Abstract
Cigarette smoking increases the risk of developing both cataract and chronic obstructive pulmonary disease (COPD). The prevalence of cataract and the clinical characteristics of COPD patients with cataract were retrospectively investigated in a 2-year observational COPD cohort. We analyzed 395 patients with complete data on ophthalmologic evaluation (319 subjects with COPD and 76 subjects at risk of COPD). There was no difference in the prevalence of cataract between COPD patients and those at risk (47.0% vs. 42.1%, p = 0.44). Age ≥ 75 years, low body mass index, and hypertension were independently associated with cataract as a comorbidity in COPD. The incidence of exacerbation within 2 years was significantly higher in COPD patients with cataract than those without cataract (36.6% vs. 18.3%, p = 0.0019). COPD patients with cataract exhibited significantly higher COPD assessment test score compared to those without cataract (13.7 ± 8.9 vs. 11.5 ± 7.2, p = 0.0240). Overall St George’s Respiratory Questionnaire score and each component were significantly worse in COPD patients with cataract compared to those without cataract. COPD patients with cataract exhibited poor health-related quality of life and frequent exacerbations. The association between cataract and exacerbations of COPD deserves further attention.
Keywords: Chronic obstructive pulmonary disease, cataract, quality of life, exacerbation, COPD assessment test
Background
Patients with chronic obstructive pulmonary disease (COPD) frequently suffer from various comorbidities, such as cachexia, lung cancer, cardiovascular disease, osteoporosis, normocytic anemia, diabetes, obstructive sleep apnea, and depression.1 In a recent multicenter cohort in Japan, called the Keio COPD Comorbidity Research (K-CCR), we reported the findings of cross-sectional and longitudinal cohort studies that showed associations between some comorbidities and various aspects of COPD.2–6 Among a variety of comorbidities, the presence of gastroesophageal reflux disease, depression, arrhythmia, and anxiety was significantly associated with a high COPD assessment test (CAT) score,2 implying the importance of a comprehensive approach to COPD comorbidities.
Cataract is the most common cause of reversible loss of useful vision7 and is a major public health problem, affecting almost 50% of adults aged >65 years.8 In the general population, several factors, such as increasing age, alcohol consumption, poor lifestyle habits, diabetes mellitus, and oral steroid use, are the risk factors for cataract.9,10 It should be noted that cigarette smoking is not only a major risk factor for COPD,11 but also for cataract.12,13 However, cataract has been largely ignored as a comorbidity of COPD.11,14 Moreover, the prevalence of cataract and the clinical characteristics of COPD patients with cataract have not been elucidated.
In this study, we focused on cataract, as a comorbidity that pulmonary physicians might routinely pay no attention to COPD. We hypothesized that COPD patients have a higher prevalence of cataract than the general population and that COPD patients with cataract exhibit worse quality of life (QOL). Therefore, we investigated the prevalence of cataract in COPD patients and the differences in clinical characteristics between COPD patients with cataract and those without cataract, as well as the association of cataract with COPD exacerbation within 2-year observation period.
Patients and methods
Study population
Our analysis was based on data collected from K-CCR, a prospective observational cohort study that investigated the clinical characteristics of COPD. The study protocol of K-CCR was described elsewhere.2–4 A total of 572 subjects were recruited at Keio University Hospital and its affiliated hospitals, including patients who had been diagnosed with COPD and those referred to the investigation of possible COPD. Inclusion criteria were: (i) age ≥ 40 years; (ii) forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 0.7; (iii) presence of emphysematous changes on chest computed tomography (CT); and (iv) chronic respiratory symptoms with significant smoking history (≥30 pack-years). The COPD group fulfilled criteria (i) and (ii), while the non-COPD group met the criteria (i) and either (iii) or (iv) without airflow limitation (FEV1/FVC ≥ 0.7). For the specific purpose of this study, the data set of 395 subjects who completed the ophthalmologic evaluation was analyzed. The group at risk of COPD fulfilled the criterion of either the presence of emphysematous changes on chest CT or the presence of chronic respiratory symptoms with significant smoking history (≥30 pack-years). The follow-up period was 2 years. Written informed consent to analyze and present their data was obtained from each patient, and the study (University Hospital Medical Information Network; UMIN000003470) was approved by the ethics committees of Keio University and its affiliated hospitals (20090008).
Assessment of clinical parameters
Upon enrolment and annually thereafter, full medical and smoking histories, as well as information about current pharmacologic treatment, were obtained based on the reviews of physicians’ medical records and prescription. All patients were assessed by spirometry and chest CT imaging. Spirometry, using an electronic spirometer, was performed in all patients in a stable condition using an electronic spirometer in accordance with the guidelines of the American Thoracic Society.15 Predicted values of spirometric measurements were derived from the guidelines issued by the Japanese Respiratory Society for pulmonary function test.16 The extent of emphysema was quantified as the ratio of low attenuation area (LAA%) on CT images using custom-made software (AZE Ltd, Tokyo, Japan).3,6 Independent investigators in the present study retrospectively judged the number and severity of exacerbations based on the reviews of physicians’ medical records, as we had previously reported.6 Mild COPD exacerbation was defined as worsening of symptoms that were self-managed (by measures such as an increase in salbutamol use) and resolved without systemic corticosteroids or antibiotics. Moderate COPD exacerbation was defined as a requirement for treatment with systemic corticosteroids or antibiotics or both. Severe COPD exacerbation was defined as hospitalization, including an emergency admission, for 24 h.
Assessment of health-related QOL
All patients were clinically stable and had no exacerbations for at least 1 month before study enrollment and the day of annual examinations. The Japanese version of CAT was applied for the assessment of COPD-specific health status,17,18 together with the St. George’s Respiratory Questionnaire (SGRQ) in Japanese for the assessment of disease specific instrument with obstructive airways disease.19–21 In addition, the Medical Outcomes Study Short-Form 36-Item (SF-36) version 2 was also used to assess the general health status.22
Assessment of cataract and other comorbidities
At enrolment, the diagnosis of cataract was made by ophthalmologic examinations. There was no information about the severity and subtypes of cataract, such as cortical cataract, nuclear cataract, and posterior subcapsular cataract. Other comorbid conditions were diagnosed based on clinical history and physical examination findings, supported by review of available medical records, as we reported previously.2,3
Statistical analysis
Data were presented as mean ± standard deviation (SD) or as median ± interquartile range. Data were compared between two groups using t-test, Mann–Whitney U test, or χ 2 test, as appropriate. Univariate and multivariate logistic regression analyses were performed to assess the factors that affect cataract and COPD exacerbations. To compare health-related QOL between the two groups, analysis of covariance (ANCOVA) using age as a covariate was performed. Differences in levels of CAT, SGRQ scores, and in rates of change over time between the two groups were estimated using mixed-effects modeling. Cochran–Armitage trend test was used to identify the age trend of cataract prevalence. Two-sided p values of <0.05 were considered significant for all tests. Data were analyzed using JMP 10 software (SAS Institute, Cary, North Carolina, USA).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (clinical trial registered with UMIN (UMIN000003470)).
Informed consent
Written informed consent to analyses and present their data was obtained from each patient
Results
Patient characteristics
The study population comprised of 319 COPD patients and 76 individuals who were at risk of COPD. The baseline characteristics of the patients are shown in Table 1. Compared with the group at risk of COPD, patients with COPD were older (p = 0.0014) and had higher pack-years of smoking (p = 0.0386). The average age of COPD patients (72 years) was older than that of a Western COPD cohort.23 At baseline, 22.9%, 46.1%, 24.1%, and 6.9% were diagnosed as COPD grade, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) I, II, III, and IV, respectively.
Table 1.
Characteristics of the study population.a
| At risk of COPD | COPD | p-Value | |
|---|---|---|---|
| N | 76 | 319 | |
| Gender, female, n (%) | 6 (7.9) | 35 (11.0) | 0.429 |
| Age (years) | 67.9 ± 11.4 | 72.4 ± 7.8 | 0.0014 |
| Smoking index (pack-years) | 46.5 ± 29.0 | 54.6 ± 29.8 | 0.0386 |
| Current smokers, n (%) | 22.6 ± 4.4 | 22.7 ± 3.4 | 0.864 |
| BMI (kg/m2) | 22.6 ± 4.4 | 22.7 ± 3.4 | 0.864 |
| FEV1/FVC (%) | 76.8 ± 5.9 | 51.7 ± 12.3 | <0.0001 |
| %FEV1 (%) | 88.6 ± 17.5 | 62.7 ± 21.7 | <0.0001 |
| GOLD grade I/II/III/IV, n (%) | 73/147/77/22 (22.9/46.1/24.1/6.9) |
BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; %FEV1: ratio of predicted FEV1; GOLD: Global Initiative for Chronic Obstructive Lung Disease; COPD: chronic obstructive pulmonary disease; SD: standard deviation.
aData are shown as numbers (%) and mean ± SD.
Prevalence of cataract
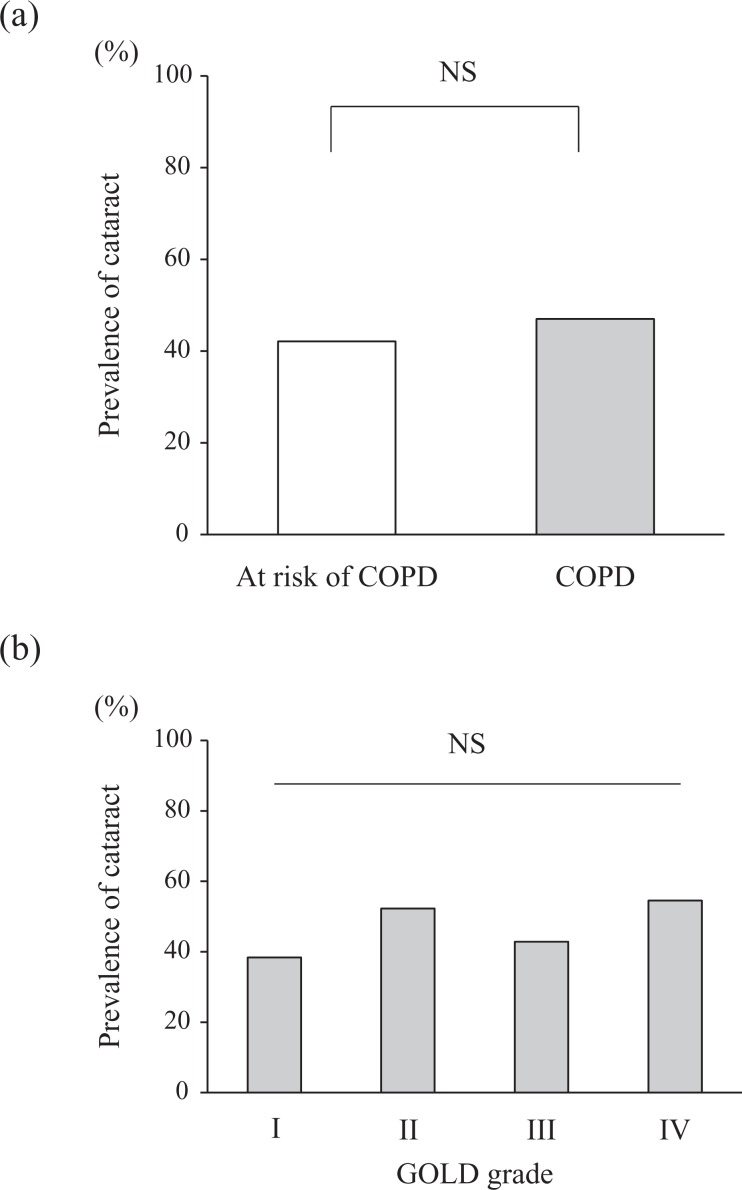
The prevalence of cataract is shown in Figure 1. The prevalence of cataract was the same between COPD patients and COPD at risk subjects (47.0% vs. 42.1%, respectively, p = 0.44; Figure 1(a)) and among the different GOLD grades of COPD (Figure 1(b)).
Figure 1.
Prevalence of cataract according to diagnosis or risk of COPD and GOLD grade. COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; NS: not significant.
Characteristics of COPD patients with cataract
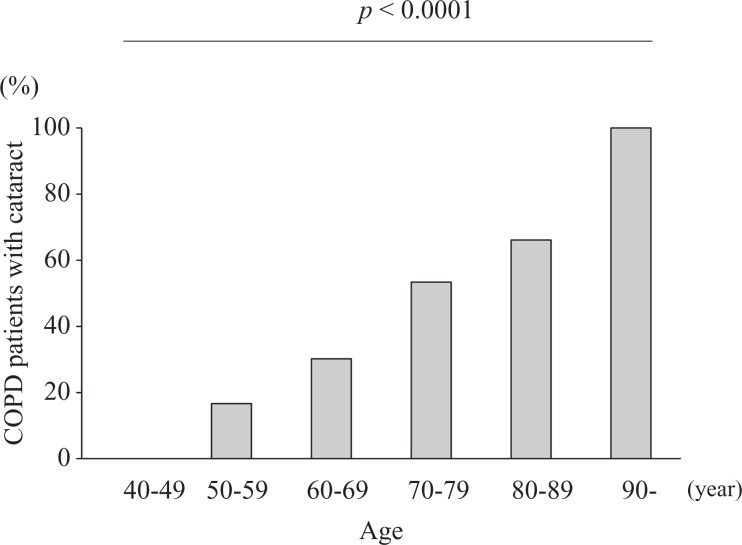
The characteristics of COPD patients stratified by the presence of cataract are shown in Table 2. Patients with cataract were significantly older than those without cataract (75.0 ± 6.8 vs. 70.1 ± 8.0, p < 0.0001). There were more women than men who had cataract (14.7% vs. 7.7%, p = 0.0467). The Cochran–Armitage trend test showed that cataract prevalence increased significantly with age (Z = 5.406 and p < 0.0001; Figure 2). The mean body mass index (BMI) was significantly lower in patients with cataract than in those without cataract (22.3 ± 3.3 vs. 23.2 ± 3.4, p = 0.017). Among the lifestyle-related diseases, hypertension had significantly higher prevalence in COPD patients with cataract than those without cataract (47.3% vs. 32.9%, p = 0.0096). There were no differences in pack-years, the ratio of the predicted FEV1(%FEV1), LAA%, and other comorbidities between the two groups. In addition, there was no differences in the ratio of prescribed inhaled corticosteroids (ICSs) and dose of ICS between the two groups.
Table 2.
Clinical characteristics of COPD patients according to the presence of cataract.a
| Patients without cataract | Patients with cataract | p-Value | |
|---|---|---|---|
| N | 169 | 150 | |
| Gender, female, n (%) | 13 (7.7) | 22 (14.7) | 0.0467 |
| Age (years) | 70.14 ± 8.0 | 75.0 ± 6.8 | <0.0001 |
| Smoking index (pack-years) | 55.3 ± 29.8 | 53.8 ± 29.8 | 0.666 |
| Current smokers, n (%) | 19 (11.7) | 14 (9.6) | 0.557 |
| BMI (kg/m2) | 23.2 ± 3.4 | 22.3 ± 3.3 | 0.017 |
| %FEV1 (%) | 64.5 ± 22.0 | 60.6 ± 21.2 | 0.111 |
| GOLD grade I/II/III/IV, n (%) | 45/70/44/10 (26.6/41.4/26.0/5.9) | 28/77/33/12 (18.7/51.3/22.0/8.0) | 0.177 |
| LAA% (%) | 10.6 (5.4–22.7) | 13.0 (5.2–22.5) | 0.817 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 29 (17.7) | 23 (15.5) | 0.612 |
| Hyperuricemia, n (%) | 13 (7.9) | 18 (12.2) | 0.212 |
| Hypertension, n (%) | 54 (32.9) | 70 (47.3) | 0.0096 |
| Dyslipidemia, n (%) | 35 (21.3) | 26 (17.6) | 0.401 |
| Coronary artery disease, n (%) | 22 (13.4) | 13 (8.8) | 0.1956 |
| Steroid use | |||
| ICS, n (%) | 49 (29.5) | 50 (33.3) | 0.172 |
| Dose of ICS,b µg/day, median | 500 | 500 | 0.125 |
| OCS, n (%) | 5 (3.0) | 5 (3.4) | 0.854 |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; %FEV1: ratio of predicted FEV1; GOLD: Global Initiative for Chronic Obstructive Lung Disease; LAA%: ratio of low attenuation area; ICS: inhaled corticosteroid; OCS, oral corticosteroids; SD: standard deviation.
aData are shown as mean ± SD and median (interquartile range).
bDose of ICS is shown as fluticasone propionate equivalent.
Figure 2.
Proportion of COPD patients with cataract according to age group, Z = 5.406 and p < 0.0001. COPD: chronic obstructive pulmonary disease.
Association of cataract and health status in COPD patients
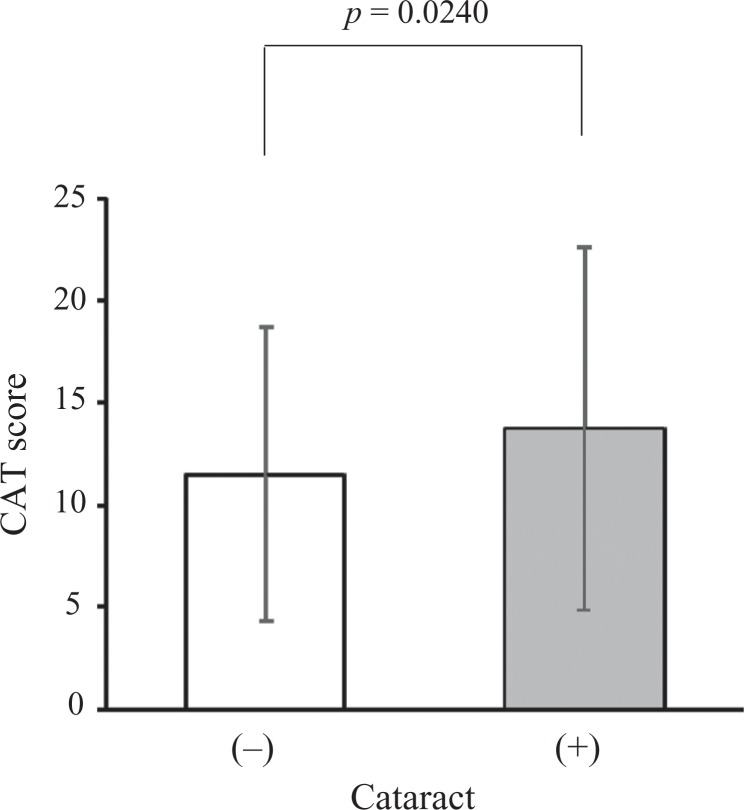
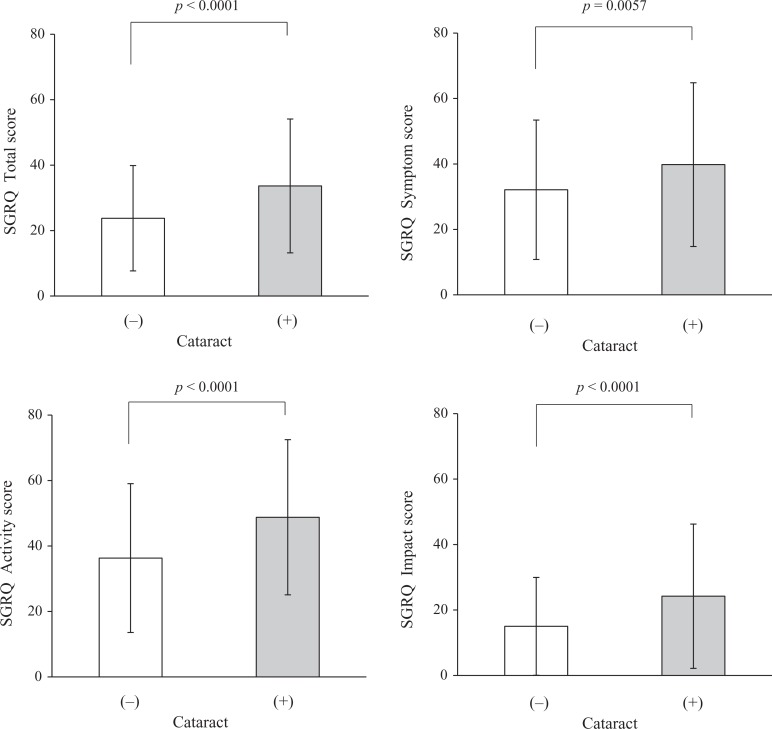
At baseline, COPD patients with cataract exhibited significantly higher total CAT score compared with those without cataract (13.7 ± 8.9 vs. 11.5 ± 7.2, p = 0.0240; Figure 3). In addition, overall SGRQ score and each of its components were significantly worse in COPD patients with cataract compared to those without cataract (Figure 4). In a similar way, SF-36 physical functioning, physical role functioning, general health perception, social role functioning, and emotional role functioning scores were worse in COPD patients with cataract compared to those without cataract (Online Supplemental Figure S1). We assessed the effect of having cataract on CAT and total SGRQ scores after the adjustment of age. ANCOVA showed significant age-adjusted differences in CAT and total SGRQ scores between two groups (CAT: p = 0.047 and SGRQ: p = 0.0003).
Figure 3.
Comparison of baseline mean CAT score in COPD patients according to the presence or absence of cataract. COPD: chronic obstructive pulmonary disease; CAT: COPD assessment test.
Figure 4.
Comparison of baseline mean overall and individual SGRQ scores in COPD according to the presence or absence of cataract. COPD: chronic obstructive pulmonary disease; SGRQ: St George’s Respiratory Questionnaire.
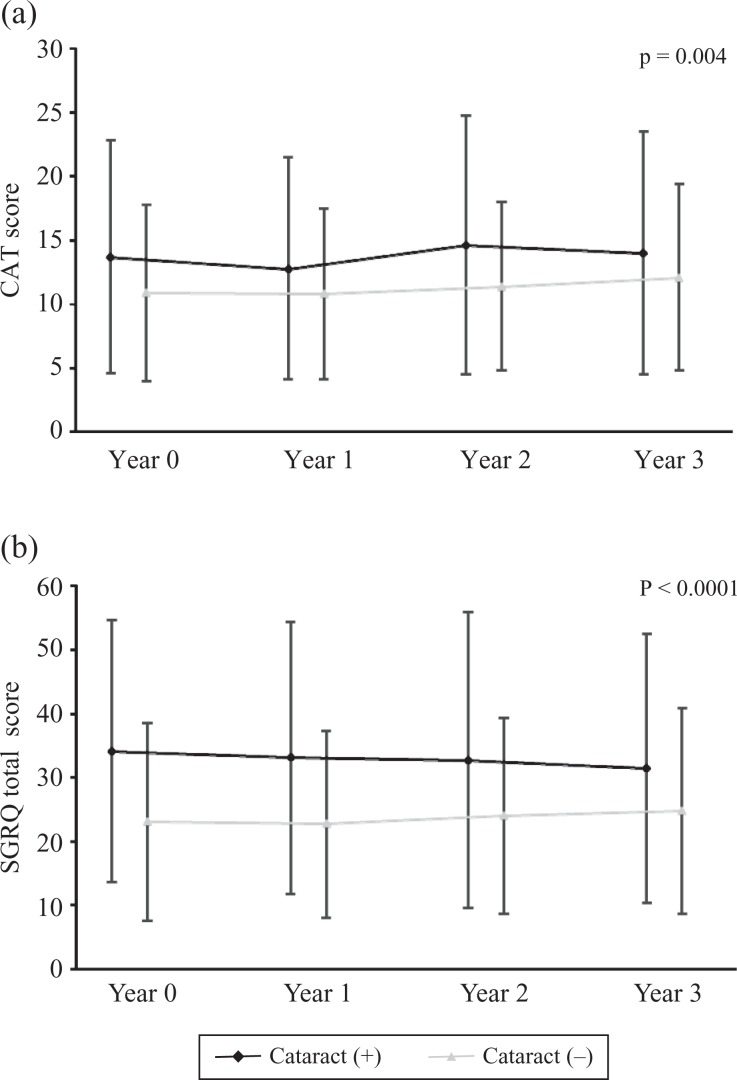
Follow-up analysis within 3 years indicated that the difference in total CAT scores and total SGRQ scores between the two groups was also significant (CAT: p = 0.004 and SGRQ: p < 0.0001; Figure 5). However, there was no significant difference in the rate of change in these scores between the two groups over 3 years (CAT: p = 0.744 and SGRQ: 0.561).
Figure 5.
Follow-up analysis of the difference in (a) CAT scores and (b) total SGRQ scores within 3 years according to the presence or absence of cataract. CAT: chronic obstructive pulmonary disease assessment test; SGRQ: St George’s Respiratory Questionnaire.
Predictors of cataract development in COPD patients
Based on the univariate logistic regression analysis, COPD patients with cataract were more likely to be ≥75 years of age, female, with low BMI, and having hypertension (Online Supplemental Table S1). Multivariate logistic regression analyses were performed based on a model of risk factors that reached the statistical significance in the univariate analyses (Table 3). Age ≥ 75 years, low BMI, and hypertension were independently associated with the presence of cataract in COPD patients.
Table 3.
Predictors of cataract by multivariate logistic regression analysis.
| Odds ratio (95% CI) | p-Value | |
|---|---|---|
| Gender, female | 1.78 (0.83–3.93) | 0.1382 |
| Age ≥ 75 years | 2.32 (1.45–3.76) | 0.0005 |
| BMI | 0.16 (0.04–0.62) | 0.0081 |
| Hypertension | 1.83 (1.12–3.00) | 0.0162 |
BMI: body mass index.
Association of cataract and COPD exacerbations
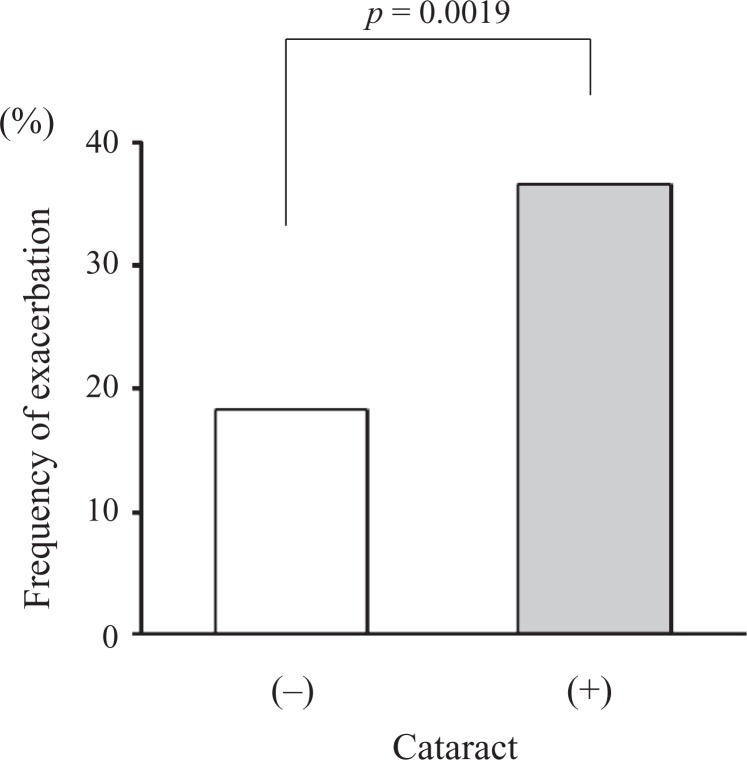
The incidence of moderate and severe exacerbations over 2 years was significantly higher in COPD patients with cataract than those without cataract (36.6% vs. 18.3%, p = 0.0019; Figure 6). After adjusting for age ≥ 75 years, gender, and %FEV1 < 50%, having cataract increased the number of COPD exacerbations over 2 years (odds ratio = 2.99, 95% confidence interval: 1.57–5.90, and p = 0.0008). In the COPD patients, who experienced moderate or severe exacerbations over 2 years, there was no significant difference in the oral steroid use between the patients with cataract and those without cataract (1/23 vs. 5/34, p = 0.21).
Figure 6.
Frequency of COPD exacerbations within 2 years according to the presence or absence of cataract. COPD: chronic obstructive pulmonary disease.
Discussion
To the best of our knowledge, this was the first and largest scale study that investigated the prevalence of cataract in COPD and the clinical characteristics of COPD patients with cataract. There were some previous studies, which showed that cigarette smoking increased the risk of cataract.12,13 However, the relationship between cataract and COPD had been largely overlooked. In addition, the possible risk factors of cataract development in COPD patients and the influence of cataract on health-related QOL of COPD patients had not been investigated.
Visually significant cataract is present in approximately 42.8% of Americans aged 70–79 years.24 This prevalence was completely in line with the findings of a Japanese age-related cataract epidemiologic study on a general population.25 In this study, ophthalmologic examinations objectively revealed that cataract prevalence was 53.38% in COPD patients aged 70 to 79 years; this prevalence was equivalent to that of subjects at risk of COPD. In the general population, older age, cigarette smoking, female, steroid use, and diabetes mellitus are well-established risk factors for cataract.7,9,10,12,13 In the present study, older age, low BMI, and hypertension were the independent risk factors for cataract in COPD patients; however, smoking, gender, steroid use, and diabetes mellitus were not associated with cataract in this present cohort of COPD patients. In addition, COPD severity according to pulmonary function or degree of emphysema was not related to the presence of cataract, suggesting that cataract was not a comorbidity that developed along with the progression of COPD.
Our study provided two novel observations of potential relevance. First, cataract in COPD patients was associated with worse QOL measures, not only SF-36, but also CAT and SGRQ. In the general population, visual impairment caused by eye diseases, such as cataract, had worse impact on SF-36.26 Our findings suggested that the treatment of cataract may improve visual acuity, aspects of daily living, and overall QOL. The definitive treatment for cataract is the surgical removal and replacement of the opacified eye lens to restore the transparency of the visual axis. Modern surgical techniques have been safely performed with few major complications.27 Second, exacerbations were more frequent in COPD patients with cataract than those without cataract during the 2 years of follow-up. Frequent exacerbation is a major driver of worsening health status6,28 in COPD and is an important cause of hospital admission29 and death.30 The pathophysiology of COPD exacerbation is complex.31–33 In the present study, having cataract turned out to be an independent risk factor for future exacerbations, even after adjusting the known risk factors of exacerbations.33,34 Among the COPD patients, there was no significant difference in inhaled steroid dose between patients with cataract and those without. In the COPD patients, who experienced moderate or severe exacerbation, there was also no difference in the ratio of oral steroid use between the group of patients with cataract and those without. At least in this population, steroid use was not associated with the presence of cataract. The specific cause and effect relationship between cataract and exacerbations is unclear. However, the development of cataract and COPD exacerbation may include a common pathogenesis, such as robust oxidative stress.35,36 In addition, the poor health-related QOL of COPD patients with cataract may be indirectly related to frequent exacerbations.
Some studies showed that the use of higher doses and longer duration of ICS is associated with the prevalence of cataracts in COPD patients.1,2 However, there were no differences in ICS use between COPD patients with cataracts and those without in this study. The reason of the discrepancy might be lower dosage of daily ICS and relatively smaller number of subjects in our study compared to the previous studies.37,38
One of the major strengths of this study was the comprehensive assessment of comorbid factors in the K-CCR cohort study and the longitudinal follow-up.6,39 In this study, we analyzed only data from patients who had completed the ophthalmologic examinations at enrollment. The development of age-related cataract is sometimes asymptomatic, but progresses by age. Lens opacity can only be confirmed by a fundus examination using the direct ophthalmoscope. In some previous studies, the presence of cataract was defined as any database code for cataract without ophthalmologic examinations.40,41 Therefore, it might be possible that patients who had incomplete cataract with slightly opaque lens and clear cortex were not considered as having cataract in those previous studies and might have affected the results. Nevertheless, increasing the awareness of both patients and pulmonary physicians about cataract as a comorbid condition of COPD should be emphasized.
There were several limitations of the present study. First, this study did not obtain information on surgery, types, grades of cataract,7,42 and proportion of patients with visual impairment. Differentiation among the several sites of cataract formation (i.e. central lens location and granular opacification) is important, because posterior subcapsular cataract is more likely to result in visual impairment at an earlier stage and would be more likely to impair function and require early surgical intervention.7,43,44 Second, Japan has become a super-aged society before other countries did45; the average age of patients who participated in our study was higher than that of other COPD clinical studies conducted in Western countries. Increasing age has been constantly associated with nuclear and cortical opacities; therefore, when clinicians apply the results of this study to a general COPD patient population, aging factor should be kept in mind.
Conclusions
COPD patients with cataract exhibited worse health-related QOL, and cataract was shown to be related to frequent COPD exacerbations. The pathophysiologic association between cataract and COPD exacerbation deserves further investigation.
Supplemental material
supplemental_fig1 for Impact of cataract on health-related quality of life in a longitudinal Japanese chronic obstructive pulmonary cohort by Hidehiro Irie, Shotaro Chubachi, Minako Sato, Mamoru Sasaki, Naofumi Kameyama, Takashi Inoue, Yoshitaka Oyamada, Hidetoshi Nakamura, Koichiro Asano, Tomoko Betsuyaku, and On behalf of the Keio COPD Comorbidity Research (K-CCR) Group in Chronic Respiratory Disease
Acknowledgments
The authors would like to thank Kazuo Tsubota of the Department of Ophthalmology, Keio University School of Medicine, for helping in the ophthalmologic examinations; Chiyomi Uemura for helping with data collection; and all the members of the Keio COPD Comorbidity Research group, who participated in this study, including Saiseikai Utsunomiya Hospital, Eiju General Hospital, Tokyo Saiseikai Central Hospital, Sano Public Welfare General Hospital, Nihon Kokan Hospital, Saitama Social Insurance Hospital, Kawasaki City Ida Hospital, Saitama City Hospital, Tokyo Medical Center, Tokyo Dental College Ichikawa General Hospital, Tokyo Electric Power Company Hospital, and the International Medical Welfare College Shioya Hospital.
Footnotes
Author contributions: HI participated in the design of the study, performed the statistical analyses, and was a major contributor in writing the manuscript. SC planned the study design, contributed to the interpretation of results, and helped in drafting the manuscript. HN, KA, and TB conceived the study, participated in its design and coordination, and helped in drafting the manuscript. MSat, MSas, NK, TI, and YO contributed to the collection of data, interpretation of results, and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Shotaro Chubachi  http://orcid.org/0000-0002-5046-3762
http://orcid.org/0000-0002-5046-3762
Supplemental material: Supplemental material for this article is available online.
References
- 1. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33: 1165–1185. DOI: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 2. Miyazaki M, Nakamura H, Chubachi S, et al. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res 2014; 15: 13 DOI: 10.1186/1465-9921-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chubachi S, Nakamura H, Sasaki M, et al. Polymorphism of LRP5 gene and emphysema severity are associated with osteoporosis in Japanese patients with or at risk for COPD. Respirology 2015; 20: 286–295. DOI: 10.1111/resp.12429. [DOI] [PubMed] [Google Scholar]
- 4. Miyazaki M, Nakamura H, Takahashi S, et al. The reasons for triple therapy in stable COPD patients in Japanese clinical practice. Int J Chron Obstruct Pulmon Dis 2015; 10: 1053–1059. DOI: 10.2147/COPD.S79864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haraguchi M, Nakamura H, Sasaki M, et al. Determinants of chronic obstructive pulmonary disease severity in the late-elderly differ from those in younger patients. BMC Res Notes 2016; 9: 7 DOI: 10.1186/s13104-015-1810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato M, Chubachi S, Sasaki M, et al. Impact of mild exacerbation on COPD symptoms in a Japanese cohort. Int J Chron Obstruct Pulmon Dis 2016; 11: 1269–1278. DOI: 10.2147/COPD.S105454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asbell PA, Dualan I, Mindel J, et al. Age-related cataract. The Lancet 2005; 365: 599–609. DOI: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 8. Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiol Rev 1995; 17: 336–346. [DOI] [PubMed] [Google Scholar]
- 9. West SK, Valmadrid CT. Epidemiology of risk factors for age-related cataract. Surv Ophthalmol 1995; 39: 323–334. [DOI] [PubMed] [Google Scholar]
- 10. Lindblad BE, Håkansson N, Philipson B, et al. Metabolic syndrome components in relation to risk of cataract extraction: a prospective cohort study of women. Ophthalmology 2008; 115: 1687–1692. DOI: 10.1016/j.ophtha.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 11. Vestbo J, Agusti A, Wouters EF, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med 2014; 189: 1022–1030. DOI: 10.1164/rccm.201311-2006PP. [DOI] [PubMed] [Google Scholar]
- 12. Christen WG, Manson JE, Seddon JM, et al. A prospective study of cigarette smoking and risk of cataract in men. JAMA 1992; 268: 989–993. [PubMed] [Google Scholar]
- 13. Ye J, He J, Wang C, et al. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci 2012; 53: 3885–3895. DOI: 10.1167/iovs.12-9820. [DOI] [PubMed] [Google Scholar]
- 14. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 728–735. DOI: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 15. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. DOI: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16. Committee of Pulmonary Physiology, the Japanese Respiratory Society: The Japanese Respiratory Society Guidelines for pulmonary function tests: spirometry, flow-volume curve, diffusion capacity of the lung (in Japanese). Tokyo, 2004. [PubMed] [Google Scholar]
- 17. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J 2009; 34: 648–654. DOI: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 18. Kwon N, Amin M, Hui DS, et al. Validity of the COPD assessment test translated into local languages for Asian patients. Chest 2013; 143: 703–710. DOI: 10.1378/chest.12-0535. [DOI] [PubMed] [Google Scholar]
- 19. Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. DOI: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 20. Hajiro T, Nishimura K, Tsukino M, et al. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158: 1185–1189. DOI: 10.1164/ajrccm.158.4.9802091. [DOI] [PubMed] [Google Scholar]
- 21. Hajiro T, Nishimura K, Tsukino M, et al. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 785–790. DOI: 10.1164/ajrccm.157.3.9703055. [DOI] [PubMed] [Google Scholar]
- 22. Fukuhara S, Bito S, Green J, et al. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol 1998; 51: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 23. Cho MH, Castaldi PJ, Hersh CP, et al. A genome-wide association study of emphysema and airway quantitative imaging phenotypes. Am J Respir Crit Care Med 2015; 192: 559–569. DOI: 10.1164/rccm.201501-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson J, Lakhani N. Cataracts. Prim Care 2015; 42: 409–423. DOI: 10.1016/j.pop.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 25. Sasaki K. Cataract epidemiology performed with Scheimpflug documentation. Ophthalmic Res 1999; 31: 75–85. DOI: 55517. [DOI] [PubMed] [Google Scholar]
- 26. Chia EM, Mitchell P, Rochtchina E, et al. Unilateral visual impairment and health related quality of life: the Blue Mountains Eye Study. Br J Ophthalmol 2003; 87: 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clark A, Morlet N, Ng JQ, et al. Whole population trends in complications of cataract surgery over 22 years in Western Australia. Ophthalmology 2011; 118: 1055–1061. DOI: 10.1016/j.ophtha.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 28. Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004; 59: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsui MS, Lun FC, Cheng LS, et al. Risk factors for hospital readmission for COPD after implementation of the GOLD guidelines. Int J Tuberc Lung Dis 2016; 20: 396–401. DOI: 10.5588/ijtld.15.0256. [DOI] [PubMed] [Google Scholar]
- 30. Almagro P, Soriano JB, Cabrera FJ, et al. Short- and medium-term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest 2014; 145: 972–980. DOI: 10.1378/chest.13-1328. [DOI] [PubMed] [Google Scholar]
- 31. Hoogendoorn M, Feenstra TL, Hoogenveen RT, et al. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis 2010; 5: 435–444. DOI: 10.2147/COPD.S13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. DOI: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 33. de Torres JP, Casanova C, Hernández C, et al. Gender and COPD in patients attending a pulmonary clinic. Chest 2005; 128: 2012–2016. DOI: 10.1378/chest.128.4.2012. [DOI] [PubMed] [Google Scholar]
- 34. Donaldson GC, Wedzicha JA. COPD exacerbations .1: epidemiology. Thorax 2006; 61: 164–168. DOI: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mallia P, Johnston SL. Mechanisms and experimental models of chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc 2005; 2: 361–366; discussion 371–372. DOI: 10.1513/pats.200504-025SR. [DOI] [PubMed] [Google Scholar]
- 36. Sutherland ER, Crapo JD, Bowler RP. N-acetylcysteine and exacerbations of chronic obstructive pulmonary disease. COPD 2006; 3: 195–202. [DOI] [PubMed] [Google Scholar]
- 37. Nath T, Roy SS, Kumar H, et al. Prevalence of steroid-induced cataract and glaucoma in chronic obstructive pulmonary disease patients attending a tertiary care center in India. Asia Pac J Ophthalmol (Phila) 2017; 6: 28–32. DOI: 10.22608/APO.201616. [DOI] [PubMed] [Google Scholar]
- 38. Weatherall M, Clay J, James K, et al. Dose-response relationship of inhaled corticosteroids and cataracts: a systematic review and meta-analysis. Respirology 2009; 14: 983–990. DOI: 10.1111/j.1440-1843.2009.01589.x. [DOI] [PubMed] [Google Scholar]
- 39. Chubachi S, Sato M, Kameyama N, et al. Identification of five clusters of comorbidities in a longitudinal Japanese chronic obstructive pulmonary disease cohort. Respir Med 2016; 117: 272–279. DOI: 10.1016/j.rmed.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 40. Jick SS, Vasilakis-Scaramozza C, Maier WC. The risk of cataract among users of inhaled steroids. Epidemiology 2001; 12: 229–234. [DOI] [PubMed] [Google Scholar]
- 41. Miller DP, Watkins SE, Sampson T, et al. Long-term use of fluticasone propionate/salmeterol fixed-dose combination and incidence of cataracts and glaucoma among chronic obstructive pulmonary disease patients in the UK General Practice Research Database. Int J Chron Obstruct Pulmon Dis 2011; 6: 467–476. DOI: 10.2147/COPD.S14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitchell P, Cumming RG, Attebo K, et al. Prevalence of cataract in Australia: the Blue Mountains Eye Study. Ophthalmology 1997; 104: 581–588. [DOI] [PubMed] [Google Scholar]
- 43. Adamsons I, Muñoz B, Enger C, et al. Prevalence of lens opacities in surgical and general populations. Arch Ophthalmol 1991; 109: 993–997. [DOI] [PubMed] [Google Scholar]
- 44. Panchapakesan J, Mitchell P, Tumuluri K, et al. Five year incidence of cataract surgery: the Blue Mountains Eye Study. Br J Ophthalmol 2003; 87: 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. United Nations Department of Economic and Social Affairs Population Division. World population prospects: the 2010 revision, volume I Comprehensive Tables. 2011. http://esa.un.org/wpp/documentation/pdf/WPP2010_Volume-I_Comprehensive-Tables.pdf. (accessed 22 July 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplemental_fig1 for Impact of cataract on health-related quality of life in a longitudinal Japanese chronic obstructive pulmonary cohort by Hidehiro Irie, Shotaro Chubachi, Minako Sato, Mamoru Sasaki, Naofumi Kameyama, Takashi Inoue, Yoshitaka Oyamada, Hidetoshi Nakamura, Koichiro Asano, Tomoko Betsuyaku, and On behalf of the Keio COPD Comorbidity Research (K-CCR) Group in Chronic Respiratory Disease