Visual Abstract
Key Words: cardiac efficiency, db/db, glucose oxidation, ketone oxidation
Abbreviations and Acronyms: ANOVA, analysis of variance; ATP, adenosine triphosphate; LV, left ventricular; PDH, pyruvate-dehydrogenase; ßOHB, β-hydroxybutyrate
Highlights
-
•
This study evaluated cardiac energy production and bioenergetics in an experimental model of diabetes treated with the SGLT2 inhibitor empagliflozin.
-
•
Rates of glucose oxidation, fatty acid oxidation, ketone oxidation, glycolysis, and cardiac function were measured in diabetic (db/db) mice treated with or without empagliflozin.
-
•
Rates of glucose and ketone oxidation in the hearts of untreated db/db mice were markedly decreased, whereas fatty acid oxidation was increased with a significant overall reduction in cardiac ATP production compared to nondiabetic mice.
-
•
Empagliflozin treatment increased overall cardiac ATP production by ∼30% and prevented cardiac failure; this effect was due to an increase in the rate of glucose and fatty acid oxidation, but with no a change in the rate of ketone oxidation.
-
•
The authors conclude that the SGLT2 inhibitor empagliflozin enhances the cardiac energy pool by increasing cardiac energy production from glucose and fatty acid oxidation, but not ketone oxidation.
Summary
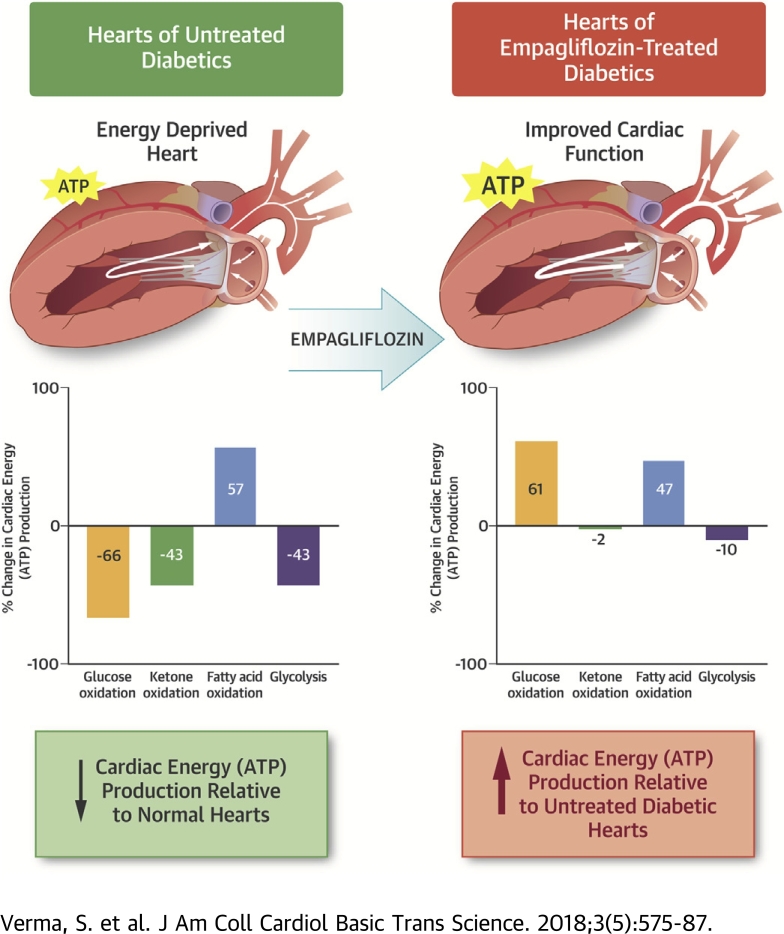
SGLT2 inhibitors have profound benefits on reducing heart failure and cardiovascular mortality in individuals with type 2 diabetes, although the mechanism(s) of this benefit remain poorly understood. Because changes in cardiac bioenergetics play a critical role in the pathophysiology of heart failure, the authors evaluated cardiac energy production and substrate use in diabetic mice treated with the SGTL2 inhibitor, empagliflozin. Empagliflozin treatment of diabetic db/db mice prevented the development of cardiac failure. Glycolysis, and the oxidation of glucose, fatty acids and ketones were measured in the isolated working heart perfused with 5 mmol/l glucose, 0.8 mmol/l palmitate, 0.5 mmol/l ß-hydroxybutyrate (ßOHB), and 500 μU/ml insulin. In vehicle-treated db/db mice, cardiac glucose oxidation rates were decreased by 61%, compared with control mice, but only by 43% in empagliflozin-treated diabetic mice. Interestingly, cardiac ketone oxidation rates in db/db mice decreased to 45% of the rates seen in control mice, whereas a similar decrease (43%) was seen in empagliflozin-treated db/db mice. Overall cardiac adenosine triphosphate (ATP) production rates decreased by 36% in db/db vehicle-treated hearts compared with control mice, with fatty acid oxidation providing 42%, glucose oxidation 26%, ketone oxidation 10%, and glycolysis 22% of ATP production in db/db mouse hearts. In empagliflozin-treated db/db mice, cardiac ATP production rates increased by 31% compared with db/db vehicle-treated mice, primarily due to a 61% increase in the contribution of glucose oxidation to energy production. Cardiac efficiency (cardiac work/O2 consumed) decreased by 28% in db/db vehicle-treated hearts, compared with control hearts, and empagliflozin did not increase cardiac efficiency per se. Because ketone oxidation was impaired in db/db mouse hearts, the authors determined whether this contributed to the decrease in cardiac efficiency seen in the db/db mouse hearts. Addition of 600 μmol/l ßOHB to db/db mouse hearts perfused with 5 mmol/l glucose, 0.8 mmol/l palmitate, and 100 μU/ml insulin increased ketone oxidation rates, but did not decrease either glucose oxidation or fatty acid oxidation rates. The presence of ketones did not increase cardiac efficiency, but did increase ATP production rates, due to the additional contribution of ketone oxidation to energy production. The authors conclude that empagliflozin treatment is associated with an increase in ATP production, resulting in an enhanced energy status of the heart.
SGLT2 inhibitors are antihyperglycemic agents that have profound and precocious effects on the prevention of heart failure and cardiovascular mortality in individuals with type 2 diabetes who are at high cardiovascular risk 1, 2, 3, 4, 5, 6. In 2 recently completed U.S. Food and Drug Administration–mandated cardiovascular outcome studies, the SGLT2 inhibitors empagliflozin and canagliflozin were associated with an ∼35% reduction in the rates of hospitalization for heart failure in participants with either established cardiovascular disease or with cardiovascular risk factors. Importantly, the benefits of empagliflozin and canagliflozin therapy emerged almost immediately, with very rapid separation of the Kaplan-Meier curves for both hospitalization for heart failure and cardiovascular mortality. Although various mechanisms have been suggested 7, 8, 9, 10, 11, including osmotic diuresis and natriuresis, the potential impact of SGLT2 inhibitors on cardiac bioenergetics has not been reported.
Alterations in myocardial substrate use and cardiac energy production play a critical role in the pathophysiology of heart failure 12, 13, 14, 15, 16, 17, 18, 19. To fulfill the exceptional metabolic demands, the myocardium is capable of incredible metabolic flexibility and can use a variety of energy substrates such as fatty acids, glucose, ketone bodies, and amino acids for the generation of adenosine triphosphate (ATP). Marked and aberrant changes in metabolic flexibility and substrate use play an early and permissive role in the development of heart failure and are believed to promote adverse remodeling and progression to severe heart failure in both experimental and clinical settings 12, 13, 20.
It has been hypothesized that SGLT2 inhibitors may prevent heart failure through improving ATP generation from ketone body oxidation, thereby enhancing cardiac efficiency (cardiac work/O2 consumed) 21, 22. However, this hypothesis remains controversial (23), and to date, no cogent evidence to support this proposed beneficial mechanism has been provided. Because SGLT2 inhibitors are known to increase the production of ketone bodies (such as β-hydroxybutyrate [ßOHB]), it has been suggested that an increase in myocardial ketone body oxidation may serve to improve cardiac ATP production as a preferred substrate to fatty acids or glucose in the diabetic heart. Whereas ketone bodies have been shown to be a source of energy supply in the hypertrophied failing heart (24), the relationship that exists between ketone bodies, glucose, and fatty acid oxidation in the diabetic heart remains unclear, particularly as it relates to SGLT2 inhibition.
We sought to shed light on the potential effects of SGLT2 inhibitors on myocardial energetics and energy substrate use. Mouse surrogates for diabetes (db/db mice) were treated with 10 mg/kg/day empagliflozin or the vehicle for 4 weeks before the rates of glycolysis, fatty acid, glucose, and ketone oxidation were evaluated in isolated perfused working hearts harvested from these mice.
Methods
Experimental animals
The experimental procedures described herein were approved by the University of Alberta Institutional Animal Care and Use Committee and conform to the guidelines of the Canadian Council of Animal Care. At 18 weeks of age, male db/db (Leprdb/J, The Jackson Laboratory, Bar Harbor, Maine) mice were treated with either empagliflozin (10 mg/kg/day via their food) or the vehicle for 4 weeks. Concomitantly, 20-week-old male C57BL/6J mice were treated for 4 weeks with the vehicle via their food.
A separate group of C57BL/6J and db/db mice was treated with a single 10 mg/kg dose of empagliflozin via oral gavage before being subjected to a 24-h fast. Lastly, 23-week-old naive male db/db mice were used to study the short-term effects of ketones on the db/db mouse heart.
Transthoracic echocardiography
The cardiac function of anesthetized (3% isoflurane) mice was assessed with a Vevo 3100 high-resolution imaging system that was coupled to a 30-MHz transducer (RMV-707B; VisualSonics, Toronto, Canada). Systolic and diastolic parameters were assessed as previously described (25).
Blood metabolite levels
For both fed and fasted protocols, total plasma ketones levels were measured using a 2-part kit from Wako Diagnostics (Cat: 415-73301 and 411-73491, Wako Diagnostics, Mountain View, California), blood glucose levels were measured using a glucometer, and plasma fatty acid levels were measured using a kit from Roche (Cat: 11383175001, Roche, Basel, Switzerland).
Isolated working heart perfusion
Male 22-week to 23-week-old db/db mice and age-matched control C57BL/6J mice were sacrificed with sodium pentobarbital after their hearts were excised and blood collected for plasma isolation. The hearts were perfused for 60 min in the isolated working mode as previously described (26) with Krebs-Henseleit solution containing 2.5 mmol/l Ca2+, 5 mmol/l [5-3H/U-14C]glucose, 0.8 mmol/l palmitate (pre-bound to 3% albumin), and 500 μmol/l βOHB in the presence or absence of 500 μU/ml insulin. A second set of hearts were perfused under identical conditions, but with 5 mmol/l glucose, 0.8 mmol/l [9,10-3H]palmitate (pre-bound to 3% albumin), and 500 μmol/l [3-14C]βOHB. To study the short-term effect of ketones on the hearts from db/db mice, these hearts were acutely perfused in the absence or presence of 600 μmol/l β-OHB. At the end of the perfusion protocol, the left ventricles were snap-frozen with liquid nitrogen and stored at −80°C.
Immunoblotting
Frozen left ventricular (LV) tissue samples (30 mg) were homogenized, resolved by SDS-PAGE, and transferred onto nitrocellulose. The membranes were blocked in 5% fat-free milk for 1 h before being probed with primary antibodies that included: anti–acetyl-lysine (Millipore, AB3879, MilliporeSigma, St. Louis, Missouri), anti–β-hydroxyacyl-CoA-dehydrogenase (βHAD) (ab37673, Abcam, Cambridge, United Kingdom), anti–long-chain-acyl-CoA-dehydrogenase (LCAD) (ab129711, Abcam), anti–pyruvate-dehydrogenase (PDH) (2784, Cell Signaling Technology, Danvers, Massachusetts), and anti–phospho-PDH (PDH ε1a [Ser293]) (Calbiochem, AP1062, MilliporeSigma). The membranes were then incubated with the appropriate secondary antibodies (goat anti-rabbit, catalog sc-2054; goat anti-mouse, catalog sc-2055; and goat anti-chicken, catalog sc-2901; Santa Cruz Biotechnology, Dallas, Texas). Protein bands were visualized with enhanced chemiluminescence and semiquantified via densitometric analysis using the Image J 1.50i software (NIH, Bethesda, Maryland). Tubulin (catalog T6074; MilliporeSigma) acted as the loading control to normalize any variation in protein loading.
Immunoprecipitation
Lysates (300 μg) were pre-cleared with 20 μl of protein A/G agarose beads, incubated overnight at 4°C with anti–acetyl lysine (2 μl/300 μg lysate, EMD Millipore, catalog AB3879; MilliporeSigma) before the acetylated proteins were pulled down with A/G agarose beads (15). The heavy chain of IgG was used as the loading control.
Myocardial malonyl-CoA levels
An assay to determine malonyl-CoA levels from frozen myocardial tissue was performed based on a previously described modified ultra high–pressure liquid chromatography procedure (27).
Statistical analysis
The data are presented as mean ± SEM and were either analyzed by one-way analysis of variance (ANOVA) and the least significant difference post hoc test or repeated one-way ANOVA and the least significant difference post hoc test. A p value of <0.05 was considered significant.
Results
Long-term empagliflozin treatment improves cardiac function in db/db mice
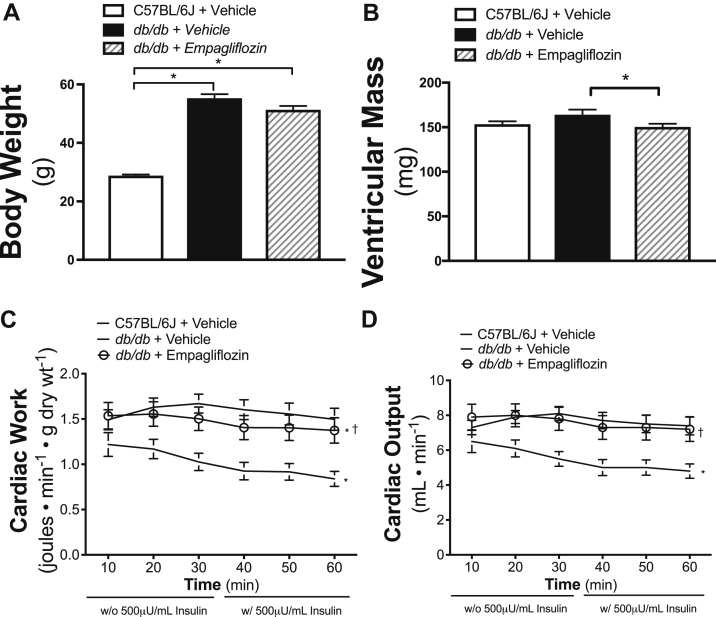
C57BL/6J mice were significantly lighter than the db/db mice (Figure 1A). Empagliflozin treatment did not appreciably affect the weights of db/db mice. Ventricular mass, a surrogate measurement of hypertrophy, was higher for vehicle-treated db/db mice relative to that for C57BL/6J mice. As shown in Figure 1B, mean ventricular mass for empagliflozin-treated db/db mice was lower than that for vehicle-treated db/db mice and comparable to that for C57BL/6J mice. Empagliflozin significantly lowered fasting blood glucose levels (Table 1). Although plasma ketone levels in the vehicle-treated fed and fasted db/db mice were not different from those of the C57BL/6J mice, significantly higher levels of fed and fasted ketone levels were detected in the empagliflozin-treated db/db mice (Table 1). Free fatty acid levels, generally higher in db/db mice relative to C57BL/6J mice, were unaffected by empagliflozin treatment (Table 1).
Figure 1.
Body Weight, LV Mass, and Cardiac Function in Empagliflozin-Treated db/db Mice
Body weight (n = 16 to 19/group) (A) and ventricular mass measured after sacrifice (n = 12 to 13/group) (B) are shown. Cardiac work presented in J ∙ min−1 ∙ gram dry weight−1(C) and cardiac output in ml ∙ min−1(D) (n = 19 to 20/group) determined by isolated working heart perfusion are shown. Data are presented as mean ± SEM. Data were analyzed by 1-way analysis of variance (ANOVA) followed by least significant difference (LSD) post hoc test. Cardiac work and cardiac output were analyzed by repeated 1-way ANOVA followed by LSD post hoc test. *p < 0.05 was considered as a significantly different comparison with C57BL/6J + Vehicle. †p < 0.05 was considered as a significantly different comparison with db/db + Vehicle.
Table 1.
Physiologic and Echocardiographic Parameters
| C57BL/6J + Vehicle | db/db + Vehicle | db/db + Empagliflozin | |
|---|---|---|---|
| Blood metabolites, mM | |||
| Fed group glucose | 9.0 ± 0.2 | 11.3 ± 1.7 | 7.6 ± 0.3† |
| Fed group ketones | 0.10 ± 0.02 | 0.11 ± 0.01 | 0.22 ± 0.03† |
| Fed group FFA | 0.38 ± 0.06 | 0.87 ± 0.11∗ | 1.00 ± 0.19 |
| Fasted group glucose | 7.2 ± 0.6 | 21.4 ± 1.8∗ | 11.8 ± 1.3† |
| Fasted group ketones | 0.59 ± 0.07 | 0.47 ± 0.14 | 1.20 ± 0.19† |
| Fasted group FFA | 0.51 ± 0.04 | 1.00 ± 0.09∗ | 0.94 ± 0.04 |
| Echocardiography data | |||
| Fractional shortening, % | 31.1 ± 1.0 | 38.8 ± 1.2∗ | 35.9 ± 1.3∗ |
| Ejection fraction, % | 59.4 ± 1.5 | 69.5 ± 1.5∗ | 65.7 ± 1.8∗ |
| MV E, mm/s | 586 ± 21 | 616 ± 35 | 507 ± 38† |
| MV A, mm/s | 482 ± 25 | 498 ± 33 | 423 ± 35 |
| MV E/E' | 25.6 ± 1.0 | 27.1 ± 2.2 | 26.3 ± 2.4 |
| IVS;d, mm | 0.95 ± 0.04 | 0.97 ± 0.02 | 0.92 ± 0.03 |
| IVS;s, mm | 1.31 ± 0.05 | 1.50 ± 0.04∗ | 1.42 ± 0.04 |
| LVID;d, mm | 3.77 ± 0.06 | 3.83 ± 0.08 | 3.81 ± 0.07 |
| LVID;s, mm | 2.65 ± 0.07 | 2.38 ± 0.08∗ | 2.47 ± 0.09 |
| LVPW;d, mm | 0.88 ± 0.05 | 0.91 ± 0.02 | 0.91 ± 0.03 |
| LVPW;s, mm | 1.29 ± 0.06 | 1.32 ± 0.02 | 1.29 ± 0.04 |
Values are mean ± SD. Mice in the fed group were treated for 4 weeks with empagliflozin (10 mg/kg/day) or vehicle. For the fasting measurements, an independent set of mice were given a single 10 mg/kg dose of empagliflozin or vehicle and then subjected to a 24-h fast. Blood metabolite levels are shown in the fed group for glucose (n = 16 per group), ketones (n = 14 to16 per group), and FFA (n = 8 to 15 per group), and in the fasted group (n = 8 to 10 per group). Fractional shortening and ejection fraction of the left ventricle (n = 14 to 19 per group) and mitral valve (MV) E, MV A, and MV E/E' (n = 14 to19 per group) are shown. Ventricular measurements, including interventricular septum at end diastole/systole (IVS;d, IVS;s), left ventricular internal diameter at end diastole/systole (LVID;d, LVID;s) and left ventricular posterior wall at end diastole/systole (LVPW;d, LVPW;s) are included. Data are presented as mean ± SEM and analyzed by One-Way ANOVA followed by LSD post hoc test.
ANOVA = analysis of variance; FFA = free fatty acids; IVS = interventricular septum; LSD = least significant difference; LVID = left ventricular internal diameter; LVPW = left ventricular posterior wall; MV = mitral valve.
p<0.05 was considered as significantly different compared to C57BL/6J + Vehicle.
p<0.05 was considered as significantly different compared to db/db + Vehicle.
Cardiac function, as measured in the isolated working heart model, was significantly compromised in the hearts of db/db mice when compared with that of hearts from C57BL/6J mice (Figures 1C and 1D). This agrees with previous reports 28, 29. Of note, the cardiac function of hearts from empagliflozin-treated db/db mice was significantly improved compared with db/db vehicle-treated mice. The differences in cardiac function between the empagliflozin- and vehicle-treated db/db mice were not as dramatic when assessed by in vivo echocardiography and did not have a significant impact on individual LV dimensions (Table 1).
Empagliflozin improves cardiac ATP production
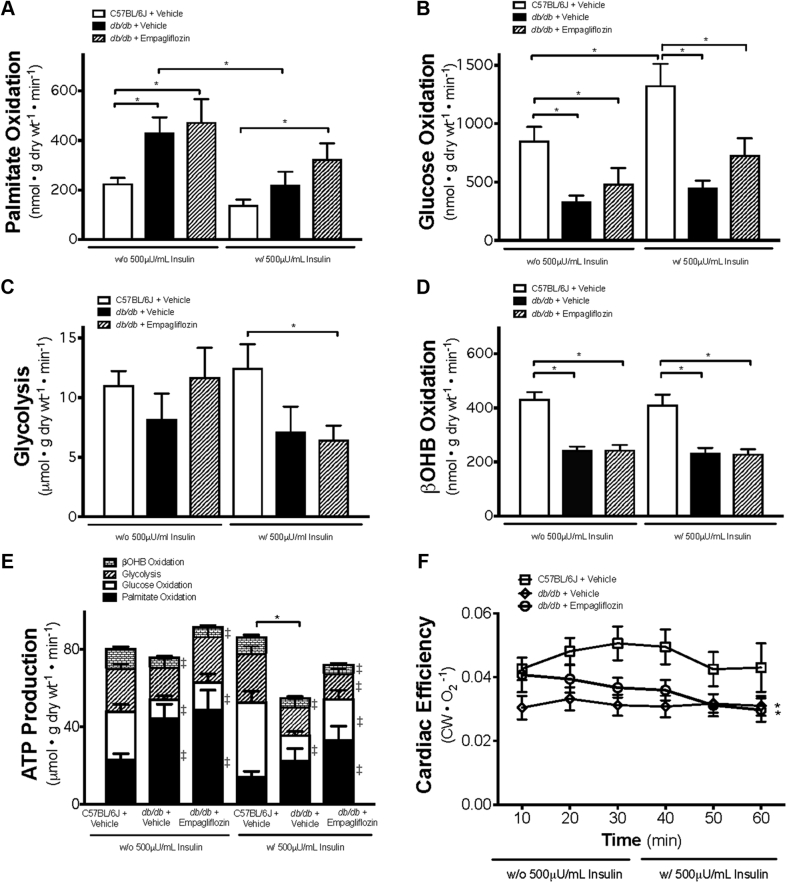
Fatty acid oxidation rates in the hearts of vehicle-treated db/db mice were significantly higher than those measured in the hearts of C57BL/6J mice (Figure 2A). Mean palmitate oxidation rate, in the presence of insulin, in the hearts of empagliflozin-treated db/db mice was 133% that of hearts from C57BL/6J mice (Figure 2A). Conversely, glucose oxidation rates in the hearts of vehicle-treated db/db mice, in the presence and absence of insulin, were significantly lower compared with those of hearts from C57BL/6J mice (Figure 2B). Although the presence of insulin increased glucose oxidation in the hearts of C57BL/6J mice, the insulin-associated effect was absent in hearts from db/db mice, which is consistent with the presence of cardiac insulin resistance 28, 29. Glycolysis rates measured in the presence of insulin were lower in the empagliflozin-treated db/db mouse hearts compared with C57BL/6J hearts (Figure 2C). As shown in Figure 2D, the mean cardiac ketone oxidation rate in the hearts of db/db mice was approximately 43% lower than that for hearts from C57BL/6J mice (Figure 2D). Notably, this outcome was apparent regardless of whether insulin was present or not. Of further interest is the observation that empagliflozin had no influence on the ketone oxidation rates of hearts from db/db mice (Figure 2D).
Figure 2.
Absolute Metabolic Rates and Cardiac Efficiency in Ex Vivo Isolated Working Hearts From Empagliflozin-Treated db/db Mice
Metabolic rates are shown for palmitate oxidation (n = 8 to 10/group) (A), glucose oxidation (n = 8 to 10/group) (B), glycolysis (8 to 9/group) (C), β-hydroxybutyrate (βOHB) oxidation (n = 8 to 9/group) (D), and total adenosine triphosphate (ATP) production (8 to 10/group) (E). Cardiac efficiency calculated as cardiac work/O2 consumed (n = 9 to 12/group) (F) is also shown. Data are presented as mean ± SEM. Data were analyzed by 1-way ANOVA to determine differences within the same insulin status and between groups in the presence or absence of insulin followed by LSD post hoc test. Four separate 1-way ANOVAs were performed to determine each substrate’s contribution to ATP production. Cardiac efficiency was analyzed by repeated 1-way ANOVA followed by LSD post hoc test. *p < 0.05 was considered as a significantly different comparison with C57BL/6J + Vehicle. For E, ‡p < 0.05 was considered as a significantly different in comparison with C57BL/6J + Vehicle within the same insulin status. Abbreviations as in Figure 1.
Overall cardiac ATP production rates in the hearts of vehicle-treated db/db mice were 36% lower than those measured in the C57BL/6J mice, with fatty acid oxidation providing 42%, glucose oxidation 26%, ketone oxidation 10%, and glycolysis 22% of energy production in the presence of insulin (Figure 2E). In empagliflozin-treated db/db mice, cardiac ATP production rates increased by 31%, such that they were restored to levels similar to those measured for the hearts from C57BL/6J mice (Figure 2E).
Cardiac efficiency (cardiac work/O2 consumed) decreased by 28% in the hearts of vehicle-db/db mice compared with that of the hearts from vehicle-treated C57BL/6J mice (Figure 2F). Under our experimental condition, isolated hearts from empagliflozin-treated db/db mice did not exhibit an increase in cardiac efficiency compared with db/db mice treated with vehicle.
EX VIVO addition of ketones adds an additional source of ATP production, but does not improve cardiac efficiency in the diabetic mouse heart
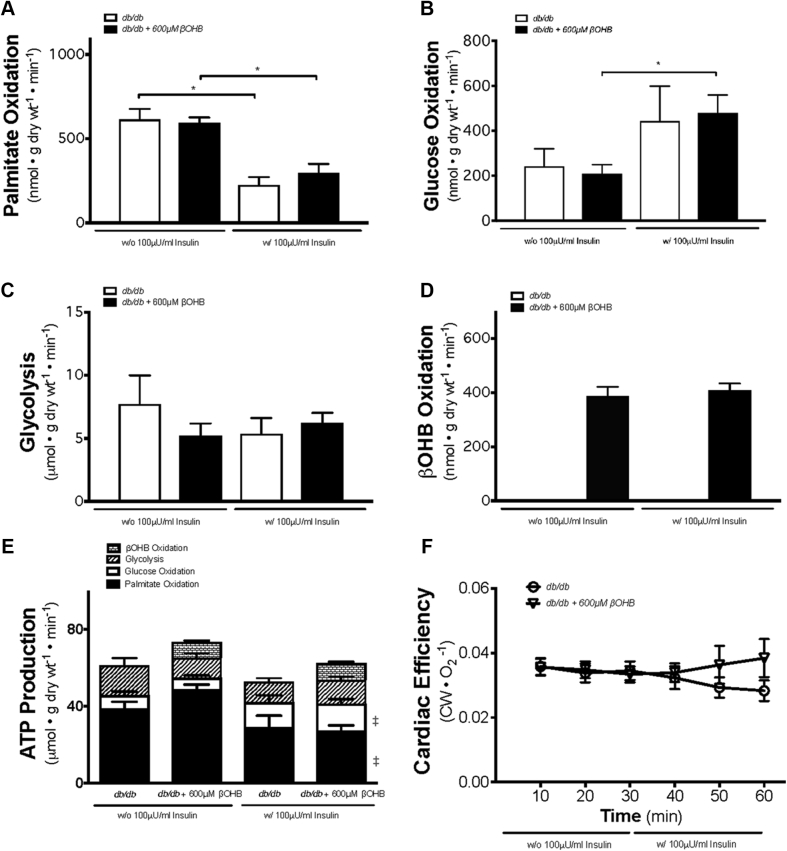
Given that empagliflozin has been shown to increase circulating blood ketone levels and that the “thrifty” fuel hypothesis suggests that ketones may be responsible for the cardiovascular benefits observed with empagliflozin 21, 22, we conducted studies to determine whether the ex vivo addition of ketones to db/db mice hearts would improve cardiac energetic status and efficiency. In db/db mice treated with 600 μmol/l βOHB, there was an insulin-dependent increase in glucose oxidation, accompanied with a drop in palmitate oxidation (Figures 3A and 3B). The addition of 600 μmol/l βOHB to isolated perfused hearts from db/db mice did not affect palmitate oxidation, glucose oxidation, or glycolysis (Figures 3A and 3C). However, addition of βOHB resulted in increased ketone oxidation (Figure 3D), which was independent of insulin. Interestingly, the addition of βOHB increased total energy (ATP) production (Figure 3E) without impairing glucose oxidation or exhibiting any sort of significant substrate competition. However, the additional presence of ketones, representing only a few percent of total calories from available fuels, did not result in an increase in cardiac efficiency and cardiac work in the hearts of db/db mice (Figure 3F, Supplemental Figure 1).
Figure 3.
Cardiac Metabolic Rates, Energy Production, and Cardiac Efficiency in db/db Mouse Hearts Perfused With or Without 600 μmol/l βOHB
Working heart perfusion derived palmitate (fatty acid) oxidation (n = 7 for db/db, n = 6 for db/db + 600 μM βOHB) (A), glucose oxidation (n = 5 for db/db, n = 8 for db/db + 600 μM βOHB) (B), and glycolysis (n = 4 to 5 for db/db, n = 7 to 8 for db/db + 600 μM βOHB) (C) levels. βOHB (ketone body) oxidation levels (n = 8 for db/db + 600 μM βOHB) (D) are also shown. Cardiac ATP production and comparison between contribution from glycolysis and the oxidation of glucose, palmitate, and βOHB (n = 5 to 8 for all groups) (E). Cardiac efficiency of the ex vivo heart as determined by normalizing cardiac work to oxygen consumption (n = 10 for db/db, n = 15 for db/db + 600 μM βOHB) (F). Data are presented as mean ± SEM. Data were analyzed by 1-way ANOVA followed by LSD post hoc test. Three separate 1-way ANOVAs were performed to determine each substrate’s contribution to ATP production. *p < 0.05 was considered as a significantly different comparison to the insulin-absent levels of the same group. For E, ‡p < 0.05 was considered as significantly different in comparison with the same group in the absence of insulin. Abbreviations as in Figures 1 and 2.
Cardiac oxidative enzyme expression and acetylation were maintained with empagliflozin treatment
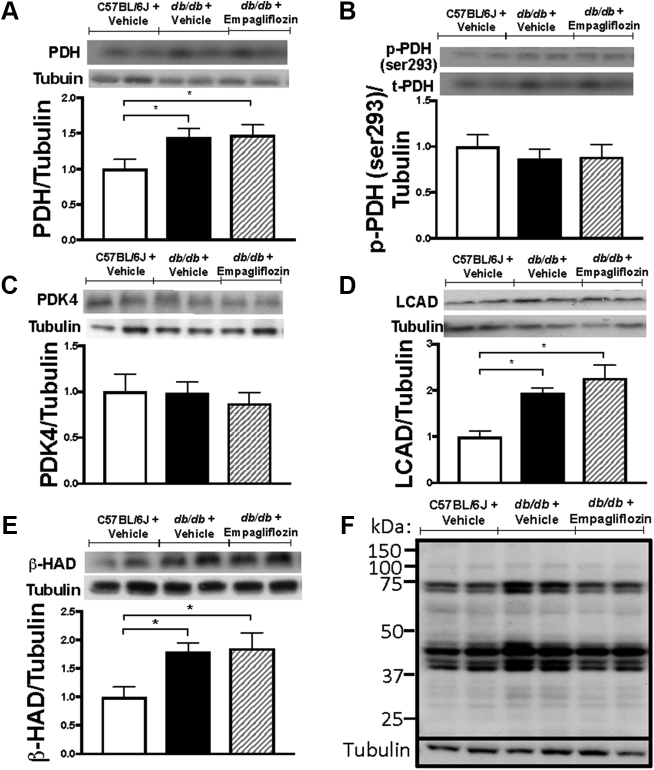
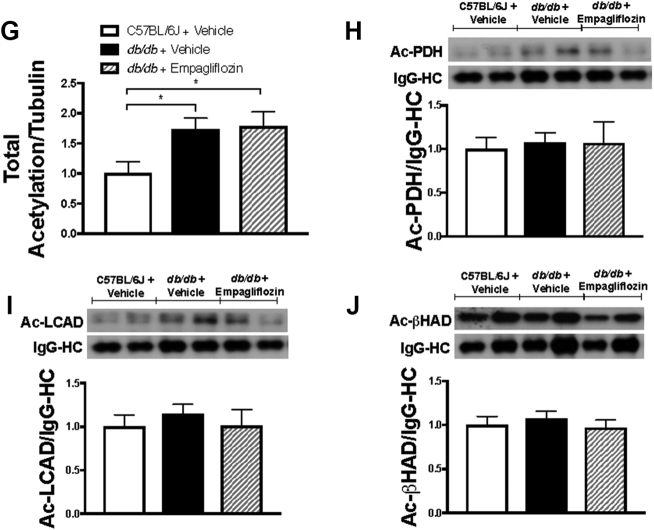
PDH is the rate-limiting component of glucose oxidation. Protein levels of PDH were significantly higher in the hearts of vehicle-treated db/db mice relative to those in the hearts of vehicle-treated C57BL/6 mice. Notably, PDH levels in the hearts of db/db mice were unaffected by empagliflozin treatment (Figure 4A). The phosphorylation and deactivation of PDH on serine residue 293 and the expression of pyruvate dehydrogenase kinase 4 (PDK4) were similar across the 3 groups of mice studied (Figures 4B and 4C). Cardiac protein expression of LCAD and β-HAD were significantly increased in db/db mice treated with vehicle, whereas empagliflozin treatment in db/db mice did not result in altered expression (Figures 4D and 4E). We also looked at cardiac acetylation because an increase in acetylation is positively associated with increased fatty acid oxidation 15, 30. Total acetylation significantly increased in db/db mice, although this was not significantly different in empagliflozin-treated db/db mice (Figures 4F and 4G). Lysine acetylation of PDH, LCAD, and β-HAD were also not significantly different between the groups (Figures 4H and 4J). Malonyl CoA, a known inhibitor of fatty acid oxidation (31), was significantly higher in db/db mice treated with vehicle compared with C57BL/6J, although this was not altered with empagliflozin treatment (12.3 ± 0.8 nmol/g dry weight in C57BL/6J, 17.9 ± 1.6 nmol/g dry weight in the hearts of vehicle-treated db/db mice, and 16.8 ± 1.6 nmol/g dry weight in the hearts from empagliflozin-treated db/db mice).
Figure 4.
Cardiac Protein Expression and Regulation of Glucose and Fatty Acid Oxidation Enzymes and Its Regulation by Acetylation in Empagliflozin-Treated db/db Mice
Cardiac protein expression of glucose oxidation enzyme pyruvate dehydrogenase (PDH) (n = 6–7/group) (A), phosphorylation of PDH at the serine 293 residue (n = 6 to 7/group) (B), protein expression of pyruvate dehydrogenase kinase 4 (PDK4) (n = 6 to 7/group) (C), fatty acid β-oxidation enzymes long chain acyl CoA dehydrogenase (LCAD) (D), and β-hydroxyacyl CoA dehydrogenase (β-HAD) (E) (n = 6 to 7/group) are shown. Total cardiac lysine acetylation blot (F) with quantification (G) (n = 7/group) along with lysine acetylation levels of PDH, LCAD, and β-HAD normalized to the anti-acetyllysine immunoglobulin heavy chain (n = 5 to 6/group) (H–J) are shown. Data are presented as mean ± SEM. Data were analyzed by 1-way ANOVA followed by LSD post hoc test. *p < 0.05 was considered as a significantly different comparison with C57BL/6J + Vehicle. Abbreviations as Figures 1 and 2.
Discussion
The current study was undertaken to characterize the effects of empagliflozin on myocardial energetics and substrate use in diabetes with the specific aim of determining if the impact of SGLT2 inhibitors on the prevention of heart failure are related to improvements in cardiac energy production. Several important and translational insights have been gained in this regard. First and foremost, as has been shown previously, we observed that the decline in cardiac function in db/db mice was associated with a reduction in cardiac ATP production. This was due to a combined decrease in glucose oxidation and fatty acid oxidation rates (17). We also provide the novel observation that ketone oxidation is dramatically inhibited in the db/db mouse heart, contributing to the decrease in ATP production in these hearts. It is well known that in diabetes, decreased insulin stimulation of glucose oxidation in the failing heart contributes to both an impairment of heart efficiency and the development of cardiac failure 12, 17, 18, 19. In support of this concept, we have demonstrated that the low glucose oxidation rates in the db/db mouse hearts was accompanied by a decrease in cardiac efficiency. We also observed that compared with the hearts of C57BL/6J mice, db/db mouse hearts had an ∼36% reduction in cardiac ATP production, with fatty acid oxidation providing 42%, glucose oxidation 26%, ketone oxidation 10%, and glycolysis 22% of total ATP production. By contrast, in empagliflozin-treated db/db mice, cardiac ATP production rates increased by 31% compared with vehicle-treated db/db mice. This was not accompanied by a significant change in overall cardiac efficiency, suggesting that the cardiac benefits associated with empagliflozin were associated with an increase in cardiac ATP production. Furthermore, when db/db mouse hearts were perfused with higher ketone levels (which was seen in vivo with empagliflozin treatment), overall cardiac ATP production was increased, supporting the concept that the beneficial effects of empagliflozin are associated with an increased ATP production in the heart.
Of note, rates of ketone oxidation were markedly depressed in db/db mouse hearts. Intriguingly, this phenomenon did not seem to be responsible for the observed decrease in cardiac efficiency because infusion of db/db hearts with βOHB did not change the rates of either glucose or fatty acid oxidation. Importantly, exogenous βOHB infusion was associated with an overall increase in ATP production in db/db mouse hearts, not through changes in glucose or fatty acid oxidation, but rather via an independent increase in ATP production through ketone oxidation. Finally, empagliflozin treatment did not affect the rates of ketone oxidation in db/db hearts. Taken together, these data suggest that empagliflozin treatment is associated with an increase in overall cardiac ATP production, largely through a mechanism of increased glucose oxidation and fatty acid oxidation versus changes in glycolysis, or rates of ketone oxidation. Whereas empagliflozin does not alter rates of myocardial ketone oxidation per se, it is plausible that an increase in serum ketone bodies that occurs with empagliflozin may serve as an additional source of cardiac ATP production without changing/inhibiting rates of glucose or fatty acid oxidation.
A decrease in the glucose oxidation contribution to ATP production was evident in the db/db mouse hearts. The decrease in glucose oxidation did not appear to be due to an increase phosphorylation and inhibition of PDH, the rate-limiting enzyme for glucose oxidation (Figure 4B). The decreased glucose oxidation was also not the result of an increased acetylation of PDH (Figure 4H) (which inhibits PDH [32] and glucose oxidation). Rather, it appears the decrease in glucose oxidation observed in db/db mouse hearts was due to an increased expression of fatty acid oxidative enzymes (Figures 4D and 4E), which would decrease glucose oxidation secondary to an increase in fatty acid oxidation (i.e., the Randle Cycle [33]). Interestingly, the permissive effect empagliflozin exerts on ATP production from glucose oxidation did not appear to result from altered fatty acid oxidation enzyme expression in db/db mouse hearts.
In addition to the changes in myocardial energetics noted in this study, various other mechanisms by which SGLT2 inhibition confers cardiovascular benefits have been suggested 7, 8, 34. These include indirect effects to improve filling conditions, through a reduction in preload and afterload, effects on attenuating the expression of cardiac sodium-hydrogen exchanger (NHE) 7, 8, 34, 35, changes in adipokines, inflammatory biomarkers, natriuretic peptides, and epicardial adipose tissue volume (7). Importantly, the benefits empagliflozin exert on cardiac function appear to be independent of hyperglycemia. In animal models of heart failure that do not factor in diabetes (induced by transverse aortic constriction), empagliflozin has been reported to prevent the decline in ejection fraction (11). In this study, the benefits of empagliflozin were observed in addition to extrinsic factors that regulate cardiac function, such as hemodynamics and ketone oxidation, because these benefits were observed in the setting of matching preload and afterload, and in the presence of similar concentrations of insulin, fatty acids, glucose, or ketones. These data suggest that outcomes mediated by empagliflozin may be over and above indirect hemodynamic effects, a hypothesis that is supported by our current study demonstrating an effect to improve overall cardiac ATP generation through increases in rates of glucose oxidation. Recent data suggest that empagliflozin may inhibit myocardial NHE activity (36), in part through binding with the extracellular domain of NHE. In addition to reducing intracellular sodium and calcium, inhibition of NHE has been suggested to exhibit a protective effect on mitochondrial function, attenuating mitochondrial permeability transition pore opening and improving cardiac respiratory function (37).
Study limitations
First, we did not include an empagliflozin-treated C57BL/6J group because our primary focus was on the effects of empagliflozin in a diabetic setting. In future studies, the addition of an empagliflozin-treated C57BL/6J group would help assess whether empagliflozin increases cardiac glucose oxidation and fatty acid oxidation in a model in which glucose oxidation is not suppressed as in the db/db mouse heart. Second, we also did not include a C57BL/6J group in the ex vivo ketone study. Accordingly, it would be important in future investigations to investigate the short-term effect of ketones in a healthy heart. Third, we used 5 mmol/l of glucose in the diabetic mouse heart perfusions. This is subphysiological in a diabetic setting. However, 5 mmol/l glucose is within the normal glucose levels for both the healthy model and an empagliflozin-treated model, and was maintained throughout all groups for consistency. Finally, the concentration of insulin added to the ex vivo heart buffer 30 min after the initiation of perfusion was lower in the ex vivo ketone study versus the chronic empagliflozin feeding study, which should be considered when making comparisons between these 2 studies.
Conclusions
The present study provides important translational clues on the effects of empagliflozin on cardiac energetics in experimental diabetes. We conclude that the salutatory effects of SGLT2 inhibitors on cardiac failure may be, in part, due to an increase in cardiac ATP production via an increase in rates of cardiac oxidation of glucose and fatty acids, and increased supply and oxidation of ketones by the heart. We observed that overall rates of ketone oxidation were dramatically depressed in experimental diabetes, and remained unchanged with empagliflozin treatment, but could be increased by increasing ketone supply to the heart. This suggests that the ability of empagliflozin to increase circulating ketone levels may provide the heart with an additional source of energy to sustain contractile function.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Although SGLT2 inhibitors have been shown to reduce the rates of heart failure hospitalizations in individuals with type 2 diabetes, the underlying mechanisms by which these benefits occur remain unclear. Cardiac failure is characterized by changes in myocardial fuel metabolism and bioenergetics, and changes in substrate use have been demonstrated to play a causal and permissive role in the development and natural history of heart failure. There has been a growing interest in the hypothesis that SGLT2 inhibitors improve cardiac function through an effect on cardiac energy production, in part through increasing ketone body production/oxidation. To this aim, the present study provides a characterization of the cardiac energetics and fuel metabolic flux in an experimental model of diabetes treated with and without the SGLT2 inhibitor, empagliflozin.
TRANSLATIONAL OUTLOOK: We observed that experimental diabetes led to a decrease in cardiac function, coincident with a reduction in overall cardiac ATP production. This was due to a reduction in rates of glucose and ketone oxidation, with a concomitant increase in fatty acid oxidation. Empagliflozin treatment prevented the decrease in cardiac function and increased cardiac ATP production without changing overall cardiac efficiency. This increase in cardiac energy production was ascribed to a combined increase in glucose oxidation and fatty acid oxidation rates, without changes in the rates of glycolysis or ketone oxidation. However, increasing ketone supply to the heart may also contribute to the beneficial effects of empagliflozin on increasing cardiac ATP production. These data provide translational clues as to how SGLT2 inhibitors may prevent cardiac failure, through augmenting glucose and fatty acid oxidation. Contrary to prior hypotheses, increased rates of cardiac ATP production, as opposed to increased cardiac efficiency, may explain the beneficial effects of SGLT2 inhibitors on improving cardiac function in diabetes.
Footnotes
This work was supported through an unrestricted grant from Boehringer Ingelheim, and operating grants from the Canadian Institutes of Health Research to Drs. Verma and Lopaschuk. Dr. Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and has received speaker honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, NovoNordisk, and Sanofi; and received research support from Amgen, AstraZeneca, Boehringer Ingelheim, and Eli Lilly. Dr. Oudit has received speaker honoraria from Sanofi, Novartis, and Amgen. Dr. Lopaschuk is a shareholder in Metabolic Modulators Research Ltd; and has received grant support from Servier, Boehringer Ingelheim, Sanofi, and REMED Biopharmaceuticals. Dr. Marx has received support for clinical trial leadership from Boehringer Ingelheim; has served as a consultant to Amgen, Bayer, Boehringer Ingelheim, Sanofi, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, NovoNordisk; has received grant support from Boehringer Ingelheim and Merck Sharp & Dohme; and has served as speaker for Amgen, Bayer, Boehringer Ingelheim, Sanofi, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Lilly, and NovoNordisk. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Zinman B., Wanner C., Lachin J.M. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Neal B., Perkovic V., Mahaffey K.W. CANVAS Program Collaborative Group, Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 3.Farkouh M.E., Verma S. Prevention of heart failure with SGLT-2 inhibition: insights from CVD-REAL. J Am Coll Cardiol. 2018;71:2507–2510. doi: 10.1016/j.jacc.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 4.Cavender M.A., Norhammar A., Birkeland K.I., CVD-REAL Investigators and Study Group SGLT-2 inhibitors and cardiovascular risk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018;71:2497–2506. doi: 10.1016/j.jacc.2018.01.085. [DOI] [PubMed] [Google Scholar]
- 5.Verma S., Mazer C.D., Al-Omran M. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–407. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
- 6.Verma S., Mazer C.D., Fitchett D. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME(R) randomised trial. Diabetologia. 2018;61:1712–1723. doi: 10.1007/s00125-018-4644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018 doi: 10.1007/s00125-018-4670-7. In press. [DOI] [PubMed] [Google Scholar]
- 8.Verma S., McMurray J.J.V., Cherney D.Z.I. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol. 2017;2:939–940. doi: 10.1001/jamacardio.2017.1891. [DOI] [PubMed] [Google Scholar]
- 9.Shi X., Verma S., Yun J. Effect of empagliflozin on cardiac biomarkers in a zebrafish model of heart failure: clues to the EMPA-REG OUTCOME trial? Mol Cell Biochem. 2017;433:97–102. doi: 10.1007/s11010-017-3018-9. [DOI] [PubMed] [Google Scholar]
- 10.Verma S., Garg A., Yan A.T. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39:e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 11.Byrne N.J., Parajuli N., Levasseur J.L. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. J Am Coll Cardiol Basic Trans Science. 2017;1:347–354. doi: 10.1016/j.jacbts.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 13.Wende A.R., Brahma M.K., McGinnis G.R., Young M.E. Metabolic origins of heart failure. J Am Coll Cardiol Basic Trans Science. 2017;2:297–310. doi: 10.1016/j.jacbts.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh K.K., Shukla P.C., Yanagawa B. Regulating cardiac energy metabolism and bioenergetics by targeting the DNA damage repair protein BRCA1. J Thorac Cardiovasc Surg. 2013;146:702–709. doi: 10.1016/j.jtcvs.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima A., Zhang L., Huqi A. Acetylation contributes to hypertrophy-caused maturational delay of cardiac energy metabolism. JCI Insight. 2018;3:99239. doi: 10.1172/jci.insight.99239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karwi Q.G., Uddin G.M., Ho K.L., Lopaschuk G.D. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. 2018;5:68. doi: 10.3389/fcvm.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori J., Patel V.B., Abo Alrob O. Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail. 2014;7:327–339. doi: 10.1161/CIRCHEARTFAILURE.113.000672. [DOI] [PubMed] [Google Scholar]
- 18.Zhabyeyev P., Gandhi M., Mori J. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc Res. 2013;97:676–685. doi: 10.1093/cvr/cvs424. [DOI] [PubMed] [Google Scholar]
- 19.Mori J., Alrob O.A., Wagg C.S., Harris R.A., Lopaschuk G.D., Oudit G.Y. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol. 2013;304:H1103–H1113. doi: 10.1152/ajpheart.00636.2012. [DOI] [PubMed] [Google Scholar]
- 20.Huss J.M., Kelly D.P. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrannini E., Mark M., Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 22.Mudaliar S., Alloju S., Henry R.R. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? a unifying hypothesis. Diabetes Care. 2016;39:1115–1122. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 23.Lopaschuk G.D., Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metab. 2016;24:200–202. doi: 10.1016/j.cmet.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Aubert G., Martin O.J., Horton J.L. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu R., Oudit G.Y., Wang X. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol. 2009;297:H2096–H2108. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 26.Larsen T.S., Belke D.D., Sas R. The isolated working mouse heart: methodological considerations. Pflugers Arch. 1999;437:979–985. doi: 10.1007/s004240050870. [DOI] [PubMed] [Google Scholar]
- 27.Lopaschuk G.D., Witters L.A., Itoi T., Barr R., Barr A. Acetyl-CoA carboxylase involvement in the rapid maturation of fatty acid oxidation in the newborn rabbit heart. J Biol Chem. 1994;269:25871–25878. [PubMed] [Google Scholar]
- 28.Buchanan J., Mazumder P.K., Hu P. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 29.Mazumder P.K., O'Neill B.T., Roberts M.W. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 30.Alrob O.A., Sankaralingam S., Ma C. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res. 2014;103:485–497. doi: 10.1093/cvr/cvu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyck J.R., Barr A.J., Barr R.L., Kolattukudy P.E., Lopaschuk G.D. Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol. 1998;275:H2122–H2129. doi: 10.1152/ajpheart.1998.275.6.H2122. [DOI] [PubMed] [Google Scholar]
- 32.Ozden O., Park S.H., Wagner B.A. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med. 2014;76:163–172. doi: 10.1016/j.freeradbiomed.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randle P.J., Garland P.B., Hales C.N., Newsholme E.A. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;281:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 34.Sherman S.E., Bell G.O., Teoh H. Canagliflozin improves the recovery of blood flow in an experimental model of severe limb ischemia. J Am Coll Cardiol Basic Trans Science. 2017;3:327–329. doi: 10.1016/j.jacbts.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Packer M., Anker S.D., Butler J., Filippatos G., Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2:1025–1029. doi: 10.1001/jamacardio.2017.2275. [DOI] [PubMed] [Google Scholar]
- 36.Uthman L., Baartscheer A., Bleijlevens B. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61:722–726. doi: 10.1007/s00125-017-4509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javadov S., Huang C., Kirshenbaum L., Karmazyn M. NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J Mol Cell Cardiol. 2005;38:135–143. doi: 10.1016/j.yjmcc.2004.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.