Abstract
The aim of the study was to determine if there was a difference in 6-minute walk distance (6MWD) when two 6-minute walk tests (6MWTs) were performed at the initial assessment prior to attendance at the pulmonary hypertension (PH) clinic and at the 6-month follow-up. Two 6MWTs were performed at both visits on a 32-m continuous track in the physiotherapy hospital outpatient setting using standard instructions and encouragement. Two hundred and fourteen participants completed two 6MWTs at the initial assessment and 71 participants at the 6-month follow-up (mean (standard deviation) age: 57 (16) years; body mass index: 27 (6) kg/m2). Using the better 6MWT, the mean distances walked were 429 (136) and 447 (130) m, respectively. There was a significant increase in 6MWD when a second 6MWT was performed at initial assessment (mean difference [95% confidence interval (CI)]: 19 m (14–24), p < 0.001) and at the follow-up (mean difference [95% CI]: 19 m (10–27), p < 0.001) but not in those who walked <300 m at the initial assessment (mean difference [95% CI]: 9 m (−5 to 22), p = 0.208). There were no adverse events during testing. Prior to attendance at the PH Clinic when people are asked to perform the 6MWT for the first time and at the 6-month follow-up, two walk tests should be performed in order to eliminate a learning effect and to ensure accuracy of measurement.
Keywords: Pulmonary hypertension, 6-minute walk test
Background
Pulmonary hypertension (PH) is defined as a rise in mean pulmonary artery pressure (≥ 25 mmHg) at rest during right heart catheterization.1–6 The practical management of PH requires accurate assessment of disease severity7 and should be based on the assessment of a number of factors including symptoms, other possible causes of breathlessness, and measurement of exercise tolerance.8 No single tool can be relied upon to assess disease severity or to predict prognosis.6,9–13 The 6-minute walk test (6MWT) has been shown to reflect exercise capacity in people with PH and the 6-minute walk distance (6MWD) has a strong, independent association with mortality.14,15
The 6MWT is a simple, inexpensive, and reliable measure of functional exercise capacity16,17 and is frequently used to assess prognosis and response to therapy in a wide range of pulmonary disorders18 including pulmonary arterial hypertension.9,19
When the 6MWT is used, there are a number of factors that need to be standardized to ensure that the test is performed accurately.16,17,20,21 The factors that influence the distance walked in the 6MWT include the number of tests performed,21–23 instructions and encouragement given,17 the layout of the walking track,17,24 the administration of bronchodilators,20,25 and the use of supplemental oxygen.26 When a second 6MWT was performed in people with chronic obstructive pulmonary disease (COPD) and heart failure (HF), there was a significant increase in the distance walked of between 7%21 and 15%26 and 13 m (p < 0.001).23 This increase in 6MWD is known as a “learning effect” and has been attributed to factors such as patient motivation,27,28 familiarity with the walking track, overcoming anxiety, feeling more confident, improved coordination, and adjusting to levels of dyspnoea.24 It is unclear if the learning effect demonstrated when people perform the 6MWTs for the first time persists at 3- and 6-month follow-ups. In people with COPD who performed two 6MWTs 3 months following the completion of pulmonary rehabilitation, there was a learning effect demonstrated of 16 m. However, there is no information about the presence of a learning effect when two 6MWTs are performed for the first time or at 6-month follow-up assessments in people with PH.
Therefore, the aim of the study was to determine if there was a difference in 6MWD when two 6MWTs were performed prior to attendance at the Royal Prince Alfred Hospital (RPAH) PH Clinic and at the 6-month follow-up.
Methods
Study protocol
This was a retrospective review of patient data collected from 2005 to 2011. All participants were naïve to the 6MWT and performed two 6MWTs at the initial assessment (6MWT1 and 6MWT2) and at the 6-month follow-up (6MWT3 and 6MWT4).
Participants
Participant data were included if they were over 18 years with suspected PH and had performed two 6MWTs for the first time, prior to attending the PH Clinic at RPAH and again at the 6-month follow-up. Participant data were not included if they were unable to perform two 6MWTs. The study was approved by the Ethics Committee of the Sydney Local Health District (Protocol No X12-0018 and HREC/12/RPAH/11).
Six-minute walk test
The 6MWT was carried out according to the ATS/ERS Guidelines.29 On all testing occasions, participants rested for at least 10 minutes before performing the first 6MWT and for at least 30 minutes between tests or until oxygen saturation (SpO2), heart rate (HR), and dyspnoea had returned to resting levels (within 2% or one point on the Borg scale and 2 bpm, respectively).15 All 6MWTs were performed in the physiotherapy gymnasium on a continuous 32-m track marked with black tape for easy visibility. Standardized instructions were given before each test, with encouragement given each minute throughout the test. Participants were asked to walk as far as they could in 6 minutes, to do the best they could, and to cover as much ground as possible. Every minute, participants were made aware of the time and were given standardized encouragement, such as you are doing well—you have 5 minutes to go! This was alternated each minute with keep up the good work—you have (insert number of minutes) remaining! If the participants needed to stop, they could do so, but were asked every 15 seconds to commence walking as soon as they felt able.
Before and immediately after the 6MWT, SpO2 and HR were recorded using a portable pulse oximeter with a forehead probe (RAD-5v; Masimo Corp, Irvine, California, USA), and dyspnoea was measured using the modified Borg scale (0–10).30 The test was terminated if participants felt that they could not continue or if they experienced chest pain, evolving mental confusion, lack of coordination, light-headedness, intolerable dyspnoea, or extreme fatigue.
Statistical analysis
All data are presented as mean and standard deviation (SD). The differences between 6MWT1 and 6MWT2 and 6MWT3 and 6MWT4 were analyzed using paired t-tests. In addition, participants with 6MWD < 300 m in test 1 (6MWTa) and test 2 (6MWTb) at initial assessment were analyzed as a subgroup using paired t-tests. For all analyses, a p value of <0.05 was taken to be statistically significant. Data were analyzed using SPSS Version 23. The individual mean difference between the first and second 6MWT at both time points was examined using the Bland–Altman analysis, to plot the individual difference in test distance against the individual test mean distance and the coefficient of repeatability (defined as 1.96 times the SD of the difference between the first 6MWT and the second 6MWT at both time points).31
Results
At the initial assessment, 214 of 307 (70%) people completed two 6MWTs (6MWT1 and 6MWT2) and at the 6-month follow-up, 71 of 97 (73%) people completed two 6MWTs (6MWT3 and 6MWT4). Participant characteristics are described in Table 1 and the classification of PH37 for participants who performed the walk tests is described in Table 2. No firm diagnosis had been made at the time of the 6MWT. There were a number of participants who did not complete two 6MWTs for reasons unrelated to their medical condition, for example, time and staffing. There was a mean 6MWD (SD) of 429 (136) m at initial assessment and 447 (130) m at the 6-month follow-up. Of the total number of participants who performed the tests at initial assessment, 46 participants (21%) walked <300 m at initial assessment (6MWTa and 6MWTb) with a mean 6MWD (SD) of 222 (69).
Table 1.
Baseline characteristics (n = 214).
| Mean (SD) | |
|---|---|
| Age (yrs) | 57 (16) |
| BMI (kg/m2) | 27 (6) |
| Initial 6MWD (m) | 429 (136) |
| Initial 6MWD (% predicted) | 87 (24) |
| Initial 6MWD < 300 m (%) | 47 (22) |
| SpO2 rest (%) | 97 (4) |
| HR rest (bpm) | 81 (4) |
n: numbers; yrs: years; SD: standard deviation; BMI: body mass index; kg/m2: kilograms per meters squared; 6MWD: 6-minute walk distance; m: meters; %: percentage; HR: heart rate; bpm: beats per minute; SpO2: oxygen saturation; 6MWD % predicted calculated using equation.36
Table 2.
Classification of pulmonary hypertensiona for participants who performed two 6MWTs at initial assessment.
| Classification | Total n = 214 (%) | 6MWD > 300 m (n = 168 (%)) | 6MWD < 300 m (n = 46 (%)) | |
|---|---|---|---|---|
| 1 | PAH | |||
| (a) IPAH | 42 (19) | 34 (20) | 8 (17) | |
| (b) Connective tissue disease | 71 (33) | 57 (33) | 14 (30) | |
| (c) Congenital heart disease | 11 (5) | 10 (6) | 1 (2) | |
| (e) Portal hypertension | 3 (1) | 3 (2) | Nil | |
| 2 | Left heart disease | 30 (14) | 20 (12) | 10 (21) |
| 3 | Lung disease | 28 (13) | 19 (11) | 9 (19) |
| 4 | Thromboembolic disease | 12 (6) | 11 (6) | 1 (2) |
| 5 | Unclear multi factorial mechanisms | 20 (9) | 16 (9) | 4 (9) |
PAH: pulmonary artery hypertension; IPAH: idiopathic pulmonary artery hypertension; n: number; %: percentage; 6MWD: 6-minute walk distance; PH: pulmonary hypertension.
aClassification of pulmonary hypertension by Simonneau et al.37
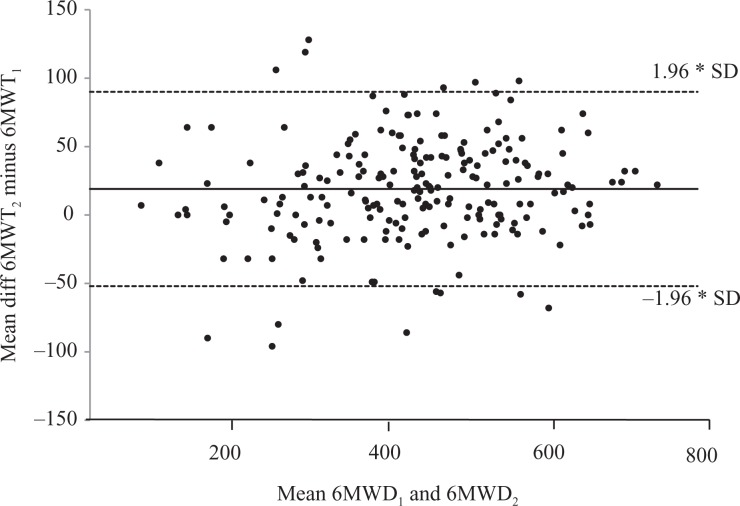
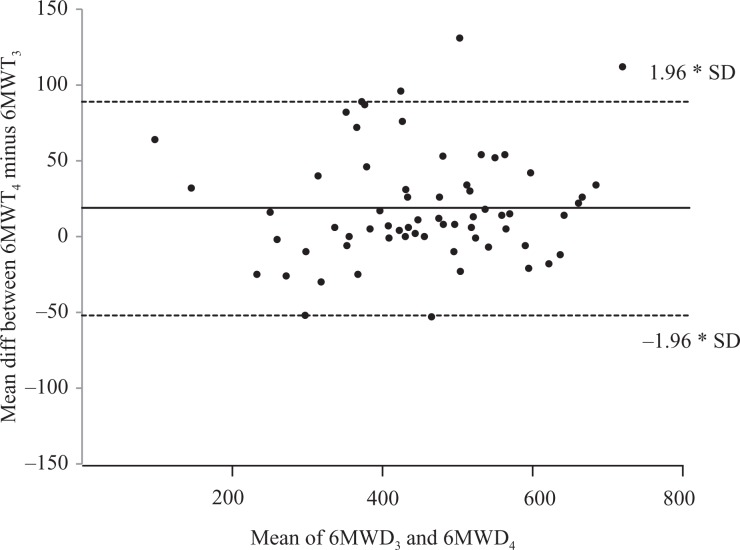
At the initial assessment, for the 214 participants, there was a significant increase in distance walked when 6MWT2 was compared with 6MWT1 (mean difference [95% confidence interval (CI)]: 19 m (14–24), p < 0.001), with 66% of the participants walking further in 6MWT2 than 6MWT1 (Table 3 and Figure 1). For the 46 participants who walked <300 m in the two initial walk tests (6MWTa and 6MWTb), there was no significant increase in distance walked when 6MWTb was compared with 6MWTa (mean difference [95% CI]: 9 m (−5 to 22), p = 0.208), with 55% of participants walking further in 6MWTb than 6MWTa (Table 4 and Figure 2). At the 6-month follow-up, for the 71 participants, there was a significant increase in distance walked when 6MWT4 was compared with 6MWT3 (mean difference [95% CI]: 19 m (10–27), p < 0.001), with 69% of the participants walking further in 6MWT4 than 6MWT3 (Table 3 and Figure 3).
Table 3.
6MWD, SpO2, HR, and dyspnoea at initial assessment and at 6-month follow-up (including those who walked <300 m).
| Measures | Initial 6MWT1 mean (SD) n = 214 | Initial 6MWT2 mean (SD) n = 214 | Mean difference 6MWT2 and 6MWT1 (95% CI) | Six-month 6MWT3 mean (SD) n = 71 | Six-month 6MWT4 mean (SD) n = 71 | Mean difference 6MWT4 and 6MWT3 (95% CI) |
|---|---|---|---|---|---|---|
| 6MWD (m) | 419 (135) | 438 (140) | 19 (14–24) | 438 (130) | 457 (132) | 19 (10 to 27) |
| HR rest (bpm) | 81 (15) | 83 (13) | 79 (14) | 77 (14) | ||
| HR end ex (bpm) | 107 (24) | 107 (24) | 106 (21) | 107 (20) | ||
| SpO2 rest (%) | 97 (4) | 97 (5) | 97 (3) | 97 (4) | ||
| SpO2 end ex (%) | 92 (10) | 91 (9) | 91 (13) | 90 (11) | ||
| Dyspnoea end ex | 3 (1) | 3 (1) | 3 (1) | 3 (1) |
n: numbers; 6MWT: 6-minute walk test; 6MWT1 and 6MWT2: performed at initial assessment; 6MWT3 and 6MWT4: performed at the 6-month follow-up; 6MWD: 6-minute walk distance; m: meters; HR: heart rate; bpm: beats per minute; ex: exercise; %: percentage; SpO2: oxygen saturation; SD: standard deviation; 95% CI: 95% confidence interval.
Figure 1.
Bland–Altman plots for the difference between two 6MWTs at the initial assessment. 6MWT: 6-minute walk test; m: meters; 6MWD: distance walked in 6-minute walk test; 6MWT1 and 6MWT2—first and second 6MWT performed at initial assessment; unbroken line: mean difference; dashed line: co-efficient of variability (±1.96*SD); x-axis: mean 6MWT; y-axis: difference between 6MWTs; SD: standard deviation.
Table 4.
6MWD, SpO2, HR, and dyspnoea at initial assessment for patients who walked < 300 m).
| Measures | Initial 6MWTa mean (SD) n = 46 | Initial 6MWTb mean (SD) n = 46 | Mean difference 6MWTb and 6MWTa (95% CI) |
|---|---|---|---|
| 6MWD (m) | 228 (69) | 237 (72) | 9 (−5 to 22) |
| HR rest (bpm) | 79 (19) | 76 (17) | |
| HR end ex (bpm) | 99 (25) | 99 (26) | |
| SpO2 rest (%) | 95 (5) | 94 (5) | |
| SpO2 end ex (%) | 85 (12) | 85 (13) | |
| Dyspnoea end ex | 4 (1) | 4 (2) |
n: numbers; 6MWT: 6-minute walk test; 6MWTa and 6MWTb: performed at initial assessment; 6MWD: 6-minute walk distance; m: meters; HR: heart rate; bpm: beats per minute; ex: exercise; %: percentage; SpO2: oxygen saturation; SD: standard deviation; 95% CI: 95% confidence interval.
Figure 2.
Bland–Altman plots for the difference between two 6MWTs at initial assessment in those who walked <300 m. 6MWT: 6-minute walk test; m: meters; 6MWD: distance walked in 6-minute walk test; 6MWTa and 6MWTb—first and second 6MWT at initial assessment in those who walked <300m; unbroken line: mean difference; dashed line: co-efficient of variability (±1.96*SD); x-axis: mean 6MWT; y-axis: difference between 6MWTs; SD: standard deviation.
Figure 3.
Bland–Altman plots for the difference between two 6MWTs at the 6-month follow-up. 6MWT: 6-minute walk test; m: meters; 6MWD: distance walked in 6-minute walk test; 6MWT3 and 6MWT4—first and second 6MWT performed at the 6-month follow-up; unbroken line: mean difference; dashed line: co-efficient of variability (±1.96*SD); x-axis: mean 6MWT; y-axis: difference between 6MWTs. SD: standard deviation.
Results for SpO2 and HR at rest and end 6MWT and for dyspnoea at end 6MWT are shown in Tables 3 and 4, respectively. The mean (SD) end-exercise SpO2 was 92% (10) with 66 (30%) of participants desaturating below 90%. For the 46 participants who walked <300 m, the mean (SD) end-exercise SpO2 was 85% (10) with 30 (75%) of these participants desaturating below 90%.
Ninety-seven percent of participants (n = 207) reported dyspnoea at the completion of the 6MWT, with a mean dyspnoea score of 3 (“moderate” on the modified Borg 1–10 scale). Thirty-four participants (16%) reported symptoms other than dyspnoea at the completion of the 6MWT with the most common symptom being joint pain, occurring in 10% of patients (Table 5).
Table 5.
Symptoms other than dyspnoea reported at the end of the 6MWT at initial assessment (n = 34/214).
| Symptoms | n |
| Chest pain | 4 |
| Dizziness | 2 |
| Joint pain | 22 |
| Fatigue | 2 |
| Irregular HR | 2 |
| Claudication | 2 |
n: number; chest pain: reported as <2/10 on the visual analogue scale; HR: heart rate.
Discussion
This study demonstrated a significant increase in the distance walked when two 6MWTs were performed for the first time in people attending the PH Clinic at RPAH and again at the 6-month follow-up. However, in people who walked <300 m on the first 6MWT, there was no significant increase in the distance walked on the second test.
In this study, the increase in 6MWD when two 6MWTs were performed at both time points demonstrated that a learning effect was present. The difference between the first and second 6MWT was 19 m, which was greater than the 13 m increase in 6MWD reported when two 6MWTs were performed for the first time in people with HF.23 On the other hand, a larger difference was reported in the distance walked when two 6MWTs were performed for the first time in people with COPD.21,22 There are a number of factors that could have contributed to the learning effect in our study, such as a lack of confidence, unfamiliarity with the walking track, and testing procedures.32 The improvement in 6MWD between 6MWT1 and 6MWT2 and 6MWT3 and 6MWT4 was not accompanied by any change that would suggest better effort as the HR, dyspnoea score, and SpO2 were nearly identical, despite the longer distance walked (Tables 3 and 4). This supports the view that the increased distance walked by participants in the second 6MWT (i.e. 6MWT2 and 6MWT4) was due to a learning effect and greater confidence of participants in performing the second 6MWT rather than an increased physiological effort. It was surprising that when participants completed two 6MWTs at the 6-month follow-up, there was still a learning effect demonstrated. One would have thought that having performed the walk test twice at the initial assessment, participants may not have displayed a learning effect again at the 6-month time point. Therefore, it appears that 6 months may be long enough for familiarity with the walking tests to be lost.
As the participants in the current study were naive to the 6MWT, the second test was likely to better reflect the participant’s true functional exercise capacity by eliminating the learning effect. At 6-month follow-up, it appeared that participants were again naïve to the walk test showing that two walk tests were again necessary to accurately assess distance walked. The performance of two 6MWTs at initial assessment and at the 6-month follow-up is essential to accurately monitor patient progress and the effects of medication in people with PH. This raises the important issue of the time required by the clinician and the patient to perform two walk tests and in day-to-day clinical practice, this may not be feasible. However, due to the fact that in Australia and other countries, stability or improvement in 6MWD is required as a condition for continued access to publically funded supply of specific PH medications, accurate documentation of baseline and follow-up 6MWD is critical. Therefore, we recommend that future international guidelines encourage clinicians where possible to perform two 6MWTs at initial and 6-month follow-up assessments to ensure accurate patient monitoring.
Dyspnoea has been reported as the main clinical manifestation in people with PH,8,33 and this was the main symptom affecting the distance walked by participants in our study. A previous study reported that 75% of people with PH reported breathlessness at rest and with mild activity by the time of diagnosis.33 In our study, all participants reported an increase in their level of dyspnoea at end of the 6MWT with the mean Borg score reported as 3 of 10 (“moderate breathlessness”). We may have expected higher Borg scores at the end of the 6MWTs considering that dyspnoea was the most commonly reported symptom. However, joint pain was reported as the second most common symptom (10%) during the 6MWT with 33% of participants having PH associated with connective tissue disease. Therefore, joint pain may have prevented participants reaching higher Borg scores.
For the participants who walked <300 m (21%) on their first 6MWT, there was no significant increase in 6MWD demonstrated when performing a second 6MWT at initial assessment, despite a 30-minute recovery period between walks tests. Poor functional exercise capacity and desaturation below 90% may have been contributing factors to no increase in 6MWD in the second 6MWT. Similar results were observed by Adsett et al.23 who reported no significant increase in 6MWD when two 6MWTs were performed in people with HF who walked <300 m. Previous reports have observed that a 6MWD of <350 m has been associated with a higher mortality in people with PH.34 It appears that in participants who walk <300 m, it may not be necessary to perform two 6MWTs as there was no significant increase in the walk distance demonstrated when a second 6MWT was performed.
For the purpose of this study, an adverse event was considered to be severe chest pain, evolving mental confusion, lack of coordination or light-headedness, intolerable dyspnoea, or extreme fatigue. There were no adverse events reported in the 428 walk tests that were performed as part of this study. All tests were performed in a hospital physiotherapy department by experienced physiotherapists. However, a previous larger study of 6MWT in people with PH reported that there were a number of adverse events during testing including cardiac arrest. As a result, the authors suggested that performing the 6MWT was unsafe and that ECG monitoring may be necessary during the 6MWT in people with PH.35 However in our study, there were no adverse events and, after dyspnoea, the commonest symptom reported was joint pain.
There are a number of limitations to the study. The study was a retrospective review of patients who were referred for assessment at the PH clinic at RPAH and therefore diagnosis had not been confirmed. The nadir SpO2 and HR may not have been accurately captured because minute by minute oxygen and HR information were not collected during the 6MWT protocol. At the time of testing, only resting and end-exercise SpO2, HR, and dyspnoea were part of the testing protocol. Based on the recent publication of the ATS/ERS systematic review and technical standards16–17,38, the new recommendations for 6MWT are that minute by minute SpO2 and HR should be recorded to ensure nadir SpO2 is observed.
Conclusion
There was a significant increase in the distance walked when a second 6MWT was performed both at initial assessment and at the 6-month follow-up in people referred for evaluation of PH and who were naïve to the 6MWT. Both at initial assessment and at the 6-month follow-up, the second 6MWT was more likely to reflect the participant’s true functional exercise capacity by taking into account the learning effect and as such, would be a more valid comparison with subsequent 6MWTs used for ongoing monitoring. The authors recommend that all people referred for assessment of suspected PH should perform two 6MWTs at initial assessment and at the 6-month follow-up and that the second walk test be used as the true measure of functional exercise capacity.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Pristera N, Musarra R, Schilz R, et al. The role of echocardiography in the evaluation of pulmonary arterial hypertension. Echocardiograpy 2016; 33(1): 105–116. [DOI] [PubMed] [Google Scholar]
- 2. Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr 2013; 26(1): 1–14. [DOI] [PubMed] [Google Scholar]
- 3. Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30(20): 2493–2537. [DOI] [PubMed] [Google Scholar]
- 4. Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(Suppl 25): D42–50. [DOI] [PubMed] [Google Scholar]
- 5. Montani D, Gunther S, Dorfmuller P, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis 2013; 8: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta H, Ghimire G, Naeije R. The value of tools to assess pulmonary arterial hypertension. Eur Respir Rev 2011; 20(122): 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howard LS. Prognostic factors in pulmonary arterial hypertension: assessing the course of the disease. Eur Respir Rev 2011; 20(122): 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009; 34(6): 1219–1263. [DOI] [PubMed] [Google Scholar]
- 9. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American college of cardiology foundation task force on expert consensus documents and the American heart association: developed in collaboration with the American college of chest physicians, American thoracic society, Inc., and the pulmonary hypertension association. Circulation 2009; 119(16): 2250–2294. [DOI] [PubMed] [Google Scholar]
- 10. Vachiery JL, Yerly P, Huez S. How to detect disease progression in pulmonary arterial hypertension. Eur Respir Rev 2012; 21(123): 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J, Mehta S, Mura M. Early decline in 6-minute walk distance from the time of diagnosis predicts clinical worsening in pulmonary arterial hypertension. Respiration 2015; 89(5): 365–373. [DOI] [PubMed] [Google Scholar]
- 12. Badesch DB, Champion HC, Sanchez MAG, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54(Suppl 1): S55–66. [DOI] [PubMed] [Google Scholar]
- 13. Gaine S, Simonneau G. The need to move from 6-minute walk distance to outcome trials in pulmonary arterial hypertension. Eur Respir Rev 2013; 22(130): 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farber HW, Miller DP, McGoon MD, et al. Predicting outcomes in pulmonary arterial hypertension based on the 6-minute walk distance. J Heart Lung Transplant 2015; 34(3): 362–368. [DOI] [PubMed] [Google Scholar]
- 15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000; 161(2 Pt 1): 487–492. [DOI] [PubMed] [Google Scholar]
- 16. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European respiratory society/American thoracic society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1447–1478. [DOI] [PubMed] [Google Scholar]
- 17. Holland AE, Spruit MA, Troosters T, et al. An official European respiratory society/American thoracic society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1428–1446. [DOI] [PubMed] [Google Scholar]
- 18. Rasekaba T, Lee AL, Naughton MT, et al. The six-minute walk test: a useful metric for the cardiopulmonary patient. Int Med J 2009; 39(8): 495–501. [DOI] [PubMed] [Google Scholar]
- 19. Deboeck G, Niset G, Vachiery JL, et al. Physiological response to the six-minute walk test in pulmonary arterial hypertension. Eur Respir J 2005; 26(4): 667–672. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax 1984; 39(11): 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spencer LM, Alison JA, McKeough ZJ. Six-minute walk test as an outcome measure: are two six-minute walk tests necessary immediately after pulmonary rehabilitation and at three-month follow-up? Am J Phys Med Rehabil 2008; 87(3): 224–228. [DOI] [PubMed] [Google Scholar]
- 22. Jenkins S, Cecins NM. Six-minute walk test in pulmonary rehabilitation: do all patients need a practice test? Respirology 2010; 15(8): 1192–1196. [DOI] [PubMed] [Google Scholar]
- 23. Adsett J, Mullins R, Hwang R, et al. Repeat six-minute walk tests in patients with chronic heart failure: are they clinically necessary? Eur J Cardiovasc Prev Rehabil 2011; 18(4): 601–606. [DOI] [PubMed] [Google Scholar]
- 24. Sciurba F, Criner GJ, Lee SM, et al. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med 2003; 167(11): 1522–1527. [DOI] [PubMed] [Google Scholar]
- 25. Hay JG, Stone P, Carter J, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J 1992; 5(6): 659–664. [PubMed] [Google Scholar]
- 26. Leach RM, Davidson AC, Chinn S, et al. Portable liquid oxygen and exercise ability in severe respiratory disability. Thorax 1992; 47(10): 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stevens D, Elpern E, Sharma K, et al. Comparison of hallway and treadmill six-minute walk tests. Am J Respir Crit Care Med 1999; 160(5 Pt 1): 1540–1543. [DOI] [PubMed] [Google Scholar]
- 28. Eiser N, Willsher D, Dore CJ. Reliability, repeatability and sensitivity to change of externally and self-paced walking tests in COPD patients. Respir Med 2003; 97(4): 407–414. [DOI] [PubMed] [Google Scholar]
- 29. American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1): 111–117. [DOI] [PubMed] [Google Scholar]
- 30. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14(5): 377–381. [PubMed] [Google Scholar]
- 31. Bland JM, Altman DG. Statistical methods for assessing agreement between methods of clinical measurement. Lancet 1986; 1: 301–310. [PubMed] [Google Scholar]
- 32. Mainguy V, Provencher S, Maltais F, et al. Assessment of daily life physical activities in pulmonary arterial hypertension. PloS One 2011; 6(11): e27993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(12 Suppl S): 13S–24S. [DOI] [PubMed] [Google Scholar]
- 34. Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005; 95(2): 199–203. [DOI] [PubMed] [Google Scholar]
- 35. Morris NR, Seale H, Harris J, et al. Serious adverse events during a 6-minute walk test in patients with pulmonary hypertension. Eur Respir J 2015; 45(4): 1179–1182. [DOI] [PubMed] [Google Scholar]
- 36. Camarria B, Eastwood P, Cecins N, et al. Six minute walk distance in healthy subjects aged 55–75 years. Respir Med 2006; 100: 658–665. [DOI] [PubMed] [Google Scholar]
- 37. Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl D): D35–D41. [DOI] [PubMed] [Google Scholar]
- 38. Swigris JJ, Zhou X, Wamboldt FS, et al. Exercise peripheral oxygen saturation (SpO2) accurately reflects arterial oxygen saturation (SaO2) and predicts mortality in systemic sclerosis. Thorax 2009; 64(7): 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]