Abstract
OBJECTIVE:
This study was aimed to assess the effect of unilateral common carotid artery occlusion on brain pathophysiology in rats pretreated with subchronic hypoxia.
MATERIALS AND METHODS:
Rats (200 ± 20 g) were randomized into three groups: Group 1 served as sham, Group 2 were normoxic (21% O2 and 79% N2), and Group 3 were hypoxia preconditioned (10% O2 and 90% N2) for 21 days before left common carotid artery occlusion (LCCAO). The LCCAO was done for 75 min followed by reperfusion for 12 h. Neurological scores were recorded. Serum malondialdehyde (MDA) and nitric oxide (NO) levels at pre- and 12 h post-LCCAO were measured. Brain histopathological assessments were also done.
RESULTS:
Higher neurological deficits scores in Group 2 as compared to Group 3 rats were noticed. Serum MDA and NO levels at 12 h post-LCCAO in Group 2 rats showed significant elevation as compared to preocclusion levels. Group 3 rats did not show such elevations. On histopathology of left and right cerebral hemispheres of Group 1 (sham) did not show any specific changes. In Group 2 rats, the right cerebral hemisphere (nonoccluded) showed no areas of ischemia-induced brain changes, but in the left side (occlusive), there were features of ischemic brain damage including cerebral edema. In the case of Group 3 rats, there were less ischemic damages in the left occluded side as compared to the left side of the Group 2 rats.
CONCLUSION:
This study clearly demonstrates that subchronic hypoxia pretreatment can reduce ischemic brain injury by unilateral common carotid artery occlusion in rats.
Keywords: Brain histopathology, cerebral edema, left common carotid artery occlusion, neurological scores, pretreated hypoxia
Introduction
The significance of cerebrovascular ischemia in medical practices made medical scientists to take up preparation of various experimental ischemic models on rodents.[1] It is reported that after occlusion of extracranial or common carotid artery the functional competence of the intracranial collateral circulation through internal carotid artery and basilar artery protects further ischemic brain damages except some preexisting vascular irregularities or exposure of hypoxia.[2] It is also evidenced that the duration to tolerate cerebral ischemia depends on the severity of ischemia, duration of reperfusion, and many other conditions including metabolic reserves. It is further stated that different areas of brain differentially tolerate ischemia.[3] Studies reported that rats engaged in exercise for 2 weeks before transient forebrain experimental ischemia show marked improvement in neuronal damages.[4] Hypoxia leads to altered intracellular chemical microenvironment by increasing calcium concentration, lipooxygenase, lipid peroxidation, and cyclooxygenase.[5] Cells exposed to hypoxia follow adaptive changes for its survival. One such response is to activate hypoxia-inducible factors 1α. Hypoxia microenvironment stimulates cell signaling mechanism to stabilize many proteins such as signal transducer and activator of transcription (STAT3) and protein kinase B (Akt) to adapt against ischemic assaults.[6]
The study was aimed to evaluate the consequences of the left common carotid artery occlusion for 75 min and subsequent reperfusion for 12 h on brain histopathology in normoxic (21% O2) and subchronic hypoxia pretreated (10% O2 for 21 days) rats.
Materials and Methods
Animals
Adult male Wistar-strain albino rats (Rattus norvegicus; 180–200 g, b.wt.) from central animal house of BLDE Deemed to be University Vijayapur, Karnataka, were obtained. Rats were divided into the three groups (n = 6 in each group) under 12 h light: 12 h dark cycle to avoid diurnal rhythm. Metabolic wire cage (60 cm × 30 cm × 20 cm) is used to keep maximum 3 rats in each one. Standard pellets (Hindustan Lever, Mumbai, India) were used as normal diet, and drinking water were provided ad libitum during the entire experimental protocol.
Experimental groups
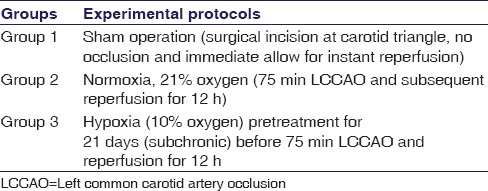
Table 1 shows the study design of experimental groups of rats which are divided into three groups with six rats in each group.
Table 1.
Experimental groups of rats (m=6 in each group)
Subchronic hypoxia exposure
The hypoxia was set up with the inflow of a combination of air (oxygen 10% and nitrogen 90%) that was regulated by an oxygen analyzer (model 175518A, Gold Edition, Vacuum Med, Sanchung, Taipei). CO2 was absorbed by soda lime (27 granules), and excess humidity was removed by a desiccators. The room temperature was maintained at 24°C–26°C. Twice in a week for 1 h, the hypoxia chamber was opened to clean the cages and provide food and water. The exposure to low oxygen in all the rats of Group 3 was continued for 21 days.[7]
Experimental protocol
Group 1 served as sham, Group 2 was normoxic, and Group 3 served as hypoxic. Electrocardiography (ECG) and pneumogram were recorded using Biopac (MP 45) before, during the surgical procedure and after 12 h of reperfusion. Data were evaluated and analyzed with SPSS version 16.0 (SPSS Inc., Chicago, USA).
Group 1 (sham) was taken as a control group; Group 2 (normoxic) was exposed with normal atmospheric oxygen under normal pressure, and Group 3 (hypoxic) was exposed to subchronic sustained hypoxia by keeping the rats in 10% oxygen and 90% nitrogen for 21 days as per the standard protocol before left CCA occlusion. The occlusion (genuine and sham) of the left common carotid artery at carotid triangle level was performed in all the rats of Group 1, Group 2, and Group 3 under proper anesthesia (Ketamine, 60 mg/kg b.wt and Xylazine, 6 mg/kg b.wt.).[8] Sham operations in Group 1 rats were performed by similar left common carotid artery occlusion (LCCAO) technique except occlusion and allowing instant reperfusion.[9] Once the left carotid artery at the carotid triangle was exposed, it was occluded for 75 min in all the rats of Group 2 and Group 3 [Figure 1]. After 75 min, carotid occlusion was slowly released, surgical incision was closed with catgut, and reperfusion was allowed for 12 h till sacrifice.[10] Utmost postoperative care was taken for each rat. At the end of 12 h, all three groups of rats were euthanized by deep anesthesia. Before the sacrifice, blood samples were collected from retro-orbital plexus. Blood samples were also collected in a similar way before LCCAO from all the rats of Group 1, Group 2, and Group 3. The brain was carefully dissected out and immersion fixed in 10% formalin for 2 days. Serum malondialdehyde (MDA) levels as a marker of oxidative stress were assessed in all the three groups of rats before the occlusion and after 12 h of reperfusion by Kei Satoh method.[11] Similarly, serum nitric oxide (NO), a nitrosative stress marker, and also a regulator of oxidative stress were estimated using Griess reagents spectrophotometrically.[12]
Figure 1.
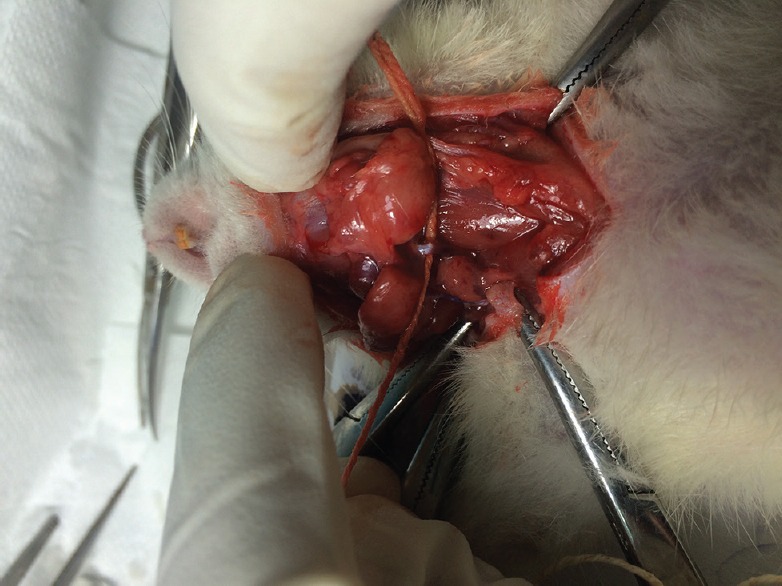
Ligation of the left common carotid artery with silicon thread
Neurological scores
Neurologic examinations were performed on all the rats in Group 1, Group 2, and Group 3 in just before the sacrifice. Neurological scores were evaluated as per standard protocol.[13]
Histopathological procedure
Cerebral hemispheres were sliced into coronal slices of 2-mm thickness. The brain stem was cut vertical to its long axis. The cerebellum also cut into two slices perpendicularly to the folia of the dorsal angle of each hemisphere. Bilateral blocks of the brain were embedded in paraffin wax and sections were cut on a rotary microtome from 2.0 μ to 3.0 μ and stained with routine hematoxylin and eosin (H and E) stains. The brain sections of all animals were examined under a conventional light microscopy (Olympus CH20i) with Samsung Digital Color Camera, Model No. SDC-242, N. J 07094, U. S. A.
Statistical analysis
Results were obtained as mean ± standard deviation values for each group. To determine the significance of intergroup differences, one-way analysis (ANOVA) followed by “post hoc t-test” were done.
Ethics
Animal experiments were conducted as per the ethical norms approved by CPCSEA, the Ministry of Social Justice and Empowerment, Government of India (Reg. No. 1076/PO/ERs/S/07 CPCSEA dated Aug 20.,2014), and Institutional Animal Ethical Committee, BLDE University, Vijayapur (BLDE/BPC/641/2016-17 dated Aug 22, 2016).
Results and Discussion
Physiological and biochemical parameters
The ECG pattern (heart rate [HR]), pneumogram (respiratory rate [RR]), and blood pressure (systolic blood pressure [BP], diastolic BP, and mean arterial pressure) were also found within normal ranges during the entire experimental protocol in all the animals of all the groups till sacrifice [Table 2]. Results suggested that no vital parameters such as HR, BP, and RR were affected in all the three groups during the entire experimental procedure. After 12 h of reperfusion and just before sacrifice, we observed cognitive and neurological impairments in Group 2 and Group 3 rats [Table 3]. Results showed the maximum neurological deficit scores in Group 2 (normoxia) rats (35/60; 58%) followed by Group 3 (subchronic hypoxia pretreatment) rats (26/60; 43%). Oxidative and nitrosative stress levels were measured by assessing serum MDA, and NO in Group 2 (normoxic) rats following 12 h of reperfusion showed a significant increase compared to preocclusion levels. In the case of subchronic hypoxia pretreated rats (Group 3), no such significant alteration of MDA and NO levels (pre vs post) were observed [Figures 2 and 3]. Higher MDA and NO levels in Group 3 rats before surgery (pre-LCCAO) may be due to hypoxia-induced lipid peroxidation, and subsequently, the formation of peroxynitrites due to exposure of subchronic hypoxia but such preconditioning actually facilitate greater adaptation from cerebral ischemic stress (post-LCCAO) induced lipid peroxidation. The increase of lipid peroxidation or rise of reactive oxygen species and reactive nitrogen species may probably induce neuronal injury from apoptosis and further contribute to ischemic brain injuries. Our results indicate a greater protection from oxidative stress-induced inflammatory response due to LCCAO in rats preconditioned with hypoxia.[14]
Table 2.
Cardiovascular and respiratory parameters of all the group of rats
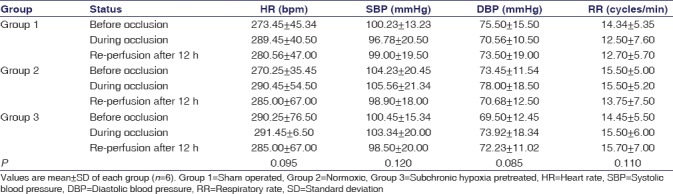
Table 3.
Neurological deficit scores after 12 h reperfusion and before sacrifice

Figure 2.
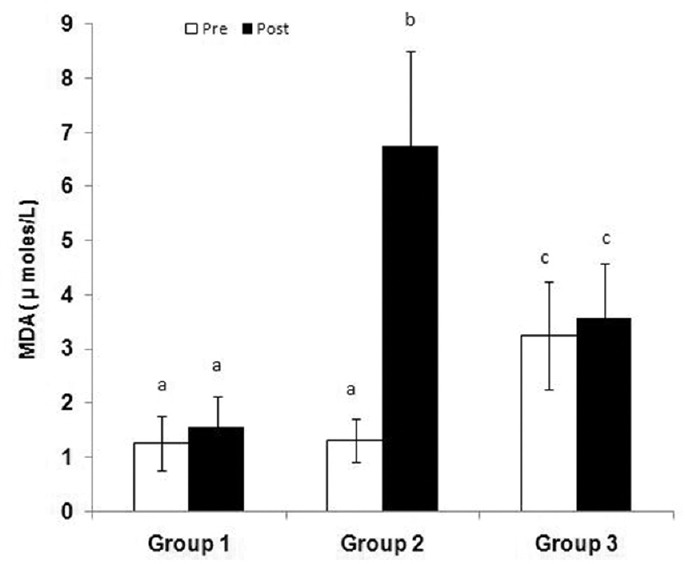
Serum malondialdehyde levels in Group 1 (sham), Group 2 (normoxia), and Group 3 (subchronic hypoxia pretreated) rats before (pre) left common carotid artery occlusion and 12 h after reperfusion (post).n= 6 rats in each group. Values with different superscripts (a and b) are significantly differ from each other (P< 0.05)
Figure 3.
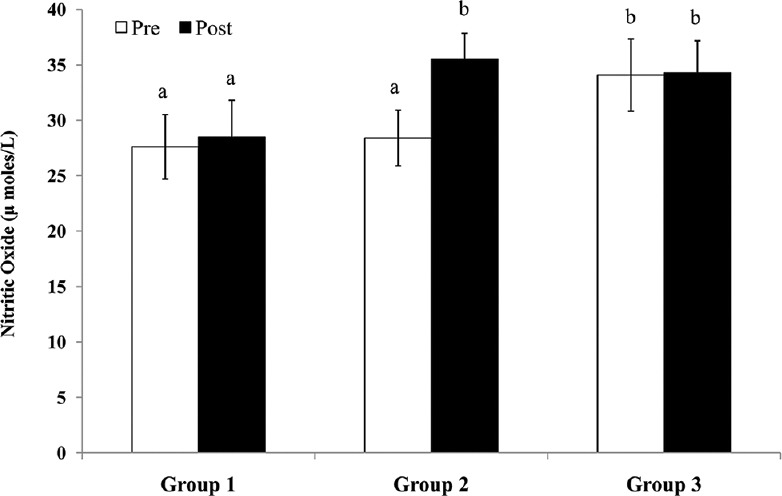
Serum nitric oxide levels in Group 1 (sham), Group 2 (normoxia), and Group 3 (subchronic hypoxia pretreated) rats before (pre) left common carotid artery occlusion and 12 h after reperfusion (post).n= 6 rats in each group. Values with different superscripts (a and b) are significantly differ from each other (P< 0.05)
Histopathological observations in cerebral cortex
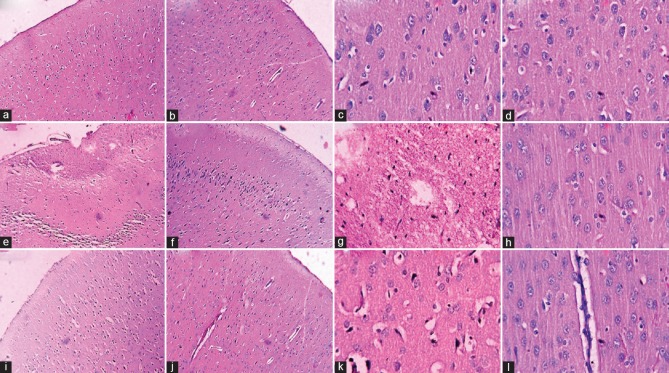
Sections of all the three groups under H and E stain were studied serially on the left and right cerebral hemispheres of the brain (n = 6), from the frontoparietal cortex and dorsolateral portion of the neostriatum. Figure 4a to l shows H- and E-stained sections of the left and right cerebral hemispheres of the brain. The sections from sham animals showed normal cerebral cortex consisting of gray matter made of cell bodies of neurons and glial cells along with their processes blood vessels and white matter made up of bundles of axons. No visible pathology was noted in the group. The blood vessels appeared normal on both sides of the hemispheres [Figure 4c and d]. Group 2 (normoxic with left-CCA occlusion) reveals histopathological changes in gray matter of left cerebral cortex with a decrease in number of pyramidal cells and stellate (granular) cells which appear to be small, multipolar, and vacuolated to eosinophilic cytoplasm and pyknotic nuclei [Figure 4e]. There were foci of wedge-shaped areas of cerebral infarcts with diffuse interstitial edema of the cerebrum Figure 4g]. The junction between gray and white matter was blurred. Right cerebral hemisphere of the brain (nonoccluded side) showed normal brain parenchyma [Figure 4f and h]. Group 3 (subchronic hypoxia pretreated rats with left-CCA occlusion subsequently sacrifice after 12 h) shows in the gray matter of cerebral cortex, a decrease in number of pyramidal cells, small multipolar stellate (granular) neurons, and vacuolated eosinophilic cytoplasm with pyknotic nuclei having features of early cellular damage (Red cells) in the left cerebral hemisphere [Figure 4i]. There were also foci of interstitial edema of the left cerebrum but without the features of cerebral ischemia which were observed in case of Group 2 experimental animals [Figure 4k]. Besides these findings, blurring of the junction between gray and white matter was also noticed in Group 3 rats (subchronic hypoxia pretreated). Right cerebral hemisphere of brain (nonoccluded side) of Group 3 rats showed normal brain parenchyma. Dilated blood vessels, lined with prominent blood vessels cells on the right side of the hemispheres, were also observed in Group 3 rats [Figure 4j and l].
Figure 4.
Hematoxylin and Eosin stain showing (a) sham's left cerebral cortex (×10), (b) sham's right (×10), (c) sham's left cerebral cortex (×40), (d) sham's right (×40), (e) normoxic left (occluded) cerebral cortex (×10), (f) normoxic right (nonoccluded) (×10), (g) normoxic left (occluded) cerebral cortex (×40), (h) normoxic right (nonoccluded) (×40), (i) hypoxia pretreated left (occluded) cerebral cortex (×10), (j) hypoxia pretreated right (non occluded) (×10), (k) hypoxia pretreated left (occluded) cerebral cortex (×40), and (l) hypoxia pretreated right (nonoccluded) (×40)
Histopathological observations in subcortex
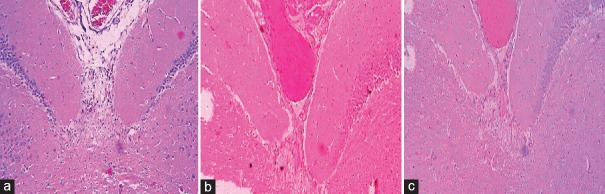
There were no changes in the subcortical structures of sham-operated rats [Figure 5a]. In case of Group 2 (normoxic), most of the caudate nuclei, internal capsule, globus pallidus, substantia nigra, and putamen of basal ganglia showed small lacunar infarcts [Figure 5b], whereas Group 3 (subchronic hypoxia pretreated) rats such small lacunar infarcts were found relatively less as compared to normoxic rats. This group showed pallor of staining and vacuolization of the white matter [Figure 5c].
Figure 5.
Photomicrograph stained with hematoxylin and eosin stain showing subcortical structures mainly the caudate nuclei, internal capsule, globus pallidus, substantia nigra, and putamen of basal ganglia (×10) of (a); sham's left and right side, (b); normoxic left (occluded side) and right (nonoccluded) side, and (c); subchronic hypoxia pretreated left (occluded side) and right (nonoccluded) side
The partial/incomplete infarct section, defined as the area with lack of staining (pallor or failure to perfuse) of the cortex, brain edema, cell body degeneration, and suggestive of early focal neuronal damage (red neurons), was determined from serially cut sections from the frontoparietal cortex and dorsolateral portion of the neostriatum of both left (ischemia-induced) and right brain hemispheres of all the sham, normoxic, and subchronic hypoxia pretreated rats (n = 6). In normoxic rats, there were no areas of ischemia-induced brain changes in the right side of the (nonoccluded) cerebral hemisphere and left side (left occluded side), there were features of diffuse global ischemic brain damage involving mainly frontal, frontoparietal regions of the cortex, and basal ganglia (striatum), particularly in the lateral segment of the caudate.[15] The striking feature in both sides of the brain in subchronic hypoxia pretreated rat was the dilated blood vessels lined by prominent endothelial cells signifying a compensatory mechanism in response to subchronic hypoxia. The reduced brain damage in subchronic hypoxic pretreated rats was well correlated morphologically with vascular adaptive changes whereas the changes of occipital cortex and medial striatum were concerned only uncommonly and variable elsewhere. In contrast to the left side of the subchronic hypoxia pretreated rats with less ischemic damages, the left side of the normoxic rats showed more extensive damages.[16]
Our study demonstrated a reduction in brain edema associated with reduced infarct volume in animals preconditioned (21 days) to subchronic hypoxia by comparing the infarct size difference in brain between the left (ischemic site) and right (nonischemic) hemispheres [Figure 4e-k]. Hypoxia preconditioned for 21 days before cerebral ischemia possibly reduced the ischemic alterations and decreased ischemic brain damage after focal ischemia.[17] These observations are further supported by neurological deficit scores and serum MDA and NO levels while comparing in both Group 2 and Group 3 rats. Results also showed that subchronic hypoxia preconditioning (Group 3) increases both serum MDA and NO levels at pre-LCCA occlusions as compared to respective normoxic Group 2 experimental animals [Figures 2 and 3]. Interestingly, both serum MDA and NO levels of Group 3 did not further change after occlusion from preocclusion values at the end of 12 h reperfusion (post) period. Nonsignificant changes of both serum MDA and NO between pre- and post-LCCA occlusion in Group 3 (subchronic hypoxia pretreated) reflects lesser LCCA occlusion induced oxidative and nitrosative stresses in Group 3 (subchronic hypoxia pretreated) as compared to Group 2 (normoxia). Some studies on mice showed acute hypoxia preconditioning protect against transient focal cerebral ischemia, but our study demonstrated for the first time that subchronic preconditioning with hypoxia protects the brain injury against transient focal ischemia induced by unilateral carotid artery occlusion.[18]
This hypoxic model could serve as a helpful plan to remodel ischemic brain injury from cerebral ischemia including stroke. The exact mechanism underlying this neuroprotective effect of preconditioned/pretreated subchronic hypoxia remains obscure. Although further studies are needed to establish true cerebrovascular pathophysiology on unilateral common carotid artery occlusion with reference to subchronic hypoxia pretreatment, it may be considered as a possible protective strategy to ameliorate ischemic brain injury from cerebral focal ischemia or stroke. Further, it has been reported from a clinical study that hypoxia preconditioning provides more protective cardiometabolic profile.[19] Hypoxia preconditioning was also found to be beneficial to promote mesenchymal stem cells proliferation which facilitates intrastriatal transplantation and support therapeutically to fight against Parkinson's disease.[20]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The first author deeply acknowledges Life Sciences Research Board, DRDO, Ministry of Defence, Government of India (R and D/81/48222/LSRB-285/EPB/2014 dated 18/7/2014) and VGST, Government of Karnataka (VGST-KFIST/1230/2015-16 Dated 22/6/2016) for providing a research grant to him.
References
- 1.Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006;28:184–9. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- 2.Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 3.Payabvash S, Souza LC, Wang Y, Schaefer PW, Furie KL, Halpern EF, et al. Regional ischemic vulnerability of the brain to hypoperfusion: The need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke. 2011;42:1255–60. doi: 10.1161/STROKEAHA.110.600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovasc Res. 1998;39:106–20. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 5.Stummer W, Weber K, Tranmer B, Baethmann A, Kempski O. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke. 1994;25:1862–9. doi: 10.1161/01.str.25.9.1862. [DOI] [PubMed] [Google Scholar]
- 6.Das KK, Saha S. Hypoxia, lead toxicities and oxidative stress: Molecular interactions and antioxidant (Vitamin C) defense. Curr Signal Transduct Ther. 2014;9:113–22. [Google Scholar]
- 7.Das KK, Jargar JG, Saha S, Yendigeri SM, Singh SB. A-tocopherol supplementation prevents lead acetate and hypoxia-induced hepatic dysfunction. Indian J Pharmacol. 2015;47:285–91. doi: 10.4103/0253-7613.157126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronner G, Mitchell K, Welsh FA. Cerebrovascular adaptation after unilateral carotid artery ligation in the rat: Preservation of blood flow and ATP during forebrain ischemia. J Cereb Blood Flow Metab. 1998;18:118–21. doi: 10.1097/00004647-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Engel O, Kolodziej S, Dirnagl U, Prinz V. Modeling stroke in mice - middle cerebral artery occlusion with the filament model. J Vis Exp. 2011:pii: 2423. doi: 10.3791/2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanvi B, Robinson T. Complete occlusion of extracranial internal carotid artery: Clinical features, pathophysiology, diagnosis and management. Postgrad Med J. 2007;83:95–9. doi: 10.1136/pgmj.2006.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 12.Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: A critical evaluation. Clin Chem. 1995;41:892–6. [PubMed] [Google Scholar]
- 13.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 14.Shi S, Yang W, Tu X, Chen C, Wang C. Ischemic preconditioning reduces ischemic brain injury by suppressing nuclear factor kappa B expression and neuronal apoptosis. Neural Regen Res. 2013;8:633–8. doi: 10.3969/j.issn.1673-5374.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uluç K, Miranpuri A, Kujoth GC, Aktüre E, Başkaya MK. Focal cerebral ischemia model by endovascular suture occlusion of the middle cerebral artery in the rat. J Vis Exp. 2011:pii: 1978. doi: 10.3791/1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das KK, Das S, Ambekar JG. Hypoxia and oxidative stress: Cell signalling mechanisms and protective role of Vitamin C and cilnidipine. In: Catala A, editor. Lipid Peroxidation: Inhibition, Effects and Mechanisms. 1st ed. New York: Nova Publishers; 2017. pp. 249–62. [Google Scholar]
- 17.Guo M, Cox B, Mahale S, Davis W, Carranza A, Hayes K, et al. Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience. 2008;151:340–51. doi: 10.1016/j.neuroscience.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P, et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Glazachev O, Kopylov P, Susta D, Dudnik E, Zagaynaya E. Adaptations following an intermittent hypoxia-hyperoxia training in coronary artery disease patients: A controlled study. Clin Cardiol. 2017;40:370–6. doi: 10.1002/clc.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Yang J, Li H, Wang X, Zhu L, Fan M, et al. Hypoxia promotes dopaminergic differentiation of mesenchymal stem cells and shows benefits for transplantation in a rat model of parkinson's disease. PLoS One. 2013;8:e54296. doi: 10.1371/journal.pone.0054296. [DOI] [PMC free article] [PubMed] [Google Scholar]