Abstract
Purpose
Sleep deprivation induces depressive symptoms. Dexmedetomidine is a α2-adrenoreceptor agonist and this drug possesses sedative, anxiolytic, analgesic, and anesthetic-sparing effect. In this study, the action of dexmedetomidine on sleep deprivation-induced depressive behaviors was investigated using mice.
Methods
For the inducing of sleep deprivation, the mice were placed inside a water cage containing 15 platforms and filled with water up to 1 cm below the platform surface for 7 days. One day after sleep deprivation, dexmedetomidine at the respective dosage (0.5, 1, and 2 μg/kg) was intraperitoneally treated into the mice, one time per a day during 6 days. Then, forced swimming test and tail suspension test were conducted. Immunohistochemistry for tyrosine hydroxylase (TH), 5-hydroxytryptamine (5-HT; serotonin), tryptophan hydroxylase (TPH) and western blot for D1 dopamine receptor were also performed.
Results
Sleep deprivation increased the immobility latency in the forced swimming test and tail suspension test. The expressions of TPH, 5-HT, and D1 dopamine receptor were decreased, whereas, TH expression was increased by sleep deprivation. Dexmedetomidine decreased the immobility latency and increased the expressions of TPH, 5-HT, and D1 dopamine receptor, whereas, HT expression was decreased by dexmedetomidine treatment.
Conclusions
In our results, dexmedetomidine alleviated sleep deprivation-induced depressive behaviors by increasing 5-HT synthesis and by decreasing dopamine production with up-regulation of D1 dopamine receptor.
Keywords: Sleep deprivation, Dexmedetomidine, Depression, Serotonin, Dopamine
• HIGHLIGHTS
- Selective α2-adrenoreceptor agonist dexmedetomidine acts as an analgesic, sedative, and anesthetic-sparing agent.
- Dexmedetomidine alleviated sleep deprivation-induced depressive behaviors.
- The effect of dexmedetomidine was achieved by increasing serotonin synthesis and by decreasing dopamine production.
INTRODUCTION
Sleep deprivation is common in health care professionals and night shifts, and sleep deprivation causes anxiety, depressive symptoms, and impaired judgment [1]. Depressive symptoms are potent risk factor causing sleep disorders, and depression is also considered as one of the major complications in insomnia patients. Serotoninergic nervous system is associated with the modulation of sleep and wakefulness. Depression induces a functional decrease of central serotoninergic neurotransmission and depression is associated with the specific alterations of sleep, notably insomnia [2].
Serotonin (5-hydroxytryptamine, 5-HT) is implicated in many physiological functions, such as mood control, feeding, and sleep. Tryptophan hydroxylase (TPH) catalyzed 5-HT synthesis from tryptophan, which initially producing 5-hydroxytryptophan. Aromatic amino acid decarboxylase is involved in the decarboxylation of 5-hydroxytryptophan into 5-HT. Because TPH is known as the rate-limiting enzyme for the 5-HT production, the level of TPH has been used as an indicator for 5-HT synthesis. Dysfunction of 5-HT and its synthetic enzyme TPH is closely associated with depression or anxiety [3-5].
Dopamine neurons originate from the substantia nigra (SN) and project to the cerebral forebrain structures, such as prefrontal cortex (PFC) and striatum. In particular, striatum is a part of the anatomic network that aids in the function of dorsolateral PFC. Tyrosine hydroxylase (TH) catalyzes the production of L-dihydroxyphenylalanine, which is the rate-limiting step for the dopamine synthesis [6]. Functional loss of dopaminergic nervous control in humans causes diverse sleep disorders [7]. Pharmacologic drugs targeting to the dopaminergic neurotransmission are clinically used for the treatment of many neuropsychiatric diseases [8]. Acute sleep deprivation elevated TH expression in the ventral tegmental area, nucleus accumbens, and hypothalamus [9].
Dopamine receptors are one of the families included in the G-protein linked receptors. D1-like dopamine receptors (D1 dopamine receptor and D5 dopamine receptor) regulate cyclic AMP level positively [10]. D1 dopamine receptor is exclusively located at the postsynaptic site. On the other hand, D2-like dopamine receptors (D2 dopamine receptor, D3 dopamine receptor, and D4 dopamine receptor) are known to inhibit adenylate cyclase activity. D2 dopamine receptor and D3 dopamine receptor are presynaptically and postsynaptically present in both [11]. Dopamine receptors mediate all physiological actions by dopamine, from voluntary movement to hormonal regulation [8]. Of these, D1 dopamine receptor is associated with the action of antidepressants, and D1 dopamine receptor agonists have been considered as the potential antidepressants [8,12].
Selective α2-adrenoreceptor agonist dexmedetomidine acts as an analgesic, sedative, and anesthetic-sparing agent [13]. Dexmedetomidine has been reported to exert neuroprotective effects against various brain insults through inhibiting neuronal apoptosis [14,15]. Dexmedetomidine induces sedation similar to natural sleep, and dexmedetomidine is a safe agent that does not induce apoptosis under the normal conditions [16,17].
In this study, we investigated whether dexmedetomidine is effective on sleep deprivation-induced depression. For this experiment, forced swimming test, tail suspension test, immunohistochemcal staining for 5-HT, TPH, TH, and Western blot analysis for D1 dopamine receptor were conducted using mice.
MATERIALS AND METHODS
Animal Treatments
Male ICR mice, weighing 30±2 g (15 weeks in age), were purchased for this experiment. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP[SE]-16-021), and performed in accordance with the National Institute of Health Council for the management and use of laboratory animals.
The mice were bred at the controlled conditions (23℃±2℃ room temperature, 8:00 AM to 8:00 PM lighting). The animals were classified as the following five groups (n=10 for each group): control, sleep deprivation, sleep deprivation and 0.5 μg/kg dexmedetomidine-treated, sleep deprivation and 1 μg/kg dexmedetomidine-treated, and sleep deprivation and 2 μg/kg dexmedetomidine-treated groups. One day after sleep deprivation, dexmedetomidine at the respective dose (0.5, 1, and 2 μg/kg in 200 μL NaCl) was intraperitoneally injected to the mice, one time per a day during 6 days.
Induction of Sleep Deprivation
Animal model of sleep deprivation was made using the method described previously [18,19]. Sleep deprivation was induced by placing the mice inside water cage (90 cm×60 cm×40 cm high) containing 15 platforms (3 cm in diameter, 15 cm in height) and filled with water up to 1 cm beneath the platform surface, during 7 consecutive days. The mice were able to move or jump from one platform to the other inside of the water cage. Food and water were supplied into the grid placed on top of the water cage.
Forced Swimming Test
As the method described previously [3,20], depression-like behavior was determined by the forced swimming test. Six days after sleep deprivation, a preliminary test was performed during 15 minutes. One day after a preliminary test, test was conducted for 5 minutes. Individually, the mice were located in a vertical plexiglass cylinder (40 cm height, 20 diameter) filled with 30 cm water at 25℃±2℃. For the forced swimming test, a Smart version 2.5 video tracking system (Panlab, Barcelona, Spain) calculated immobility. Immobility defines the limb and trunk as completely immobile. Following test, the mice were dried using a towel and placed in a separate cage, and were heated by a lamp until the mice were completely dry.
Tail Suspension Test
As the method described previously [21,22], the tail suspension test is based on the action that the animal does not move if the animal gets stress that could not be avoided in a short period of time. Seven days after sleep deprivation, mouse was hung from a tube by its tail for 5 minutes approximately 10 cm away from the ground. During this time, the mouse tried to escape and reach to the ground. The time it takes until it remains immobile was measured.
Tissue Preparation
As the method described previously [3,20], the mice were sacrificed immediately after tail suspension test. Zoletil 50 (10 mg/kg; Vibac Laboratories, Carros, France) was used to anesthetize the mice, and then 50mM phosphate-buffered saline was transcardially perfused to the mice, and the mice were fixed by 4% paraformaldehyde in 100mM phosphate buffer (pH, 7.4). After that, the brains were removed, stored during overnight in the same fixation solution, and then treated with 30% sucrose for cryoprotection. Using a cryostat (Leica, Wetzlar, Germany), the slices were coronal sectioned with 40-μm thickness. For the immunohistochemical staining, region spanning from Bregma 1.98 to 1.70 mm was selected for the PFC, region spanning from Bregma 0.74 to 0.38 mm was selected for the striatum, region spanning from Bregma -2.70 to -3.28 mm was selected for the SN, and region spanning from Bregma -4.36 to -4.72 mm was selected for the dorsal raphe. The 10 average sections were selected from each mouse.
Immunohistochemistry
As the method described previously [20,23], we performed immunohistochemistry to detect the expression of 5-HT, TPH, and TH. The tissue sections were treated with mouse anti-TH, mouse anti-TPH, and rabbit anti-5-HT antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) during overnight. The sections were treated with biotinylated anti-mouse TH, anti-mouse TPH secondary antibody and with biotinylated antirabbit 5-HT secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) during 1 hour. Next, avidin-biotin-peroxidase complex (Vector Laboratories) was treated to the section during 1 hour at 25℃. The sections were treated with a reaction mixture consisting of 0.03% 3,3’-diaminobenzidine and 0.03% hydrogen peroxide during 5 minutes. After the slides were dried under the room conditions, Permount (Fisher Scientific, Fair Lawn, NJ, USA) was used for the coverslips mounting.
Western Blotting
As the method described previously [20,23], Western blotting was conducted to evaluated D1 dopamine receptor expression in the PFC, striatum, and SN. After dissecting PFC, striatum, and SN, these tissues were collected. The tissues were treated with the lysis buffer composing 150mM NaCl, 10% glycerol, 1% Triton X-100, 1mM phenylmethanesulfonyl fluoride, 1mM EGTA, 1.5mM MgCl2 ·6H2O, 1mM sodium orthovanadate, 100mM sodium fluoride, and 50mM HEPES (pH, 7.5). We used colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA) to measure protein content in each sample. After separating protein samples (30 μg) using sodium dodecyl sulfate-polyacrylamide gel, these samples were moved onto the nitrocellulose membrane. This membrane was treated with 5% skim milk in Tris-buffered saline with 0.1% Tween-20, and then the membrane was treated with following primary antibodies during overnight at 4℃: rabbit anti-GAPDH antibody and goat anti-D1 dopamine receptor antibody (1:1,000; Santa Cruz Biotechnology). After that, the membrane was treated with secondary antibody (1:2,000; Vector Laboratories) during 1 hour. We used enhanced chemiluminescence detection kit (Santa Cruz Biotechnology) for the band detection.
Data Analysis
Densitometric analysis by an Image-Pro Plus image analysis system (Media Cybernetics Inc., Silver Spring, MD, USA) was conducted to confirm the expression of D1 dopamine receptor. Under the light microscope (Olympus, Tokyo, Japan), we counted the number of TH-positive cells in the SN and the number of TPH and 5-HT-positive cells in the dorsal raphe. By an image analyzer (Multiscan, Fullerton, CA, USA), we calculated the optical density of TH-immunoreactive fibers in the PFC and striatum at 100-×100-μm area. Optical density of TH was corrected by the nonspecific background density, in which was completely denervated PFC and striatum. The formulation of optical density of TH-positive fibers in the PFC and striatum is as follows: optical density in the lesion side/optical density in the intact side.
One-way analysis of variance following by Duncan’s post-hoc test was used for the data analysis. Mean±standard error of the mean was presented for the results, and P<0.05 was considered as significance.
RESULTS
Immobility Latency in Forced Swimming Test and Tail Suspension Test
Fig. 1 represents immobility latency of the forced swimming test and tail suspension test. In our results, immobility latency of the forced swimming test and tail suspension test was increased by sleep deprivation. Dexmedetomidine treatment shortened immobility latency in the forced swimming test (F=10.730, P<0.001) and in the tail suspension test (F=12.490, P<0.001) of the sleep deprived mice.
Fig. 1.
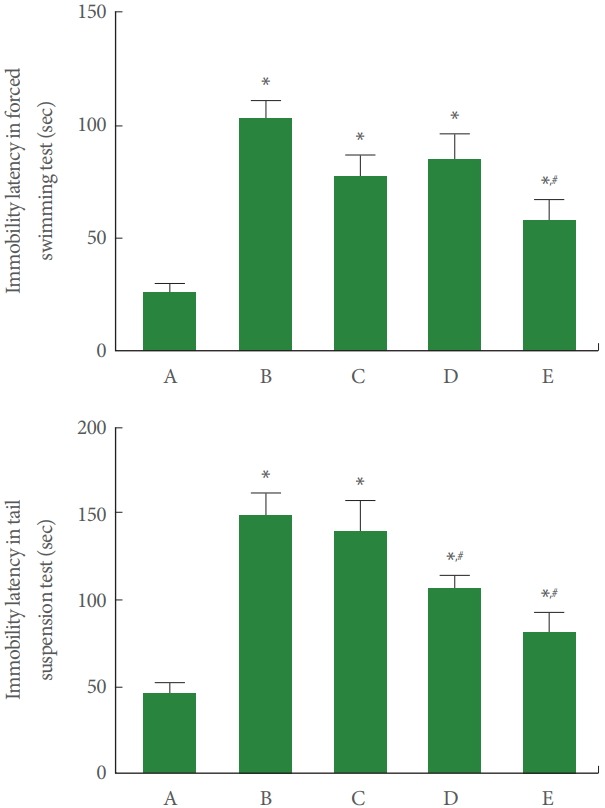
Effect of dexmedetomidine on immobility latency in the forced swimming and tail suspension test. Upper panel: Immobility latency of forced swimming test. Lower panel: Immobility latency of tail suspension test. A, control group; B, sleep deprivation group; C, sleep deprivation and 0.5 μg/kg dexmedetomidine-treated group; D, sleep deprivation and 1 μg/kg dexmedetomidine-treated group; E, sleep deprivation and 2 μg/kg dexmedetomidine-treated group. *P<0.05 compared to the control group. # P<0.05 compared to the sleep deprivation group.
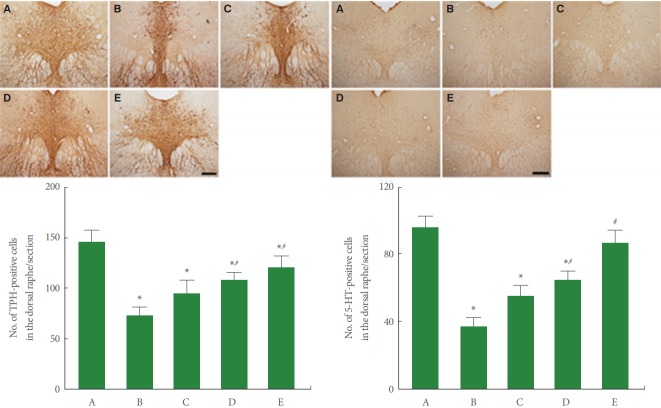
TPH and 5-HT Expression in Dorsal Raphe
Fig. 2 is photomicrographs of TPH-positive and 5-HT-positive cells in the dorsal raphe. In our results, sleep deprivation decreased TPH and 5-HT expression in the dorsal raphe. Dexmedetomidine treatment enhanced TPH (F=5.799, P<0.01) and 5-HT (F=11.861, P<0.001) expression in the dorsal raphe of the sleep deprived mice.
Fig. 2.
Effect of dexmedetomidine on tryptophan hydroxylase (TPH) and 5-hydroxytryptamine (5-HT) expressions in the dorsal raphe. Upper panel: Photomicrographs of THP-positive cells (left) and 5-HT-positive cells (right) in the dorsal raphe. The scale bars represent 200 μm. Lower panel: Number of TPH-positive (left) and 5-HT-positive cells (right) in each group. A, control group; B, sleep deprivation group; C, sleep deprivation and 0.5 μg/kg dexmedetomidine-treated group; D, sleep deprivation and 1 μg/kg dexmedetomidine-treated group; E, sleep deprivation and 2 μg/kg dexmedetomidine-treated group. *P<0.05 compared to the control group. # P<0.05 compared to the sleep deprivation group.
TH Expression in PFC, Striatum, and SN
Fig. 3 is photomicrographs of TH expression in the PFC, striatum, and SN. In our results, sleep deprivation increased the TH expression in the PFC, striatum, and SN. Dexmedetomidine treatment suppressed TH expression in the PFC (F=12.490, P<0.001), striatum (F=35.878, P<0.001), and SN (F=9.952, P<0.001) of the sleep deprived mice.
Fig. 3.
Effect of dexmedetomidine on tyrosine hydroxylase (TH) expressions in the pre-frontal cortex (PFC), striatum, and substantia nigra (SN). Upper panel: Photomicrographs of TH-immunoreactivity fiber in the PFC (left), striatum (middle), and SN (right). The scale bars represent 200 μm. Lower panel: Density of TH immunoreactivity fiber in each group. A, control group; B, sleep deprivation group; C, sleep deprivation and 0.5 μg/kg dexmedetomidine-treated group; D, sleep deprivation and 1 μg/kg dexmedetomidine-treated group; E, sleep deprivation and 2 μg/kg dexmedetomidine-treated group. *P<0.05 compared to the control group. # P<0.05 compared to the sleep deprivation group.
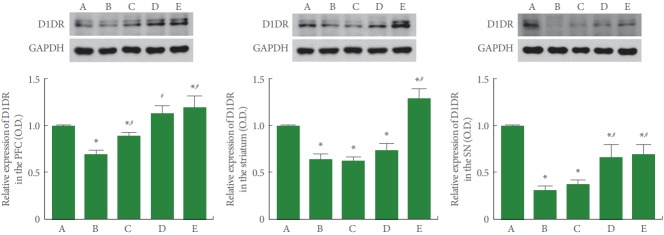
D1 Dopamine Receptor Expression in PFC, striatum, and SN
Fig. 4 is relative expression of D1 dopamine receptor in the PFC, striatum, and SN. In our results, sleep deprivation decreased the D1 dopamine receptor expression in the PFC, striatum, and SN. Dexmedetomidine treatment enhanced D1 dopamine receptor expression in the PFC (F=8.253, P<0.001), striatum (F=22.758, P<0.001), and SN (F=13.587, P<0.001) of the sleep deprived mice.
Fig. 4.
Effect of dexmedetomidine on D1 dopamine receptor (D1DR) in the pre-frontal cortex (PFC), striatum, and substantia nigra (SN). Upper panel: The results of band detection using the enhanced chemiluminescence detection kit in PFC (left), striatum (middle), and SN (right). Lower panel: The relative expression of D1 dopamine receptor in each group. A, control group; B, sleep deprivation group; C, sleep deprivation and 0.5 μg/kg dexmedetomidine-treated group; D, sleep deprivation and 1 μg/kg dexmedetomidinetreated group; E, sleep deprivation and 2 μg/kg dexmedetomidine-treated group. *P<0.05 compared to the control group. # P<0.05 compared to the sleep deprivation group.
DISCUSSION
One of the most widely known tests for determining antidepressant activity is the forced swimming test [3,20]. The forced swimming test is centered on a rodent’s response to the threat of drowning (immobility behaviors), and the results are interpreted according to the measured susceptibility to negative mood. Likewise, the tail suspension test is an experimental method used to measure depressive behavior in rodents [21,22]. Sleep deprivation increased depressive behaviors such as immobility latency of the forced swimming test or tail suspension test in animals [24,25].
In our study, the immobility latency of both forced swimming test and tail suspension test was increased by sleep deprivation, indicating that sleep deprivation caused depressive behaviors in mice. Dexmedetomidine suppressed immobility latency of the forced swimming test and tail suspension test, suggesting that dexmedetomidine ameliorated sleep deprivationinduced depressive behaviors. Dexmedetomidine exerted antidepressant action via α2-adrenoreceptor in mice [26].
The development of rapid eye movement (REM) sleep is caused by a decrease of serotoninergic tone in the brain stem structure, thus antidepressants that increase serotoninergic tone inhibit REM sleep [2]. Sleep deprivation for 8 hours caused a gradual decline in extracellular fluid serotonin level in the hippocampus and prefrontal cortex [27]. Sleep restriction during one week evoked a blunted pituitary adrenocorticotropin response, and this blunted pituitary response might be associated with the decreased sensitivity to serotonin-1A receptor [18]. They concluded that chronic sleep deprivation could alter neurotransmitter receptor system and neuroendocrine responses similar to those seen in depression [18]. Repeated separation of their pups increased immobility latency in the forced swim test of the maternal rats, and 5-HT and TPH expressions in the dorsal raphe were declined in the depressive maternal rats [5]. Decreased levels of 5-HT and TPH expression were observed in the dorsal raphe of the depressive rat pups caused by maternal separation [3] and depressive rats with intracerebral hemorrhage [4].
In our study, the levels of 5-HT and TPH expression in the dorsal raphe were inhibited by sleep deprivation, indicating that suppression of 5-HT level in the dorsal raphe by sleep deprivation is closely related with depressive symptoms in mice. Dexmedetomidine enhanced 5-HT and TPH expression in the dorsal raphe, suggesting that dexmedetomidine restored sleep deprivation-induced decrease in 5-HT level. Enhanced 5-HT and TPH expression in the dorsal raphe suggests that depressive symptoms were ameliorated [4,20].
Sleep deprivation potentiated TH level in the sex reply-associated dopaminergic pathways [28]. TH level in the ventral tegmental area, nucleus accumbens, and hypothalamus was enhanced by sleep deprivation [9]. Mesolimbic dopaminergic system is closely related with the development of neuropsychiatric disorders, including adulthood depression [12]. Increased TH activity means enhanced dopamine synthesis, in contrast, decreased TH activity is associated with the injury of dopaminergic neurons and fibers [23,29].
In our study, the expression of TH in the PFC, striatum, and SN was enhanced by sleep deprivation, indicating that sleep deprivation increased dopamine synthesis in mice. Dexmedetomidine suppressed expression of TH in the PFC, striatum, and SN, suggesting that dexmedetomidine alleviated sleep deprivation-induced overproduction of dopamine. Dexmedetomidine is known to decrease extracellular dopamine level, and this decrement of dopamine by dexmedetomidine was mediated through nucleus accumbens [30].
Sleep deprivation was accompanied by decrease of D1 dopamine receptor immunoreactivity and increase of D2 dopamine receptor immunoreactivity in the caudate nucleus [31]. D1 dopamine receptor level was decreased, in contrast, D2 dopamine receptor level was not changed, and D3 dopamine receptor level was increased in the striatum of mice by sleep deprivation. They suggested that dopaminergic circuits in the brain could be remodeled by sleep deprivation [19]. Long-term awakening inhibits the acquisition of short-term memory in mammals, while D1 dopamine receptor is essential for the rescue of sleep-deprivation-induced learning impairments [32]. D1 dopamine receptor mediates the anti-depressant-like effect [12]. High density level of the D1 dopamine receptor is appeared in the striatum, nucleus accumbens, substantia nigra, olfactory bulb, amygdala, and frontal cortex [8].
In our study, sleep deprivation decreased D1 dopamine receptor expression in the PFC, striatum, and SN, indicating that sleep deprivation down-regulated D1 dopamine receptor in mice. The down-regulating effect of sleep deprivation on D1 dopamine receptor might be ascribed to the overproduction of dopamine caused by sleep deprivation. Dexmedetomidine enhanced this D1 dopamine receptor expression.
Dexmedetomidine showed anti-apoptotic effect in various brain diseases without any adverse effects [14,15]. Direct injection of 40-μg/kg dexmedetomidine into the brachial plexus did not show apoptosis and degeneration [16]. In our study, dexmedetomidine alleviated sleep deprivation-induced depressive behaviors by increasing 5-HT synthesis and by decreasing dopamine production with up-regulation of D1 dopamine receptor. Based on our study, dexmedetomidine can be considered as a safe drug for patients with depression due to insomnia.
Footnotes
Fund/Grant Support
This work was supported by a grant from Kyung Hee University in 2015 (grant number: KHU-20151261).
Research Ethics
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP[SE]-16-021), and performed in accordance with the National Institute of Health Council for the management and use of laboratory animals.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: EJM, JWY
·Study concept and design: BJL, JWY
·Acquisition of data: IGK, SEK, JJJ, LH
·Analysis and interpretation of data: IGK, HA
·Drafting of the manuscript: EJM, IGK
·Critical revision of the manuscript for important intellectual content: BJL, JWY
·Statistical analysis: IGK, SEK
·Obtained funding: JWY
·Administrative, technical, or material support: JJJ, LH
·Study supervision: CJK, JWY
REFERENCES
- 1.Veasey S, Rosen R, Barzansky B, Rosen I, Owens J. Sleep loss and fatigue in residency training: a reappraisal. JAMA. 2002;288:1116–24. doi: 10.1001/jama.288.9.1116. [DOI] [PubMed] [Google Scholar]
- 2.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6:341–51. [PubMed] [Google Scholar]
- 3.Baek SS, Jun TW, Kim KJ, Shin MS, Kang SY, Kim CJ. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain Dev. 2012;34:45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Roh JH, Ko IG, Kim SE, Lee JM, Ji ES, Kim JH, et al. Treadmill exercise ameliorates intracerebral hemorrhage-induced depression in rats. J Exerc Rehabil. 2016;12:299–307. doi: 10.12965/jer.1632692.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung YH, Shin MS, Cho S, Baik HH, Jin BK, Chang HK, et al. Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neurosci Lett. 2010;5;470:86–90. doi: 10.1016/j.neulet.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 6.Haavik J, Toska K. Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol. 1998;16:285–309. doi: 10.1007/BF02741387. [DOI] [PubMed] [Google Scholar]
- 7.Rye DB. Parkinson’s disease and RLS: the dopaminergic bridge. Sleep Med. 2004;5:317–28. doi: 10.1016/j.sleep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 9.Azogu I, de la Tremblaye PB, Dunbar M, Lebreton M, LeMarec N, Plamondon H. Acute sleep deprivation enhances avoidance learning and spatial memory and induces delayed alterations in neurochemical expression of GR, TH, DRD1, pCREB and Ki67 in rats. Behav Brain Res. 2015;279:177–90. doi: 10.1016/j.bbr.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–32. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 11.Deransart C, Landwehrmeyer GB, Feuerstein TJ, Lucking CH. Upregulation of D3 dopaminergic receptor mRNA in the core of the nucleus accumbens accompanies the development of seizures in a genetic model of absence-epilepsy in the rat. Brain Res Mol Brain Res. 2001;94:166–77. doi: 10.1016/s0169-328x(01)00240-6. [DOI] [PubMed] [Google Scholar]
- 12.Amiri S, Amini-Khoei H, Mohammadi-Asl A, Alijanpour S, Haj-Mirzaian A, Rahimi-Balaei M, et al. Involvement of D1 and D2 dopamine receptors in the anti-depressant-like effects of selegiline in maternal separation model of mouse. Physiol Behav. 2016;163:107–14. doi: 10.1016/j.physbeh.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 13.Panzer O, Moitra V, Sladen RN. Pharmacology of sedative-analgesic agents: dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral Mu antagonists. Anesthesiol Clin. 2011;29:587–605. doi: 10.1016/j.anclin.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Choi IY, Hwang L, Jin JJ, Ko IG, Kim SE, Shin MS, et al. Dexmedetomidine alleviates cerebral ischemia-induced short-term memory impairment by inhibiting the expression of apoptosis-related molecules in the hippocampus of gerbils. Exp Ther Med. 2017;13:107–16. doi: 10.3892/etm.2016.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang L, Choi IY, Kim SE, Ko IG, Shin MS, Kim CJ, et al. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. Int J Mol Med. 2013;31:1047–56. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- 16.Han JH, Kim DO, Yi JW, Park SW, Kang WJ, Chol YK, et al. Dexmedetomidine, α2-adrenoceptor agonist, does not induce apoptosis in the brachial plexus of rats. Anim Cells Syst. 2014;18:407–15. [Google Scholar]
- 17.Park JH, Ko IG, Kim SE, Jin JJ, Hwang L, Kim CJ, et al. Dexmedetomidine oral mucosa patch for sedation suppresses apoptosis in hippocampus of normal rats. Int Neurourol J. 2017;21(Suppl 1):S39–47. doi: 10.5213/inj.1734884.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novati A, Roman V, Cetin T, Hagewoud R, den Boer JA, Luiten PG, et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31:1579–85. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim MM, Xu J, Holtzman DM, Mach RH. Sleep deprivation differentially affects dopamine receptor subtypes in mouse striatum. Neuroreport. 2011;22:489–93. doi: 10.1097/WNR.0b013e32834846a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji ES, Lee JM, Kim TW, Kim YM, Kim YS, Kim K. Treadmill exercise ameliorates depressive symptoms through increasing serotonin expression in postpartum depression rats. J Exerc Rehabil. 2017;13:130–5. doi: 10.12965/jer.1734968.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes A, Li Q, Koenig EA, Gold E, Stephenson D, Yang RJ, et al. Phenotypic assessment of galanin overexpressing and galanin receptor R1 knockout mice in the tail suspension test for depression-related behavior. Psychopharmacology (Berl) 2005;178:276–85. doi: 10.1007/s00213-004-1997-1. [DOI] [PubMed] [Google Scholar]
- 22.Rosa PB, Ribeiro CM, Bettio LE, Colla A, Lieberknecht V, Moretti M, et al. Folic acid prevents depressive-like behavior induced by chronic corticosterone treatment in mice. Pharmacol Biochem Behav. 2014;127:1–6. doi: 10.1016/j.pbb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Ko IG, Kim SE, Kim TW, Ji ES, Shin MS, Kim CJ, et al. Swimming exercise alleviates the symptoms of attention-deficit hyperactivity disorder in spontaneous hypertensive rats. Mol Med Rep. 2013;8:393–400. doi: 10.3892/mmr.2013.1531. [DOI] [PubMed] [Google Scholar]
- 24.Daniele TM, de Bruin PF, Rios ER, de Bruin VM. Effects of exercise on depressive behavior and striatal levels of norepinephrine, serotonin and their metabolites in sleep-deprived mice. Behav Brain Res. 2017;332:16–22. doi: 10.1016/j.bbr.2017.05.062. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira RA, Cunha GM, Borges KD, de Bruin GS, dos Santos-Filho EA, Viana GS, et al. The effect of venlafaxine on behaviour, body weight and striatal monoamine levels on sleep-deprived female rats. Pharmacol Biochem Behav. 2004;79:499–506. doi: 10.1016/j.pbb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Stone EA, Lin Y, Sarfraz Y, Quartermain D. Antidepressant-like action of intracerebral 6-fluoronorepinephrine, a selective full α-adrenoceptor agonist. Int J Neuropsychopharmacol. 2011;14:319–31. doi: 10.1017/S1461145710000507. [DOI] [PubMed] [Google Scholar]
- 27.Bjorvatn B, Grønli J, Hamre F, Sørensen E, Fiske E, Bjørkum AA, et al. Effects of sleep deprivation on extracellular serotonin in hippocampus and frontal cortex of the rat. Neuroscience. 2002;113:323–30. doi: 10.1016/s0306-4522(02)00181-1. [DOI] [PubMed] [Google Scholar]
- 28.Damasceno F, Skinner GO, Cordeiro JF, Ferraz MR, Almeida OM. Sleep deprivation affects sexual behavior and tyrosine hydroxylase (TH) levels in sexually experienced male rats. Physiol Behav. 2008;94:405–11. doi: 10.1016/j.physbeh.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Shin MS, Kim TW, Lee JM, Ji ES, Lim BV. Treadmill exercise alleviates nigrostriatal dopaminergic loss of neurons and fibers in rotenone-induced Parkinson rats. J Exerc Rehabil. 2017;13:30–5. doi: 10.12965/jer.1734906.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittington RA, Virág L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analg. 2006;10:448–55. doi: 10.1213/01.ane.0000195234.07413.5a. [DOI] [PubMed] [Google Scholar]
- 31.Oganesyan GA, Aristakesyan EA, Romanova IV, Belova VA, Artamokhina IV. The dopaminergic nigrostriatal system in sleep deprivation in cats. Neurosci Behav Physiol. 2008;38:785–92. doi: 10.1007/s11055-008-9047-9. [DOI] [PubMed] [Google Scholar]
- 32.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–7. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]