Abstract
Background
The mcr-1 gene is a transferable resistance determinant against colistin, a last-resort antimicrobial for infections caused by multi-resistant Gram-negatives.
Aim
To study carriage of antibiotic-resistant bacteria in healthy school children as part of a helminth control and antimicrobial resistance survey in the Bolivian Chaco region.
Methods
From September to October 2016 we collected faecal samples from healthy children in eight rural villages. Samples were screened for mcr-1- and mcr-2 genes. Antimicrobial susceptibility testing was performed, and a subset of 18 isolates representative of individuals from different villages was analysed by whole genome sequencing (WGS).
Results
We included 337 children (mean age: 9.2 years, range: 7–11; 53% females). The proportion of mcr-1 carriers was high (38.3%) and present in all villages; only four children had previous antibiotic exposure. One or more mcr-1-positive isolates were recovered from 129 positive samples, yielding a total of 173 isolates (171 Escherichia coli, 1 Citrobacter europaeus, 1 Enterobacter hormaechei). No mcr-2 was detected. Co-resistance to other antimicrobials varied in mcr-positive E. coli. All 171 isolates were susceptible to carbapenems and tigecycline; 41 (24.0%) were extended-spectrum β-lactamase producers and most of them (37/41) carried bla CTX-M-type genes. WGS revealed heterogeneity of clonal lineages and mcr-genetic supports.
Conclusion
This high prevalence of mcr-1-like carriage, in absence of professional exposure, is unexpected. Its extent at the national level should be investigated with priority. Possible causes should be studied; they may include unrestricted use of colistin in veterinary medicine and animal breeding, and importation of mcr-1-positive bacteria via food and animals.
Keywords: polymyxin, South America, Bolivia, mcr-1, mcr-2, mcr-1.5, rural communities, antimicrobial resistance, AMR, susceptibility testing, whole genome sequencing, WGS
Background
The mcr-1 gene is a transferable colistin resistance determinant that was first described among enterobacterial strains isolated from animals and humans in China. The gene encodes a phosphoethanolamine transferase that modifies the colistin target by addition of phosphoethanolamine to the 1’ or 4’ phosphate group of lipid A, which reduces its affinity to colistin [1,2]. Discovery of mcr-1 was considered highly alarming, given the role that colistin has recently regained as a last-resort antibiotic for treatment of infections caused by multi-resistant Gram-negative pathogens such as carbapenem-resistant Enterobacterales and Acinetobacter baumannii [1,3].
Subsequent to its discovery, several studies have revealed a global distribution of mcr-1, with an overall higher prevalence among Escherichia coli and Salmonella enterica, and occasional occurrence in other enterobacterial species. Most mcr-1-positive strains were of animal origin, and farm animals were identified as the principal reservoir of mcr-1 genes [1,4]. Investigation of archival strains dated the presence of mcr-1 back to at least the 1980s [5]. As with other resistance genes, minor allelic variants of mcr-1 have been detected [6]. More recently, additional transferable mcr genes (mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7 and mcr-8) have been reported, for which the global epidemiology remains to be clarified [7-13].
In South America, mcr-1 genes have been reported from several countries in isolates from humans, animals and food [14-27]. Recently, the Pan American Health Organisation (PAHO) section of the World Health Organization (WHO) recommended to implement and strengthen surveillance and epidemiological investigation of plasmid-mediated transferable colistin resistance in its Member States [14]. In Bolivia, mcr-1 has thus far been reported in a Citrobacter braakii that was isolated from a ready-to-eat food sample [21], as well as in a few clinical isolates of E. coli referred from various departments to the National Institute of Health Laboratories (INLASA) (data not shown).
During the last two decades we carried out several surveillance studies in the Bolivian Chaco region, documenting a high prevalence of resistance to old and more recent antibiotics in commensal and pathogenic bacteria from humans [21,28-32].
In 2016, a new surveillance study was carried out in a population of healthy school children from several rural communities in this region to investigate the prevalence of intestinal parasites and the carriage of antibiotic-resistant bacteria. Here we report about an unexpected and high rate of faecal carriage of mcr-1-positive Enterobacterales in this population.
Methods
Study population and setting
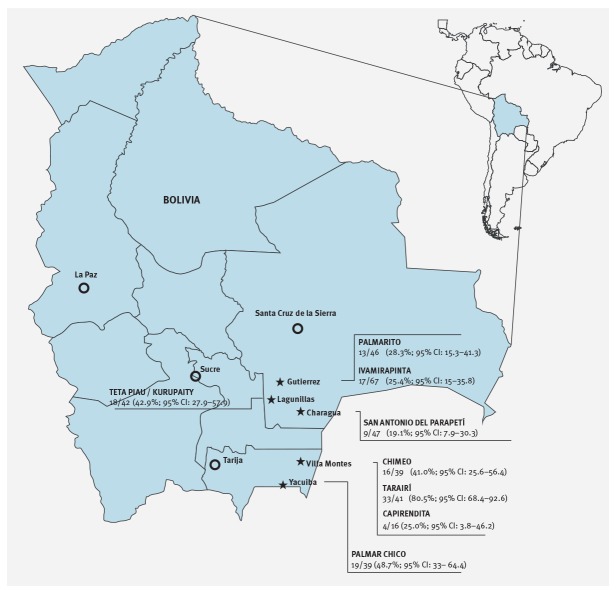
The study population consisted of healthy school children living in eight rural communities of the Chaco region, in south-eastern Bolivia (between longitude 63°66 and 63°18 east and latitude 19°49 and 21°88 south, Figure 1). In these communities, the population lives in houses mostly constructed of mud and sticks, with packed earth floors and straw or corrugated metal roofs. There is no wired electricity and no sewage system. The main water sources are small ponds, in which animals also bathe and drink, and outdoor taps. The economy is mostly based on subsistence farming and local animal husbandry.
In each community, children were selected among those attending primary school, starting from the third year and possibly including the upper years, to achieve a number of ca 50 individuals per site whenever possible. This sample size corresponded to that recommended by WHO for cluster sampling in helminth control programmes in healthy school children [33].
Previous use of antibiotics during the last 15 days was investigated by a questionnaire administered to parents.
Laboratory analyses
Screening for mcr-1- and mcr-2-positive strains in faecal samples
One faecal sample for each child was collected during a two-month period from September to October 2016; the samples were transferred to the Laboratories of Camiri or Villa Montes Hospitals within 6 hours and were plated onto MacConkey agar. After incubation at 35 °C for 24 hours, the bacterial growth (representative of the total enterobacterial microbiota) was collected with a sterile swab in an Amies transport medium and was shipped to Italy. Each sample was then subcultured on MacConkey agar again, and the bacterial growth was resuspended in Brain Heart Infusion broth plus 20% (v/v) glycerol and stored at -70 °C pending further analyses.
To screen for the presence of mcr-1- and mcr-2-positive strains, the preserved suspensions of total enterobacterial microbiota were thawed and 10 μl were inoculated onto McConkey supplemented with colistin (2 mg/L, MCC medium). After incubation at 35 °C for 24 hours, a loopful of the bacterial growth (taken either from confluent growth or from isolated colonies of different morphologies) was resuspended in 300μl of normal saline, and half of the bacterial suspension was used to prepare a crude DNA extract by heating at 99 °C for 15 minutes. The crude extracts were then screened for the presence of mcr-1 and mcr-2 genes by real-time (RT) PCR, as described previously [34]. In the case of a positive result, the remaining bacterial suspension was used to inoculate the MCC medium to obtain isolated colonies, and all isolated colonies of different morphology were then tested for the presence of mcr genes by RT-PCR. The mcr-positive isolates were identified using MALDI-TOF mass spectrometry (Vitek MS, bioMérieux, Marcy-l’Etoile, France).
When a sample yielded two or more mcr-1-positive isolates of the same species, clonal relatedness of the isolates was investigated by random amplification of polymorphic DNA (RAPD) profiling, as described previously [35]. The three mcr-positive isolates that were colistin susceptible were subjected to mcr gene amplification and sequencing using previously described primers and conditions [34].
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was carried out using reference broth microdilution [36]. Minimum inhibitory concentration (MIC) results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints [36].
Analysis of extended-spectrum β-lactamases
All isolates showing a ceftazidime and/or cefotaxime MIC > 1 mg/L were screened for extended-spectrum β-lactamases (ESBL) production by a combination disk test using ceftazidime and cefotaxime as substrates and clavulanic acid as an inhibitor [37]. ESBL-positive isolates by phenotypic testing were subjected to RT-PCR for the detection of bla CTX-M ESBL genes, as described previously [38].
Whole genome sequencing
A subset of 18 mcr-1-positive isolates were subjected to whole genome sequencing (WGS) analysis. This subset comprised two E. coli isolates per community: from randomly selected individuals co-colonised by two different mcr-1-positive E. coli or from two randomly selected individuals if co-colonisations were not detected and the two non-E. coli isolates bore mcr-1. For the latter, species identification was carried out by the analysis of housekeeping genes [39,40]. Bacterial genomic DNA of these 18 selected mcr-positive isolates, extracted using the phenol-chloroform method [41], was subjected to WGS with a MiSeq platform (Illumina, Inc., San Diego, California, United States (US)) using a 2x300 paired-end approach. Raw reads were assembled using SPAdes 3.5 [42]. An average of 120 contigs per strain was obtained, with an average N50 of 163 Kb. Draft genomes have been deposited in the National Center for Biotechnology Information (NCBI) WGS database under the BioProject PRJNA427943 (accession numbers: PQTO00000000; PQTN00000000; PQTM00000000; PQTL00000000; PQTK00000000; PQTJ00000000; PQTI00000000; PQTH00000000; PQTG00000000; PQTF00000000; PQTE00000000; PQTD00000000; PQTC00000000; PQTB00000000; PQTA00000000; PQSZ00000000; PQSY00000000; PQSX00000000). Resistance genes and plasmid content were investigated using the ResFinder and PlasmidFinder tools available at the Center for Genomic Epidemiology at https://cge.cbs.dtu.dk/services/ResFinder/. Clonal relatedness was investigated by in silico determination of the multilocus sequence typing (MLST) profile obtained by the MLST 1.8 software (available at https://cge.cbs.dtu.dk/services/MLST/) using the assembled WGS as input data.
Statistical analysis
Statistical analysis of the data was performed with STATA 11.0 (StataCorp, College Statio, Texas, US). Frequencies and percentages with 95% confidence intervals (CI) for categorical variables, medians and interquartile ranges (IQR) for continuous variables were calculated. Mann–Whitney test was used to compare median age. Chi-squared test was used to investigate the association of mcr-1 carriage with sex and prior antibiotic use. Results were considered significant when the p value was ≤ 0.05.
Ethical statement
Written informed consent was always obtained from parents or legal guardians. The investigation was planned and carried out within a collaboration agreement between the Ministry of Health of the Plurinational State of Bolivia and the University of Florence, Italy, and with the support of the Guaraní political organisation (Asamblea del Pueblo Guaraní). Ethical approval for the study was obtained from the above-mentioned institutions (see Acknowledgements section).
Results
Faecal specimens were obtained from 337 healthy school children in eight rural communities of the Bolivian Chaco region (Figure 1). Children (179 females; 53%;) were aged 7 to 11 years (mean: 9.2 years). Previous antibiotic exposure was only reported for four children.
Figure 1.
Geographical locations of the surveyed communities and the proportion of mcr-1-positive samples, Chaco, Boliva, September–October 2016 (n = 8 communities)
CI: confidence interval.
The surveyed communities are located in the Bolivian Chaco, in rural areas of five municipalities (indicated with stars). For each community, the proportion of mcr-1-positive samples versus the total number of collected samples is reported, along with 95% CI. Major Bolivian cities are indicated by circles.
mcr-1 carriage
All 337 samples of enterobacterial microbiota yielded some growth (from scanty to vigorous) on the MCC medium, and 129 (38.3%) yielded a positive result for mcr-1. Positive samples were detected in children from each village, although at variable rates (range: 19.1–80.5%; Figure 1). No mcr-2 genes were detected. One or more mcr-1-positive isolates were recovered from each of the 129 samples, yielding a total of 173 positive isolates, including 171 E. coli, one Citrobacter spp. and one Enterobacter spp.. Multiple mcr-1-positive isolates from the same sample consisted of either two or three E. coli isolates of different colonial morphology and RAPD profile (in 32 and 5 samples, respectively), or in an E. coli plus an Enterobacter spp. (in one sample).
No differences were found in the demographic characteristics, sex or age, of children carrying mcr-1-positive Enterobacterales or children without mcr-1 carriage (Table 1), nor were there any differences in the living conditions of the communities with different proportions of carriers (data not shown).
Table 1. Features of the study population, stratified by mcr-1-positive and -negative children, Chaco, Bolivia, September–October 2016.
| Characteristics | Total (n/N) |
% |
mcr-1-negative (n/N) |
% |
mcr-1-positive (n/N) |
% | P value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 158/337 | 47 | 100/208 | 48 | 58/129 | 45 | 0.58 |
| Female | 179/337 | 53 | 108/208 | 52 | 71/129 | 55 | |
| Age (years) | |||||||
| Mean (95% CI) | 9.3 (9.1–9.4) | NA | 9.3 (9.1–9.5) | NA | 9.2 (9.0–9.5) | NA | 0.81 |
| Median (IQR) | 9 (8–10) | NA | 9 (8–10) | NA | 9 (8–10) | NA | |
| Prior antibiotic usea | 4/337 | 1 | 3/208 | 1 | 1/129 | 1 | 0.58 |
CI: confidence interval; IQR: interquartile range; NA: not applicable.
aIn the last 15 days.
Antimicrobial susceptibility of mcr-1-positive isolates
Colistin susceptibility testing showed that the majority (n = 170; 98.3%) of the mcr-1-positive isolates were resistant to colistin (MIC range: 4–> 8 mg/L), while only three E. coli (from different villages) were colistin-susceptible (all with an MIC of 2 mg/L) (Table 2). Sequencing of mcr amplicons from the latter isolates showed identity with mcr-1, suggesting that the colistin susceptible phenotype was not due to mutations inactivating the gene. Variable resistance rates to other antimicrobial agents were observed, including fluoroquinolones, expanded-spectrum cephalosporins, β-lactamase plus inhibitor combinations and gentamicin. All isolates were susceptible to carbapenems and tigecycline (Table 2).
Table 2. Susceptibility of mcr 1-positive Escherichia coli isolates to various antimicrobials, Chaco, Bolivia, September–October 2016 (n = 171).
| AMC | PTZ | CAZ | CTX | FEP | MEM | ERT | GEN | CIP | TIG | COL | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| 72 | 42.1 | 171 | 100 | 130 | 76.0 | 128 | 74.9 | 130 | 76.0 | 171 | 100 | 171 | 100 | 138 | 80.7 | 62 | 36.3 | 171 | 100 | 168 | 1.7 | 171 | 100 |
AMC: amoxicillin/clavulanate (clavulanate at fixed concentration of 4 mg/L); CAZ: ceftazidime; CIP: ciprofloxacin; COL: colistin; CTX: cefotaxime; ERT: ertapenem; FEP: cefepime; GEN: gentamicin; MEM: meropenem; PTZ: piperacillin/tazobactam (tazobactam at fixed concentration of 4 mg/L); TIG: tigecycline.
Numbers and percentages of susceptible isolates are given.
All 41 isolates showing a ceftazidime and/or cefotaxime MIC > 1 mg/L were positive for ESBL production by combination disk synergy test, and 37 of them were positive for the presence of a bla CTX-M-type ESBL gene.
Diversity of the mcr-1-positive isolates
WGS analysis of the subset of 18 mcr-1-positive isolates confirmed the identification of the two non-E. coli isolates as Citrobacter europaeus and Enterobacter hormaechei, respectively (Table 3), two species in which mcr-1 was not previously reported.
Table 3. Features of mcr-1-positive isolates subjected to whole genome sequencing analysis, Chaco, Bolivia, September–October 2016 (n = 18).
| Community | Isolate code | Subject code | Species | Additional resistance trait(s)a | Acquired resistance genesb | STc | mcr variant and genetic contextd | mcr contig size (bp) |
|---|---|---|---|---|---|---|---|---|
| Palmarito | 12A | 1 | Escherichia coli | AMC; GEN; CIP | blaTEM-1B; aac (3)-IV; aph (4)-Ia; fosA3; floR; qnrB19; tet(A) | 48 | mcr-1-pap (IncI2) | 61,600 |
| 12B | 1 | E. coli | GEN; CIP | blaTEM-1B; aac (3)-IV; aph(3’)-Ia; aph (4)-Ia; strA; strB; catA1; floR; oqxA; oqxB; sul2; tet(A) | 744 | mcr-1-pap (IncI2) | 60,992 | |
| Ivamirapinta | 155A | 2 | E. coli | FSe | ND | 10 | mcr-1-pap (IncI2) | 60,547 |
| 155B | 2 | E. coli | AMC; CIP | blaTEM-1A; aadA1; aadA2; strA; strB; cmlA1; floR; qnrB19; sul2; sul3; tet(A); dfrA8 | 206 | mcr-1-unkf | 2,943 | |
| Tetapiau/Kurupaity | 86A | 3 | E. coli | AMC; CIP | blaTEM-1B; strA; strB; floR; qnrB19; sul2; tet(A); dfrA1 | 2,705 | mcr-1-unkf | 2,942 |
| 86B | 3 | E. coli | AMC; CIP | blaTEM-1B; aadA1; aadA2; strA; strB; cmlA1; floR; sul1; sul2; sul3; tet(A); tet(B); dfrA1; dfrA12 | 2,936 | mcr-1.5-pap-ISApl1 (IncHI1) | 13,7897 | |
| 67A | 4 | Citrobacter europaeus | AMC | qnrB19; qnrB28 | NA | mcr-1-pap (IncI2) | 60,321 | |
| San Antonio del ParapetÍ | 173A | 5 | E. coli | AMC; CIP; GEN; CAZ; CTX; FEP; (ESBL) | blaCTX-M-55; aadA1; aadA2; aadA5; rmtB; fosA3; cmlA1; floR; qnrB19; sul3; tet(A); dfrA17 | 1,286 | mcr-1-unkf | 6,134 |
| 173B | 5 | E. coli | AMC; CIP; GEN; CAZ; CTX; FEP; (ESBL) | blaCTX-M-55; blaTEM-1B; aadA1; aadA2; rmtB; cmlA1; floR; qnrB19; sul3; tet(A) | 1,286 | mcr-1-unkf | 2,863 | |
| TarairÍ | 224A | 6 | E. coli | AMC | aadA1; aadA2; strA; strB; cmlA1; floR; qnrB19; sul2; sul3; tet(A); tet(B); dfrA14 | 2,705 | mcr-1-pap (IncI2) | 59,561 |
| 224B | 6 | E. coli | AMC; CIP | blaTEM-1B; aadA1; aadA2; strA; strB; cmlA1; floR; QnrB19; sul2; sul3; tet(A); tet(B); dfrA14 | 7,570 | ∆ISApl1-mcr-1-pap-ISApl1 (IncHI1) | 52,737 | |
| Palmar Chico | 306A | 7 | E. coli | AMC | blaTEM-1B; aadA5; strA; strB; sul1; sul2; dfrA17 | 69 | mcr-1-pap (IncI2) | 63,921 |
| 306B | 7 | E. coli | AMC; CIP | blaTEM-1B; blaOXA-1; aadA1; sul1; tet(X) | 10 | mcr-1-pap (IncI2) | 64,425 | |
| 301B | 8 | Enterobacter hormaechei | FSe | ND | - | mcr-1-pap (IncI2) | 63,943 | |
| Capirendita | 286A | 9 | E. coli | AMC | blaTEM-1B; aadA1; floR; sul3; tet(A); tet(C); dfrA1 | 117 | mcr-1-pap (IncI2) | 59,748 |
| 295B | 10 | E. coli | FSe | ND | 711 | mcr-1-pap (IncI2) | 56,317 | |
| Chimeo | 274A | 11 | E. coli | AMC; GEN; CIP | blaTEM-1B; aac (3)-IV; aadA1; aadA2; aph(3’)-Ia; cmlA1; floR; qnrB19; sul2; sul3; tet(A); tet(M); dfrA12 | 7,571 | mcr-1-unkf | 2,943 |
| 274B | 11 | E. coli | AMC; CAZ; CTX; FEP (ESBL) | blaCTX-M-55; blaTEM-1B; blaOXA-10; aac(6')Ib-cr; aacA4; aadA1; strA; strB; fosA3; cmlA1; floR; qnrB19; qnrVC4; sul2; tet(A); dfrA14 | 3,056 | mcr-1-pap (IncI2) | 60,652 |
AMC: amoxicillin/clavulanate (clavulanate at fixed concentration of 4 mg/L); CAZ: ceftazidime; CIP: ciprofloxacin; COL: colistin; CTX: cefotaxime; ESBL: extended-spectrum β-lactamase; FEP: cefepime; FS: fully susceptible; GEN: gentamicin; NA: not applicable; ND: none detected; ST: sequence type; unk: unknown.
aAll isolates were resistant to colistin; additional resistance traits referred to the panel of tested drugs reported in Table 2.
bAcquired resistance genes as determined by analysis with the ResFinder software.
cSequence-types were assigned using the Warwick scheme (http://enterobase.warwick.ac.uk/species/index/ecoli).
dIf the gene was linked with a known plasmid backbone, the plasmid replicon type is reported in brackets.
eThe isolate was susceptible to all tested agents except colistin.
fIn these cases it was not possible to reveal the nature of flanking regions due to the presence of repeated sequences flanking the gene.
For each isolate, the epidemiological data, additional resistance profile, acquired resistance genes content, sequence type and mcr genetic context are reported.
In silico MLST analysis of the 16 E. coli isolates revealed a considerable diversity, with only a few isolates from different villages belonging to the same sequence type (ST). All but one of the couples isolated from the same individual belonged to different STs (Table 3).
Analysis of the acquired resistance genes showed a remarkable diversity and a variety of patterns (Table 3). The number of known acquired resistance genes varied from 0 to 16 (median: 9). Overall, the resistance gene content was consistent with the susceptibility profile. The three ESBL-positive E. coli isolates carried the bla CTX-M-55 variant previously reported in Bolivia [30].
Analysis of the mcr-1 carrying contigs revealed that in 13 isolates the mcr-1 gene was linked to backbone regions typical of IncI2 or IncHI1 plasmids, suggesting a plasmid location, with some plasmid diversity. In the remaining five isolates, it was not possible to determine the nature of flanking regions due to the presence of repeated sequences flanking the gene (Table 3).
Discussion
Our study revealed a very high prevalence of carriage of mcr-1-positive strains among healthy children living in rural communities of the Bolivian Chaco. Carriage of mcr-1-positive strains in healthy humans has been investigated in a limited number of studies, mostly from Asian countries [41-53]. The prevalence rates detected in such studies have usually been low (< 5%), except in a group of chicken farmers from Vietnam, where a 34.7% carriage rate of mcr-1-positive E. coli was detected and attributed to professional exposure to mcr-1-positive animals [45]. Therefore, to our best knowledge, we present the highest rate of mcr-1 carriage thus far reported in healthy humans.
In our study, professional exposure could be excluded as a reason for the high prevalence of mcr-1 carriage, as well as human use of colistin. Overall, only four children had prior exposure to antibiotics and the use of colistin in Bolivia is occasional and limited to infections by some multi-drug resistant pathogens in large urban hospitals (data not shown). However, colistin is available with no restrictions for veterinary use and in animal breeding [54], and we hypothesise that this could have played a major role in the selection of colistin-resistant strains in the animal population and the environment. Moreover, the introduction of mcr-positive strains via imported food and/or food-producing animals from countries where their prevalence was found to be high (e.g. Brazil) [15,22] could also represent a source of such strains. Poor sanitation and close contact with animals, which characterise the studied setting, may lead to a high level of environmental contamination and facilitate cross-transmission of colistin-resistant strains and colistin resistance genes between different environments, resulting in a high prevalence in humans who are not directly exposed to the drug.
In our case, only a minority of the mcr-positive isolates showed resistance to other antimicrobials, and no carbapenem resistance was detected, leaving a number of therapeutic options in case of infection. However, the potential risk of spread of the mcr-1 gene to extensively resistant isolates through transferable plasmids mechanisms should not be underestimated.
Genomic analysis of a subset of the mcr-1-positive E. coli isolates, representative of different communities and of different isolates from the same child, revealed a remarkable heterogeneity in terms of clonal lineages and genetic supports. Therefore, the observed epidemiological scenario could not be ascribed to the expansion of a single mcr-1-positive clone, nor even to the spread of a single plasmid. The diversity of the genetic background of the mcr-1 genes underlined the ability of this gene to transfer itself among different clones (and even different species) and plasmids. Interestingly, we detected for the second time in South America the mcr-1.5 variant, previously described in an E. coli strain from Argentina [23].
Our study has some limitations. First, the presence of animal or environmental reservoirs of mcr-positive isolates and the direct transmission between humans and animals/environment could not be demonstrated, since we did not collect any samples from animals or the environment. Second, apart from mcr-2, we did not search for other recently described mcr-variants that could be responsible for resistance observed in other isolates. Third, the study was designed as a cross-sectional survey, in which one sample from each individual was collected. It would be interesting to investigate the prevalence of mcr-1 carriage in adults and the duration of carriage over time to understand if and how much humans could represent a major reservoir in this setting. It would also be interesting to further characterise, in more detail, the plasmid supports of the mcr-1 and other resistant determinants. Investigations on these aspects are underway.
In conclusion, our findings prompt the need to rapidly monitor the extent of human and animal carriage rates and environmental contamination by mcr genes with a one-health approach, and to introduce policies banning the non-therapeutic use of colistin. This was also recently highlighted by the PAHO/WHO, which encouraged the implementation of animal-human surveillance, as well as actions to prevent and control the spread of mcr-positive microorganisms, such as the monitoring of colistin use in human food production [14]. In Europe, knowledge of mcr carriage among healthy individuals is still limited [47,53]. While available data suggest a very low occurrence, it will be interesting to study human and animal carriage rates and environmental contamination in different countries and settings.
Acknowledgements
The Bolivian Ministry of Health and the Regional Health Departments approved the study design, including its ethical aspects; the Guaraní political organization (Asamblea del Pueblo Guaraní) supported the field work and conducted the interviews.
Conflict of interest: None declared.
Authors’ contributions: TG and SS analysed the data and drafted the manuscript; AA, VDP, CN did the molecular analysis and genome sequencing; TM, AM and LP produced phenotypic data and handled the samples; MS, MM, FC, JM, PC, DBV, ED, SM and RR collected the samples and participated in the coordination of the survey; AB and GMR coordinated the survey and edited the manuscript.
References
- 1. Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30(2):557-96. 10.1128/CMR.00064-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161-8. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 3. Giamarellou H. Epidemiology of infections caused by polymyxin-resistant pathogens. Int J Antimicrob Agents. 2016;48(6):614-21. 10.1016/j.ijantimicag.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 4. Schwarz S, Johnson AP. Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother. 2016;71(8):2066-70. 10.1093/jac/dkw274 [DOI] [PubMed] [Google Scholar]
- 5. Shen Z, Wang Y, Shen Y, Shen J, Wu C. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect Dis. 2016;16(3):293. 10.1016/S1473-3099(16)00061-X [DOI] [PubMed] [Google Scholar]
- 6. Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018;23(6):17-00672. 10.2807/1560-7917.ES.2018.23.6.17-00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21(27):30280. 10.2807/1560-7917.ES.2016.21.27.30280 [DOI] [PubMed] [Google Scholar]
- 8. Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio. 2017;8(4):e01166-17. 10.1128/mBio.01166-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017;22(31):30589. 10.2807/1560-7917.ES.2017.22.31.30589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother. 2017;72(12):3317-24. 10.1093/jac/dkx327 [DOI] [PubMed] [Google Scholar]
- 11. AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, Nunez-Garcia J, et al. mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother. 2017;72(10):2745-9. 10.1093/jac/dkx286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73(7):1791-5. 10.1093/jac/dky111 [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, et al. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7(1):122. 10.1038/s41426-018-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan American Health Organization (PAHO)/World Health Organization (WHO). Epidemiological Alert: Enterobacteriaceae with plasmid-mediated transferable colistin resistance, public health implications in the Americas, 10 June 2016. Washington, D.C./Geneva: PAHO/WHO; 2016. Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=35007&lang=en
- 15. Fernandes MR, Moura Q, Sartori L, Silva KC, Cunha MP, Esposito F, et al. Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Euro Surveill. 2016;21(17):30214. 10.2807/1560-7917.ES.2016.21.17.30214 [DOI] [PubMed] [Google Scholar]
- 16. Rapoport M, Faccone D, Pasteran F, Ceriana P, Albornoz E, Petroni A, et al. MCR Group. First description of mcr-1-mediated colistin resistance in human infections caused by Escherichia coli in Latin America. Antimicrob Agents Chemother. 2016;60(7):4412-3. 10.1128/AAC.00573-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delgado-Blas JF, Ovejero CM, Abadia-Patiño L, Gonzalez-Zorn B. Coexistence of mcr-1 and blaNDM-1 in Escherichia coli from Venezuela. Antimicrob Agents Chemother. 2016;60(10):6356-8. 10.1128/AAC.01319-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalmolin TV, Martins AF, Zavascki AP, de Lima-Morales D, Barth AL. Acquisition of the mcr-1 gene by a high-risk clone of KPC-2-producing Klebsiella pneumoniae ST437/CC258, Brazil. Diagn Microbiol Infect Dis. 2018;90(2):132-3. 10.1016/j.diagmicrobio.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 19. Saavedra SY, Diaz L, Wiesner M, Correa A, Arévalo SA, Reyes J, et al. Genomic and Molecular characterization of clinical isolates of Enterobacteriaceae harboring mcr-1 in Colombia, 2002 to 2016. Antimicrob Agents Chemother. 2017;61(12):e008441-17. 10.1128/AAC.00841-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ortega-Paredes D, Barba P, Zurita J. Colistin-resistant Escherichia coli clinical isolate harbouring the mcr-1 gene in Ecuador. Epidemiol Infect. 2016;144(14):2967-70. 10.1017/S0950268816001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sennati S, Di Pilato V, Riccobono E, Di Maggio T, Villagran AL, Pallecchi L, et al. Citrobacter braakii carrying plasmid-borne mcr-1 colistin resistance gene from ready-to-eat food from a market in the Chaco region of Bolivia. J Antimicrob Chemother. 2017;72(7):2127-9. 10.1093/jac/dkx078 [DOI] [PubMed] [Google Scholar]
- 22. Monte DF, Mem A, Fernandes MR, Cerdeira L, Esposito F, Galvão JA, et al. Chicken meat as a reservoir of colistin-resistant Escherichia coli strains carrying mcr-1 genes in South America. Antimicrob Agents Chemother. 2017;61(5):e02718-16. 10.1128/AAC.02718-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tijet N, Faccone D, Rapoport M, Seah C, Pasterán F, Ceriana P, et al. Molecular characteristics of mcr-1-carrying plasmids and new mcr-1 variant recovered from polyclonal clinical Escherichia coli from Argentina and Canada. PLoS One. 2017;12(7):e0180347. 10.1371/journal.pone.0180347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandes MR, Sellera FP, Esposito F, Sabino CP, Cerdeira L, Lincopan N. Colistin-resistant mcr-1-positive Escherichia coli on public beaches, an infectious threat emerging in recreational waters. Antimicrob Agents Chemother. 2017;61(7):e00234-17. 10.1128/AAC.00234-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aires CAM, da Conceição-Neto OC, Tavares E Oliveira TR, Dias CF, Montezzi LF, Picão RC, et al. Emergence of the plasmid-mediated mcr-1 gene in clinical KPC-2-producing Klebsiella pneumoniae Sequence Type 392 in Brazil. Antimicrob Agents Chemother. 2017;61(7):e00317-17. 10.1128/AAC.00317-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sellera FP, Fernandes MR, Sartori L, Carvalho MPN, Esposito F, Nascimento CL, et al. Escherichia coli carrying IncX4 plasmid-mediated mcr-1 and blaCTX-M genes in infected migratory Magellanic penguins (Spheniscus magellanicus). J Antimicrob Chemother. 2017;72(4):1255-6. [DOI] [PubMed] [Google Scholar]
- 27. Rossi F, Girardello R, Morais C, Cury AP, Martins LF, da Silva AM, et al. Plasmid-mediated mcr-1 in carbapenem-susceptible Escherichia coli ST156 causing a blood infection: an unnoticeable spread of colistin resistance in Brazil? Clinics (Sao Paulo). 2017;72(10):642-4. 10.6061/clinics/2017(10)09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bartoloni A, Cutts F, Leoni S, Austin CC, Mantella A, Guglielmetti P, et al. Patterns of antimicrobial use and antimicrobial resistance among healthy children in Bolivia. Trop Med Int Health. 1998;3(2):116-23. 10.1046/j.1365-3156.1998.00201.x [DOI] [PubMed] [Google Scholar]
- 29. Bartoloni A, Pallecchi L, Riccobono E, Mantella A, Magnelli D, Di Maggio T, et al. Relentless increase of resistance to fluoroquinolones and expanded-spectrum cephalosporins in Escherichia coli: 20 years of surveillance in resource-limited settings from Latin America. Clin Microbiol Infect. 2013;19(4):356-61. 10.1111/j.1469-0691.2012.03807.x [DOI] [PubMed] [Google Scholar]
- 30. Bartoloni A, Sennati S, Di Maggio T, Mantella A, Riccobono E, Strohmeyer M, et al. Antimicrobial susceptibility and emerging resistance determinants (blaCTX-M, rmtB, fosA3) in clinical isolates from urinary tract infections in the Bolivian Chaco. Int J Infect Dis. 2016;43:1-6. 10.1016/j.ijid.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 31. Bartoloni A, Riccobono E, Magnelli D, Villagran AL, Di Maggio T, Mantella A, et al. Methicillin-resistant Staphylococcus aureus in hospitalized patients from the Bolivian Chaco. Int J Infect Dis. 2015;30:156-60. 10.1016/j.ijid.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 32. Pallecchi L, Malossi M, Mantella A, Gotuzzo E, Trigoso C, Bartoloni A, et al. Detection of CTX-M-type beta-lactamase genes in fecal Escherichia coli isolates from healthy children in Bolivia and Peru. Antimicrob Agents Chemother. 2004;48(12):4556-61. 10.1128/AAC.48.12.4556-4561.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO)/Department of control of neglected tropical diseases. Helminth control in school-aged children, a guide for managers of control programs. Geneva: WHO; 2012. Available from:http://www.who.int/neglected_diseases/resources/9789241548267/en/
- 34.Coppi M, Cannatelli A, Antonelli A, Baccani I, Di Pilato V, Sennati S, et al. A simple phenotypic method for screening of MCR-1-mediated colistin resistance. Clin Microbiol Infect. 2018;24(2):S1198-743X(17)30457-3. [DOI] [PubMed]
- 35. Pacheco AB, Guth BE, Soares KC, Nishimura L, de Almeida DF, Ferreira LC. Random amplification of polymorphic DNA reveals serotype-specific clonal clusters among enterotoxigenic Escherichia coli strains isolated from humans. J Clin Microbiol. 1997;35(6):1521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0. Växjö: EUCAST; 1 Jan 2018. Available from:http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf
- 37.European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST guideline for the detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 2.0. Växjö: EUCAST; July 2016. Available from:http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf
- 38. Giani T, Antonelli A, Caltagirone M, Mauri C, Nicchi J, Arena F, et al. Evolving beta-lactamase epidemiology in Enterobacteriaceae from Italian nationwide surveillance, October 2013: KPC-carbapenemase spreading among outpatients. Euro Surveill. 2017;22(31):30583. 10.2807/1560-7917.ES.2017.22.31.30583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ribeiro TG, Novais Â, Branquinho R, Machado E, Peixe L. Phylogeny and comparative genomics unveil independent diversification trajectories of qnrB and genetic platforms within particular Citrobacter species. Antimicrob Agents Chemother. 2015;59(10):5951-8. 10.1128/AAC.00027-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chavda KD, Chen L, Fouts DE, Sutton G, Brinkac L, Jenkins SG, et al. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. MBio. 2016;7(6):e02093-16. 10.1128/mBio.02093-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1989. [Google Scholar]
- 42. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455-77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bi Z, Berglund B, Sun Q, Nilsson M, Chen B, Tärnberg M, et al. Prevalence of the mcr-1 colistin resistance gene in extended-spectrum β-lactamase-producing Escherichia coli from human faecal samples collected in 2012 in rural villages in Shandong Province, China. Int J Antimicrob Agents. 2017;49(4):493-7. 10.1016/j.ijantimicag.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 44. Purohit MR, Chandran S, Shah H, Diwan V, Tamhankar AJ, Stålsby Lundborg C. Antibiotic resistance in an Indian rural community: a ‘One-Health’ observational study on commensal coliform from humans, animals, and water. Int J Environ Res Public Health. 2017;14(4):386-13. 10.3390/ijerph14040386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trung NV, Matamoros S, Carrique-Mas JJ, Nghia NH, Nhung NT, Chieu TTB, et al. Zoonotic transmission of mcr-1 colistin resistance gene from small-scale poultry farms, Vietnam. Emerg Infect Dis. 2017;23(3):529-32. 10.3201/eid2303.161553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhong LL, Zhang YF, Doi Y, Huang X, Zhang XF, Zeng KJ, et al. Co-production of MCR-1 and NDM-1 by colistin-resistant Escherichia coli isolated from a healthy individual. Antimicrob Agents Chemother. 2016;61(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zurfluh K, Stephan R, Widmer A, Poirel L, Nordmann P, Nüesch HJ, et al. Screening for fecal carriage of MCR-producing Enterobacteriaceae in healthy humans and primary care patients. Antimicrob Resist Infect Control. 2017;6(28):28. 10.1186/s13756-017-0186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu X, Hu Y, Luo M, Zhou H, Wang X, Du Y, et al. MCR-1.6, a New MCR variant carried by an IncP plasmid in a colistin-resistant Salmonella enterica Serovar Typhimurium isolate from a healthy individual. Antimicrob Agents Chemother. 2017;61(5):e02632-16-13. [DOI] [PMC free article] [PubMed]
- 49. Wang Y, Tian GB, Zhang R, Shen Y, Tyrrell JM, Huang X, et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet Infect Dis. 2017;17(4):390-9. 10.1016/S1473-3099(16)30527-8 [DOI] [PubMed] [Google Scholar]
- 50. Zhang X-F, Doi Y, Huang X, Li H-Y, Zhong L-L, Zeng K-J, et al. Possible transmission of mcr-1-harboring Escherichia coli between companion animals and human. Emerg Infect Dis. 2016;22(9):1679-81. 10.3201/eid2209.160464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang R, Huang Y, Chan EW, Zhou H, Chen S, Zhang R, et al. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis. 2016;16(3):291-2. 10.1016/S1473-3099(16)00062-1 [DOI] [PubMed] [Google Scholar]
- 52.Chen K, Chan EW, Xie M, Ye L, Dong N, Chen S. Widespread distribution of mcr-1-bearing bacteria in the ecosystem, 2015 to 2016. Euro Surveill. 2017;22(39):17-00206. [DOI] [PMC free article] [PubMed]
- 53. Gröndahl-Yli-Hannuksela K, Lönnqvist E, Kallonen T, Lindholm L, Jalava J, Rantakokko-Jalava K, et al. The first human report of mobile colistin resistance gene, mcr-1, in Finland. APMIS. 2018;126(5):413-7. 10.1111/apm.12834 [DOI] [PubMed] [Google Scholar]
- 54.Servicio Nacional de Sanidad Agropecuaria e Inocuidad Alimentaria (SENASAG). [National Service of Agricultural Health and Food Safety]. Registro de Productos de Uso Veterinario e Insumos Pecuarios. [Registration of Products for Veterinary Use and Livestock Supplies]. Bolivia: SENASAG; 2018. Spanish. Available from: http://190.129.48.189/egp/productosVeterinarios.html