Abstract
BACKGROUND:
From the past five decades, metronidazole and tinidazole have been used for treating nonresistant and resistant giardiasis and trichomoniasis. However, due to the occurrence of drug resistance to standard therapy idealizes us to explore some additional therapies which is cost-effective, easy accessibility, and natural which has least side effects. Manuka honey obtained from Leptospermum scoparium is well known for its antibacterial and wound healing properties and is thought to be a better option as an additional therapy.
OBJECTIVE:
The present study was conducted to find out the effect of manuka honey on anaerobic protozoans that includes Giardia and Trichomonas under in vitro conditions in comparison to metronidazole and tinidazole.
MATERIALS AND METHODS:
Axenic culture of Giardia lamblia strain Portland 1 and Trichomonas vaginalis strain 413 was used for drug sensitivity assay to tinidazole, metronidazole, and manuka honey with the highest concentration of 17.1 μg/ml, 24.7 μg/ml, and 50%v/v by using (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole). For this, head-to-head comparison has been done and IC 50 of the standard drug as well as manuka honey was calculated.
RESULTS:
The result showed that percentage inhibition on the growth of both the parasites is dependent on concentration as well as exposure time of the drug. The calculated IC 50 was found to be 5.6%v/v and 1.5%v/v for manuka honey with respect to G. lamblia and T. vaginalis.
CONCLUSION:
The present study suggests that manuka honey can be used as an additional therapy for the patient with giardiasis or trichomoniasis. However, in vivo study in the near future will elucidate more about the effectiveness of honey in treating parasitic infections.
Keywords: (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Giardia lamblia; in vitro; manuka honey; metronidazole; tinidazole; Trichomonas vaginalis
Introduction
Anaerobic parasites especially Giardia lamblia and Trichomonas vaginalis are the major cause of other crucial diseases such as dysentery, inflammatory diseases, and sexually transmitted infections that are affecting millions of people each year all over the globe.[1,2] Geographical distribution reveals the worldwide distribution of both the parasites, in which giardiasis infects about 6%–8% of children and 2% of adult in developed countries. Furthermore, about 33% of population in developing countries are having giardiasis.[3] Same time global incidence of trichomoniasis is in between 170 and 180 million cases annually.[4]
At present, metronidazole and tinidazole are the most optimum drug therapy for treating both giardiasis and trichomoniasis.[5,6] However, resistant clinical isolates to metronidazole have been consistently notified,[7] which suggest that these parasites can easily acquire the resistance to metronidazole.[8] In spite of this, in some of the countries, metronidazole is only the licensed drug for trichomoniasis and desirable drug for giardiasis.[6] Metronidazole often leads to unusual side effects such as vertigo, headache, nausea with a pinch of metallic taste, and in some extreme cases, it may responsible for causing toxicity inside the brain and pancreatitis, over prolonged duration of treatment.[5] Moreover, studies also reported for the mutagenic and carcinogenic effect of this particular drug on bacteria and rodent model if taken for longer duration[9] and therefore all together enforces to look around some other alternative therapies.
Manuka honey has been derived from the nectar of manuka (Leptospermum scoparium) which is known to be indigenous shrubs of New Zealand. The intrinsic quality of manuka honey is reflected in its broad-spectrum ability to inhibit a wide variety of bacterial and yeast pathogens including multidrug-resistant bacteria.[10] Beside this, manuka honey is also found to be preventive in the biofilms' formation as well as in disrupting preformed S. pyogenes[11] and Clostridium difficile[12] biofilms. Moreover, till now, there is no evidence of the development of resistance to manuka honey under in vitro conditions as compared to other standard antibiotic.[10] It has been seen that the presence of various active compounds not solely permits most honey to be unambiguously extensive spectrum, but at the same time, it diminishes the capacity of many microbial populations for evolving as a resistant one.[13] In addition, honey has potential for immune system stimulation and can promote wound healing.[14] Hence, here in this study, we tried to find out whether the manuka honey has some inhibitory effects on anaerobic protozoans mainly G. lamblia and T. vaginalis or not, with two well-known standard antiprotozoal drugs, i.e., metronidazole and tinidazole. Therefore, a head-to-head comparison has been done among all three compounds on two important anaerobic protozoans, and to our best knowledge, till now, there were no similar studies that reported for antiprotozoal effect of manuka honey which have been published.
Materials and Methods
Antiprotozoal drug and manuka honey
Tinidazole and metronidazole were procured from Sigma-Aldrich and stock solution of both the drugs at the concentration of 1 μg/ml were prepared by dissolving it in dimethylsulfoxide (Sigma-Aldrich) and finally stored it at − 20°C. Manuka honey 20+ was dissolved in distilled water; subsequently, different concentrations of honey expressed as %v/v were prepared at the same day of experiment. A starting concentration that was used in all the experiments was 200 μM, which yielded a maximum concentration of 17.1 μg/ml metronidazole and 24.7 μg/ml tinidazole.
Culture
The G. lamblia Portland 1 and T. vaginalis 413 strain wereused throughout the experiment which were perpetuated axenically in medium, i.e., Diamond's modified TYI-S-33 by incubating it at 37°C,[15] and is boosted with 10% of heat-inactivated fetal bovine serum and two antibiotics, i.e., penicillin and streptomycin. Both Giardia and Trichomonas trophozoites were routinely subcultured after every 72 h. In all experiments, trophozoites were used at their log phase of growth.
In vitro drug-sensitive assay
Drug sensitivity has been performed to all above compounds using (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay with slight modification.[16] Briefly, Giardia and Trichomonas culture were harvested from previous 24 h old cultures and resuspended into the fresh medium. The total count of parasite was arranged to 3 × 105 parasites/ml in medium by hemocytometer. Trophozoites of each parasite were detached for the preparation of inoculum which was achieved through constant chilling and shaking the cultures on ice for 15–20 min. Final concentrations of drug in rows from A to H were chiefly 100, 50, 25, 12.5, 6.3, 3.2, 1.6, and 0.8 μM, respectively. Each test of the study included control, i.e., without drug and blank wells, i.e., medium only. Double dilutions were carried out down the plate, and the left 100 μl from each of the wells of row H was discarded. A 100 μl amount of culture consisting both media as well as parasites was added to each of the rows starting from A to H. Absorbance was taken at wavelength of 540 nm, and Elisa Reader was used to take reading of each experiment. Each experiment has been performed three times, and in each plate, two wells were of the same concentration of drug.
Percentage inhibition at each drug concentration was calculated using formula:
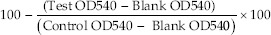
Statistical analysis
SPSS version 22 (IBM Corp., Armonk, NY) was used for statistical analysis of data. Data are presented as a mean ± standard deviation. Paired and unpaired Student's t-test were applied to collate the variables. P < 0.05 was considered to be significant, P < 0.005 more significant, and P < 0.0005 highly significant.
Results
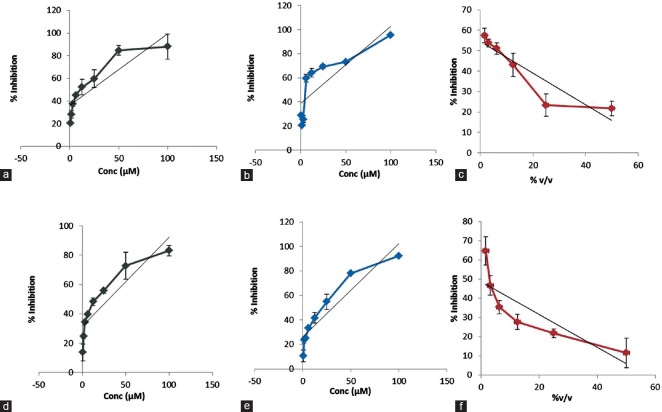
The comparative effects of manuka honey, metronidazole, and tinidazole on the growth of the parasite were observed by exposing axenic culture of G. lamblia and T. vaginalis shown in Figure 1 to different concentrations of drug for the period of 24 h and 48 h. Percentage growth inhibition versus different drug concentration has been plotted to compute IC50 of individual drug which is shown in Figure 2 and Table 1. The IC50 was found to be 5.6%v/v for manuka honey, 21 μM for metronidazole, and 17.86 μM for tinidazole on G. lamblia. However, having same time exposure, the IC50 value was 1.5%v/v, 30.75 μM, and 31.37 μM for manuka honey, metronidazole, and tinidazole, respectively, on T. vaginalis.
Figure 1.
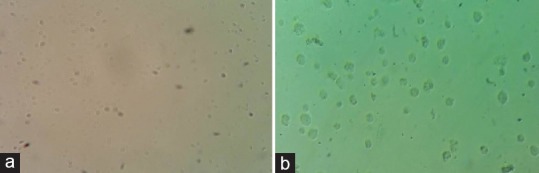
(a) Growth of axenic culture of Giardia lamblia (×40 magnification). (b) Growth of axenic culture of Trichomonas vaginalis (×40 magnification)
Figure 2.
Graph plot for computing IC50 value of each compound (manuka honey as test drug and metronidazole and tinidazole as standard drug) used in the study after 24 h of drug incubation for Giardia lamblia and Trichomonas vaginalis. (a) Giardia metronidazole (24 h), (b) Giardia tinidazole (24 h), (c) Giardia manuka honey (24 h), (d) Trichomonas metronidazole (24 h), (e) t richomonas tinidazole (24 h), (f) Trichomonas manuka honey (24 h)
Table 1.
Comparison of IC50 of manuka honey and standard drug (metronidazole and tinidazole) on Fh ‘qch’ ‘K lah’ and Sqhbgnlnm’r u‘fhm’khr after 24 h drug exposure by sensitivity assay

Head-to-head comparisons have been done to demonstrate percentage growth inhibition of both the parasite at two different times of drug exposure. Intra- and inter-drug variability has been checked on Giardia and Trichomonas by exposing the axenic culture to various concentrations of metronidazole, tinidazole, and manuka honey for 24 and 48 h which is shown in Figures 3-8.
Figure 3.
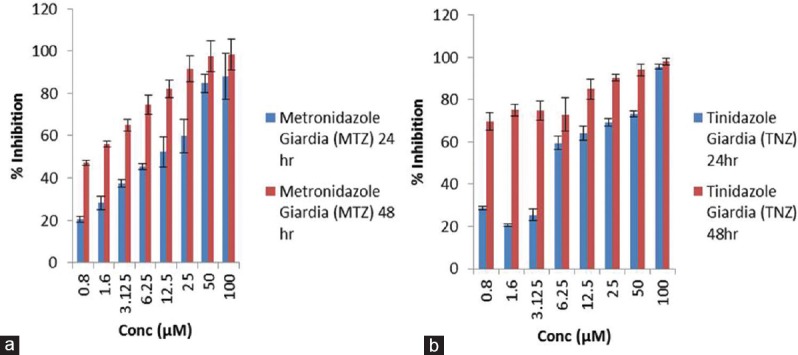
Graph plot to show variation in percentage inhibition of metronidazole (a) and tinidazole (b) on Giardia lamblia at two time points, i.e., 24 and 48 h. Data were expressed as mean ± standard deviation
Figure 8.
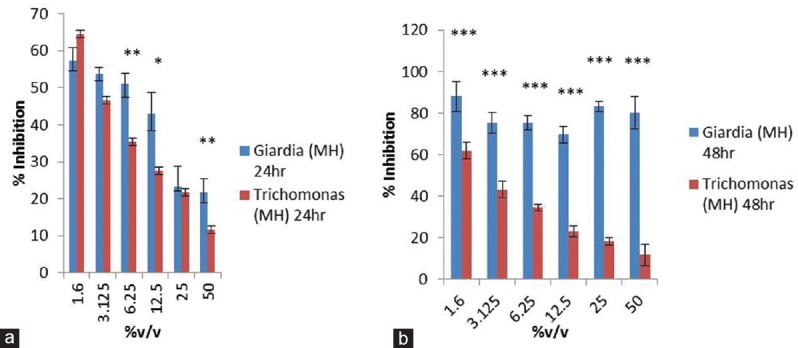
Graph plot to show intervariation in percentage inhibition of Giardia lamblia and Trichomonas vaginalis after manuka honey exposure for 24 (a) and 48 h (b). Data were expressed as mean ± standard deviation (*) =p< 0.05, (**) =p< 0.005, (***) =p< 0.0005
Figure 4.
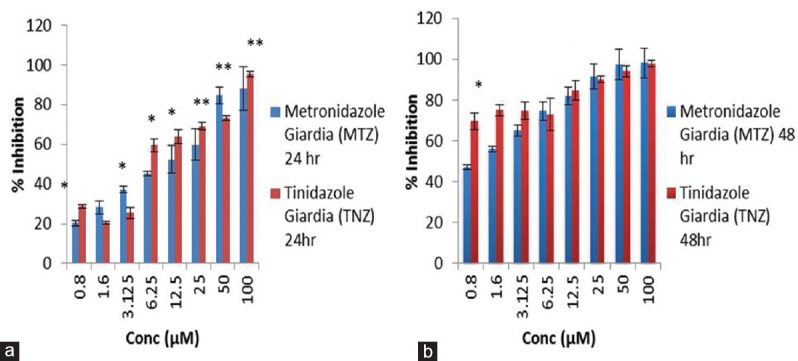
Graph plot to show intervariation in percentage inhibition between metronidazole and tinidazole on Giardia lamblia at two time points, i.e., 24 h (a) and 48 h (b). Data were expressed as mean ± standard deviation (*) =p< 0.05, (**) =p< 0.005, (***) =p< 0.0005
Figure 5.
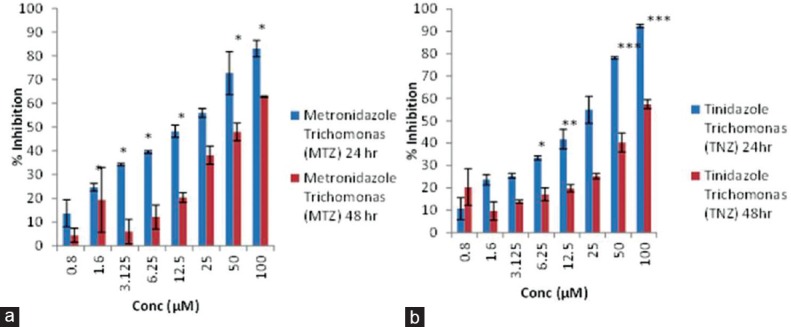
Graph plot to show variation in percentage inhibition of metronidazole (a) and tinidazole (b) on Trichomonas vaginalis at two time points, i.e., 24 and 48 h. Data were expressed as mean ± standard deviation (*) =p< 0.05, (**) =p< 0.005, (***) =p< 0.0005
Figure 6.
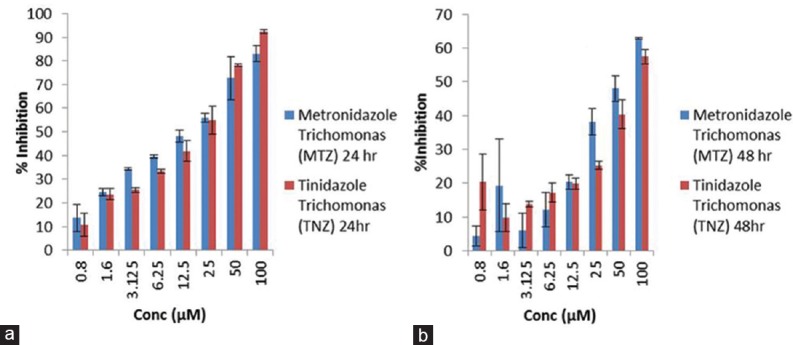
Graph plot for to show variation in percentage inhibition between metronidazole and tinidazole on Trichomonas vaginalis at two time points, i.e., 24 (a) and 48 h (b). Data was expressed as mean ± standard deviation
Figure 7.
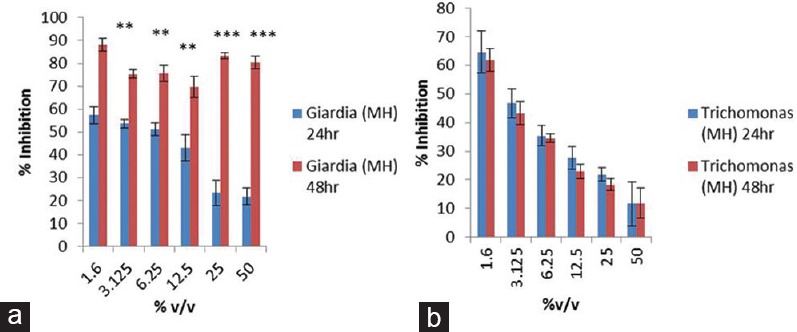
Graph plot to show intravariation in percentage inhibition of manuka honey on Giardia lamblia (a) and Trichomonas vaginalis (b) at different time points, i.e., 24 and 48 h. Data were expressed as mean ± standard deviation (*) =p< 0.05, (**) =p< 0.005, (***) =p< 0.0005
Looking into variation in percentage inhibition of metronidazole and tinidazole on G. lamblia at two time points, i.e., 24 and 48 h reveals that metronidazole has >95% of inhibitory effect on Giardia when exposed for 48 h as compared to 24 h which has < 90% at maximum drug concentration and also inhibition is dependent on concentration. However, almost similar pattern of inhibition on the growth of Giardia has been obtained by treating it with tinidazole, whereas, in case of T. vaginalis, the vice versa effect has been observed in respect of G. lamblia.
Moreover, comparison in percentage inhibition between metronidazole and tinidazole on G. lamblia at two time points illustrated that both the drugs have more inhibitory effect at 48 h drug exposure in a concentration-dependent manner. However, in others, i.e., T. vaginalis, the inhibitory effect of metronidazole, is more prominent than tinidazole at 24 h till 12.5 μM. However, mixed response of both the drug has been obtained after 48 h exposure.
Consequences of manuka honey on the growth of both the parasites reveals inhibition in Giardia growth i.e., >57% at 24 h and >88% at 48 h at the lowest concentration of the manuka honey (1.6%v/v) which was analyzed in the experiment. However, inhibition in the growth was also obtained at higher concentration (>80%) after 48 h exposure, while >60% inhibition in the growth of Trichomonas was obtained at 1.6%v/v of the honey at both the time points. Furthermore, percentage inhibitory effect of manuka honey was >57% and 64% on Giardia and Trichomonas, respectively, during 24 h exposure as compared to 48 h exposure which was >88% and 61%.
Discussion
The study was accomplished to analyze the outcome of manuka honey on the growth of two parasites that is G. lamblia and T. vaginalis, and the study illustrates that manuka honey has growth inhibitory effect on both the parasites. However, concentration and time exposure were the two major outcomes which came out of the study which shows that less concentrated honey has more inhibitory effect on both Giardia and Trichomonas as compared to more. This finding is consistent with the previous study in which study on clinical isolates under in vitro condition of methicillin-susceptible and methicillin-resistant staphylococci were delineated to be an equivalence susceptibility of manuka honey, having minimum inhibitory concentrations reported as < 3%v/v.[17] Inhibition on growth of Giardia trophozoites was found to be highly significant (p < 0.0002) when exposed to highest concentration of both the drugs for 24 h which is contrary to 48 h drug exposure, p < 0.01 at lowest drugs concentration. Also the result is consistence with recently published study showing time-dependent inhibition of parasitic growth in metronidazole-treated cultures where significant ≥90% G. lamblia trophozoites' growth inhibition was observed after 42, 48, and 72 h.[18] Furthermore, trophozoites of Giardia and Trichomonas were found to be more sensitive to manuka honey after 48 h exposure in comparison to 24 h exposure which suggests that density of the honey might be the reason that is responsible for penetration inside the cell membrane of the parasite. Looking into intervariation in percentage inhibition of growth in Giardia and Trichomonas, it illustrates that Giardia is more susceptible as compared to Trichomonas after manuka honey exposure.
Intravariation in percentage inhibition of manuka honey on G. lamblia at two time points unveil that inhibition was significantly higher, p < 0.0000002, p < 0.000004, p < 0.003, p < 0.0007, p < 0.0001, and p < 0.0002, at 50%, 25%, 12.5%, 6.25%, 3.12%, and 1.6%, concentration of manuka honey as it was compared at 24 and 48 h. Nevertheless, statistically, no significant difference was observed at two different time points in case of T. vaginalis. Intervariation in percentage inhibition of G. lamblia and T. vaginalis after manuka honey exposure tells that though percentage inhibition in the growth (57.36 ± 3.58 and 64.66 ± 2.75) was achieved at lowest concentration of manuka honey, i.e., 1.6%v/v, statistically significant difference in inhibition pattern at 24 h between both the protozoans has been achieved at 50%v/v and 6.25%v/v, (p < 0.002 and p < 0.003). In contrast, highly significant difference was obtained at each concentration by looking the same at 48 h of exposure on Giardia and Trichomonas but also has same inhibition concentration (1.6%v/v) that shows maximum percentage inhibition of 88.07 ± 7.28 for Giardia and 61.95 ± 3.95 for Trichomonas which finally illustrates the importance of manuka honey in successfully inhibiting the growth of anaerobic protozoans by incorporating the correct exposure time and appropriate concentration. Furthermore, G. lamblia is found to be more responsive to manuka honey killing as compared to Trichomonas.
Natural health products, such as nutraprevention and probiotics adaptogenic plants (echinacea and ginseng) are giving encouraging results, in improving the overall health of a human which limits the benefits of allopathic medicine.[19] Recently, garlic available commercially as Tomex was tested for its efficacy against metronidazole under in vitro conditions on T. vaginalis, and the results observed on the parasite growth inhibition were found to be in proportion to incubation time and different concentration of Tomex and finally concluded for its potential for phytotherapeutic agent.[20] In the present study also, appropriate concentration and exposure time were found to be important factor in inhibiting growth of both the parasites. Due to inadequacy in expansion of novel antibiotic classes to cure various infections that are mostly the outcome of resistant microorganisms, natural product like honey is progressively appreciated for having diversified range of antibacterial effect and also for its amazing treating capacity during chronic wound infections.[16] Recent studies also elucidated about immunomodulatory properties of honey which significantly affect the production of cytokine, i.e., tumor necrosis factor-α thus, plays an important role in innate immunity[17] which is consistent with other natural products which enhances the immune system by participating as immune modulatory agents. Although the mechanism of action by which manuka honey employs its antimicrobial effects remains an area of active study, most of the studies illustrate that manuka honey activity is mainly because of methylglyoxal (MGO) existence, which produced from nonenzymatically conversion of dihydroxyacetone, a component found in manuka nectar.[21] Hence, the antiparasitic activity shown by manuka honey may be due to the presence of MGO.
Conclusion
The present study shows that manuka honey is effective in inhibiting the growth of both anaerobic protozoans when it is least concentrated. There are lots of individual factors in honey which contributes to its antimicrobial activity which needs to be explored in case of parasitic growth; however, association of MGO of manuka honey with antiprasitic growth is an interest in need. Furthermore, further study is needed to evaluate efficacy of manuka honey under in vivo condition that enumerates much more about its efficacy as well as its mechanism of action. Apart, as there were no such similar studies that demonstrate the effect of manuka honey on Giardia lamblia and T. vaginalis provides preliminary data for further studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24:110–40. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cudmore SL, Delgaty KL, Hayward-McClelland SF, Petrin DP, Garber GE. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin Microbiol Rev. 2004;17:783–93. doi: 10.1128/CMR.17.4.783-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epidemiology & Risk Factors. Centers for Disease Control And Prevention. [Last accessed on 2015 Jul 21]. Available from: http://www.cdc.gov/parasites/giardia/epi.html .
- 4.World Health Organization. WHO/HIV_AIDS/2001.02. Geneva: World Health Organization; 2001. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overviews and Estimates. [Google Scholar]
- 5.Escobedo AA, Cimerman S. Giardiasis: A pharmacotherapy review. Expert Opin Pharmacother. 2007;8:1885–902. doi: 10.1517/14656566.8.12.1885. [DOI] [PubMed] [Google Scholar]
- 6.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–64. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tejman-Yarden N, Millman M, Lauwaet T, Davids BJ, Gillin FD, Dunn L, et al. Impaired parasite attachment as fitness cost of metronidazole resistance in Giardia lamblia. Antimicrob Agents Chemother. 2011;55:4643–51. doi: 10.1128/AAC.00384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali V, Nozaki T. Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by “amitochondriate” protozoan parasites. Clin Microbiol Rev. 2007;20:164–87. doi: 10.1128/CMR.00019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindmark DG, Müller M. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob Agents Chemother. 1976;10:476–82. doi: 10.1128/aac.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair SE, Cokcetin NN, Harry EJ, Carter DA. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis. 2009;28:1199–208. doi: 10.1007/s10096-009-0763-z. [DOI] [PubMed] [Google Scholar]
- 11.Maddocks SE, Lopez MS, Rowlands RS, Cooper RA. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology. 2012;158:781–90. doi: 10.1099/mic.0.053959-0. [DOI] [PubMed] [Google Scholar]
- 12.Hammond EN, Donkor ES, Brown CA. Biofilm formation of Clostridium difficile and susceptibility to manuka honey. BMC Complement Altern Med. 2014;14:329. doi: 10.1186/1472-6882-14-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Carter DA, Turnbull L, Rosendale D, Hedderley D, Stephens J, et al. The effect of New Zealand Kanuka, manuka and clover honeys on bacterial growth dynamics and cellular morphology varies according to the species. PLoS One. 2013;8:e55898. doi: 10.1371/journal.pone.0055898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannabathula S, Skinner MA, Rosendale D, Greenwood JM, Mutukumira AN, Steinhorn G, et al. Arabinogalactan proteins contribute to the immunostimulatory properties of New Zealand honeys. Immunopharmacol Immunotoxicol. 2012;34:598–607. doi: 10.3109/08923973.2011.641974. [DOI] [PubMed] [Google Scholar]
- 15.Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–8. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 16.Bénéré E, da Luz RA, Vermeersch M, Cos P, Maes L. A new quantitative in vitro microculture method for Giardia duodenalis trophozoites. J Microbiol Methods. 2007;71:101–6. doi: 10.1016/j.mimet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J R Soc Med. 1999;92:283–5. doi: 10.1177/014107689909200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed SE, Kabbashi AS, Koko WS, Ansari MJ, Adgaba N, Al-Ghamdi A. In vitro activity of some natural honeys against Entamoeba histolytica and Giardia lamblia trophozoites. Saudi J Biol Sci. 2017 doi: 10.1016/j.sjbs.2017.06.004. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Alternat Med. 2005;2:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim AN. Comparison of in vitro activity of metronidazole and garlic-based product (Tomex®) on Trichomonas vaginalis. Parasitol Res. 2013;112:2063–7. doi: 10.1007/s00436-013-3367-6. [DOI] [PubMed] [Google Scholar]
- 21.Cokcetin NN, Pappalardo M, Campbell LT, Brooks P, Carter DA, Blair SE, et al. The antibacterial activity of Australian Leptospermum honey correlates with methylglyoxal levels. PLoS One. 2016;11:e0167780. doi: 10.1371/journal.pone.0167780. [DOI] [PMC free article] [PubMed] [Google Scholar]