Abstract
Since 2012, tick-borne encephalitis (TBE) is a notifiable in the European Union. The European Centre for Disease Prevention and Control annually collects data from 28 countries plus Iceland and Norway, based on the EU case definition. Between 2012 and 2016, 23 countries reported 12,500 TBE cases (Ireland and Spain reported none), of which 11,623 (93.0%) were confirmed cases and 878 (7.0%) probable cases. Two countries (Czech Republic and Lithuania) accounted for 38.6% of all reported cases, although their combined population represented only 2.7% of the population under surveillance. The annual notification rate fluctuated between 0.41 cases per 100,000 population in 2015 and 0.65 in 2013 with no significant trend over the period. Lithuania, Latvia and Estonia had the highest notification rates with 15.6, 9.5 and 8.7 cases per 100,000 population, respectively. At the subnational level, six regions had mean annual notification rates above 15 cases per 100,000 population, of which five were in the Baltic countries. Approximately 95% of cases were hospitalised and the overall case fatality ratio was 0.5%. Of the 11,663 cases reported with information on importation status, 156 (1.3%) were reported as imported. Less than 2% of cases had received two or more doses of TBE vaccine.
Keywords: Europe, epidemiology, tick-borne encephalitis (TBE), surveillance, tick-borne diseases, vector-borne infections, viral encephalitis
Background
Tick-borne encephalitis (TBE) is an infectious disease of the central nervous system caused by a flavivirus and usually transmitted by the bite of infected Ixodes spp. These ticks can be found from western Europe to Japan [1]. Less frequently, humans can be infected by drinking contaminated milk. Many vertebrate species can be infected by the TBE virus but ticks are the main reservoir for the virus. There are three subtypes of the TBE virus: the European subtype (TBEV-Eu) is mainly transmitted by I. ricinus while both the Far-eastern (TBEV-FE) and Siberian (TBEV-Sib) subtypes are mainly transmitted by I. persulcatus. Recent findings from Finland suggest that I. ricinus can also transmit TBEV-Sib [2]. In Europe, most cases are infected by TBEV-Eu but cases infected with TBEV-FE were reported in Estonia and Latvia [1] and with TBEV-Sib in Estonia [3] and Finland [4].
The typical course of the disease is biphasic. After a median incubation period of 8 days, the first stage consists of a few days of non-specific symptoms such as fever, fatigue and body pain. After a symptom-free week, approximately one-third of infected persons can develop neurological conditions [5], ranging from mild meningitis to severe encephalitis [1]; increasing age is a known risk factor for severe TBE. Infection with TBEV-FE is associated with more severe disease with case fatality as high as 20–40% compared with 1–2% with TBEV-Eu [6].
There is no curative treatment for TBE but a vaccine is available. This vaccine is highly immunogenic [7] and the impact of mass vaccination in Austria is suggestive of good effectiveness [8]. Vaccine schedules for the two vaccines licensed in Europe based on TBEV-Eu strains require three doses followed by boosters [1]. In a position paper on TBE vaccination published in 2011, the World Health Organization (WHO) recommended that TBE vaccination should be offered to all age groups in highly endemic areas (i.e. areas with TBE incidence above 5 cases per 100,000 population) [9].
In Europe, most cases occur during June-September [10]. Ixodes spp. are found in large parts of Europe but areas at risk for TBE are mainly located in central and eastern Europe and the Baltic and Nordic countries [11]. Between 2000–2010, the annual number of TBE cases reported in the European Union and European Economic Area (EU/EEA) fluctuated between 2,000–3,500 cases [11,12]. Spikes in cases of TBE have occurred in some years, e.g. 2006, but this was likely a result of changes in human behaviour based on suitable weather conditions (e.g. increased outdoor recreational activities) [13]. More recently, some countries, e.g. Belgium and the Netherlands, reported possible new endemic foci having found antibodies to the TBE virus in roe deer and cattle [14,15] and in 2016, the Netherlands reported their first locally-acquired human case [16]. The mapping of endemic foci is essential to make recommendations for vaccination programme and travel advice [17]. In 2011, the first attempt to collect TBE surveillance data at the EU/EEA level underlined the need for an agreed case definition and systematic data collection [11]. Therefore, in 2012, the European Commission included TBE in the list of notifiable diseases in the EU/EEA [17].
Here, we describe TBE cases reported in the EU/EEA between 2012 and 2016.
Methods
Since 2012, the European Centre for Disease Prevention and Control (ECDC) requires all 28 EU Member States, plus Iceland and Norway, to annually report their TBE data to the European Surveillance System (TESSy) database using the EU case definition (Box) [18]. More detailed information on surveillance systems is available elsewhere [10]. We included all cases reported during the years 2012–2016 meeting the EU case definition in the analysis.
Box. European Union case definition for tick-borne encephalitis.
A confirmed case is defined as any person meeting the clinical criteria i.e. symptoms of inflammation of the central nervous system
AND
• Has laboratory-confirmation i.e. at least one of the following five:
• Tick-borne encephalitis (TBE) specific IgM and IgG antibodies in blood.
• TBE specific IgM antibodies in cerebrospinal fluid.
• Sero-conversion or fourfold increase of TBE-specific antibodies in paired serum samples.
• Detection of TBE viral nucleic acid in a clinical specimen.
• Isolation of TBE virus from clinical specimen.
A probable case is defined as any person meeting the clinical criteria and the laboratory criteria for a probable case i.e. detection of TBE-specific IgM-antibodies in a unique serum sample
OR
Any person meeting the clinical criteria with exposure to a common source (unpasteurised dairy products).
TBE Information received included age, sex, date of disease onset, probable place of infection, place of residence, importation status, hospitalisation status, vaccination status, and clinical outcome. Coded values for variables with geographical information (probable place of infection and place of residence) followed the nomenclature of territorial units for statistics (NUTS) of the EU [19].
We used population denominator data provided by the Statistical Office of the EU (Eurostat) for calculating rates (data extracted on 22 September 2017). We compared continuous variables by the Mann–Whitney U test and categorical variables using the chi-squared test. We estimated annual rates of change and their 95% confidence intervals (CI) using a log-linear regression of notification rates over the period 2012–2016. We assessed goodness of fit of linear regressions using F statistics. We used Stata software release 14 (StataCorp. LP, United States) for all data management and statistical analyses.
Results
Case classification and notification rate
Over the 2012–2016 period, 23 countries reported 12,500 TBE cases (Ireland and Spain reported no cases), of which 11,622 (93.0%) were confirmed cases and 878 (7.0%) probable cases (Table 1). We excluded 31 cases with unknown classification (11 cases for Austria, 15 cases for Lithuania, four cases for Poland and one case for Slovenia). Cyprus, Iceland, Malta, and Portugal had no TBE surveillance and Denmark did not report any data.
Table 1. Number of reported casesa of tick-borne encephalitis, percentage of imported cases, notification rate per 100,000 population and trend, in 25 countries, European Union and European Economic Area, 2012–2016 (n = 12,500).
| Country | 2012 | 2013 | 2014 | 2015 | 2016 | Total cases | Trend | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases | Notification rate | Number of cases | Notification rate | Number of cases | Notification rate | Number of cases | Notification rate | Number of cases | Notification rate | Number of cases | Imported cases n (%)b |
Notification rate | Annual variation (%) | 95% CI | |
| Austria | 38 | 0.45 | 100 | 1.18 | 81 | 0.95 | 79 | 0.92 | 95 | 1.09 | 393 | 6 (1.7) | 0.92 | 15.2 | - 19.6 to 49.9 |
| Belgium | 2 | 0.02 | 1 | 0.01 | 0 | 0.00 | 1 | 0.01 | 1 | 0.01 | 5 | 3 (100) | 0.01 | NA | NA |
| Bulgaria | 0 | 0.00 | 2 | 0.03 | 0 | 0.00 | 2 | Unknown | < 0.01 | NA | NA | ||||
| Croatia | 45 | 1.05 | 44 | 1.03 | 23 | 0.54 | 26 | 0.62 | 6 | 0.14 | 144 | Unknown | 0.68 | - 45.1 | - 90.5 to 0.3 |
| Czech Republic | 573 | 5.45 | 625 | 5.94 | 410 | 3.90 | 349 | 3.31 | 565 | 5.35 | 2,522 | 16 (0.6) | 4.79 | - 6.2 | - 33.0 to 20.6 |
| Estonia | 178 | 13.43 | 114 | 8.64 | 83 | 6.31 | 116 | 8.82 | 81 | 6.16 | 572 | 2 (0.3) | 8.68 | - 15.4 | - 39.0 to 8.2 |
| Finland | 39 | 0.72 | 38 | 0.70 | 47 | 0.86 | 68 | 1.24 | 61 | 1.11 | 253 | Unknown | 0.93 | 14.4 | 0.7 to 28.1 |
| France | 1 | < 0.01 | 2 | < 0.01 | 9 | 0.01 | 10 | 0.02 | 22 | 0.03 | 44 | 9 (21.4) | 0.01 | 77.3 | 42.0 to 112.7 |
| Germany | 195 | 0.24 | 419 | 0.52 | 264 | 0.33 | 219 | 0.27 | 348 | 0.42 | 1,445 | 47 (3.5) | 0.36 | 4.6 | - 31.1 to 40.2 |
| Greece | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 | 2 | 0 | < 0.01 | NA | NA |
| Hungary | 44 | 0.44 | 53 | 0.53 | 31 | 0.31 | 24 | 0.24 | 19 | 0.19 | 171 | 2 (1.2) | 0.35 | - 24.5 | - 42.9 to - 6.1 |
| Ireland | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0 | 0 | NA | NA |
| Italy | 0 | 0.00 | 0 | 0.00 | 5 | 0.01 | 48 | 0.08 | 53 | 3 (6.3) | 0.02 | NA | NA | ||
| Latvia | 229 | 11.20 | 230 | 11.36 | 149 | 7.44 | 141 | 7.10 | 204 | 10.36 | 953 | 0 | 9.51 | - 6.3 | - 30.2 to 17.7 |
| Lithuania | 494 | 16.45 | 487 | 16.39 | 353 | 11.99 | 336 | 11.50 | 633 | 21.91 | 2,303 | 18 (0.9) | 15.64 | 2.2 | - 28.3 to 32.7 |
| Luxembourg | NA | NA | NA | NA | 0 | 0.00 | 1 | 0.18 | 0 | 0.00 | 1 | 1 (100) | 0.06 | NA | NA |
| The Netherlands | NA | NA | NA | NA | NA | NA | NA | NA | 4 | 0.02 | 4 | 2 (50.0) | 0.02 | NA | NA |
| Norway | 7 | 0.14 | 6 | 0.12 | 13 | 0.25 | 9 | 0.17 | 12 | 0.23 | 47 | 7 (15.9) | 0.18 | 13.7 | - 13.9 to 41.3 |
| Poland | 187 | 0.49 | 226 | 0.59 | 195 | 0.51 | 149 | 0.39 | 283 | 0.75 | 1,040 | 8 (0.8) | 0.55 | 4.2 | - 22.4 to 30.7 |
| Romania | 3 | 0.01 | 3 | 0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 7 | 0 | < 0.01 | NA | NA |
| Slovakia | 102 | 1.89 | 163 | 3.01 | 116 | 2.14 | 84 | 1.55 | 173 | 3.19 | 638 | 5 (0.8) | 2.36 | 3.8 | - 31.2 to 38.9 |
| Slovenia | 164 | 7.98 | 307 | 14.91 | 100 | 4.85 | 62 | 3.01 | 83 | 4.02 | 716 | 1 (0.2) | 6.95 | - 29.7 | - 78.6 to 19.2 |
| Spain | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0 | 0 | NA | NA |
| Sweden | 287 | 3.03 | 209 | 2.19 | 178 | 1.85 | 268 | 2.75 | 238 | 2.42 | 1,180 | 21 (1.8) | 2.44 | - 2.2 | - 24.3 to 19.9 |
| UK | 3 | < 0.01 | 0 | 0.00 | 2 | < 0.01 | 0 | 0.00 | 0 | 0.00 | 5 | 5 (100) | < 0.01 | NA | NA |
| EU/EEA | 2,591 | 0.64 | 3,027 | 0.65 | 2,056 | 0.43 | 1,950 | 0.41 | 2,876 | 0.58 | 12,500 | 156 (1.3) | 0.54 | - 6.6 | - 29.1 to 16.0 |
CL: confidence interval; EU/EEA: European Union and European Economic Area; NA: not available; UK: United Kingdom.
a Number of cases includes both confirmed and probable cases.
b cases with known importation status.
Most countries (18/23) reported over 90% of cases as confirmed. Slovakia (552/638; 86.5%), France (36/44; 81.8%), Hungary (131/171; 76.6%), Latvia (683/953; 71.7%), and Poland (712/1,040; 68.5%), classified the lowest proportions of their cases as confirmed. The mean annual notification rate was 0.54 cases per 100,000 population.
Importation
Of the 11,664/12,500 cases reported with information on importation status, 156 (1.3%) were reported as imported (Table 1). Importation status was missing for cases reported by Bulgaria, Croatia, and Finland. All cases reported in Belgium, Luxembourg, and the United Kingdom (UK) were imported. Information on the probable country of infection was available for 152 of these imported cases (97.4%). Top destinations for travel-associated TBE were Austria (32 cases, 21.1% of all imported cases), Sweden (19 cases, 12.5%) and Finland (18 cases, 11.8%). Four countries (the Czech Republic, Germany, Lithuania, and Sweden) reported 102/156 (65.3%) of all imported cases. Imported cases were slightly younger than locally-acquired cases (median age for imported cases: 46 years; locally-acquired cases: 48 years; p = 0.03) and more likely to be male (imported cases: 71% males; locally-acquired cases: 59% males; p < 0.01).
Geographical distribution
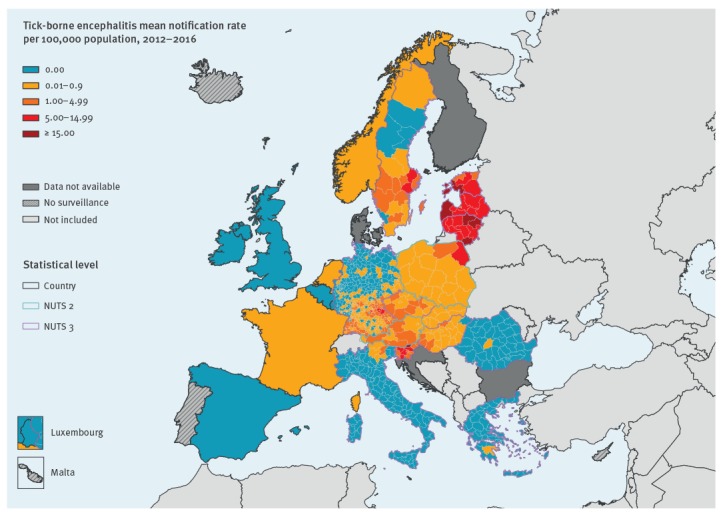
Two countries (Czech Republic and Lithuania) accounted for 4,825/12,500 (38.6%) of all reported cases (Table 1). Of the 23 countries that reported cases, 16 had mean notification rates below one case per 100,000 population. Over the 2012–2016 period Lithuania, Latvia and Estonia had the highest notification rates with 15.6, 9.5 and 8.7 cases per 100,000 population, respectively (Table 1 and Figure 1). Among the 23 countries that reported cases, 17 had locally-acquired cases. Of these, 12 provided geographical information at NUTS3 level, two at NUTS2 (Austria and Poland), and three did not have information at subnational level (France, the Netherlands, and Norway) (Figure 1). At the subnational level, six regions had mean annual notification rates above 15 cases per 100,000 population: Utena county, Lithuania (44.5), Lääne-Eesti, Estonia (27.7), Kurzeme, Latvia (23.8), Alytus county, Lithuania (22.1), Panevėžys county, Lithuania (19.1) and Carinthia, Slovenia (15.1) (Figure 1).Twenty-nine regions in seven countries (Estonia, Germany, Latvia, Lithuania, Poland, Slovenia and Sweden) had notification rates above five cases per 100,000 population. In Lithuania, Telšiai County was the region with the lowest mean annual notification rate (5.6).
Figure 1.
Rate of locally acquired tick-borne encephalitis per 100,000 population, by place of infection, European Union and European Economic Area countries, 2012–2016
ECDC. Map produced on: 19 July 2018
Trend and Seasonality
The overall annual notification rate fluctuated between a minimum of 0.41 cases per 100,000 population in 2015 and a maximum of 0.65 in 2013 with no significant trend over the period (annual variation of - 6.6% (95% CI: - 29.1 to 16; p = 0.4) (Table 1). We observed significant trends for three countries: the TBE notification rate increased at an annual rate of 14.4% (95%CI: 0.7 to 28.1) in Finland and 77.3% (95%CI: 42.0 to 112.7) in France and decreased at an annual rate of 24.5% (95%CI: 6.1 to 42.9) in Hungary.
Of the 11,397 cases reported with onset date, 10,632 (93.3%) had an onset month May–October and 135 (1.2%) had an onset month December–March (off-season) (Figure 2). We observed a comparable seasonality in the 12 countries reporting at least 100 cases over the period with onset month (Figure 3). There were peaks in 2012 (Estonia and Sweden), 2013 (Germany, Hungary, Slovakia, Czech Republic and Slovenia), 2014 (Austria), 2015 (Austria, Estonia, Finland, Sweden), and 2016 (Czech Republic, Germany, Lithuania, Poland and Slovakia).
Figure 2.
Number of reported tick-borne encephalitis cases by month of onset, and 12-month moving average, 19 European Union and European Economic Area countries, 2012–2016
Includes data from: Austria, Czech Republic, Estonia, France, Germany, Greece, Hungary, Italy, Latvia, Latvia, Lithuania, Luxembourg, Netherlands, Norway, Poland, Romania, Slovakia, Slovenia and Sweden.
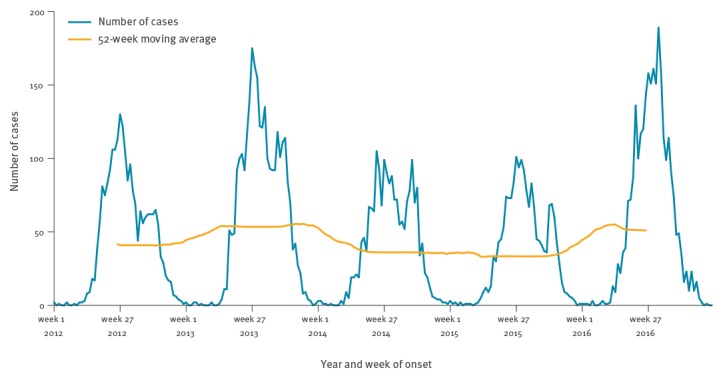
Figure 3.
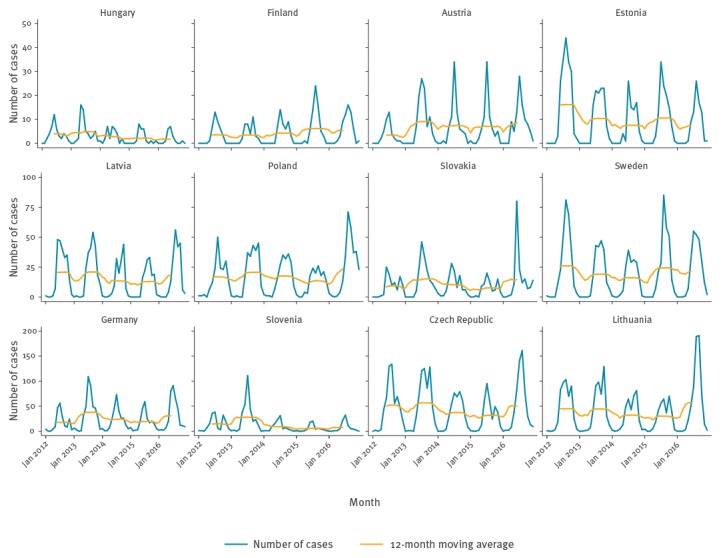
Number of reported tick-borne encephalitis cases by week of onset and 52-week moving average, 12 European Union and European Economic Area countries, 2012–2016
Demographics
Of the 12,470 cases reported with information on age, 6,782 (54.4%) were in the 40–69 years old group (Table 2). TBE was more common in males with a male-to-female rate ratio of 1.5:1. Notification rates increased with age in both sexes, peaking at 0.89 cases per 100,000 population in males aged 60–69 years, and then decreased in older age groups (Figure 4). At date of disease onset, females (median 51 years, interquartile ratio (IQR): 35–62) were older than males (median 47 years, IQR: 31–61) (p < 0.01).
Table 2. Main characteristics of reported cases of tick-borne encephalitis, European Union and European Economic Area countries, 2012−2016 (n = 12,500).
| Characteristics | Number of cases | Percent | Notification rate per 100,000 persons |
|---|---|---|---|
| Total | 12,500 | 100 | 0.54 |
| Age group (years) | |||
| < 20 | 1,402 | 11.2 | 0.29 |
| 20–29 | 1,257 | 10.1 | 0.44 |
| 30–39 | 1,575 | 12.6 | 0.50 |
| 40–49 | 2,200 | 17.6 | 0.65 |
| 50–59 | 2,474 | 19.8 | 0.77 |
| 60–69 | 2,108 | 16.9 | 0.80 |
| 70–79 | 1,183 | 9.5 | 0.63 |
| ≥ 80 | 271 | 2.2 | 0.23 |
| Unknown | 30 | NA | NA |
| Sex | |||
| Female | 5,118 | 40.9 | 0.43 |
| Male | 7,381 | 59.1 | 0.65 |
| Unknown | 1 | NA | NA |
| Importation status | |||
| Imported | 156 | 1.3 | NA |
| Locally-acquired | 11,507 | 98.7 | NA |
| Unknown | 837 | NA | NA |
| Hospitalisation | |||
| Yes | 7,672 | 94.9 | NA |
| No | 409 | 5.1 | NA |
| Unknown | 4,419 | NA | NA |
| Outcome | |||
| Alive | 9,594 | 97.0 | NA |
| Dead | 48 | 0.5 | NA |
| Neurological complications | 247 | 2.5 | NA |
| Unknown | 2,611 | NA | NA |
| Vaccination status | |||
| Four doses | 24 | 0.5 | NA |
| Three doses | 36 | 0.7 | NA |
| Two doses | 27 | 0.5 | |
| One dose | 33 | 0.6 | NA |
| Vaccinated unknown doses | 19 | 0.4 | NA |
| Not vaccinated | 5,066 | 97.3 | NA |
| Unknown | 7,295 | NA | NA |
NA: not available.
Figure 4.
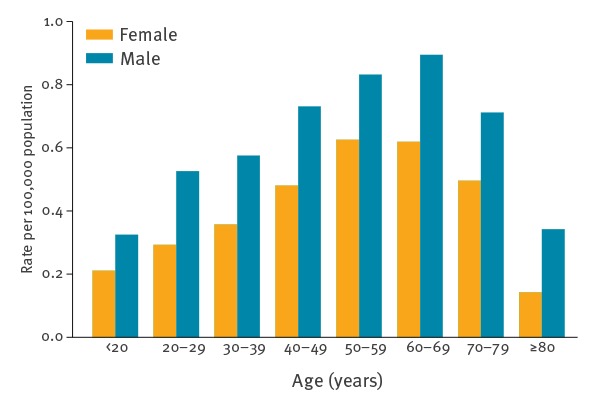
Notification rates of tick-borne encephalitis per 100,000 population, by sex and age group and male-to-female rate ratio by age group, European Union and European Economic Area countries, 2012–2016
Outcome
Of the 8,081 cases reported with hospitalisation status, 7,672 (94.9%) were admitted to hospital (Table 2). Of the 9,889 cases reported with known outcome, 48 (0.5%) died and 247 (2.5%) had neurological sequelae. The case fatality ratio did not differ significantly by sex (0.5% in males vs 0.4% in females, p = 0.30). The case fatality ratio was higher in older age groups (3.1% in cases aged 80 years or older, 2.0% in cases aged 70–79 years and < 0.5% in cases aged below 70 years).
Vaccination
Of the 5,205 cases with known vaccination status, 5,066 (97.3%) were not vaccinated, 60 had received one or two doses (1.2%), 60 (1.2%) three doses or more and 19 (0.4%) an unknown number of doses (Table 2). Of the 20 cases with fatal outcome and known vaccination status, 19 were not vaccinated and one had received one dose of the vaccine. The proportion of cases that received two doses or more of vaccine was higher in the extreme age groups compared with the other groups (2.5% in both cases aged 20 years or younger and 70 years or older). No imported cases had received more than one vaccine dose.
Discussion
The European TBE surveillance data suggest a stable trend over the years 2012–2016 with no reported changes in national surveillance systems; continuing the long-term trend observed in Europe since the mid-1990s [12]. The number of TBE cases reported in Europe, excluding Russia, increased over the years 1990–1994, probably reflecting the start of surveillance in many countries [12]. Over the following 15 years (1995–2009), the trend was stable with an annual number of TBE cases fluctuating between 2,000 and 4,000 cases. Peaks occurred when a set of countries reported unusually high numbers of TBE cases, e.g. 2006 and 2009 [12]. In 2013, several European countries experienced a peak in TBE cases, which resulted in the highest number of TBE cases (> 3,000) observed in Europe that year. An analysis carried out in eight European countries suggested that human behaviour in response to good weather conditions, e.g. increased outdoor recreational activities, was the main explanation for the 2006 spike rather than tick abundance [13].
The overall stable trend observed in TBE surveillance data is mainly driven by a few countries reporting the majority of cases, potentially masking important disparities both between and within countries. For example, two countries (Czech Republic and Lithuania) accounted for 38.6% of all reported TBE cases, although their combined population represented only 2.7% of the population of the 25 countries included in this analysis. All countries with average annual notification rate above one TBE case per 100,000 population had a stable trend over the period. We only observed an increase in Finland and France. In France, the notification rate almost tripled in 2016 compared with previous years in the Alsace region where most cases occurred [20]; some newly identified foci such as the Alpine region could also have contributed to the upsurge in cases. However, the reasons behind this increase are yet to be determined. In Finland, the emergence of new foci reported during 1995–2013 could partly explain the increase [21]. A decrease in TBE cases was observed in Hungary over the years 2012–2016, to our knowledge there is no explanation as to why. Trends at country level, such as these, may mask changes at local level as TBE endemicity is very focal and countries do not have a uniform risk across all territories/regions/counties etc. In Lithuania, which had the highest average annual notification rate, there was an eightfold difference between counties with highest and counties lowest TBE incidence. An analysis of epidemiological patterns of TBE in Lithuania suggested different trends across counties with more pronounced increases in eastern and northern parts of the country [22]. Similarly, diverging trends across regions were reported in Austria [23]. Decreasing trend were observed in north-east of Lower Austria whilst the alpine regions in the west of Austria became highly endemic.
Independently of what happens in animal reservoirs, we can classify factors driving TBE incidence in three groups: (i) tick abundance, (ii) population at risk, (iii) surveillance characteristics. Factors related to tick abundance are multiple (e.g. land, weather, reservoirs etc.) and can be very focal. The impact of climate change is debated with possibly different effects in different settings. A study carried out in Sweden suggested that milder winters were associated with increased TBE incidence in the mid-1980s [24]. Yet, a general circulation model predicted that TBE transmission could be disrupted by climate change with a contraction of TBE areas to higher altitudes in central Europe and northern latitudes in Scandinavia [25]. This would result in a decreased incidence in the coming decades but such change would probably not be captured over a 5–year period. Changes in human behaviour (e.g. increase of at-risk outdoor recreational activities) can put people at greater risk of exposure to ticks and thus TBE. However, with increased vaccine coverage such risk could be improved. Finally, better clinical awareness, testing and reporting would improve the ability of the surveillance system to detect cases.
The geographical granularity of our data (at best NUTS3) does not allow fine monitoring of TBE foci, which countries are best placed to perform. However, during the first effort to collect TBE data at the EU/EEA level most of the recommendations were followed [11]. We implemented standard EU case definition for TBE and initiated routine collection of surveillance data from EU/EEA countries, to at least NUTS-3 geographical level for most of the countries. ECDC encourages all countries to report their cases at subnational level.
The reported TBE cases followed a pronounced seasonality with most cases occurring during the warmer months May–October, which is likely due to human habits with people spending a greater amount of time outdoors in areas e.g. forests where ticks populations are high [1]. Cases infected during colder months are possible, however, especially in central Europe.
Cases of TBE are more common in older age groups, with the highest number of cases occurring in those aged 40-69 years. The highest notification rate, in those aged 60-69 years, most likely reflects high exposure to tick populations at an age where individuals have increased time for outdoor recreational activity, but also fall into the known higher severity seen in older age groups [26].
Almost 95% (7,672/8,081) of reported TBE cases were admitted to hospital, which is not unexpected given that the clinical criteria used in the case definition selects severe cases. Even though the overall case fatality was relatively low, it was far from negligible in older age groups at ca 2–3% above 70 years of age. Previous reviews suggested that a third of patients could suffer long-lasting sequelae [1]. Our analysis found a much lower proportion but it is likely that our data could not capture long-term sequelae that would be diagnosed later in time from acute infection and thus not reported.
Vaccination remains the most effective protective measure against TBE [27]. However, studies have reported vaccine failures, especially in older age groups [28]. We found that 87/5,205 (1.7%) of cases were supposedly vaccinated (at least two doses of vaccine), mostly in extreme age groups. This would be in line with results from studies suggesting that age and number of vaccine doses were the most important factors determining the immunological response to vaccination [29]. The extended period between doses may mean that people are less likely to comply to the recommendations as shown in Germany where compliance after the first dose was low [30]. Another reason for not receiving or completing TBE vaccination is cost. TBE vaccination is not reimbursed in most EU/EEA countries and the willingness to pay for vaccination may not be sufficient to ensure uptake in residents or visitors frequenting areas considered high risk for tick populations and TBE [31]. A survey published in 2008, reported that Austria, Finland, Germany, Hungary, Latvia and Slovenia included TBE in their routine vaccination programme at least for some specific groups or areas [32].
In this study, we only found a few TBE cases in international travellers. Cases that are resident in countries with little or no risk of TBE are less likely to be vaccinated or diagnosed [33]. Increased awareness of TBE is required to improve vaccination coverage in travellers and promote the best practices to avoid tick bites. Currently, the WHO recommends vaccination of travellers who are at risk of TBE exposure during outdoors activities in rural endemic areas during the period of transmission [34].
Conclusion
The overall TBE notification rate remained stable during 2012–2016. Surveillance at EU/EEA level helped provide reliable and comparative data allowing better mapping of the disease risk both at the national and subnational level. Countries with regions where the disease is highly endemic should consider strengthening information campaigns on preventive measures against tick bites as well as introducing TBE vaccine recommendations if these are not already proposed. ECDC encourages countries to report better quality and more complete data on TBE diagnoses, particularly on the sub-national geographic distribution and on imported cases.
Acknowledgements
We would like to thank all people involved in the surveillance of TBE in the participating countries as well as the data managers at ECDC, without whom surveillance of TBE at EU/EEA level would not be possible. In addition, we would like to thank the following persons for their contribution to TBE surveillance in Europe: Heidemarie Holzmann and Karin Stiasny (Austria); Tinne Lernout, Vanessa Suin and Marjan Van Esbroeck, (Belgium); Sanja Kurecic Filipovic, Iva Pem Novosel and Goranka Petrovic (Croatia); Bohumír Kříž Marek Malý and Helena Šebestová (Czech Republic); Markku Kuusi, Jussi Sane and Pirjo Turtiainen (Finland); Isabelle Leparc-Goffart (France); Doris Altmann and Wiebke Hellenbrand (Germany); Theano Georgakopoulou, Kassiani Gkolfinopoulou, Anna Papa, and Danai Pervanidou (Greece); Jeff Connell and Sarah Jackson (Ireland); Giovanni Rezza, Caterina Rizzo and Giulietta Venturi (Italy); Antra Bormane, Tatjana Klemjacionoka and Irina Lucenko (Latvia); Saulius Čaplinskas, Aidas Spiečius and Milda Žygutienė (Lithuania); Eelco Franz and Agnetha Hofhuis (The Netherlands); Solveig Jore and Heidi Lange (Norway); Cornelia Ceianu, Romana Rebreanu and Anca Sirbu (Romania); Edita Staroňová and Elena Tichá (Slovakia); Marta Grgic-Vitek, and Maja Socan (Slovenia). We are also grateful to Silviu Lucian Ionescu for his support in GIS.
Conflict of interest: None declared.
Authors’ contributions: EWP and HZ initiated the work. GS and JB ran the analysis. All authors contributed to the interpretation of the findings. JB wrote the first draft of the manuscript. All authors revised the manuscript, providing substantial intellectual input.
References
- 1. Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371(9627):1861-71. 10.1016/S0140-6736(08)60800-4 [DOI] [PubMed] [Google Scholar]
- 2. Jääskeläinen A, Tonteri E, Pieninkeroinen I, Sironen T, Voutilainen L, Kuusi M, et al. Siberian subtype tick-borne encephalitis virus in Ixodes ricinus in a newly emerged focus, Finland. Ticks Tick Borne Dis. 2016;7(1):216-23. 10.1016/j.ttbdis.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 3. Golovljova I, Vene S, Sjölander KB, Vasilenko V, Plyusnin A, Lundkvist A. Characterization of tick-borne encephalitis virus from Estonia. J Med Virol. 2004;74(4):580-8. 10.1002/jmv.20224 [DOI] [PubMed] [Google Scholar]
- 4. Jääskeläinen AE, Sironen T, Murueva GB, Subbotina N, Alekseev AN, Castrén J, et al. Tick-borne encephalitis virus in ticks in Finland, Russian Karelia and Buryatia. J Gen Virol. 2010;91(11):2706-12. 10.1099/vir.0.023663-0 [DOI] [PubMed] [Google Scholar]
- 5. Taba P, Schmutzhard E, Forsberg P, Lutsar I, Ljøstad U, Mygland Å, et al. EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur J Neurol. 2017;24(10):1214-e61. 10.1111/ene.13356 [DOI] [PubMed] [Google Scholar]
- 6. Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. Tick-borne encephalitis virus - a review of an emerging zoonosis. J Gen Virol. 2009;90(8):1781-94. 10.1099/vir.0.011437-0 [DOI] [PubMed] [Google Scholar]
- 7. Demicheli V, Debalini MG, Rivetti A. Vaccines for preventing tick-borne encephalitis. Cochrane Database Syst Rev. 2009; (1):CD000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinz FX, Stiasny K, Holzmann H, Grgic-Vitek M, Kriz B, Essl A, et al. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis. 2013;19(1):69-76. 10.3201/eid1901.120458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86(24):241-56. [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control. Tick-borne encephalitis. In: ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018. Available from: https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2016-TBE.pdf
- 11.European Centre for Disease Prevention and Control (ECDC). Epidemiological situation of tick-borne encephalitis in the European Union and European Free Trade Association countries. Stockholm: ECDC; 2012.Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/TBE-in-EU-EFTA.pdf
- 12. Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis. 2011;2(1):2-15. 10.1016/j.ttbdis.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 13. Randolph SE, Asokliene L, Avsic-Zupanc T, Bormane A, Burri C, Gern L, et al. Variable spikes in tick-borne encephalitis incidence in 2006 independent of variable tick abundance but related to weather. Parasit Vectors. 2008;1(1):44. 10.1186/1756-3305-1-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jahfari S, de Vries A, Rijks JM, Van Gucht S, Vennema H, Sprong H, et al. Tick-Borne Encephalitis Virus in Ticks and Roe Deer, the Netherlands. Emerg Infect Dis. 2017;23(6):1028-30. 10.3201/eid2306.161247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roelandt S, Suin V, Riocreux F, Lamoral S, Van der Heyden S, Van der Stede Y, et al. Autochthonous tick-borne encephalitis virus-seropositive cattle in Belgium: a risk-based targeted serological survey. Vector Borne Zoonotic Dis. 2014;14(9):640-7. 10.1089/vbz.2014.1576 [DOI] [PubMed] [Google Scholar]
- 16. de Graaf JA, Reimerink JH, Voorn GP, Bij de Vaate EA, de Vries A, Rockx B, et al. First human case of tick-borne encephalitis virus infection acquired in the Netherlands, July 2016. Euro Surveill. 2016;21(33):30318. 10.2807/1560-7917.ES.2016.21.33.30318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amato-Gauci A, Zeller H. Tick-borne encephalitis joins the diseases under surveillance in the European Union. Euro Surveill. 2012;17(42):20299. [PubMed] [Google Scholar]
- 18.European Commission. Commission Regulation (EC). Commission Implementing Decision 2012/506/EU of 8 August 2012 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Luxembourg: Publication Office of the European Union. Available from: https://publications.europa.eu/en/publication-detail/-/publication/10ed460f-0711-11e2-8e28-01aa75ed71a1/language-en
- 19.European Commission. Commission regulation (EC). No 1059/2003 establishing the common classification of territorial units for statistics (NUTS). Luxembourg: Publication Office of the European Union. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1535630753511&uri=CELEX:32003R1059
- 20. Velay A, Solis M, Kack-Kack W, Gantner P, Maquart M, Martinot M, et al. A new hot spot for tick-borne encephalitis (TBE): A marked increase of TBE cases in France in 2016. Ticks Tick Borne Dis. 2018;9(1):120-5. 10.1016/j.ttbdis.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 21. Tonteri E, Kurkela S, Timonen S, Manni T, Vuorinen T, Kuusi M, et al. Surveillance of endemic foci of tick-borne encephalitis in Finland 1995-2013: evidence of emergence of new foci. Euro Surveill. 2015;20(37):30020. 10.2807/1560-7917.ES.2015.20.37.30020 [DOI] [PubMed] [Google Scholar]
- 22. Radzišauskienė D, Žagminas K, Ašoklienė L, Jasionis A, Mameniškienė R, Ambrozaitis A, et al. Epidemiological patterns of tick-borne encephalitis in Lithuania and clinical features in adults in the light of the high incidence in recent years: a retrospective study. Eur J Neurol. 2018;25(2):268-74. 10.1111/ene.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heinz FX, Stiasny K, Holzmann H, Kundi M, Sixl W, Wenk M, et al. Emergence of tick-borne encephalitis in new endemic areas in Austria: 42 years of surveillance. Euro Surveill. 2015;20(13):9-16. 10.2807/1560-7917.ES2015.20.13.21077 [DOI] [PubMed] [Google Scholar]
- 24. Lindgren E, Gustafson R. Tick-borne encephalitis in Sweden and climate change. Lancet. 2001;358(9275):16-8. 10.1016/S0140-6736(00)05250-8 [DOI] [PubMed] [Google Scholar]
- 25. Randolph SE, Rogers DJ. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proc Biol Sci. 2000;267(1454):1741-4. 10.1098/rspb.2000.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindquist L. Tick-borne encephalitis. Handb Clin Neurol. 2014;123:531-59. 10.1016/B978-0-444-53488-0.00025-0 [DOI] [PubMed] [Google Scholar]
- 27. Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25(43):7559-67. 10.1016/j.vaccine.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 28. Andersson CR, Vene S, Insulander M, Lindquist L, Lundkvist A, Günther G. Vaccine failures after active immunisation against tick-borne encephalitis. Vaccine. 2010;28(16):2827-31. 10.1016/j.vaccine.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 29. Lindblom P, Wilhelmsson P, Fryland L, Matussek A, Haglund M, Sjöwall J, et al. Factors determining immunological response to vaccination against tick-borne encephalitis virus in older individuals. PLoS One. 2014;9(6):e100860. 10.1371/journal.pone.0100860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacob L, Kostev K. Compliance with vaccination against tick-borne encephalitis virus in Germany. Clin Microbiol Infect. 2017;23(7):460-3. 10.1016/j.cmi.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 31. Slunge D. The Willingness to Pay for Vaccination against Tick-Borne Encephalitis and Implications for Public Health Policy: Evidence from Sweden. PLoS One. 2015;10(12):e0143875. 10.1371/journal.pone.0143875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donoso Mantke O, Schädler R, Niedrig M. A survey on cases of tick-borne encephalitis in European countries. Euro Surveill. 2008;13(17):18848. [PubMed] [Google Scholar]
- 33. Haditsch M, Kunze U. Tick-borne encephalitis: a disease neglected by travel medicine. Travel Med Infect Dis. 2013;11(5):295-300. 10.1016/j.tmaid.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 34. Steffen R. Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. J Travel Med. 2016;23(4):taw018. [DOI] [PubMed] [Google Scholar]


