Abstract
Vitamin D supplementation prevents acute respiratory infections and, through modulating innate and adaptive immunity, could have a potential role in bronchiectasis management. The primary aims of this pilot study were to assess serum 25-hydroxyvitamin D (25(OH)D) levels in New Zealand adults with bronchiectasis, and their 25(OH)D levels after vitamin D3 supplementation. Adults with bronchiectasis received an initial 2.5 mg vitamin D3 oral loading dose and 0.625 mg vitamin D3 weekly for 24 weeks. The primary outcome was serum 25(OH)D levels before and after vitamin D3 supplementation. Secondary outcomes (time to first infective exacerbation, exacerbation frequency, spirometry, health-related quality of life measures, sputum bacteriology and cell counts and chronic rhinosinusitis) were also assessed. This study is registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12612001222831). The initial, average 25(OH)D level was 71 nmol/L (95% confidence interval (CI): [58, 84]), rising to 218 nmol/L (95% CI: [199, 237]) at 12 weeks and 205 nmol/L (95% CI: [186, 224]) at 24 weeks. The initial serum cathelicidin level was 25 nmol/L (95% CI: [17, 33]), rising to 102 nmol/L (95% CI: [48, 156]) at 12 weeks and 151 nmol/L (95% CI: [97, 205]) at 24 weeks. Over the 24-week study period, we observed statistically significant changes of 1.11 (95% CI: [0.08, 2.14]) in the Leicester Cough Questionnaire and −1.97 (95% CI: [−3.71, −0.23]) in the Dartmouth COOP charts score. No significant adverse effects were recorded. Many New Zealand adults with bronchiectasis have adequate 25(OH)D levels. Weekly vitamin D3 supplementation significantly improved 25(OH)D levels.
Keywords: Bronchiectasis, chronic rhinosinusitis, Dartmouth COOP charts, quality of life, Leicester Cough Questionnaire, vitamin D
Introduction
Bronchiectasis is a chronic inflammatory respiratory disease characterized by bronchial dilatation, and symptoms of productive cough, dyspnoea and repeated respiratory infections requiring frequent courses of antibiotics.1 Infective exacerbations are important insults to the lungs that may contribute to incremental loss of lung function and affect patients’ quality of life2–4 and survival.5–7 The mainstay of treatment for patients with a bronchiectasis exacerbation is antibiotic treatment and physiotherapy. Current Cochrane reviews indicate a paucity of evidence for the efficacy of treatments given to patients with non-cystic fibrosis bronchiectasis,8 although long-term low-dose macrolide maintenance therapy is effective in preventing exacerbations.9–13
Vitamin D has an important role in modulating innate immunity. In recent years, vitamin D supplementation has been shown to reduce the incidence of acute asthma exacerbations,14 acute respiratory infections15 and chronic obstructive pulmonary disease (COPD) exacerbations.16 Bronchiectasis severity is associated with low 25-hydroxyvitamin D (25(OH)D) levels.17 The role of vitamin D supplementation in bronchiectasis management is not known.
The primary aims of this pilot study were to assess serum 25(OH)D levels in a cohort of New Zealand adults with bronchiectasis, and the effect of vitamin D3 supplementation on their 25(OH)D levels.
Methods
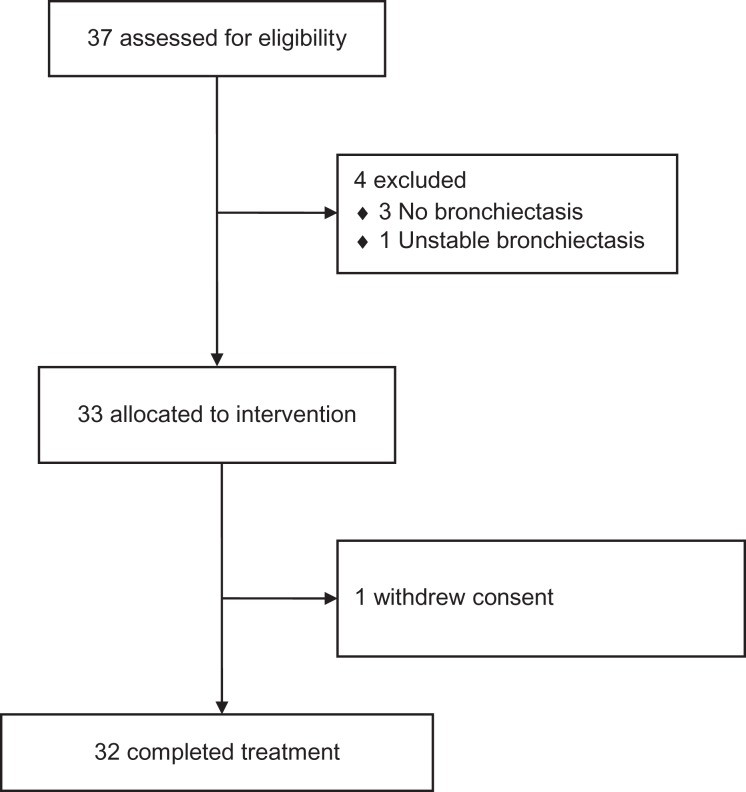
We enrolled patients in an open-label trial in Auckland (latitude 36°S), New Zealand between 14 April 2014 and 23 March 2015 (Figure 1). Patients were eligible for inclusion if they were aged ≥18 and ≤80 years, were clinically stable over the previous 4 weeks, had had at least one pulmonary exacerbation requiring antibiotic treatment in the past year and had a diagnosis of bronchiectasis defined by high-resolution chest computed tomography (CT) scan (<2 years old). Bronchiectasis severity on high-resolution chest CT scans was scored using the Bhalla index18 and CT scan sinus disease (<2 years old) was scored using the Lund–Mackay system.19
Figure 1.
CONSORT diagram showing the flow of participants through each stage of the pilot study.
Exclusion criteria were a diagnosis of cystic fibrosis, hypogammaglobulinaemia or allergic bronchopulmonary aspergillosis; a positive culture of non-tuberculous mycobacteria in the past 2 years or at screening; macrolide treatment for more than 3 months in the previous 6 months; current vitamin D supplementation (including cod liver oil) of >600 IU per day; history of renal stones or hypercalcaemia; consumption of medications that affect vitamin D metabolism (e.g. anti-epileptic or tuberculosis medication) or unstable arrhythmia. Participants provided written informed consent. The study was approved by the Northern A Health and Disability Ethics Committee.
Patients had an initial screening visit (Table 1) when forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) before and after bronchodilation were assessed. Serum 25(OH)D levels were assessed at the screening visit and 12 and 24 weeks after treatment initiation. Blood samples were collected and stored at −70°C until serum 25(OH)D levels (primary outcome) were measured.
Table 1.
Schedule of visits and procedures.
| Screen – 4 weeks | Baseline | 12 weeks | 24 weeks | |
|---|---|---|---|---|
| Eligibility criteria | X | |||
| Pre/post-spirometry | X | X | X | X |
| Nasal endoscopy | X | X | ||
| Blood tests (FBC, CRP, Ca++, U+E, LFT) | X | X | X | |
| Serum 25(OH) vitamin D | X | X | X | |
| Serum cathelicidin | X | X | ||
| Sputum analysis (including cell counts and culture) | X | X | ||
| HRCT chest + CT sinuses | X | |||
| Leicester Cough Questionnaire | X | X | X | |
| Dartmouth COOP charts | X | X | X | |
| SNOT-20 Questionnaire | X | X | X | |
| Record exacerbations | X | X | X | X |
| Record adverse events | X | X | X | |
| Phone contact | Weeks – 2, 4, 8, 16 and 20 | |||
CRP: C-reactive protein; SNOT-20: 20-Item Sino-Nasal Outcome Test; CT: computed tomography.
Secondary outcomes included: serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and neutrophil count assessed at the initial screening visit and at 12 and 24 weeks. Nasal endoscopy19,20 was performed (JB), and sputum samples for 25(OH)D levels, cathelicidin levels, cell counts and culture of respiratory pathogens were obtained at the initial screening visit and at 24 weeks (Table 1). Every sputum sample was dispersed with dithiothreitol and filtered, and total cell count and viability assessed. The cell suspensions were centrifuged, and cytospins prepared. Serum and sputum 25(OH)D levels were measured using a competitive electro chemiluminescence assay kit (Elecsys, Roche Diagnostics, Burgess Hill, UK) and cathelicidin in serum and sputum was measured using the Human LL-37 ELISA Test Kit (Hycult Biotechnology, HK321, Uden, The Netherlands) according to manufacturer instructions. The products were manufactured at ISO certified facilities. All assays were performed in duplicate and included internal standards used to construct standard curves for analyte concentration assessment.
Four weeks later at the baseline visit, patients received an initial oral loading dose of 2.5 mg (100,000 IU) vitamin D3. Patients were given capsules of 0.625 mg (25,000 IU) vitamin D3 every week for 24 weeks. Patients confirmed the intake of test medication in their diary card. If patients missed a dose, they were instructed to take the missed dose on the following day and to make a note of this in their diary card. FEV1 and FVC before and after bronchodilation were repeated. Two health-related quality of life (HRQL) measures – the Leicester Cough Questionnaire (LCQ)21 and the Dartmouth COOP charts22 and the 20-Item Sino-Nasal Outcome Test (SNOT-20)19 were administered and repeated at 24 weeks (Table 1).
The patients were contacted regularly by telephone at 2 weeks before treatment and 4, 8, 16 and 20 weeks after treatment had been initiated (Table 1). The time to first exacerbation and exacerbation frequency over 24 weeks was also assessed. An event-based exacerbation was defined as an increase in or new onset of more than one pulmonary symptom (sputum volume, sputum purulence or dyspnoea) for 48 hours or more, requiring treatment with antibiotics. This study is registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12607000641493).
Statistical analysis
Primary outcomes
The primary outcome (serum 25(OH)D level) was first analysed using McNemar’s test. A linear mixed model was then fitted to produce estimated differences in mean concentration from baseline and each subsequent time point that serum 25(OH)D was measured.
Correlations at baseline
Correlations between all measurements at the screening and baseline visits were estimated and tested against a null value of 0. Nominal 95% confidence intervals (CIs) were produced, along with False Discovery Rate (FDR) – adjusted p values.
Secondary outcomes – repeated measures
A linear mixed model was fitted to secondary outcomes, which were measured at more than one-time point post-baseline (Table 1). All repeated measures were regressed on time point (included as a factor). The model included a random effect for each participant, accounting for dependence between observations made on the same individual. The structure of the random effects and covariance was selected using Akaike’s information criterion, and the preferred model allows for the estimation of two separate variances: one for time points at which patients were under vitamin D supplementation, and one for the baseline. For continuous outcomes measured at just the screening visit and 24 weeks (Table 1), a two-sided t-test was used to test whether the difference between measurements taken at baseline and 24 weeks is equal to 0.
Exacerbation outcomes
Exacerbation frequency (post-vitamin D3 supplementation) was regressed on supplemented, serum 25(OH)D levels using a Poisson regression model, adjusting for the number of exacerbations in the 12 months prior to baseline. Follow-up time was included in the model as an offset. A time-averaged value of 25(OH)D was calculated as the average of the 12- and 24-week assessments. Exacerbation severity was regressed on serum 25(OH)D concentration using a mixed logistic regression model. Each exacerbation was coded as 0 = mild or 1 = moderate/severe. Odds ratios for moderate/severe versus mild exacerbations were obtained, as well as their likelihood-based 95% CIs from the fitted model. A time-averaged value of serum 25(OH)D concentration at the time of each exacerbation was calculated for use in the model.
Missing baseline and outcome data
Missing baseline and outcome data resulted in case-wise deletion of the record, and analyses were performed using a complete-case analysis. Baseline was modelled as a repeated measure in the mixed models; these models accommodate case-wise deletion by default.
Results
Thirty-three patients were recruited to the pilot study (Table 2), completing an initial screening visit at 4 weeks and follow-up assessment at baseline, 12 and 24 weeks. Two patients with a bronchiectasis diagnosis did not have bronchiectasis on high-resolution chest CT scans. One enrolled patient dropped out of the study prior to the 12-week assessment. The average non-missing adherence (pills taken/target) was 93% at 12 weeks and 92% at 24 weeks. The proportion of missing data on adherence was 22% (7 participants) at visit 3 and 16% (5 participants) at visit 4. No significant adverse effects were recorded.
Table 2.
Initial characteristics of patients (total n =32); data are n (%) or mean (SD).a
| Men n (%) | 15 (44) |
| Age (years) | 60.1 (12) |
| Ethnic origin | |
| European | 12 (36) |
| Maori | 7 (21) |
| Pacific Islander | 13 (39) |
| Asian | 1 (3) |
| Body-mass index (kg/m2) | 29.2 (6.7) |
| Smoking status m (%) | |
| Current smokers | 4 (12.1) |
| Ex-smokers | 14 (42.4) |
| Asthma n (%) | 7 (21.0) |
| Chronic rhinosinusitis n (%) | 12 (38) |
| Bronchiectasis severity (Bhalla index) | 37.41 (13.0) |
| Sinusitis severity (Lund–Mackay score) | 6.9 (5.2) |
| Pre-bronchodilation FEV1(%) | 66.1 (21.4) |
| Post-bronchodilation FEV1(L) | 69.9 (21.4) |
| Pre-bronchodilation FVC (L) | 77.5 (13.7) |
| Post-bronchodilation FVC (L) | 80.0 (12.4) |
| Dartmouth COOP charts score | 22.2 (5.4) |
| LCQ score | 15.0 (4.2) |
| SNOT-20 score | 26.3 (17.2) |
| Serum 25(OH)D (nmol/L) | 70.5 (35.3) |
| Serum cathelicidin (ng/ml) | 24.9 (21.9) |
| Lund–Kennedy score | 2.6 (2.1) |
| Sputum 25(OH)D (nmol/L) | 19.4 (20.7) |
| Sputum cathelicidin (ng/ml) | 1615.1 (3254.4) |
| Serum CRP (mg/L) | 4.8 (6.8) |
| Serum ESR (mm/hr) | 27.1 (18.6) |
| Neutrophil count (109/L) | 3.9 (1.4) |
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; 25(OH)D: 25-hydroxyvitamin D; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; LCQ: Leicester Cough Questionnaire; SNOT-20: 20-Item Sino-Nasal Outcome Test.
aThe Dartmouth COOP charts, LCQ and chronic rhinosinusitis symptoms score (SNOT-20) were administered at treatment initiation. FEV1 and FVC, which were performed at the initial screening and at treatment initiation, are averaged.
Correlations at baseline
No significant correlations between 25(OH)D levels and FEV1 before (nominal 95% CI: [0.10, 0.56], FDR-adjusted p = 0.86) and after bronchodilation (95% CI: [0.13, 0.55], p = 0.95), and FVC before (95% CI: [0.16, 0.53], p = 0.88) and after bronchodilation (95% CI: [0.13, 0.55], p = 0.88), LCQ (95% CI: [0.30, 0.41], p = 0.98), bronchiectasis severity (95% CI: [0.41, 0.30], p = 0.98) and sinus CT scan disease (<2 years old) (95% CI: [−0.34, 0.37], p = 0.98) were observed.
Primary outcome analysis
The initial, average 25(OH)D level was 71 nmol/L. Vitamin D3 supplementation significantly increased serum 25(OH)D levels (p < 0.001). Two subjects had a 25(OH)D level <25 nmol/L (severe deficiency), six subjects had 25(OH)D levels between 25 and 49.9 nmol/L (deficiency) and 11 subjects had 25(OH)D levels between 50 and 74.9 nmol/L (insufficiency). The proportion of participants below the 75 nmol/L threshold decreased from 61.3% (95% CI: [44.2%, 78.4%]) to 3.2% at 24 weeks (95% CI: [0.1%, 16.7%]). Estimated mean serum 25(OH)D concentrations at the screening visit, 12 and 24 weeks, were 71 nmol/L (95% CI: [58, 84]), 218 nmol/L (95% CI: [199, 237]) and 205 nmol/L (95% CI: [186, 224]) respectively (Table 3).
Table 3.
Change in secondary outcomes from initial screening visit/baseline visit to the 24-week follow-up visit.a
| Estimated change | 95% CI | Pr(>|t|) | |
|---|---|---|---|
| Pre-bronchodilation FEV1(L) | 2.17% | [−0.23, 4.53] | 0.08 |
| Post-bronchodilation FEV1(L) | 3.5% | [−1.55, 8.51] | 0.17 |
| Pre-bronchodilation FVC (L) | 1.0% | [−1.65, 3.56] | 0.47 |
| Post-bronchodilation FVC (L) | 1.7% | [−0.97, 4.44] | 0.21 |
| Dartmouth COOP charts score | −1.97 | [−3.71, −0.23] | 0.03 |
| LCQ score | 1.11 | [0.08, 2.14] | 0.03 |
| SNOT-20 score | 1.34 | [−4.17, 6.86] | 0.64 |
| Serum 25(OH)D (nmol/L) | 134 | [114.40, 153.93] | 0.00 |
| Serum cathelicidin (ng/ml) | 126 | [72.46, 179.07] | 0.00 |
| Lund–Kennedy endoscopy score | −1.27 | [−1.54, −0.99] | 0.00 |
| Sputum 25(OH)D (nmol/L) | 2.66 | [−8.78, 14.1] | 0.63 |
| Sputum cathelicidin (ng/ml) | 526.62 | [−527.42, 1580.67] | 0.31 |
| Serum CRP (mg/L) | 2.0 | [−0.90, 4.85] | 0.17 |
| Serum ESR (mm/h) | 1.56 | [−1.05, 4.16] | 0.22 |
| Neutrophil count (109/L) | −0.05 | [−0.53, 0.42] | 0.81 |
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; 25(OH)D: 25-hydroxyvitamin D; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; LCQ: Leicester Cough Questionnaire; SNOT-20: 20-Item Sino-Nasal Outcome Test; CI: confidence interval.
aThe LCQ, Dartmouth COOP charts and chronic rhinosinusitis symptoms score (SNOT-20) were administered at treatment initiation. FEV1 and FVC, which were performed at the initial screening and at treatment initiation, were averaged. The p values are for the change between the screening visit/treatment initiation and after 24 weeks of treatment.
Secondary outcomes analysis
From baseline to 24 weeks, no significant change occurred in pre-bronchodilator FEV1 2.1% (95% CI: [−0.23, 4.53]) and post-bronchodilator FEV1 3.5% (95% CI: [−1.55, 8.51]), pre-bronchodilator FVC 1.0% (95% CI: [−1.65, 3.56]) and post-bronchodilator FVC 1.7% (95% CI: [−0.97, 4.44]). Serum cathelicidin increased significantly at 12 and 24 weeks. The estimated increase from the screening visit to 12 weeks was 77 ng/ml (95% CI: [24, 130]) and at 24 weeks 126 ng/ml (95% CI: [72, 179]). No significant change was detected in sputum 25(OH)D, sputum cathelicidin, serum CRP, serum ESR or neutrophil count from the screening visit to week 24. Twenty-nine patients produced sputum for analysis at the screening visit and 26 patients produced sputum for analysis at 24 weeks. The initial microbiology profile cultured was Haemophilus influenzae (10), Moraxella catarrhalis (1), Streptococcus pneumoniae (1), Pseudomonas aeruginosa (3) and Klebsiella oxytoca (2). At 24 weeks, the microbiology profile cultured was H. influenzae (12), M. catarrhalis (1), S. pneumoniae (1), P. aeruginosa (1) and K. oxytoca (0). Three new patients cultured H. influenzae.
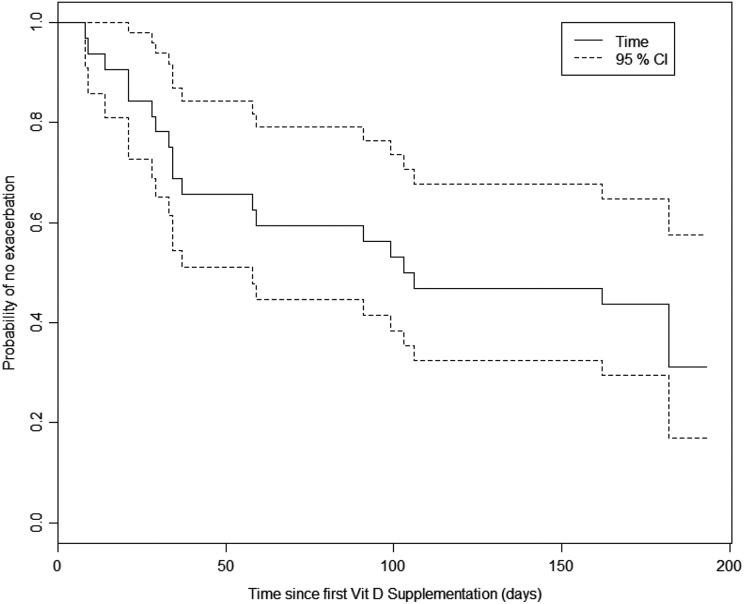
There were a total of 37 respiratory exacerbations over the 24 weeks (Figure 2). No significant association was found between supplemented serum 25(OH)D levels and frequency or severity of exacerbations. The relative risk of experiencing an exacerbation, given a 50-unit increase in serum 25(OH)D was 0.965 (95% CI: [0.674, 1.42]).
Figure 2.
Kaplan-Meier survival curves for time to first pulmonary exacerbation.
The fitted model (Table 3) shows a statistically significant improvement in the LCQ from baseline to 24 weeks, with an increase of 1.11 (95% CI: [0.08, 2.14]) and a statistically significant decrease in the Dartmouth COOP charts score from baseline to 24 weeks, with an estimated reduction of −1.97 (95% CI: [−3.71, −0.23]). No significant change in the SNOT-20 score occurred 1.3 (95% CI: [−4.17, 6.86]; however, nasal endoscopy scores improved significantly −1.27 (95% CI: [−1.54, −0.99], p < 0.01).
Discussion
The primary aims of this pilot study were to assess serum 25(OH)D levels in New Zealand adults with bronchiectasis, and their 25(OH)D levels after vitamin D3 supplementation. The initial, average 25(OH)D level in this patient population was 71 nmol/L, which was higher than reported previously in the New Zealand adult population23 and in a Scottish bronchiectasis population,17 but in keeping with the average 25(OH)D level in a recent New Zealand study investigating vitamin D3 supplementation in upper respiratory infection prevention.24 An initial vitamin D3 bolus dose (100,000 IU) and weekly vitamin D3 doses (25,000 IU) increased the average 25(OH)D level to 218 nmol/L at 3 months and 205 nmol/L at 6 months, a response consistent with the literature.25 This vitamin D3 supplementation dose was chosen, because it was anticipated that the 25(OH) levels in an adult New Zealand bronchiectasis population would be lower17,23 and that with ongoing infection/inflammation, a standard oral vitamin D3 supplementation dose could be inadequate.26
A weekly vitamin D3 supplementation regimen was adopted, because initial meta-analysis evidence indicated that a monthly bolus dose was ineffective in respiratory infection prevention27 and to improve patient compliance. A recent meta-analysis has confirmed the efficacy of weekly vitamin D supplementation in respiratory infection prevention.15 Vieth has proposed that high circulating concentrations after bolus dosing may chronically cause inappropriately low 1-alpha-hydroxylase activity coupled with inappropriately high 24-hydroxylase activity, which may limit local concentrations of the active vitamin D metabolite 1,25-dihydroxyvitamin D, resulting in decreased concentrations of this metabolite in extra-renal tissues.28 Vitamin D3 may itself play an important physiological role, although the half-life of this compound is only approximately 24 hours.29
The optimal anti-infective serum 25(OH)D level remains to be determined. Among those receiving daily or weekly vitamin D, the protective effects appear stronger when 25(OH)D levels are less than 25 nmol/L although those with higher baseline 25(OH)D concentrations also experience benefit.15 A U-shaped relationship between serum 25(OH)D and the risk of tuberculosis has been reported.30 However, observational studies report that serum 25(OH)D levels >75nmol/ L or >96 nmol/L are associated with protection against acute respiratory infection.31,32
In the Third National Health and Nutrition Examination Survey, a strong association between serum 25(OH)D, FEV1 and FVC was observed. 33 In asthmatic patients, serum 25(OH)D levels are associated with decline in lung function.34 In this pilot study, no significant correlation between screening serum 25(OH)D and baseline FEV1 or FVC was observed. From baseline to 24 weeks, no significant change in pre-bronchodilator and post-bronchodilator FEV1 or FVC was detected, a finding consistent with macrolide studies in bronchiectasis.13
Although the small sample size limited statistical power, secondary outcomes were collected to help with designing a future randomized controlled trial. Secondary aims included measuring time to first exacerbation and exacerbation frequency after vitamin D3 supplementation. There were a total of 37 respiratory exacerbations across all study participants over the duration of the study. No significant association was found between serum 25(OH)D levels and frequency or severity of exacerbations, but our study was not powered to detect effects on exacerbation frequency.
Serum cathelicidin also increased significantly. The estimated increase from the screening visit to 12 weeks was 77 and 126 ng/ml from the screening visit to 24 weeks. After 24 weeks of treatment, no significant change was seen in serum ESR, serum CRP, neutrophil count, sputum 25(OH)D or cathelicidin levels. Thijs et al. observed a similar result in the assessment of vitamin D supplementation on nasal antimicrobial peptide (AMP) levels.35 After 24 weeks of treatment, no significant change was seen in sputum neutrophil levels. In non-atopic asthma, vitamin D reduces eosinophil count but not neutrophil count.36 After 24 weeks of treatment, no major change was seen in sputum microbiology.
Two previous studies have assessed the potential co-morbidity between chronic rhinosinusitis (CRS) and bronchiectasis.37,38 CRS disease severity is also associated with low 25(OH)D levels. 39 While no significant change was seen in the SNOT-20 score, the nasal endoscopy score improved significantly with a reduction of −1.27 (95% CI: [−1.54, −0.99], p < 0.01). The SNOT-20 score is a more reliable measure of sinus symptoms and response to treatment than nasal endoscopy. Interobserver variation in nasal endoscopy has been observed.20 This finding may be a spurious result, yet any such improvement in nasal endoscopic findings could be relevant clinically.
Improving patients’ HRQL is a major goal of disease management. The LCQ is validated in bronchiectasis and is responsive to change. 21,40 In our pilot study, a statistically significant improvement in the LCQ occurred, but this did not reach the threshold for a clinically important difference of 1.3.41 The Dartmouth COOP charts score is a reliable, easy to administer HRQL tool, which is valid and responsive in COPD patients.22 We found a statistically significant improvement in the Dartmouth COOP score, although this is not a validated score in bronchiectasis. Some patients appeared to have obtained clinical benefit from vitamin D3 supplementation. A small pilot study of vitamin D supplementation in cystic fibrosis patients has also shown an improvement in HRQL respiratory score.42
To our knowledge, this pilot study is the first to investigate the potential role of vitamin D3 supplementation in bronchiectasis management. New Zealand adults with bronchiectasis had higher than expected initial 25(OH)D levels and a standard oral vitamin D3 supplementation dose successfully increased their 25(OH)D levels and improved HRQL measures.
Acknowledgements
The Middlemore Clinical Trials Unit (Auckland, New Zealand), Cecelia Tong and Lorraine Grayson provided considerable assistance and support. Tiscon Corporation provided the vitamin D3 capsules.
Authors’ note: The Health Research Council of New Zealand was not involved in the design or conduct of the study; the collection, management, analysis and interpretation of the data; or the preparation, review or approval of the article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by The Health Research Council of New Zealand.
ORCID iD: Carlos A Camargo Jr  http://orcid.org/0000-0002-5071-7654
http://orcid.org/0000-0002-5071-7654
References
- 1. Barker A. Bronchiectasis. N Engl J Med 2002; 346: 1383–1393. [DOI] [PubMed] [Google Scholar]
- 2. Wilson CB, Jones PW, O’Leary CJ, et al. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. Eur Respir J 1997; 10: 1754–1760. [DOI] [PubMed] [Google Scholar]
- 3. Martínez-García M, Perpiñá-Tordera M, Román-Sánchez P, et al. Quality-of-life determinants in patients with clinically stable bronchiectasis. Chest 2005; 128: 739–745. [DOI] [PubMed] [Google Scholar]
- 4. O’Leary CJ, Wilson CB, Hansell DM, et al. Relationship between psychological well-being and lung health status in patients with bronchiectasis. Respir Med 2002; 96: 686–692. [DOI] [PubMed] [Google Scholar]
- 5. Kolbe J, Wells AU. Bronchiectasis: a neglected cause of respiratory morbidity and mortality. Respirology 1996; 1: 221–225. [DOI] [PubMed] [Google Scholar]
- 6. Keistinen T, Säynäjäkangas O, Tuuponen T, et al. Bronchiectasis: an orphan disease with a poorly-understood prognosis. Eur Respir J 1997; 10: 2784–2787. [DOI] [PubMed] [Google Scholar]
- 7. Dupont M, Gacouin A, Lena H, et al. Survival of patients with bronchiectasis after the first ICU stay for respiratory failure. Chest 2004; 125: 1815–1820. [DOI] [PubMed] [Google Scholar]
- 8. Welsh E, Evans D, Fowler S, et al. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2015; 7: CD010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380(9842): 660–667. [DOI] [PubMed] [Google Scholar]
- 10. Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309: 1251–1259. [DOI] [PubMed] [Google Scholar]
- 11. Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013; 309: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 12. Haworth CS, Bilton D, Elborn JS. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir Med 2014; 108: 1397–1408. [DOI] [PubMed] [Google Scholar]
- 13. Gao YH, Guan WJ, Xu G, et al. Macrolide therapy in adults and children with non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. PLoS One 2014; 9: e90047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martineau A, Cates C, Urashima M, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev 2016; 9: CD011511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017; 356: i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med 2015; 3: 120–130. [DOI] [PubMed] [Google Scholar]
- 17. Chalmers J, McHugh B, Docherty C, et al. Vitamin-D deficiency is associated with chronic bacterial colonisation and disease severity in bronchiectasis. Thorax 2013; 68: 39–47. [DOI] [PubMed] [Google Scholar]
- 18. Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology 1991; 179: 783–788. [DOI] [PubMed] [Google Scholar]
- 19. Fokkens W, Lund V, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps. Rhinology 2012; (Suppl 23): 1–298. [PubMed] [Google Scholar]
- 20. Raithatha R, Anand VK, Mace JC, et al. Interrater agreement of nasal endoscopy for chronic rhinosinusitis. Int Forum Allergy Rhinol 2012; 2: 144–150. [DOI] [PubMed] [Google Scholar]
- 21. Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Resp J 2009; 34: 125–131. [DOI] [PubMed] [Google Scholar]
- 22. Eaton T, Young P, Fergusson W, et al. The Dartmouth COOP Charts: a simple, reliable, valid and responsive quality of life tool for chronic obstructive pulmonary disease. Qual Life Res 2005; 14: 575–585. [DOI] [PubMed] [Google Scholar]
- 23. Rockell J, Skeaff C, Williams S, et al. Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos Int 2006; 19: 1382–1389. [DOI] [PubMed] [Google Scholar]
- 24. Murdoch D, Slow S, Chambers S, et al. Effect of vitamin D3 supplementation on upper respiratory infections in healthy adults: a randomised, double blind, placebo-controlled trial. JAMA 2012; 308: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 25. Whiting SJ, Bonjour JP, Payen FD, et al. Moderate amounts of vitamin D3 in supplements are effective in raising serum 25-hydroxyvitamin D from low baseline levels in adults: a systematic review. Nutrients 2015; 7: 2311–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shee C. Is hypovitaminosis D a consequence rather than cause of disease? Thorax 2013; 68: 679. [DOI] [PubMed] [Google Scholar]
- 27. Bergman P, Lindh AU, Björkhem-Bergman L, et al. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2013; 8: e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vieth R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res 2009; 29: 3675–3684. [PubMed] [Google Scholar]
- 29. Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013; 98: 4619–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nielsen NO, Skifte T, Andersson M, et al. Both high and low serum vitamin D concentrations are associated with tuberculosis: a case–control study in Greenland. Br J Nutr 2010; 104: 1487–1491. [DOI] [PubMed] [Google Scholar]
- 31. Ginde A, Mansbach J, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination survey. Arch Int Med 2009; 169: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sabetta JR, DePetrillo P, Cipriani RJ, et al. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One 2010; 5: e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Black P, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest 2005; 128: 3792–3798. [DOI] [PubMed] [Google Scholar]
- 34. Brumpton BM, Langhammer A, Henriksen AH, et al. Vitamin D and lung function decline in adults with asthma: the HUNT study. Am J Epidemiol 2016; 183: 739–746. [DOI] [PubMed] [Google Scholar]
- 35. Thijs W, Janssen K, van Schadewijk AM, et al. Nasal levels of antimicrobial peptides in allergic asthma patients and healthy controls: differences and effect of a short 1,25(OH)2 Vitamin D3 treatment. PLoS One 2015; 10: e0140986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Groot JC, van Roon EN, Storm H, et al. Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J Allergy Clin Immunol 2015; 135: 670–675 e3. [DOI] [PubMed] [Google Scholar]
- 37. Guilemany JM, Angrill J, Alobid I, et al. United airways again: high prevalence of rhinosinusitis and nasal polyps in bronchiectasis. Allergy 2009; 64: 790–797. [DOI] [PubMed] [Google Scholar]
- 38. Guan WJ, Gao YH, Li HM, et al. Impacts of co-existing chronic rhinosinusitis on disease severity and risks of exacerbations in Chinese adults with bronchiectasis. PLoS One 2015; 10: e0137348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stokes PJ, Rimmer J. The relationship between serum vitamin D and chronic rhinosinusitis: a systematic review. Am J Rhinol Allergy 2016; 30: 23–28. [DOI] [PubMed] [Google Scholar]
- 40. Spinou A, Fragkos KC, Lee KK, et al. The validity of health-related quality of life questionnaires in bronchiectasis: a systematic review and meta-analysis. Thorax 2016; 71: 683–694. [DOI] [PubMed] [Google Scholar]
- 41. Raj A, Pavord D, Birring S. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol 2009; 187: 311–320. [DOI] [PubMed] [Google Scholar]
- 42. Pincikova T, Paquin-Proulx D, Sandberg JK, et al. Clinical impact of vitamin D treatment in cystic fibrosis: a pilot randomized, controlled trial. Eur J Clin Nutr 2017; 71: 203–205. [DOI] [PubMed] [Google Scholar]