Highlights
-
•
Implantable rectum spacers (IRS) separate the ano-rectal wall (ARW) from the prostate.
-
•
Dose-surface maps (DSMs) are a tool to take spatio-dosimetric info into account.
-
•
Spatio-dosimetric differences in ARW DSMs exist comparing IMRT with and without IRS.
-
•
IRS reduces the lateral and longitudinal extent of areas >50 Gy.
-
•
IRS reduces these high-dose areas in anterior and superior-inferior directions.
Keywords: Prostate cancer, Radiotherapy, Rectum spacer, Dose-surface maps, Toxicity reduction
Abstract
Background and purpose
To evaluate spatial differences in dose distributions of the ano-rectal wall (ARW) using dose-surface maps (DSMs) between prostate cancer patients receiving intensity-modulated radiation therapy with and without implantable rectum spacer (IMRT+IRS; IMRT−IRS, respectively), and to correlate this with late gastro-intestinal (GI) toxicities using validated spatial and non-spatial normal-tissue complication probability (NTCP) models.
Materials and methods
For 26 patients DSMs of the ARW were generated. From the DSMs various shape-based dose measures were calculated at different dose levels: lateral extent, longitudinal extent, and eccentricity. The contiguity of the ARW dose distribution was assessed by the contiguous-DSH (cDSH). Predicted complication rates between IMRT+IRS and IMRT−IRS plans were assessed using a spatial NTCP model and compared against a non-spatial NTCP model.
Results
Dose surface maps are generated for prostate radiotherapy using an IRS. Lateral extent, longitudinal extent and cDSH were significantly lower in IMRT+IRS than for IMRT−IRS at high-dose levels. Largest significant differences were observed for cDSH at dose levels >50 Gy, followed by lateral extent at doses >57 Gy, and longitudinal extent in anterior and superior-inferior directions. Significant decreases (p = 0.01) in median rectal and anal NTCPs (respectively, Gr 2 late rectal bleeding and subjective sphincter control) were predicted when using an IRS.
Conclusions
Local-dose effects are predicted to be significantly reduced by an IRS. The spatial NTCP model predicts a significant decrease in Gr 2 late rectal bleeding and subjective sphincter control. Dose constraints can be improved for current clinical treatment planning.
1. Introduction
Gastro-intestinal (GI) toxicity is a common side-effect of external beam radiation therapy (EBRT) for prostate cancer and has a negative impact on the quality of life even many years after the EBRT [1], [2], [3]. Various devices have been introduced to spare ano-rectal structures [4]. Endo-rectal balloons are being used to increase the distance from the dorsal and lateral rectal wall to the prostate, whereas implantable rectum spacers (IRS) separate the anterior rectal wall from the prostate by injection of an absorbable hydrogel [5], a hyaluronic acid [6], a saline-filled balloon [7], or a collagen implant [8]. Several studies have confirmed that an IRS decreases the rectal dose and consequently the acute rectal toxicity rate [9], [10], [11], [12], [13], [14], [15]. Furthermore, it has been established that an IRS decreases the late rectal toxicity rates [16], [17], leading to an increased cost-effectiveness [18]. Until now, the dosimetric impact of an IRS has been assessed quantitatively by dose-volume histograms (DVHs) obtained from the planned 3D dose distributions. From these studies, consensus exits that an IRS significantly reduces the dose exposure to the ano-rectal wall (ARW). However, spatial dosimetric information of the 3D dose distribution is lost by analysing DVHs or dose-surface histograms (DSHs), and therefore hampers to investigate the correlation between the shape and location of the ARW dose distribution with clinical outcome measures. Extraction of shape-based dose measures such as spatially correlated DSHs or contiguous-dose surface histograms (cDSHs) from dose-surface maps (DSMs) has been suggested as a valuable tool for advanced dose-response studies and to support a better selection of patients likely to benefit from the IRS [19], [20], [21], [22], [23]. Buettner et al. [24] quantified correlations between measures describing the shape and location of the dose distribution and different outcomes. Furthermore, inclusion of such spatio-dosimetric features into normal-tissue complication probability (NTCP) models has been shown to increase the predictive power over models based on DVH parameters solely [25], [26]. Hence better insights into the relationship between the ano-rectal dose distribution and (late) GI toxicity in EBRT of prostate cancer can be obtained.
The primary aim of the current study was to test the hypothesis that shape-based measures of the ARW surface dose distribution reveal a significant change in size, shape and location of the local surface dose distribution in patients undergoing intensity modulated radiation therapy with an IRS (IMRT+IRS) and without IRS (IMRT−IRS). To this end, spatial features from ARW DSMs and cDSHs were compared between these two groups. Furthermore, shape-based DSM parameters were used in combination with previously published spatial NTCP models to test the hypothesis that predicted complication rates for Grade 2 GI toxicity decrease for IMRT+IRS relative to IMRT−IRS. Finally, these results were compared with Grade 2 GI toxicity decrease derived from validated NTCP models based on DVH data solely.
2. Materials and methods
2.1. Patient selection and rectum spacer implantation
After approval by the local ethics committee, 26 consecutive patients with localized prostate cancer treated between January 2011 and June 2011 were included in this study. All patients had signed an informed consent. The patient and tumour characteristics are summarized in Table 1. Prognostic risk-group stratification of the patients was defined according to the D'Amico classification [27].
Table 1.
Patient (N = 26) and tumour characteristics.
| Age (years; median [range]) | 73 [56–82] |
| Prognostic risk group*: (no. of patients) | |
| 1- Low-risk | 8 (31%) |
| 2- Intermediate-risk | 11 (42%) |
| 3- High-risk | 7 (27%) |
| Prostate volume: (cm3; median [range]) | |
| CTV | 50 [25–130] |
| PTV | 134 [75–266] |
Abbreviations: CTV = clinical target volume; PTV = planning target volume.
Low-risk: no risk factors: PSA <10 ng/ml; Gleason score <7; cT-stage <2b; Intermediate-risk: one risk factor: PSA 10–20 ng/ml or Gleason score = 7 or cT-stage = 2b/c; High-risk: two risk factors or PSA >20 ng/ml or Gleason score >7 or cT-stage >2b/c.
An IRS gel (SpaceOAR™ System, Augmenix Inc., Waltham, MA) was injected in these patients between the prostate and the rectum prior to EBRT. The injection method has been described previously [5]. The amount of injected hydrogel was limited to 10 cm3 in all patients (only the first patient received 15 cm3). It maintains space for approximately 3 months and is compression resistant. The hydrogel is cleared in approximately 6 months via renal filtration [5].
2.2. Target volume definition and organ at risk delineation
Each patient underwent two computed tomography (CT) scans in supine position with a slice thickness of 5 mm; one prior to IRS implantation and one 3–5 days after IRS implantation. The resulting 52 CT scans were imported into the Pinnacle3 treatment planning system (Version 8.0 m, Philips Medical Systems, Fitchburg, USA) to design dose distributions for IMRT−IRS and IMRT+IRS (Fig. 1). Additionally, a T2-weighted transversal magnetic resonance imaging (MRI) scan was acquired after implantation for image registration with the post-implant CT-scan to enable soft tissue delineation of the prostate, the adjacent rectal wall and the IRS. The CT images were rigidly registered with the T2-weighted MRI scans by auto matching based on soft tissue landmarks. A knee and ankle rest was used to create a reproducible setup of the leg position both for the CT and the MRI scans. Patients were asked to have a fully bladder for both the planning CT scan and MRI scan. Treatment plans for IMRT−IRS and IMRT+IRS were planned on the respective CT scans to allow for dosimetric comparison.
Fig. 1.
Color-wash dose distribution in an axial plane before (a) and after (b) IRS gel injection in the same patient, with prostate (yellow) and PTV (red). Without IRS, the high-dose region >75% (yellow) overlaps with the anterior part of the rectum (brown), while with IRS in situ the high-dose region spans the IRS (black), and not the rectum. The 40% isodose contour (purple) overlaps the entire rectum in (a), whereas it overlaps the rectum partially in (b). Abbreviations: PTV = planning target volume; IRS = implantable rectum spacer. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Depending on the prognostic risk group the clinical target volume (CTV) was defined as the prostate only (CTV1), the prostate with the base of the seminal vesicles (CTV2) corresponding to the proximal 2–4 seminal vesicle slices, or the prostate with the whole seminal vesicles (CTV3) [28].
For the planning target volume (PTV), 8 mm lateral-anterior, 5 mm superior-inferior and 4 mm posterior margins were added to the CTV, as described in an earlier study [5]. On relevant CT image slices, the bladder, femoral heads, rectum and anal-canal were delineated as solid organs. The ano-rectum structure consists of the rectum and the anal-canal. The rectum was delineated from the top of the anal-canal up to the recto-sigmoid flexure. The anal-canal was considered as the distal 3 cm of the ano-rectum [29]. Only when the last 3 cm was obviously in the lumen of the rectum, the cranial boundary was adapted as the section below the lowest section with a visible rectum lumen [30]. In order to facilitate intra-patient comparison, the contours in the treatment plans for IMRT+IRS and IMRT−IRS were delineated over the same length in superior-inferior direction. Two independent observers performed the delineations (MP and BV). A pairwise Wilcoxon rank sum test is used to test the significant differences of prostate, PTV, and anorectum volumes between the spacer and no spacer groups, because differences in delineated volumes could have impact on the dosimetric results.
2.3. Treatment planning technique
All IMRT−IRS and IMRT+IRS plans were designed by inverse treatment planning using a direct machine parameter optimization (DMPO) algorithm for step-and-shoot IMRT with 5 coplanar 15 MV photon beams (gantry angles: 45°, 105°, 180°, 255°, 315°) [31]. The treatment planning technique has been described previously [5]. The prescribed dose to the PTV was 78 Gy in 2 Gy fractions [32], requiring at least 99% of the volume to receive 95% of the prescribed dose. The same dosimetric constraints were used for IMRT−IRS and IMRT+IRS plans, based on the relevant maximum tolerance dose (indicated as Dmax) and the maximum allowed relative volume receiving a certain dose level of xx Gy (indicated as VxxGy), as published by the Radiation Therapy Oncology Group (RTOG) for rectum and bladder [33]: V40 Gy(rectum) ≤ 60%, V75 Gy(rectum) ≤ 5%, Dmax(rectum) ≤ 76 Gy, V55 Gy(bladder) ≤ 50%, V70 Gy(bladder) ≤ 30%, V50 Gy(femoral heads) ≤ 5%. Since the V40 Gy and V75 Gy are well-known DVH parameters that are predictive for late rectal toxicity these measures were used to assess the plan quality [29], [34], [35].
2.4. Spatial analysis of the ano-rectal dose distribution
For each individual patient, DSMs for both the anal-canal wall and the rectum wall were generated from the 3D dose distributions of the IMRT−IRS and IMRT+IRS treatment plans by virtual unfolding of these structures, as previously described by Buettner and colleagues [24]. A DSM represents the 2D dose distribution over the outside of the (unfolded) rectum/anal canal wall of the ARW (Fig. 2). In the current work, DSMs were produced by first extracting the dose to the surface of the ARW contour at 100 equiangular points on every CT slice of the surface of the ARW contour and subsequent virtually cutting of this contour at its most posterior location. This was implemented using an in-house developed MATLAB software tool (The MathWorks, Natick, MA, USA).
Fig. 2.
Dose-surface maps of rectal wall (a,b) and anal wall (c,d) (in Gy) without IRS (a,c) and with IRS (b,d) in the same patient. The vertical axis corresponds to the superior-inferior direction, whereas the horizontal axis represents the circumferential direction: P, R, A, L. Abbreviations: IRS = implantable rectum spacer, P = posterior (P), right (R), anterior (A), left (L).
Shape-based dose measures were extracted from the DSMs following Buettner et al. [24]. The algorithm first generated binary DSMs by thresholding the primary DSMs at 38 dose levels ranging from 5−79 Gy into dose clusters. At each dose level an ellipse was fitted to the largest dose cluster. Lateral (LAT) extent in posterior-anterior-posterior direction and longitudinal (LONG) extent in superior-inferior direction were quantified by projecting the major and minor axes of this ellipse to the main axes of the DSMs. The non-circularity of the dose clusters was described by the eccentricity (ECC) of the ellipse. Furthermore, the algorithm assessed the contiguity of the single largest ARW area of the cDSH, by determining the single largest contiguous area of the DSM, as function of the dose threshold at a given dose level of xx Gy (cDSHxxGy).
Differences in LAT extent, LONG extent, ECC and cDSHxxGy between DSMs and cDSHs from IMRT+IRS and IMRT−IRS plans were compared statistically with a one-sided paired Wilcoxon signed rank test. The statistical analyses were performed using the Statistics and Machine Learning Toolbox from MATLAB software (Version 10.0, MathWorks, Inc., Natick, USA). LAT extent of 55 Gy, 67 Gy, and 71 Gy were compared because previous studies showed these parameters to be highly predictive for late rectal bleeding [25], [26]. The significance levels were established using a permutation test accounting for multiple testing, as described in previous work [22], [24], [25], [26]. Box plots were used to visualise the summary statistics as well as the individual and group differences. Each pair of dots linked by a dotted line represents a single patient from the study cohort, allowing for a two-level comparison of the differences in dosimetric measure. A p-value <0.05 was considered statistically significant. Shown p values were corrected for multiple testing. In order to correct for multiple testing, a fee step-down resampling algorithm was applied, taking advantage of the dependence structure between the cut-points [36]. The same framework was used in previously analysis, and further details on the method can be found in previously published work [24].
2.5. NTCP prediction
Previously published models to predict the NTCP based on shape-based features of the 3D dose distribution to the ARW were shown to have a higher predictive power for late GI toxicity than NTCP models based on DVHs [25], [26]. From these studies it was found that the LAT extent at 53 Gy and 55 Gy was one of the strongest predictor for subjective sphincter control and Grade 2 (Gr 2) late rectal bleeding, respectively [25], [26]. Furthermore LAT extent of 67 Gy and 71 Gy were compared because previous studies showed these parameters are highly predictive for late rectal bleeding [25], [26]. In the present analysis, we exploited these spatial NTCP models to assess differences in predicted complication rates between IMRT+IRS and IMRT−IRS plans. These differences were compared against predictions based on the Lyman-Kutcher-Burman (LKB) NTCP model with parameters from the QUANTEC study by Michalski et al. (n = 0.09, m = 0.13, TD50 = 76.9 Gy) taking solely the DVH data into account [37], [38].
2.6. Observed toxicity assessment
The complications were recorded in terms of the Expanded Prostate Cancer Index Composite (EPIC) Questionnaire to analyze quality-of-life (QoL) changes. The questionnaire consists of 50 items concerning urinary, bowel, sexual, and hormonal domains. The EPIC questionnaire is a validated domain-specific patient-reported questionnaire, and covers much more endpoints than the two clinical ones used in the DSM evaluation. Only these two are used to compare the observed with the predicted toxicities.
3. Results
3.1. Dosimetric plan evaluation with and without IRS
The median implanted IRS volume determined on the post-implant CT scan was 10.6 cc [range: 8.3–20.4 cm3]. The volumes data for both groups for CTV (prostate), PTV, rectum, and anorectum, are summarized in Table 2. The pairwise Wilcoxon rank sum test showed that there are no significant differences (at 5% significance level) in prostate (p = 0.269), PTV (p = 0.603), rectum (p = 0.086) and anorectum (p = 0.066) volumes between the spacer and no spacer groups. The median ano-rectum V40 Gy and V75 Gy significantly reduced between IMRT−IRS and IMRT+IRS from 53.4% to 47.6% (p = 0.036), and from 3.9% to 0.4% (p < 0.001), respectively.
Table 2.
Medians [range] of prostate, PTV and anorectal volumes (cm3) show no significant difference between patients with and without spacer.
| Without spacer | With spacer | ||
|---|---|---|---|
| Prostate | 52.4 [24.1–134.4] | 50.5 [25.3–130.3] | p = 0.269 |
| PTV | 136.2 [71.7–267.1] | 134.3 [74.8–266.1] | p = 0.603 |
| Rectum | 75.5 [34.1–374.5] | 51.3 [30.9–213.2] | p = 0.086 |
| Anorectum | 88.9 [45.7–378.8] | 61.3 [46.6–216.7] | p = 0.066 |
Abbreviations: PTV = planning target volume.
3.2. DSM analysis
For the rectal wall, LAT extent, LONG extent as well as cDSH areas were significantly lower in IMRT+IRS than in IMRT−IRS at high-dose levels (Fig. 3a). The largest significant differences were observed for cDSH areas at dose levels >50 Gy, followed by LAT and LONG extent at doses >57 Gy. For these three features, no significant differences were observed for dose levels <50 Gy. For LONG extent no significant differences were found for some high dose levels (Fig. 3a). For ECC no significant differences were found over the whole dose range (Fig. 3a). For the anal wall, LAT extent, ECC as well as cDSH areas were significantly lower in IMRT+IRS than in IMRT−IRS at high-dose levels (Fig. 3b). The largest significant differences were observed for LAT extent at doses >60 Gy. The box plots shown in Fig. 4 illustrate the summary statistics and density traces for LAT extent of 55 Gy, 67 Gy, and 71 Gy of the IMRT+IRS and IMRT−IRS plans. All box plots revealed a wide spectrum of values without apparent sub-group differentiation, but still a significantly lower LAT extent of 55 Gy, 67 Gy, and 71 Gy for IMRT+IRS than IMRT−IRS, respectively p = 0.046, p = 0.002, p < 0.001.
Fig. 3.
Significance level of differences in geometrical measures between IMRT+IRS and IMRT−IRS plans as function of the dose threshold levels for the rectum (a) and the anal (b) wall. Statistically significant differences are shown in red, black points are not significant. The horizontal dotted line represents the significance level of 0.05. Abbreviations: cDSH = contiguous-dose-surface histograms; Ecc = Eccentricity; Lat = Lateral (posterior-anterior-posterior) extent; Long = longitudinal (superior-inferior) extents.
Fig. 4.
Box plots comparing the relative lateral extent for dose levels of 55, 67 and 71 Gy for IMRT+IRS versus IMRT−IRS. The lines denote to paired observations between the same patient ± IRS.
3.3. NTCP prediction
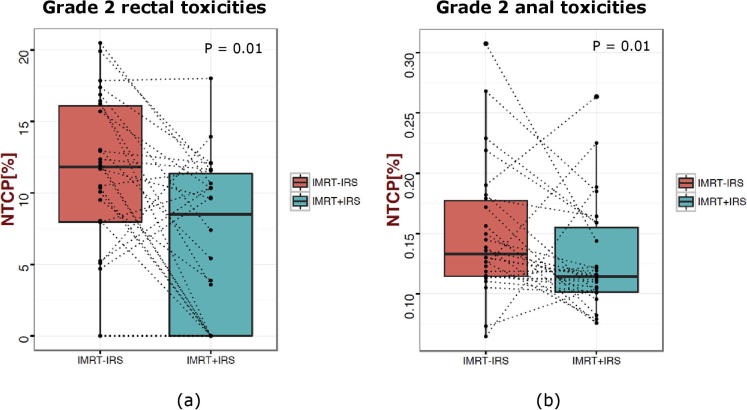
The box plots shown in Fig. 5 illustrate the predicted spatial NTCP rates for Gr 2 anal and Gr 2 rectal toxicity of IMRT−IRS and IMRT+IRS. A significant decrease in median rectal NTCP and in median anal NTCP is observed from 12% to 8.5% (p = 0.01) and from 13% to 11% (p = 0.01), respectively, when using an IRS. The median rectal NTCP for Gr 2 late rectal bleeding using the LKB-parameters were 10.1% (range: 3.9–18.6%) versus 3.8% (range: 0.1–11.8% for IMRT−IRS and IMRT+IRS, respectively.
Fig. 5.
Box plot comparing the predicted Grade 2 or more rectal toxicity rates (a) and Grade 2 or more anal toxicity rates (b) for IMRT+IRS versus IMRT−IRS using spatial NTCP models.
3.4. Observed toxicities
At the last day of EBRT uncontrolled leakage of feces, and more than once bloody stools during the last 4 weeks before the consult are observed in 12% and 19%, respectively. Two months after EBRT, these complaints are reported in 9% and 4%, respectively. Overall, after 2 and 6 years, no such problems are reported.
4. Discussion
In this study we showed that an IRS significantly changes the size, shape and location of the local surface dose distribution over ano-rectal structures in prostate cancer patients undergoing intensity modulated radiation therapy. We identified that an IRS particularly reduces the LAT and LONG extent of high-dose areas (>50 Gy) in anterior and superior-inferior directions. A correlation of these shape-based dose measures with predicted toxicity rates based on previously published NTCP models, showed that the IRS is expected to decrease the toxicity rates for Gr 2 late rectal bleeding and subjective sphincter control.
Several investigators demonstrated that minimising the volume of the ano-rectum receiving more than 70 Gy below 20% to be predictive of a very low incidence of Gr 2 late rectal bleeding [33], [34], [35]. Therefore, it is useful to prevent rectal volumes from being exposed to high radiation doses. Implantation of an IRS increases the distance between the prostate and the anterior rectal wall, and hence reduces the dose delivered to the ano-rectum [39], [40]. The current study is the first to systematically investigate shape-based differences in the ano-rectal wall dose distribution between prostate IMRT plans with and without IRS. Furthermore these spatial features were used to predict the expected NTCP reduction between IMRT+IRS and IMRT−IRS, and these were compared with the expected NTCP reduction based on DVH data solely.
A recently published prospectively randomized trial demonstrated the safety and effectiveness of a hydrogel IRS implantation in 149 consecutive patients [15]. This study showed that patients experienced 10-point declines in bowel quality of life at 15 months 11.6% and 21.4% of spacer and control patients, respectively. In contrast to this, Habl et al. reported the occurrence of 2 fistulas out of 91 patients [13]. However, there is a growing body of literature on prospective studies that supports the safety of IRSs in combination with EBRT, when practiced in experienced hands [14].
Previous studies investigated correlations between spatial 3D dose distributions to sub-regions of the ARW and (acute and late) GI toxicity [20], [21], [22], [23]. However, so far no comparative study has been performed in patients with an implanted IRS. Heemsbergen et al. described clinical evidence for a dose–effect relationship for bleeding and mucus loss within the dose to the upper 70–80% part of the ARW [20]. For soiling and faecal incontinence, they found a strong association within the dose to the inferior 40–50% to the ARW. As demonstrated in this study, an IRS reduces the dose-volume in the anterior upper and anterior inferior region of the ARW, and reduces the predicted late Gr 2 GI toxicity rates. Furthermore, Buettner et al. reported the strongest correlations between rectal bleeding and LAT extent at doses between 50 Gy and 60 Gy [26]. This confirms the importance of an IRS to reduce the LAT extent in high-dose areas. In addition, Mumbodh et al. obtained a relation between late rectal toxicity and irradiation of the upper part of the rectum [21]. An IRS decreases anterior extents in superior-inferior directions. Furthermore, Wortel et al. recently demonstrated significant relationships between acute rectal toxicity and the cranial-posterior rectal site [23], which, as we have shown in the current analysis, is decreased by an IRS.
DSM analysis is a well-known tool for advanced dose-response studies in prostate radiotherapy, which has successfully been applied to analyse radiation-induced rectal toxicity [21], [41], [42], [43], [44], [45]. Different algorithms exist to generate DSMs from the 3D dose distribution of the ARW. One of the restrictions of the used model is the fact that the DSM is constructed by cutting the rectum at the most posterior location point. However this most posterior rectum point could by chance be a long way to the left or the right of the centre of the contour, jumping between slices. This could give rise to discontinuities in the DSM. This could be corrected by using the cutting point as the point on the contour surface directly posterior to the centre of mass the centre of mass [45]. DSMs in general have some well-known limitations [24]. First, to unfold the ARW, different algorithms exist [21], [39], [40], so the same dose distribution can result in different DSMs. We used a slice-wise unfolding algorithm which has already been successfully used to examine the shape of the dose-distribution to the ARW [22], [24], [26]. Second, the DSMs were constructed on a single planning CT scan before treatment. This can lead to mismatch due to large inter-individual variations [46]. Motion of the ARW during treatment is a source of uncertainty that was not taken into account in the current study. However, we observed that in some patients a worsening of the rectal dosimetry occurred for IMRT+IRS, with consequently a worse NTCP prediction. It is well known that the rectum changes position, volume, and shape between treatment fractions. This is mainly caused by changes in rectal filling due to inclusion of gas bubbles and stool [47], [48]. In this case the distance between the prostate and ano-rectum is still increased due to IRS implantation, but the distance between the more cranial part of the rectum (above the IRS) decreased incrementally, as a consequence of which the latter part received a higher dose than the former part. Fenwick et al. concluded that setup-errors and ARW movement have only a slight impact on fits of NTCP models for a whole treatment period [49]. Furthermore, Thor and colleagues revealed a strong association with rectal morbidity at high doses (>55 Gy), for the planned and the simulated dose distributions including in particular random rectal motion [50]. Next, concerning the treatment planning technique: it is possible to reduce intermediate dose levels (30–50 Gy) in the ARW-region by an arc therapy (e.g. VMAT) with an avoidance-region near the rectum or by using strictly lateral beams to diminish the LAT and LONG extent, with consequently a decrease of Gr 2 late rectal bleeding [5]. It would also be of interest to compared spatial features between different treatment techniques (e.g. IMRT vs. VMAT). Further, no knowledge-based planning software technique based on the DVHs of previous plans with similar characteristics is used. This could be of interest to know if an additional stepwise optimization until stabilization of the OARs dose and the PTV homogeneity would provide the same results observed in this study. Finally, the models used for the spatial and non-spatial NTCP differ: a fair comparison between them is hindered by the fact that their were not derived for the same patient cohort. Nevertheless, both models have been published earlier and are used for NTCP prediction in literature. Currently, there is not enough clinical outcome data to compare the predicted and observed toxicity rates and to calibrate the models for patients who received an IRS. It is a topic of further research to find out whether the NTCP parameters derived from a patient cohort without IRS can be used for a cohort with IRS.
By using spatial NTCP models (Buettner) that were previously shown to have a higher predictive power than NTCP models based on DVH data (LKB model) [25], the current study predicts a statistically significant gain in NTCP when using an IRS in prostate cancer patients receiving IMRT. Comparing the NTCP predictions based on shape-based dose measures against those based on DVH measures a less pronounced decrease in toxicity rate was observed for the latter. This may be due to the fact that the LKB-model is more sensitive for high-dose than for intermediate-dose levels, and the relative volume receiving a high dose is smaller for the volume of the solid ano-rectum than for the 2D shape-based dose measures of the ARW (Buettner). This could explain the difference between the both models. The follow up data comparing the predicted and observed toxicity rates revealed no long toxicity rate in the observed group. Expanding patient data are needed to calibrate the NTCP models, which is beyond the scope of the current study.
Recently, decision rules were generated to support the clinician in selecting patients who are expected to benefit most from IRS implantation prior to IMRT planning [51]. This can be helpful for selecting patients for an IRS. Further systematic evaluations of dose, clinical and even genetic parameters are needed to evaluate which features are predictive to improve the benefit of an IRS, such that future treatments can be individually tailored to patients who will benefit most from IRS implantation [35], [51], [52], [53]. Spatial dose distributions in combination with the predicted GI toxicities can add extra information to be incorporated in more accurate decision support systems to further individualise prostate cancer radiotherapy treatment. Such investigations are mandatory to define the definitive role of an IRS in prostate cancer radiotherapy. Furthermore, patient decision aids can be developed with integration of the choice of an IRS to fulfil the complete personalized and participative medicine [52], [53], [54], [55], [56], [57].
In conclusion, we demonstrated statistically significant shape-based differences in ARW DSMs between IMRT+IRS and IMRT−IRS. An IRS reduces the LAT and LONG extent of high-dose areas (>50 Gy) in anterior and superior-inferior directions in 78 Gy IMRT plans. An IRS decreases the predicted toxicity rates for Gr 2 late rectal bleeding and subjective sphincter control. The extra spatial information can be added in decision support systems to optimise the decision to implant an IRS or not. The predictive power of spatial and non-spatial NTCP models has yet to be completely established for patients receiving IMRT with an IRS.
Acknowledgments
Acknowledgement
F.B. is supported by the UK Medical Research Council (MRC) via a Career Development Award (MR/M01536X/1).
Role of funding source
Part of this study was financially supported by Augmenix, Inc., Waltham, USA.
Conflict of interest statement
All authors declare to have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2018.10.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Glass A.S., Cowan J.E., Fuldeore M.J., Cooperberg M.R., Carroll P.R., Kenfield S.A. Patient demographics, quality of life, and disease features of men with newly diagnosed prostate cancer: trends in the PSA era. Urology. 2013;82(1):60–65. doi: 10.1016/j.urology.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 2.Krol R., Smeenk R.J., van Lin E.N., Hopman W.P. Impact of late anorectal dysfunction on quality of life after pelvic radiotherapy. Int J Colorectal Dis. 2013;28(4):519–526. doi: 10.1007/s00384-012-1593-5. [DOI] [PubMed] [Google Scholar]
- 3.Vanneste B.G., Van De Voorde L., de Ridder R.J., Van Limbergen E.J., Lambin P., van Lin E.N. Chronic radiation proctitis: tricks to prevent and treat. Int J Colorectal Dis. 2015;30(10):1293–1303. doi: 10.1007/s00384-015-2289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeenk R.J., Van Lin E.N.J.T. Application of anorectal sparing devices in prostate radiotherapy. Radiother Oncol. 2013;106(2):155–156. doi: 10.1016/j.radonc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Pinkawa M., Corral N.E., Caffaro M. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2011;100(3):436–441. doi: 10.1016/j.radonc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Prada P.J., Fernández J., Martinez A.A., de la Rúa A., Gonzalez J.M., Fernandez J.M. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007;69(1):95–102. doi: 10.1016/j.ijrobp.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Melchert C. Interstitial biodegradable balloon for reduced rectal dose during prostate radiotherapy: results of a virtual planning investigation based on the pre- and post-implant imaging data of an international multicenter study. Radiother Oncol. 2013;106(2):210–214. doi: 10.1016/j.radonc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Noyes W.R., Hosford C.C., Schultz S.E. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(5):1918–1922. doi: 10.1016/j.ijrobp.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Vanneste B.G., van De Beek K., Lutgens L., Lambin P. Implantation of a biodegradable rectum balloon implant: tips, Tricks and Pitfalls. Int Braz J Urol. 2017;24:43. doi: 10.1590/S1677-5538.IBJU.2016.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanneste B.G.L., van wijk Y., Lutgens L.C., Van Limbergen E.J., van Lin E.N., van de Beek K. Dynamics of rectal balloon implant shrinkage do not affect anorectal dose and late rectal complication risk in prostate VMAT. Strahlenther Onkol. 2018;194(1):31–40. doi: 10.1007/s00066-017-1222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song D.Y. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. Int J Radiat Oncol Biol Phys. 2013;87(1):81–87. doi: 10.1016/j.ijrobp.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strom T.J. A dosimetric study of polyethylene glycol hydrogel in 200 prostate cancer patients treated with high-dose rate brachytherapy ± intensity modulated radiation therapy. Radiother Oncol. 2014;111(1):126–131. doi: 10.1016/j.radonc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Habl G. Acute toxicity and quality of life in patients with prostate cancer treated with protons or carbon ions in a prospective randomized phase II study-the IPI trial. Int J Radiat Oncol Biol Phys. 2016;95(1):435–443. doi: 10.1016/j.ijrobp.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Fagundes M., Han-Chih Chang J., Michalski J., Soffen E., Davis B., Pisansky T. In Regard to Habl et al. Int J Radiat Oncol Biol Phys. 2016;96(1):241–242. doi: 10.1016/j.ijrobp.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Mariados N. hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Hamstra D.A. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Pinkawa M., Berneking V., König L., Frank D., Bretgeld M., Eble M.J. Hydrogel injection reduces rectal toxicity after radiotherapy for localized prostate cancer. Strahlenther Onkol. 2017;193(1):22–28. doi: 10.1007/s00066-016-1040-6. [DOI] [PubMed] [Google Scholar]
- 18.Vanneste B.G., Pijls-Johannesma M., Van De Voorde L., van Lin E.N., van de Beek K., van Loon J. Spacers in radiotherapy treatment of prostate cancer: is reduction of toxicity cost-effective? Radiother Oncol. 2015;114(2):276–281. doi: 10.1016/j.radonc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Hoogeman M.S., van Herk M., de Bois J., Muller-Timmermans P., Koper P.C., Lebesque J.V. Quantification of local rectal wall displacements by virtual rectum unfolding. Radiother Oncol. 2004;70(1):21–30. doi: 10.1016/j.radonc.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Heemsbergen W.D., Hoogeman M.S., Hart G.A., Lebesque J.V., Koper P.C. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(4):1011–1018. doi: 10.1016/j.ijrobp.2004.07.724. [DOI] [PubMed] [Google Scholar]
- 21.Munbodh R., Jackson A., Bauer J., Schmidtlein C.R., Zelefsky M.J. Dosimetric and anatomic indicators of late rectal toxicity after high-dose intensity modulated. Radiation therapy for prostate cancer. Med Phys. 2008;35:2137–2150. doi: 10.1118/1.2907707. [DOI] [PubMed] [Google Scholar]
- 22.Buettner F., Gulliford S.L., Webb S., Partridge M. Using dose-surface maps to predict radiation-induced rectal bleeding: a neural network approach. Phys Med Biol. 2009;54(17):5139–5153. doi: 10.1088/0031-9155/54/17/005. [DOI] [PubMed] [Google Scholar]
- 23.Wortel R.C., Witte M.G., van der Heide U.A., Pos F.J., Lebesque J.V., van Herk M. Dose-surface maps identifying local dose-effects for acute gastrointestinal toxicity after radiotherapy for prostate cancer. Radiother Oncol. 2015;117(3):515–520. doi: 10.1016/j.radonc.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Buettner F., Gulliford S.L., Webb S., Sydes M.R., Dearnaley D.P., Partridge M. Assessing correlations between the spatial distribution of the dose to the rectal wall and late rectal toxicity after prostate radiotherapy: an analysis of data from the MRC RT01 trial (ISRCTN 47772397) Phys Med Biol. 2009;54(21):6535–6548. doi: 10.1088/0031-9155/54/21/006. [DOI] [PubMed] [Google Scholar]
- 25.Buettner F., Gulliford S.L., Webb S., Partridge M. Modeling late rectal toxicities based on a parameterized representation of the 3D dose distribution. Phys Med Biol. 2011;56(7):2103–2118. doi: 10.1088/0031-9155/56/7/013. [DOI] [PubMed] [Google Scholar]
- 26.Buettner F., Gulliford S.L., Webb S., Sydes M.R., Dearnaley D.P., Partridge M. The dose-response of the anal sphincter region–an analysis of data from the MRC RT01 trial. Radiother Oncol. 2012;103(3):347–352. doi: 10.1016/j.radonc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 27.D'Amico A.V. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 28.Makarov D.V. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters S.T., Lebesque J.V., Heemsbergen W.D., van Putten W.L., Slot A., Dielwart M.F. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64(4):1151–1161. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Vordermark D., Schwab M., Ness-Dourdoumas R., Sailer M., Flentje M., Koelbl O. Association of anorectal dose-volume histograms and impaired fecal continence after 3D conformal radiotherapy for carcinoma of the prostate. Radiother Oncol. 2003;69(2):209–214. doi: 10.1016/j.radonc.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Bratengeier K., Meyer J., Flentje M. Pre-segmented 2-Step IMRT with subsequent direct machine parameter optimisation - a planning study. Radiat Oncol. 2008;3:38. doi: 10.1186/1748-717X-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollack A., Zagars G., Starkschall G., Antolak J.A., Lee J.J., Huang E. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 33.Lawton C., Michalski J., El-Naqa I. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiorino C., Valdagni R., Rancati T., Sanguineti G. Dose-volume effects for normal tissues in external radiotherapy: pelvis. Radiother Oncol. 2009;93(2):153–167. doi: 10.1016/j.radonc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Valdagni R. Is it time to tailor the prediction of radio-induced toxicity in prostate cancer patients? Building the first set of nomograms for late rectal syndrome. Int J Radiat Oncol Biol Phys. 2012;82(5):1957–1966. doi: 10.1016/j.ijrobp.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Westfall P.H., Young S.S. Wiley; New York: 1993. Resampling-based multiple testing: examples and methods for p-value adjustment. [Google Scholar]
- 37.Rancati T. Fitting late rectal bleeding data using different NTCP models: results from an Italian multi-centric study (AIROPROS0101) Radiother Oncol. 2004;73:21–32. doi: 10.1016/j.radonc.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Michalski J.M., Gay H., Jackson A., Tucker S.L., Deasy J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mok G., Benz E., Vallee J.P., Miralbell R., Zilli T. Optimization of radiation therapy techniques for prostate cancer with prostate-rectum spacers: a systematic review. Int J Radiat Oncol Biol Phys. 2014;90(2):278–288. doi: 10.1016/j.ijrobp.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 40.Pinkawa M. Spacer application for prostate cancer radiation therapy. Future Oncol. 2014;10(5):851–864. doi: 10.2217/fon.13.223. [DOI] [PubMed] [Google Scholar]
- 41.Meijer G.J., van den Brink M., Hoogeman M.S., Meinders J., Lebesque J.V. Dose–wall histograms and normalized dose–surface histograms for the rectum: a new method to analyze the dose distribution over the rectum in conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:1073–1080. doi: 10.1016/s0360-3016(99)00270-9. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Nieto B., Fenwick J.F., Nahum A.E., Dearnaley D.P. Biological dose surface maps: evaluation of 3D dose data for tubular organs. Radiother Oncol. 2001;61(Suppl 1):S52. [Google Scholar]
- 43.Tucker S.L., Zhang M., Dong L., Mohan R., Kuban D., Thames H.D. Cluster model analysis of late rectal bleeding after IMRT of prostate cancer: a case–control study. Int J Radiat Oncol Biol Phys. 2006;64:1255–1264. doi: 10.1016/j.ijrobp.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 44.van Lin E.N.J.T., Kristinsson J., Philippens M.E.P., Philippens M.E., de Jong D.J., van der Vight L.P. Reduced late rectal mucosal changes after prostate three-dimensional conformal radiotherapy with endorectal balloon as observed in repeated endoscopy. Int J Radiat Oncol Biol Phys. 2007;67:799–811. doi: 10.1016/j.ijrobp.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 45.Shelley L.E.A., Scaife J.E., Romanchikova M., Harrison K., Forman J.R., Bates A.M. Delivered dose can be a better predictor of rectal toxicity than planned dose in prostate radiotherapy. Radiother Oncol. 2017;123(3):466–471. doi: 10.1016/j.radonc.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dréan G., Acosta O., Ospina J.D., Simon A., Haigron P., Lafond C. Identification of a rectal subregion highly predictive of rectal bleeding in prostate cancer IMRT. Radiother Oncol. 2016;119(3):388–397. doi: 10.1016/j.radonc.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Huang T.C., Chou K.T., Yang S.N., Chang C.K., Liang J.A., Zhang G. Fractionated changes in prostate cancer radiotherapy using cone-beam computed tomography. Med Dosim. 2015;40(3):222–225. doi: 10.1016/j.meddos.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z., Yang Z., Wang J., Hu W. Dosimetric impact of different bladder and rectum filling during prostate cancer radiotherapy. Radiat Oncol. 2016;2(11):103. doi: 10.1186/s13014-016-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fenwick J.D. Impact of dose-distribution uncertainties on rectal ntcp modeling. II: uncertainty implications. Med Phys. 2001;28(4):570–581. doi: 10.1118/1.1355307. [DOI] [PubMed] [Google Scholar]
- 50.Thor M., Apte A., Deasy J.O., Karlsdóttir À., Moiseenko V., Liu M. Dose/volume-response relations for rectal morbidity using planned and simulated motion-inclusive dose distributions. Radiother Oncol. 2013;109(3):388–393. doi: 10.1016/j.radonc.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanneste B.G., Hoffmann A.L., van Lin E.N., Van De Voorde L., Pinkawa M., Lambin P. Who will benefit most from hydrogel rectum spacer implantation in prostate cancer radiotherapy? A model-based approach for patient selection. Radiother Oncol. 2016 doi: 10.1016/j.radonc.2016.08.026. S0167-8140(16)34290-6. [DOI] [PubMed] [Google Scholar]
- 52.West C. The REQUITE project: validating predictive models and biomarkers of radiotherapy toxicity to reduce side-effects and improve quality of life in cancer survivors. Clin Oncol (R Coll Radiol) 2014;26(12):739–742. doi: 10.1016/j.clon.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 53.van Wijk Y., Vanneste B.G.L., Walsh S., van der Meer S., Ramaekers B., van Elmpt W. Development of a virtual spacer to support the decision for the placement of an implantable rectum spacer for prostate cancer radiotherapy: comparison of dose, toxicity and cost-effectiveness. Radiother Oncol. 2017 doi: 10.1016/j.radonc.2017.07.026. S0167-8140(17)32485-4. [DOI] [PubMed] [Google Scholar]
- 54.van Wijk Y., Vanneste B.G.L., Jochems A., Walsh S., Pinkawa M., Lambin P. Development of an iso-toxic model integrating validated genetic markers of toxicity and tumour control probability: a multifactorial decision support system for prostate cancer radiotherapy to support the decision to place an implantable rectum spacer. Acta Oncol. 2018;28:1–7. [Google Scholar]
- 55.Vanneste B.G.L., Van Limbergen E.J., van de Beek K., van Lin E., Lutgens L.C., Lambin P. A biodegradable rectal balloon implant to protect the rectum during prostate cancer radiotherapy for a patient with active Crohn disease. TIPS-RO. 2018;6:1–4. doi: 10.1016/j.tipsro.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stacey D. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;28(1) doi: 10.1002/14651858.CD001431.pub4. CD001431. [DOI] [PubMed] [Google Scholar]
- 57.Lambin P. Decision support systems for personalized and participative radiation oncology. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.01.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.