with ethanol and acetone. By the end, for template removing (n-dodecyl amine) was extracted by refluxing in acidic ethanol solution containing ethanol and hydrochloric acid (ethanol/HCl: 5/1) for 24 hours in ethanol, followed by filtering and drying in oven at 50 °C.
2.3. Extraction procedure
Stock solution of 5000 ng/ml of 5-FU in deionized water were prepared. The standards were diluted using phosphate buffer solution (PBS; composed of disodium hydrogenphosphate 1.38 g, potassium dihydrogenphosphate 0.19 g, sodium chloride 8.0 g, and purified water q.s. 1000 ml, pH of 7.4) and human plasma.
To evaluate the recovery of the 5-FU from matrices, the drug concentration of 500, 1000 and 2000 ng/ml were prepared by 1:10 dilution of the corresponding solutions with plasma, and PBS. This test was performed in triplicate for each medium. Then, HMS-NH2 nanoparticles in 5, 10 and 20 mg/ml concentration were added to samples. The mixtures were shaken for 30 min and centrifuged (10,000 g) for 10 min. The nanoparticles were re-dispersed in deionized water and to detach the drug molecules, 80 μl/ml of trichloro-acetic acid (2N in ethanol) were mixed with each sample. The samples were shaken again for 30 min and centrifuged at 10,000 g for 10 min. Finally, 100 μl of the clear supernatant was injected to the chromatographic system.
The relative recovery of the method for drug by these mesoporous nanoparticles were defined and calculated. The percent ratio of the AUC (area under the curve) of the drug samples in human plasma and PBS to the corresponding AUC after direct injection of aqueous solution of the analyte was determined as the relative recovery index of 5-FU from the mentioned matrices.
2.4. Drug assay
Determination of 5-FU was performed by HPLC method. In this regard flow rate was set on 1.0 ml/min using a C18 column (Eurospher 100-5 C18, 150 mm × 4.6 mm) within the isocratic elution with a mobile phase consisting water/methanol (95/5 v/v) as the stationary phase. The wavelength was adjusted at 261 nm for ultraviolet absorbance. The software (EZChrom Elite®, Germany) were applied for analyzing of the relevant chromatograms.
2.5. Validation test
Prior to samples measurement, validation tests were performed according to the FDA/US Food and Drug Administration, and reported procedure [35].
3. Results and discussion
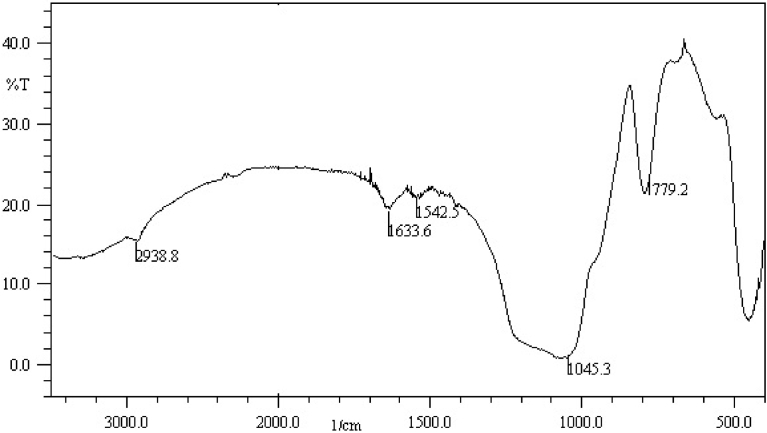
Application of mesoporous silica materials with their high surface area and tunable pore sizes afford great opportunity for setting-up new extraction methods [36]. Here, aminopropyl hexagonal mesoporous silica (HMS-NH2) was synthesized and characterized for utilization in extraction of 5-FU. In this regard, TEOS and APTES are mixed in the presence of templating agent (n-dodecylamine), and after reaction completion, the template was extracted completely to construct HMS-NH2. Structural characterization was performed by FTIR technique and showed peaks at 799 cm−1, and broad band at 1000-1200 cm−1 attributed to Si-OH and Si-O-Si stretching vibration, respectively (Fig. 2). The N-H and CH2 of aminopropyl bending vibration appeared at 1542 and 1636 cm−1. CHN analysis showed that the amount of organic layer is equal to 1.37 mmol/g.
Fig. 2.
FTIR of HMS-NH2.
The structure crystallinity was determined by XRD technique. XRD showed required crystallinity planes of HMS (Fig. 3). The XRD diagram of HMS-NH2 showed expected crystallinity of mesoporous by appearing a sharp peak at 1.5° as an indicative of 100 hkl indices of crystallographic plane in xyz directions. Moreover, the presence of amorphous silica resulted in the appearance of a broad peak around 20°.
Fig. 3.
XRD of HMS-NH2.
Morphology observation was carried out through SEM images and showed well-ordered HMS nanoparticles in hexagonal format with dimension sizes from 200 to 500 nm (Fig. 4).
Fig. 4.
SEM image of HMS-NH2.
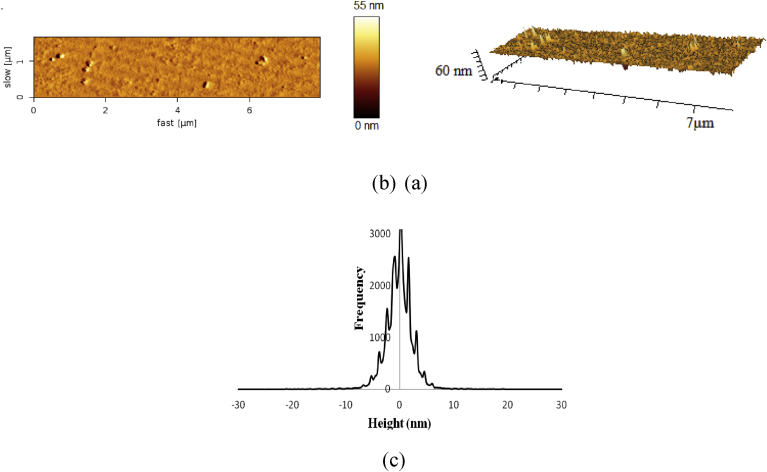
N2 adsorption, desorption test was performed to determine the specific surface area of HMS-NH2, which calculated by BET equation to be 750 m2/g. To Define particles distribution AFM analysis were performed by nanowizard instrument which provide 2-D, 3-D and size histogram (Fig. 5 a,b,c). The 2-D deflection image showed fine distribution of spherical nanoparticles with sizes less than 50 nm while 3-D image confirm this observation and also display minor agglomeration (Fig. 5 a,b). The histogram display that HMS-NH2 contains spherical particles with majority diameter sizes of 10 nm and minor part lies between 10-30 nm without agglomeration (Fig. 5 c).
Fig. 5.
AFM images of HMS-NH2; 2-D deflection image (a), 3-D image (b), and height histogram (c).
To determine hydrodynamic size of the prepared particles, DLS analysis was performed. Through this analysis the hydrodynamic sizes were measured to be in average 400 nm (Fig. 6). To illustrate, it should be emphasized that this observation which is higher than the results of TEM, SEM and AFM is due to high solvation of nanoparticles in aqueous medium.
Fig. 6.
DLS size distribution of HMS-NH2 particles.
Prior to assessment of HMS-NH2 efficiency in extraction, the HPLC method of 5-Fu characterization was validated [35]. The linearity was determined to be in the range of 25–500 ng/ml, LOQ of 25 ng/ml and LOD of 10 ng/ml.
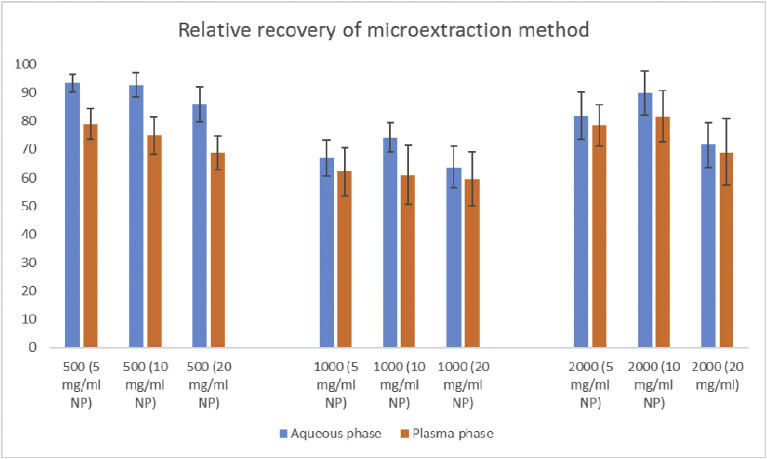
To determine the efficiencies of HMS-NH2 in extraction of 5-FU by HPLC method, a series of tests were performed (Fig. 7). The results indicate the comparative recovery of the 5-FU extraction at the concentrations of 500, 1000 and 2000 ng/ml by means of initial concentrations of dispersed HMS-NH2 nanoparticles in 5, 10 and 20 mg/ml obtained from aqueous and human plasma samples, respectively.
Fig. 7.
The comparative recovery of 5-FU extraction by HMS-NH2 nanoparticles in aqueous medium.
The outcomes of these series of tests were indicative of a proper separation efficacy while size-proportional. The relative recoveries of the developed SPE method in PBS and plasma for 5-FU by HMS-NH2 nanoparticles for various drug and particle concentration were from 63.2 to 93.4% and 59.5 to 81.7% in aqueous and plasma medium, respectively. As expected, the recovery in aqueous medium is higher than plasma medium because of possible drug-protein interactions in plasma.
Furthermore, although, no exact relationship was not found between the drug and nanoparticle concentration in recovery efficiency, it was clear that in high concentration of nanoparticles the value of recovery is reduced. This might be due to the reduction of drug molecules detachment from nanoparticles in the last step of miroextraction. Also, HMS-NH2 nanoparticles shows remarkable relative recovery due to their amino groups which are suitable functional groups to isolate and extract 5-FU selectively.
Previous methods in solid phase extraction of 5-Fu in regard with type of solid phase/determination technique/medium/linearity/limit of detection (LOD)/limit of quantification (LOQ) and recovery are summarized in (Table 1). Herein, by having HMS-NH2 as solid phase within HPLC technique in plasma and aqueous phase with LOD of 10 ng/ml, linearity of 25–500 ng/ml, LOQ of 25 ng/ml and maximum recovery of 93.4% in aqueous media and 81.7 in plasma, good to excellent results were obtained. According with Forough et al. who made an innovation by applying anchored silver nanoparticles to MCM-41-reinforced hollow fiber (AgNPs@MCM-41-HF) in solid/liquid phase extraction, the utilization of MCM-41 as mesoporous silica source made positive effect in adsorption rate, amount and selectivity.
Table 1.
Previously reported procedures for SPE of 5-Fu.
| NO. | Solid phase type | Determination technique | Medium | Linearity | LOD | LOQ | Recovery | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | OasisWax extraction cartridge | LC-MS/MS | human cultured cellular matrix | 7.5–150 (ng/ml) | - | 7.5 (ng/ml) | 85% | (37) |
| 3 | Oasis HLB (copolymer of poly(di- vinylbenzene-co-N-vinylpyrrolidone)) cartridge column | LC-MS | human plasma | 0.05–25 (μg/ml) | - | 8.4 (ng/ml) | 73.1–74.8% | (38) |
| 4 | Isolute ENV+ column | GC–MS/MS | hospital water (waste and surface water) | 2-106 (ng/L) | 0.48 (ng/L) for waste and 0.16 (ng/L) for surface water | 1.6 (ng/L) for waste and 0.54 (ng/L) for surface water | 53% for waste and 93% for surface water | (39) |
| 5 | ENV+ columns | Capillary electrophoresis | hospital waste water samples | 5–500 (μg/ml) | 1.7 (μg/ml) | 8.6 (μg/ml) | 80–96% | (40) |
| 6 | Isolute ENV+ cartridges |
GC/MS-MS | hospital waste water | 0.09–40 (μg/L) | 12 (ng/L) | 40 (ng/ml) | 93–101% | (41) |
| 7 | C18 Chem Elut Cartridge |
HPLC | human plasma | 25–1000 (ng/ml) | - | 20 (ng/ml) | 35% | (42) |
| 8 | AgNPs@MCM-41-HF | capillary electrophoresis with in-column field-amplified sample injection | Human plasma | 25–1000 (ng/ml) | - | 25 (ng/ml) | 98% | [43] |
The utilization of mesoporous silica for SPE would offer advantages such as ease of recovery with high percentage. This method could be more selective by designing engineered mesoporous silica structures.
4. Conclusion
Here, highly sensitive method based on SPE and application of HMS nanoparticles were reported for determining low concentration of 5-FU. By this mean, HMS-NH2 were synthesized by simple method, and fully characterized by mentioned techniques. The hexagonal geometry was clear in SEM image. AFM images and histogram showed highly dispersed HMS-NH2, with diameter of 10 nm. With the aim of analyte extraction, the efficiency of this material was evaluated by HPLC in extraction of 5-FU from aqueous and plasma medium. The extraction efficiency was evaluated for drug concentrations of 500, 1000 and 2000 ng/ml by means of 5, 10 and 20 mg/ml hexagonal mesoporous silica in both media. The results of recovery (for various drug and particle concentration from 63.17 to 93.38% and 59.49 to 81.69% in aqueous and plasma medium, respectively) confirm that the aminopropyl functionalized hexagonal mesoporous silica could be considered as promising device for drug bioanalysis.
Declarations
Author contribution statement
F. Farjadian, A. Azadi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. Azadi: Performed the experiments; Wrote the paper.
S. Mohammadi-Samani: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
H. Ashrafi: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Shiraz University of Medical Sciences.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Research Council of Shiraz University of Medical Sciences for support of this work (Grant NO. 1396-01-36-15113).
Contributor Information
Fatemeh Farjadian, Email: farjadian_f@sums.ac.ir.
Amir Azadi, Email: aazadi@sums.ac.ir.
References
- 1.Chabner B.A., Longo D.L. Lippincott Williams & Wilkins; 2011. Cancer Chemotherapy and Biotherapy: Principles and Practice. [Google Scholar]
- 2.Bose A., Elyagoby A., Wong T. Oral 5-fluorouracil colon-specific delivery through in vivo pellet coating for colon cancer and aberrant crypt foci treatment. Int. J. Pharm. 2014;468(1):178–186. doi: 10.1016/j.ijpharm.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald J.S. Toxicity of 5-fluorouracil. Oncology (Williston Park, NY) 1999;13(7 Suppl 3):33–34. [PubMed] [Google Scholar]
- 4.Garg M., Lincz L., Adler K., Scorgie F., Ackland S., Sakoff J. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. Br. J. Cancer. 2012;107(9):1525–1533. doi: 10.1038/bjc.2012.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline C.L.B., Schiccitano A., Zhu J., Beachler C., Sheikh H., Harvey H.A. Personalized dosing via pharmacokinetic monitoring of 5-fluorouracil might reduce toxicity in early-or late-stage colorectal cancer patients treated with infusional 5–fluorouracil-based chemotherapy regimens. Clin. Colorectal Cancer. 2014;13(2):119–126. doi: 10.1016/j.clcc.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Guichard S.M., Mayer I., Jodrell D.I. Simultaneous determination of capecitabine and its metabolites by HPLC and mass spectrometry for preclinical and clinical studies. J. Chromatogr. B. 2005;826(1):232–237. doi: 10.1016/j.jchromb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Bukkitgar S.D., Shetti N.P. Electrochemical sensor for the determination of anticancer drug 5-fluorouracil at glucose modified electrode. Chemistry Select. 2016;1(4):771–777. [Google Scholar]
- 8.Matsushima E., Yoshida K., Kitamura R., Yoshida K-i. Determination of S-1 (combined drug of tegafur, 5-chloro-2, 4-dihydroxypyridine and potassium oxonate and 5-fluorouracil in human plasma and urine using high-performance liquid chromatography and gas chromatography-negative ion chemical ionization mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1997;691(1):95–104. doi: 10.1016/s0378-4347(96)00429-x. [DOI] [PubMed] [Google Scholar]
- 9.Spietelun A., Marcinkowski Ł., de la Guardia M., Namieśnik J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A. 2013;1321:1–13. doi: 10.1016/j.chroma.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Hennion M.-C. Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography. J. Chromatogr. A. 1999;856(1-2):3–54. doi: 10.1016/s0021-9673(99)00832-8. [DOI] [PubMed] [Google Scholar]
- 11.Wen Y., Chen L., Li J., Liu D., Chen L. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. TrAC Trends Anal. Chem. (Reference Ed.) 2014;59:26–41. [Google Scholar]
- 12.Rodriguez-Mozaz S., de Alda M.J.L., Barceló D. Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography–mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J. Chromatogr. A. 2007;1152(1-2):97–115. doi: 10.1016/j.chroma.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Hamidi M., Azadi A., Ashrafi H., Rafiei P., Mohamadi-Samani S. Taguchi orthogonal array design for the optimization of hydrogel nanoparticles for the intravenous delivery of small-molecule drugs. J. Appl. Polym. Sci. 2012;126(5):1714–1724. [Google Scholar]
- 14.Liu R., Zhou J.L., Wilding A. Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extraction–gas chromatography–mass spectrometry. J. Chromatogr. A. 2004;1022(1-2):179–189. doi: 10.1016/j.chroma.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Lee H.-B., Peart T.E. Determination of 17 beta-estradiol and its metabolites in sewage effluent by solid-phase extraction and gas chromatography/mass spectrometry. J. AOAC Int. 1998;81(6):1209–1216. PMID:9850583. [PubMed] [Google Scholar]
- 16.Patrolecco L., Ademollo N., Grenni P., Tolomei A., Caracciolo A.B., Capri S. Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem. J. 2013;107:165–171. [Google Scholar]
- 17.Li J-k, Wu R-n, Hu Q-h, Wang J-h. Solid-phase extraction and HPLC determination of patulin in apple juice concentrate. Food Control. 2007;18(5):530–534. [Google Scholar]
- 18.Wu J., Lord H.L., Pawliszyn J., Kataoka H. Polypyrrole-coated capillary in-tube solid phase microextraction coupled with liquid chromatography–electrospray ionization mass spectrometry for the determination of β-blockers in urine and serum samples. J. Microcolumn Sep. 2000;12(4):255–266. [Google Scholar]
- 19.Schulz K., Schlenz K., Metasch R., Malt S., Römhild W., Dreßler J. Determination of anethole in serum samples by headspace solid-phase microextraction-gas chromatography–mass spectrometry for congener analysis. J. Chromatogr. A. 2008;1200(2):235–241. doi: 10.1016/j.chroma.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 20.Whang C.-W., Pawliszyn J. Solid phase microextraction coupled to capillary electrophoresis. Anal. Commun. 1998;35(11):353–356. [Google Scholar]
- 21.Jinno K., Kawazoe M., Saito Y., Takeichi T., Hayashida M. Sample preparation with fiber-in-tube solid-phase microextraction for capillary electrophoretic separation of tricyclic antidepressant drugs in human urine. Electrophoresis. 2001;22(17):3785–3790. doi: 10.1002/1522-2683(200109)22:17<3785::AID-ELPS3785>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Mehdinia A., Aziz-Zanjani M.O. Recent advances in nanomaterials utilized in fiber coatings for solid-phase microextraction. TrAC Trends Anal. Chem. (Reference Ed.) 2013;42:205–215. [Google Scholar]
- 23.Asefa T., Tao Z. Mesoporous silica and organosilica materials-Review of their synthesis and organic functionalization. Can. J. Chem. 2012;90(12):1015–1031. [Google Scholar]
- 24.Wu S.H., Mou C.Y., Lin H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013;42(9):3862–3875. doi: 10.1039/c3cs35405a. [DOI] [PubMed] [Google Scholar]
- 25.Beltrán-Osuna Á.A., Perilla J.E. Colloidal and spherical mesoporous silica particles: synthesis and new technologies for delivery applications. J. Sol. Gel Sci. Technol. 2016;77(2):480–496. [Google Scholar]
- 26.Macquarrie D., Jackson D., Mdoe J.G., Clark J. Organomodified hexagonal mesoporous silicates. New J. Chem. 1999;23(5):539–544. [Google Scholar]
- 27.Farjadian F., Hosseini M., Ghasemi S., Tamami B. Phosphinite-functionalized silica and hexagonal mesoporous silica containing palladium nanoparticles in Heck coupling reaction: synthesis, characterization, and catalytic activity. RSC Adv. 2015;5(97):79976–79987. [Google Scholar]
- 28.Chew T.-L., Ahmad A.L., Bhatia S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO 2) Adv. Colloid Interface Sci. 2010;153(1):43–57. doi: 10.1016/j.cis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Luque R., Balu A.M., Campelo J.M., Gracia M.D., Losada E., Pineda A. Catalytic applications of mesoporous silica-based materials. Catalysis. 2012:253–280. [Google Scholar]
- 30.Tsai C.-H. Digital Repository at Iowa State University; 2012. Development and Application of Multi-functionalized Mesoporous Silica Nanomaterials in Intracellular Drug Delivery and Heterogeneous Catalysis. [Google Scholar]
- 31.Farjadian F., Ahmadpour P., Samani S.M., Hosseini M. Controlled size synthesis and application of nanosphere MCM-41 as potent adsorber of drugs: a novel approach to new antidote agent for intoxication. Micropor. Mesopor. Mat. 2015;213:30–39. [Google Scholar]
- 32.Hosseini M., Farjadian F., Makhlouf A.S.H. Industrial Applications for Intelligent Polymers and Coatings. Springer; 2016. Smart stimuli-responsive nano-sized hosts for drug delivery; pp. 1–26. [Google Scholar]
- 33.Farjadian F., Ghasemi S., Heidari R., Mohammadi-Samani S. In vitro and in vivo assessment of EDTA-modified silica nano-spheres with supreme capacity of iron capture as a novel antidote agent. Nanomed. Nanotechnol. 2016;13(2):745–753. doi: 10.1016/j.nano.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Dave P.N., Chopda L.V., editors. Mater. Sci. Forum. Trans Tech Publ; 2014. A review on application of multifunctional mesoporous nanoparticles in controlled release of drug delivery. [Google Scholar]
- 35.Azadi S., Azadi A., Ashrafi H., Mohammadi-Samani S. A reversed-phase high performance liquid chromatography approach for analysis of 5-Fluorouracil. Trends Pharm. Sci. 2017;3(1):49–54. [Google Scholar]
- 36.Anbia M., Irannejad S. Modified SBA-15 mesoporous silica as a novel fiber coating in solid-phase microextraction and determination of BTEX compounds in water samples using gas chromatography-flame ionization detection. Anal. Methods. 2013;5(6):1596–1603. [Google Scholar]
- 37.Carli D., Honorat M., Cohen S., Megherbi M., Vignal B., Dumontet C. Simultaneous quantification of 5-FU, 5-FUrd, 5-FdUrd, 5-FdUMP, dUMP and TMP in cultured cell models by LC-MS/MS. J. Chromatogr. B. 2009;877(27):2937–2944. doi: 10.1016/j.jchromb.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y., Grem J.L. Liquid chromatography–mass spectrometry method for the analysis of the anti-cancer agent capecitabine and its nucleoside metabolites in human plasma. J. Chromatogr. B. 2003;783(1):273–285. doi: 10.1016/s1570-0232(02)00674-8. [DOI] [PubMed] [Google Scholar]
- 39.Kosjek T., Perko S., Žigon D., Heath E. Fluorouracil in the environment: analysis, occurrence, degradation and transformation. J. Chromatogr. A. 2013;1290:62–72. doi: 10.1016/j.chroma.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 40.Mahnik S.N., Rizovski B., Fuerhacker M., Mader R.M. Determination of 5-fluorouracil in hospital effluents. Anal. Bioanal. Chem. 2004;380(1):31–35. doi: 10.1007/s00216-004-2727-6. [DOI] [PubMed] [Google Scholar]
- 41.Mullot J.-U., Karolak S., Fontova A., Huart B., Levi Y. Development and validation of a sensitive and selective method using GC/MS-MS for quantification of 5-fluorouracil in hospital wastewater. Anal. Bioanal. Chem. 2009;394(8):2203–2212. doi: 10.1007/s00216-009-2902-x. [DOI] [PubMed] [Google Scholar]
- 42.Joulia J., Pinguet F., Grosse P., Astre C., Bressolle F. Determination of 5-fluorouracil and its main metabolites in plasma by high-performance liquid chromatography: application to a pharmacokinetic study. J. Chromatogr. B Biomed. Sci. Appl. 1997;692(2):427–435. doi: 10.1016/s0378-4347(96)00518-x. [DOI] [PubMed] [Google Scholar]
- 43.Forough M., Farhadi K., Molaei R., Khalili H., Shakeri R., Zamani A. Capillary electrophoresis with online stacking in combination with AgNPs@MCM-41 reinforced hollow fiber solid-liquid phase microextraction for quantitative analysis of Capecitabine and its main metabolite 5-Fluorouracil in plasma samples isolated from cancer patients. J. Chromatogr. B. 2017 1/1/;1040:22–37. doi: 10.1016/j.jchromb.2016.11.025. [DOI] [PubMed] [Google Scholar]