Abstract
Purpose
Chitosan has been shown to promote wound healing and induce bone formation. The aim of this split-mouth study was to evaluate the effectiveness of chitosan based dressing in wound healing after lower third molar extraction.
Method
Asymptomatic symmetrical mandibular third molars were extracted simultaneously in 27 patients and Chitosan dressing was placed into the extraction socket in the test side. Pain scores were recorded on VAS using a 0 to 10 pain score. Wound healing was compared between right and left side. Radiographic findings were evaluated by observing lamina dura and density of extraction socket.
Results
Test group had more pain than control at all time intervals and unerupted tooth sites showed mean pain score significantly more than erupted tooth sites. Test group was superior to control in event of wound healing. Healing was significantly better in erupted tooth than unerupted tooth. At second week 12 sites showed better radiographic findings in chitosan treated group compared to 3 sites in the control group. At third month, 14 sites showed improved bone formation in chitosan treated group compared to 4 in control group. None of the unerupted teeth group showed better radiographic finding in test side at 2 week and 3 month compared to erupted teeth group.
Conclusion
Chitosan is effective in promoting wound healing and early osteogenesis in erupted tooth socket after extraction. We recommend that chitosan dressing should be used in the sockets of erupted tooth after extraction but should be avoided in unerupted or impacted teeth cases.
Keywords: Chitosan, Extraction socket, Dry socket, Impacted third molar
1. Introduction
Mandibular third molar extraction is one of the most common procedures performed in oral surgery clinics. The complications associated with this procedure in the early postoperative phase are bleeding, pain and swelling. Delayed postoperative complications include paresthesia and dry socket.1 Since long there has been continuous efforts to reduce the complications associated with mandibular third molar extraction. Various local medicaments like tetracycline, hydrocortisone, lincomycin, clindamycin, chlorhexidine and metronidazole has been used as dressing in the extraction socket with variable success.2,3
Recent efforts have been on the use of biologically derived materials such as collagen or chitin and their derivatives that are capable of accelerating the healing processes at molecular and cellular level.4 Chitosan is a derivative of the alkaline deacetylation of chitin. It is a copolymer made of d-glucosamine and Nacetyl- d-glucosamine bonds and β bonds (1–4) in which glucosamine is the predominant repeating unit in its structure. It is the second most abundant natural polymer and is found in the shells of crustaceans and walls of fungi. Chitosan has been shown to promote wound healing by inducing bone formation and also with its inhibitory effects on microorganisms like Candida, Klebsiella, Pseudomonas, Staphylococci, and Streptococci.5
It possesses drug-delivery and antimicrobial properties and is used in various forms like fibers, films, sponges and hydrogels. Chitosan's properties of binding with red blood cells allows it to rapidly clot blood, and it has recently gained regulatory approval in the USA for use in bandages and other hemostatic agents.6,7 Recently, a new chitosan-based hemostatic oral wound dressing HemCon dental dressing (HDD; HemCon Medical Technologies, Inc, Beaverton, OR) has been developed which is derived from the US military HemCon Bandage combat wound dressing. It is manufactured from highly refined and purified insoluble shrimp shell chitin. The purpose of this study was to evaluate the efficacy of chitosan in extraction wound healing in mandibular third molars.
2. Material and methods
This was a prospective, randomized split mouth study. Ethical clearance was taken from the institutional ethical committee and written informed consent was taken from patients. Patients having asymptomatic symmetrical mandibular third molar requiring extraction with age sixteen and above were selected from the outpatient department and it involved a randomly assigned control side and a test side in each patient.
Preoperative radiographs were taken to assess the position and symmetry of mandibular third molar. Preoperative mouth rinse with 0.2% chlorhexidine gluconate was given to the patient. Mandibular third molar on both the sides was extracted simultaneously under local anesthesia in standardized manner. After extraction one of the surgical site was randomly selected as test and other as control site. In test group, HDD was placed into the extraction socket at the height of crestal bone. No suturing was done on test side and control side as it is not recommended by manufacturer in instruction manual. Pressure packs were given with sterile cotton gauze dressing on both sides. Postoperatively Paracetamol 500 mg 6 hourly was used as analgesic. No antibiotics were prescribed to the patients.
27 patients were evaluated by a blinded observer at the 24hr, 72hr, 5th day, 7th day, 2nd, 4th, 8th, 12th week postoperatively for pain and wound healing. Pain scores were recorded on visual analog scale (VAS) using a 0 to 10 pain score basis with 0 being no pain and 10 being the worst pain the patient had ever experienced. Infection was considered in the patient if pus was present and was recorded as present or absent. Wound healing was evaluated using following observation of presence or absence of blood clot, granulation tissue or fibrous tissue over the socket and condition of wound epithelium at various intervals. Wound healing was compared between right and left side and categorized into one of the three scores as −1(Worse than right), 0 (Same as right), +1(Better than right). Radiographic findings were evaluated by observing lamina dura and density of extraction socket by using scale of 0 (Clear and continuous similar to morphology of tooth), 1(Blurry but continuous), 2 (Blurry but not continuous), 3 (Totally disappeared) and was compared between right and left side and categorized into one of the three scores as −1(Worse than right), 0 (Same as right), +1(Better than right). The surgical sites were decoded at the end of study and master chart was prepared and data generated was evaluated statistically.
3. Results
Out of these 27 patients 20 had erupted (74%) and 7 unerupted (26%) teeth. Initially we placed the chitosan dressing in 7 unerupted teeth and found high complications, and hence we selected erupted teeth for dressing placement.
3.1. Pain scores
Table 1 shows mean pain score for various time intervals. Differences in pain score between test and control were statistically significant. Test group had more pain than control at all time intervals and unerupted tooth sites showed mean pain score significantly more than erupted tooth sites as shown in Table 2. Mean pain scores were also compared within erupted and unerupted teeth group in test and control side. Mean pain score were 4.0, 3.8, 2.8, 1.5 in erupted teeth group at 1st,3rd, 5th and 7th day postoperative appointments in control side and 4.6, 4.2, 3.5, 2.4 in test side and results were not significant(p > 0.05). Mean pain scores were significantly higher (p < 0.05) in test side within unerupted teeth and were 7.0, 6.1, 4.8, 4.4 as compared to 3.8, 2.5, 1.8, 1.2 in control side at 1st, 3rd, 5th and 7th day postoperative appointments.
Table 1.
Comparison of pain score between test and control site.
| Pain | Test side |
Control side |
|---|---|---|
| Mean | Mean | |
| 1st day | 5.22 ± 2.18 | 4.00 ± 1.94 |
| 3rd day | 4.70 ± 2.12 | 3.48 ± 2.00 |
| 5th day | 3.88 ± 1.88 | 2.55 ± 1.73 |
| 7th day | 2.92 ± 2.01 | 1.44 ± 1.80 |
Table 2.
Mean pain score in erupted and unerupted tooth.
| Tooth | Erupted | Unerupted |
|---|---|---|
| Pain 1 day | 4.60 ± 2.19 | 7.00 ± 0.82 |
| Pain 3rd day | 4.20 ± 2.14 | 6.14 ± 1.35 |
| Pain 5th day | 3.55 ± 2.04 | 4.86 ± 0.90 |
| Pain 7th day | 2.40 ± 2.01 | 4.43 ± 1.13 |
3.2. Infection
In 7(26%) patients infection was present in test site and in 3(11%) patients infection was present in control site. Infection was significantly higher in unerupted tooth sites than erupted tooth sites. All the unerupted teeth sockets got infected on test side whereas 3 sockets got infected on control side in erupted teeth. None of the cases on test side got infected in erupted group.
3.3. Wound healing
Statistically significant difference was found in wound healing between test and control side (Table 3). Test group was superior to control in event of wound healing. Healing was significantly better in erupted tooth than unerupted tooth in first, second, fourth and eighth week time interval. Erupted tooth was superior to unerupted tooth in event of wound healing. In first week 8 erupted teeth showed no differences in healing in test and control side whereas 12 erupted teeth showed significantly better healing in test side compared to control side. No unerupted teeth showed significantly better healing in test side (Table 4). In second and fourth week 17 erupted teeth showed significantly better healing in test side compared to control and 7 unerupted teeth significantly showed better healing in control side compared to test side (Table 4). In eighth week 15 erupted teeth showed significantly better healing compared to 7 unerupted teeth in which control side healed significantly better than test side (Table 4).
Table 3.
p - values at different time interval.
| Wound Healing | Week 1 | Week 2 | Week 4 | Week 8 |
|---|---|---|---|---|
| C > T | 2 | 8 | 8 | 8 |
| C = T | 13 | 2 | 2 | 4 |
| C < T | 12 | 17 | 17 | 15 |
| P value | 0.016 | 0.002 | 0.002 | 0.032 |
Table 4.
Comparison of wound healing in erupted and unerupted tooth.
| Wound healing First week | Tooth [n (%)] |
|
|---|---|---|
| Erupted | Unerupted | |
| C > T | 0 (0) | 2 (28.6) |
| T = C | 8 (40) | 5 (38.5) |
| T > C |
12 (60) |
0 (0) |
| Wound healing Second week | Tooth [n (%)] | |
| Erupted |
Unerupted |
|
| C > T | 1 (5) | 7 (87.5) |
| T = C | 2 (10) | 0 (0) |
| T > C |
17 (85) |
0 (0) |
| Wound healing Fourth week | Tooth [n (%)] | |
| Erupted |
Unerupted |
|
| C > T | 1 (5) | 7 (100) |
| T = C | 2 (10) | 0 (0) |
| T > C |
17 (85) |
0 (0) |
| Wound healing Eighth week | Tooth [n (%)] | |
| Erupted |
Unerupted |
|
| C > T | 1 (5) | 7 (100) |
| T = C | 4 (20) | 0 (0) |
| T > C | 15 (75) | 0 (0) |
3.4. Radiographic finding
In second week 12(44.4%) sites showed better radiographic findings in test group (Fig. 1Aand B) and in 12(44.4%) sites similar radiographic findings were present. In only 3(11.1%) sites radiographic finding was better in control as compare to test group. Statistically significant difference was found between test and control side at second week (Table 5). In third months 14(51.9%) sites showed better findings in test group (Fig. 2Aand B) and in 9(33.3%) sites similar radiographic findings were present in test and control group. Rest 4(14.8%) sites showed better findings in control. No statistically significant difference was found between test and control side at third month (Table 5). Test group was superior to control in terms of new bone formation. Radiographic finding was also compared in erupted and unerupted tooth at second week and three months after extraction (Table 6). Radiographic finding was significantly better in erupted tooth than unerupted tooth. Erupted tooth was superior to unerupted tooth in event of new bone formation. In 2nd week 12 erupted teeth showed significantly better radiographic finding in test side compared to control side and 3 unerupted teeth showed significantly better radiographic finding in control side compared to test side (Table 6). In third month 14 erupted teeth showed significantly better radiographic finding in test side compared to control side (Table 6).
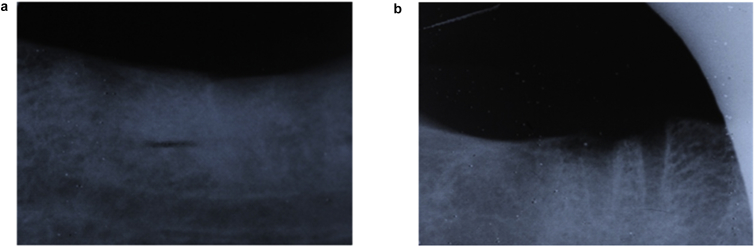
Fig. 1.
Shows blurry and non continuous lamina dura at test side (A) and clear continues lamina Dura at control side (B) at 2nd week follow up.
Table 5.
p - value at different time interval.
| Radiographic findings | 2 week | 3 Month |
|---|---|---|
| C > T | 3 | 4 |
| C = T | 12 | 9 |
| T > C | 12 | 14 |
| P value | 0.050 | 0.062 |
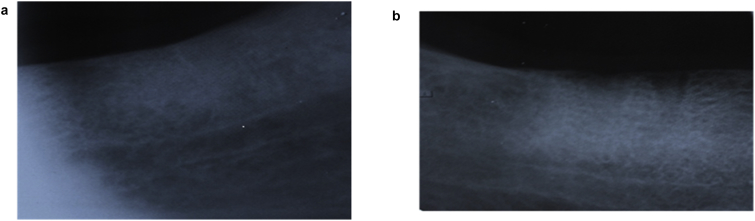
Fig. 2.
Shows completely disappeared lamina dura at test side (A) and blurry and non continuous at control side (B) at 3 month follow up.
Table 6.
Comparison of radiographic finding in erupted and unerupted tooth.
| Second week | Tooth [n (%)] |
|
|---|---|---|
| Erupted | Unerupted | |
| C > T | 0 (0) | 3 (42.9) |
| C = T | 8 (40) | 4 (57.1) |
| T > C |
12 (60) |
0 (0) |
| Three months | Tooth [n (%)] | |
| Erupted |
Unerupted |
|
| C > T | 0 (0) | 4 (57.1) |
| T = C | 6 (30) | 3 (42.9) |
| T > C | 14 (70) | 0 (0) |
C – Control group.
T – Test group.
4. Discussion
The study showed that chitosan improve healing of extraction socket in an erupted tooth whereas it has opposite effect in an impacted or unerupted tooth.
Chitosan is a polymer with a number of basic amino groups and hence possesses an overall cationic charge, especially at acidic pH. This is due to the presence of primary amines on the molecule that bind protons according to the equation: Chit-NH2 + H3O+ = Chit-NH2+ + H2O. In common with many cationic polymers, chitosan has pronounced antimicrobial effects due to destabilization of the outer membrane of Gram-negative bacteria and permeabilization of the microbial plasma membrane.8, 9, 10 In addition, chitosan modulates the functions of inflammatory cells and subsequently promotes granulation and organization.11 It could accelerate wound healing by enhancing the functions of inflammatory cells, such as polymorphonuclear leukocytes (PMN) macrophages and fibroblasts or osteolasts. As a semipermeable biological dressing, it maintains a sterile wound exudate beneath a dry scab, preventing dehydration and contamination of the wound, to optimize conditions for healing.12
Malmquist et al. evaluated the efficacy of the HDD in dental extraction socket and studied pain score at postoperative 1 week. The pain scores were lower than control but the difference was not statistically significant.13 Another observation noted was that any excess HDD material would result in small amounts of residual unreacted residual acetic acid that elevated pain scores in the initial days. This was easily corrected by reducing or trimming the material at chair side to loosely fit the extraction socket. In our study, pain scores were high in test group performed compared to control group. The increased pain could have been due to acidic pH and untrimmed margins of HDD. Additionally, mean pain score were significantly higher in unerupted tooth site on first and third day as compared erupted tooth site. Within unerupted group mean pain scores were significantly higher in test side compared to control side whereas in erupted teeth group differences in pain score between test and control side were not significant. We hypothesized that unerupted tooth site act as close anatomic space where dissolution of hemcon dental dressing was impaired and result in unreacted residual acetic acid.
Postoperative infections after third molar removal have been reported to vary from 0.8% to 4.2%. In the present study the rate of infection was 26% and 11% in test group and control group respectively. This rate was more in test group than control side. Infection occurred in all 7 test side in unerupted teeth group compared to 3 test side in erupted teeth group out of 20. The exact antimicrobial mechanism of chitosan is still uncertain but it has been proposed that interaction between positively charged chitosan molecules and negatively charged microbial cell membranes leads to the disruption of microbial membrane and subsequently the leakage of proteinaceous and other intracellular constituents. No et al. in an invitro study compared the antibacterial activities of chitosan and chitosan oligomers against both Gram-negative and Gram-positive bacteria.14 Chitosan showed stronger bactericidal effects with Gram-positive bacteria than with Gram-negative bacteria. But this study also showed that infection was significantly higher in test side in unerupted teeth group and corroborated with our findings therefore we did not include unerupted teeth further in our study and strongly recommend against putting it deeply in an unerupted or impacted teeth socket.
Chitosan and its derivatives accelerate wound healing by enhancing the functions of inflammatory cells such as polymorphonuclear leukocytes (PMN), macrophages, fibroblasts or osteolasts. It has also been reported that chitosan could increase the tensile strength of wounds.12 In the present study, 12(44.4%) sites showed better wound healing in chitosan treated group as compare to control in the first week. In second and fourth week 17(63%) sites showed better healing in chitosan treated group and in eighth week 15(55.6%) sites showed better healing in chitosan treated group as compare to control and these results were statistically significant. These findings support the wound healing property of chitosan as described in literature. Wound healing was significantly better in erupted teeth group compared to unerupted teeth at various follow up period.
Li et al. did the only other human study of chitosan based microbial fibre membrane flagyl (MF-FLA) on wound healing after tooth extraction.15 Their results showed better and early wound healing and reduced incidence of post extraction complications because of its biocompatibility, isolating and anti-inflammatory ability and supporting the formation of blood clot in the tooth socket. The results of their radiographic and histological investigations showed that MF-FLA better facilitated wound healing because of its affinity for the blood clot in the socket and its biocompatibility. The main composition of the microbial fibres from fungi is chitin, polysaccharide, and vitamins which contributed to its biocompatibility. Therefore, MF-FLA in the socket works as a scaffold along which the fibroblasts can grow. MF-FLA facilitated the proliferation of fibroblasts, accelerated the growth of granulation tissue and epithelium, and induced the proliferation of new bone. Malmquist et al. evaluated the efficacy of the HDD on haemostasis and surgical healing outcomes following oral surgical procedures and showed that approximately 32% of HDD treated sites had significantly better healing compared with control sites.
Pippi et al. evaluated the efficacy of HDD after tooth extraction in patients receiving oral anticoagulant therapy and have an INR lower than 3.5.16,17 They advocated that use of chitosan dressing is a safe procedure without discontinuation of the oral anticoagulant regimen. The hemcon dental dressing seems to reduce postoperative side effects and obtain rapid soft tissue healing. This study did not include patients who therapeutically anticoagulated though further modificatons in the study can include similar patients.
The authors are aware of the limitations of the present study. A small sample size and a slow learning curve due to lack of relevant literature led us to initially place the dressing in deeply impacted teeth sockets which showed high complications. Later on the case selection was limited to erupted tooth which showed better results. We used dressing from a single manufacturer and our results are specific to this product. There may be differences in manufacturing process and processing by the other manufacturers and results may vary. Further studies with larger sample size and chitosan products from different manufacturers are required to conclusively prove its efficacy in promoting wound healing. In conclusion, Chitosan is effective in promoting wound healing and early osteogenesis in erupted tooth socket after extraction. We recommend that chitosan dressing should be used in the sockets of erupted tooth after extraction but should be avoided in unerupted or impacted teeth cases.
Contributor Information
Akshat Gupta, Email: aksgupta18@yahoo.com.
Vidya Rattan, Email: drvidyarattan@gmail.com.
Sachin Rai, Email: drraisachin@gmail.com.
References
- 1.Bui C., Seldin E., Dodson T. Types, Frequencies and Risk factors for complications after third molar extraction. J Oral Maxillofac Surg. 2003;61:1379–1389. doi: 10.1016/j.joms.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Alexander R.E. Dental extraction wound management: a case against medicating postextraction sockets. J Oral Maxillofac Surg. 2000;58:538–551. doi: 10.1016/s0278-2391(00)90017-x. [DOI] [PubMed] [Google Scholar]
- 3.Patrick J. Dental extraction wound management. Medicating postextraction sockets. J Oral Maxillofac Surg. 2000;58:531–537. doi: 10.1016/s0278-2391(00)90016-8. [DOI] [PubMed] [Google Scholar]
- 4.Jayakumar R., Prabaharan M., Sudheesh P., Nair S., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Triplett R.G., Budinskaya O. New frontiers in biomaterials. Oral Maxillofac Surg Clin. 2017;29:105–115. doi: 10.1016/j.coms.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Kozen B.G., Kircher S.J., Henao J. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15(1):74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 7.Millner R.W., Lockhart A.S., Bird H. A new hemostatic agent: initial life-saving experience with Celox (chitosan) in cardiothoracic surgery. Ann Thorac Surg. 2009;87(2):13–14. doi: 10.1016/j.athoracsur.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Rabea E.I., Badawy M.E., Stevens C.V. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 9.Li P., Poon Y.F., Li W. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater. 2011;10(2):149–156. doi: 10.1038/nmat2915. [DOI] [PubMed] [Google Scholar]
- 10.Tang H., Zhang P., Kieft T.L. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010;6(7):2562–2571. doi: 10.1016/j.actbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno H., Mori T., Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52(2):105–115. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 12.Dai T., Tanaka M., Huang Y., Hamblin M. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9(7):857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malmquist J., Clemens S., Oien H., Wilson S. Hemostasis of oral surgery wounds with the hemCon dental dressing. J Oral Maxillofac Surg. 2008;66:1177–1183. doi: 10.1016/j.joms.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 14.No H.K., Park N.Y., Lee S.H. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74(1–2):65–72. doi: 10.1016/s0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Shan Z. Initial study on facilitating wound healing after tooth extraction by using microbial fiber membrane flagyl. J Oral Maxillofac Surg. 2011;69:994–1002. doi: 10.1016/j.joms.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Pippi R., Santoro M., Cafolla A. The effectiveness of a new method using an extra-alveolar hemostatic agent after dental extractions in older patients on oral anticoagulation treatment: an intrapatient study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:15–21. doi: 10.1016/j.oooo.2015.02.482. [DOI] [PubMed] [Google Scholar]
- 17.Pippi R., Santoro M., Cafolla A. The use of a chitosan-derived haemostatic agent for post-extraction bleeding control in patients on anti-platelet treatment. J Oral Maxillofac Surg. 2017;75(6):1118–1123. doi: 10.1016/j.joms.2017.01.005. [DOI] [PubMed] [Google Scholar]