Abstract
Background
The aim of this study was to compare magnetic resonance imaging (MRI) and hysteroscopy (HS) for assessing cervical involvement in early-stage endometrial adenocarcinoma in order to establish a more reliable screening method to aid in clinical decision-making.
Material/Methods
A retrospective analysis was performed on the clinicopathological data from 88 patients with stage I or II endometrial adenocarcinoma who underwent MRI and HS prior to surgery in the Sun Yat-sen Memorial Hospital, Sun Yat-sen University, China. Chi-square and Fisher’s exact tests were performed to compare the accuracy, sensitivity, specificity, and positive and negative predictive values in the diagnosis of cervical involvement by MRI and HS. The relationship between clinicopathological factors and the deviation of diagnosis by MRI and HS from that by pathology was also analyzed.
Results
The accuracy of assessing cervical conditions was 93.2% by MRI and 55.7% by HS. Among these variables, the accuracy, specificity, and positive predictive values of MRI were significantly different from those of HS, while the sensitivity and negative predictive values of MRI and HS were not significantly different from each other. Age, tumor size, tumor differentiation, and depth of myometrial invasion were not associated with the differences in cervical assessment between MRI and HS. However, the tumor location may affect assessment by HS.
Conclusions
MRI is better than HS for cervical assessment. The negative predictive values of both MRI and HS are high and unsatisfactory.
MeSH Keywords: Endometrial Neoplasms, Hysteroscopy, Magnetic Resonance Imaging, Neoplasm Staging
Background
Endometrial carcinoma is one of the 3 most common female malignancies, of which the most common type is endometrial adenocarcinoma [1,2]. Due to the presence of early symptoms, 90% of endometrial carcinomas are diagnosed at an early stage [3]. Stage I indicates that the cancer is confined to the uterus, while stage II indicates that the cancer has infiltrated the cervical stroma but is still confined to the uterus (involvement of endocervical glands is considered stage I). The initial treatment is surgery for patients with surgical tolerance. After hysterectomy, the uterus is examined, and, based on the presence or absence of high-risk factors for nodal metastasis, pelvic and/or para-aortic lymphadenectomy is performed. However, the extent of tissue resection surrounding the uterus should be determined by considering whether cervical involvement is observed. In stage I, when there is no cervical involvement, only an extrafascial total abdominal hysterectomy together with a bilateral salpingo-oophorectomy is needed. In stage II, when the cancer has spread to the cervix, a radical hysterectomy with a bilateral salpingo-oophorectomy is needed [1,2]. Radical hysterectomy involves a broad region, causes significant surgical trauma, and easily introduces complications. However, if only extrafascial total abdominal hysterectomy is performed in patients with stage II disease, the likelihood of postoperative radiotherapy and recurrence is higher than when radical hysterectomy is performed. Therefore, precise staging prior to surgery is critical for the selection of the appropriate surgical approach, reduction in complications, and improvement of therapeutic efficacy. Magnetic resonance imaging (MRI) has a high resolution in soft tissue, and multidimensional and multisequence images may be obtained. As a result, MRI has been used more frequently in the diagnosis of endometrial carcinoma prior to surgery [4–6]. MRI is considered a critical diagnostic method for the preoperative diagnosis of cervical involvement, and it was recommended by FIGO and NCCN for diagnosis of cervical involvement with endometrial adenocarcinoma. However, it still has a problem with sensitivity. Hysteroscopy (HS) has the advantages of a wide direct field of view, high resolution, high accuracy, and minimal invasiveness. HS has an important position in the diagnosis of intrauterine diseases, and its diagnostic value in the assessment of cervical involvement has been previously reported [7–9].
This study compared the diagnostic values of MRI and HS in the assessment of cervical involvement in endometrial carcinoma in order to assist clinicians with accurate preoperative staging and selection of appropriate surgical procedures.
Material and Methods
Subjects
A retrospective analysis was performed on the clinical, surgical, and pathological data from inpatients with endometrial adenocarcinoma who presented at the Sun Yat-sen Memorial Hospital, Sun Yat-Sen University (China) between January 1, 2010 and December 31, 2014. Patients recruited for the study met all the following criteria: 1. diagnosis of endometrial adenocarcinoma; 2. stage I or II disease; 3. treatment with surgery and availability of complete postoperative pathological data; and 4. cervical MRI and HS examinations at the Sun Yat-Sen Memorial Hospital, Sun Yat-sen University, China. Patients who received preoperative treatments and those with incomplete data were excluded.
Assessment of examination data
MRI examination
The MRI scans and reports for all participants were examined. Assessments included the location and size of the lesion, the status of the uterine junctional zone, depth of myometrial invasion, presence of cervical involvement, lymph node status, and the status of the surrounding organs. The endometrial cancer with or without cervical involvement examined by MRI were showed in Figures 1, 2.
Figure 1.
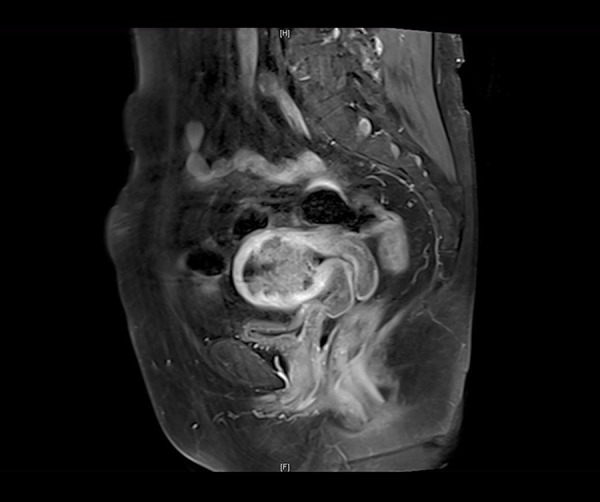
MRI of endometrial cancer without cervical involvement.
Figure 2.
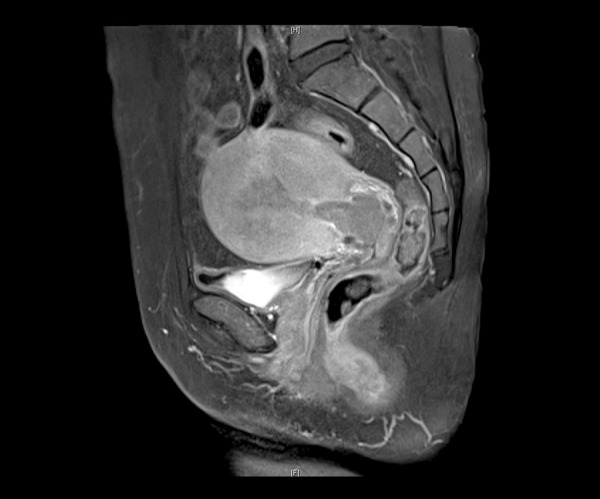
MRI of endometrial cancer with cervical involvement.
Hysteroscopy
The hysteroscopy images and reports of all participants were examined. The cervical canal, internal cervical os, uterine wall, and uterine horns were assessed, as these primarily describe the status of the cervix and endometrial thickness, as well as the tumor location and size. The endometrial cancer with or without cervical involvement examined by hysteroscopy were showed in Figures 3, 4.
Figure 3.
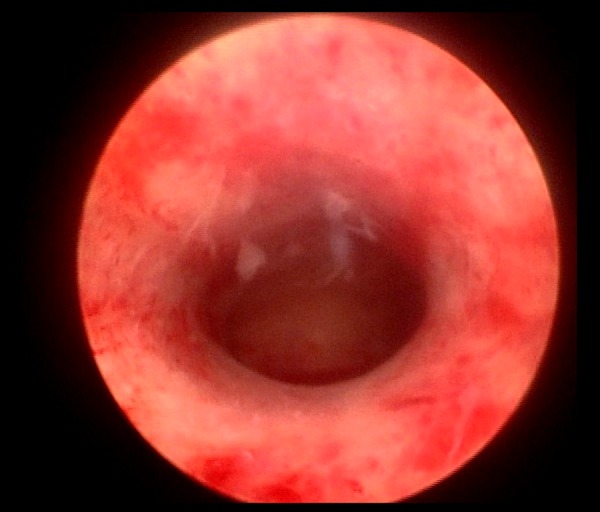
Hysteroscopy of endometrial cancer without cervical involvement.
Figure 4.
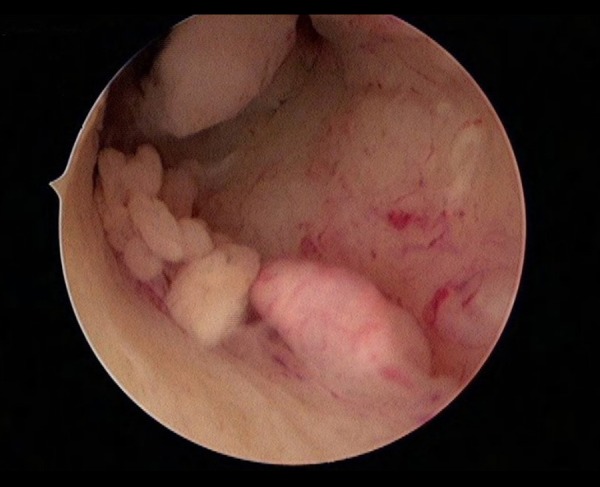
Hysteroscopy of endometrial cancer with cervical involvement.
Pathology results
The pathology results of all participants were reviewed. Pathological indices included pathological type, differentiation, tumor size, and location and evidence of cervical involvement.
Examination measures
Using the pathology results as a criterion standard, the cervical status was assessed by MRI and HS and compared with the postoperative pathology. The sensitivity, specificity, positive and negative predictive values, and accuracy of the 2 methods were also compared. Additional analysis was performed on the deviation of the MRI and HS assessments of cervical involvement from those determined by pathology and elucidated the relationship between this deviation and factors such as age, tumor size and location, differentiation, and depth of myometrial invasion.
Statistical analysis
All data were analyzed by SPSS13.0 software. The sensitivity, specificity, positive and negative predictive values, and accuracy of the MRI and HS assessments of cervical involvement were calculated. Chi-square and Fisher’s exact tests were performed to compare the diagnostic differences between MRI and HS. The relationship between the clinicopathological factors and the diagnostic deviations (between MRI and pathology and between HS and pathology) was analyzed. The difference was regarded as statistically significant when P values were less than 0.05.
Results
Basic information
In all, 88 patients with an age range of 28–77 years and an average age of 51.89±9.424 were recruited for this study. Among the patients, 52 were premenopausal and 36 were menopausal. The interval between MRI examination and surgery ranged from 0–34 days with an average of 5.22±5.706 days. The interval between HS and surgery ranged from 1 to 55 days with an average of 8.93±9.400 days. Among the participants, 18 patients underwent extrafascial total abdominal hysterectomy with bilateral salpingo-oophorectomy. Six patients underwent extrafascial total abdominal hysterectomy together with bilateral salpingo-oophorectomy and pelvic and/or para-aortic lymphadenectomy. Six patients underwent sub-radical hysterectomy together with bilateral salpingo-oophorectomy. Fourteen patients underwent sub-radical hysterectomy together with bilateral salpingo-oophorectomy and pelvic and/or para-aortic lymphadenectomy. Seven patients underwent radical hysterectomy together with bilateral salpingo-oophorectomy. Thirty-seven patients underwent radical hysterectomy together with bilateral salpingo-oophorectomy and pelvic and/or para-aortic lymphadenectomy.
Pathological examination results
The pathological type of all cases was endometrial adenocarcinoma. Among the cases, 72 were highly differentiated, 11 were moderately differentiated, and 5 were poorly differentiated. The pathology analysis showed that the tumor was restricted to the uterus in 84 cases (among them, 18 cases showed involvement of the cervical junction), while 4 cases had cervical involvement. Fifty-three cases had a tumor volume ≥2 cm, and 35 cases had a tumor volume <2 cm. Tumors were found in the uterine fundus in 17 cases, in the uterine body in 51 cases, in the lower uterine segment in 7 cases, and all around the uterus in 13 case (Table 1).
Table 1.
The clinicopathologic characteristics of patients with early stage endometrial adenocarcinoma.
| Factors | n (%) | |
|---|---|---|
| Age (years) | 51.89±9.424 | / |
| Menopause | No | 36 (40.9) |
| Yes | 52 (59.1) | |
| Stage (FIGO2009) | I | 84 (95.5) |
| II | 4 (4.5) | |
| Grade | G1 | 72 (81.8) |
| G2 | 11 (12.5) | |
| G3 | 5 (5.7) | |
| Tumor size(cm) | <2c m | 35 (39.8) |
| ≥2 cm | 53 (60.2) | |
| Tumor position | Fundus | 17 (19.3) |
| Corpus | 51 (58.0) | |
| Uterine cavity | 13 (14.8) | |
| Lower uterine | 7 (8.0) |
Predictive value of HS for the cervical status of patients with early-stage endometrial adenocarcinoma
Forty-one patients were diagnosed with cervical involvement by HS prior to surgery, and among them, only 3 were confirmed to have cervical involvement by pathology. At diagnosis, 47 patients did not have cervical involvement according to HS prior to surgery, and among them, only 1 was confirmed to have cervical involvement by pathology. The accuracy of assessing cervical status by HS was 55.7%. The sensitivity, specificity, and positive and negative predictive values for HS in the diagnosis of cervical involvement were 75%, 54.8%, 7.3%, and 97.8%, respectively (Table 2).
Table 2.
Degree of pathological correspondence between Hysteroscopy and MRI.
| Pathology | HS | MRI | ||||
|---|---|---|---|---|---|---|
| + | − | Total | + | − | Total | |
| + | 3 | 1 | 4 | 4 | 0 | 4 |
| − | 38 | 46 | 84 | 6 | 78 | 84 |
| Total | 41 | 47 | 88 | 10 | 78 | 88 |
Predictive value of MRI for the cervical status of patients with early-stage endometrial adenocarcinoma
Ten patients were diagnosed with cervical involvement by MRI prior to surgery, and among them, 4 were confirmed to have cervical involvement by postoperative pathology. At diagnosis, 78 patients did not have cervical involvement by MRI prior to surgery, and none of them had cervical involvement according to postoperative pathology. The accuracy of assessing cervical status by MRI was 93.2%. The sensitivity, specificity, and positive and negative predictive values for MRI in the diagnosis of cervical involvement were 100%, 92.9%, 40%, and 100%, respectively (Table 2).
The data above indicate that the accuracy, sensitivity, specificity, and positive and negative predictive values for MRI in the diagnosis of cervical status in early-stage endometrial adenocarcinoma were higher than those of HS. The differences in the accuracy, specificity, and positive predictive value between MRI and HS were statistically significant, while the differences in the sensitivity and negative predictive value were not statistically significant (Table 3). The relationship between the clinicopathological characteristics and the diagnostic deviation is shown in Tables 4 and 5.
Table 3.
Diagnosis value for assessment of cervical involvement in early-stage endometrial adenocarcinoma between Hysteroscopy and MRI.
| Category | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| MRI | 100% | 92.9% | 40% | 100% |
| HS | 75% | 54.8% | 7.3% | 97.8% |
| χ2 | / | 31.531 | 4.754 | / |
| P | 1.0000 | 0.0000 | 0.029 | 0.376 |
Table 4.
Relationship between clinicopathologic characteristics and diagnosis bias of hysteroscopy.
| Factors | Diagnosis bias (%) | P |
|---|---|---|
| Age (years) | ||
| <60 | 30 (42.9) | 0.586 |
| ≥60 | 9 (50.0) | |
|
| ||
| Tumor size (cm) | ||
| <2 | 13 (37.1) | 0.271 |
| ≥2 | 26 (49.1) | |
|
| ||
| Grade | ||
| G1 | 31 (43.1) | 0.234 |
| G2 | 7 (63.3) | |
| G3 | 1 (20.0) | |
|
| ||
| Position | ||
| Fundus | 5 (29.4) | 0.06 |
| Corpus | 20 (39.2) | |
| Uterine cavity | 9 (69.2) | |
| Lower uterine | 5 (71.4) | |
|
| ||
| Myometrium invasion | ||
| ≤1/2 | 34 (44.2) | 0.935 |
| >1/2 | 5 (45.5) | |
Table 5.
Relationship between clinicopathologic characteristics and diagnosis bias of MRI.
| Factors | Diagnosis bias (%) | P |
|---|---|---|
| Age (years) | ||
| <60 | 5 (7.1) | 0.812 |
| ≥60 | 1 (5.6) | |
|
| ||
| Tumor size (cm) | ||
| <2 | 2 (5.7) | 0.738 |
| ≥2 | 4 (7.5) | |
|
| ||
| Grade | ||
| G1 | 6 (8.3) | 0.489 |
| G2 | 0 (0.0) | |
| G3 | 0 (0.0) | |
|
| ||
| Position | ||
| Fundus | 1 (5.9) | 0.888 |
| Corpus | 4 (7.8) | |
| Uterine cavity | 1 (7.7) | |
| Lower uterine | 0 (0.0) | |
|
| ||
| Myometrium invasion | ||
| ≤1/2 | 5 (6.5) | 0.749 |
| >1/2 | 1 (9.1) | |
Discussion
MRI is a common supplementary examination that is used for the identification of risk factors in endometrial carcinoma and for the guidance of surgical treatment, especially for the distinction among myometrial invasion, cervical stromal involvement, and lymph node metastasis [10]. MRI has a high resolution for soft tissue and can clearly show the anatomical structure of the uterus and pelvis. MRI can also image freely in three-dimensional space and is the primary method used for the preoperative staging of endometrial carcinoma. In MRI images, the normal cervical stroma shows signals with low intensity. When the cervical stroma is invaded by cancer, the low-intensity signals are replaced by moderate signals [11]. HS used for examination and biopsy is thought to be the best method for the diagnosis of endometrial pathogenesis. HS is also commonly used to assist in the diagnosis of cervical involvement in patients with endometrial carcinoma. A recent meta-analysis showed that the sensitivity, specificity, and positive and negative predictive values for the prediction of cervical involvement by MRI were 57.0%, 94.8%, 68.7%, and 90.5%, respectively [10]. A study by Avila et al. found sensitivity, specificity, and positive and negative predictive values of 87%, 47%, 66%, and 75%, respectively, for the diagnosis of cervical involvement by HS [12]. Li et al. reported sensitivity, specificity, and positive and negative predictive values of 24.78%, 93.76%, 46.75%, and 84.95%, respectively, for the diagnosis of cervical involvement by a combination of HS and endocervical curettage [13], while these same values in another study were 79.48%, 88.05%, 56.36%, and 95.67%, respectively [8]. However, few studies have compared MRI and HS. Our study shows that the accuracy of assessing cervical conditions is 93.2% by MRI, which is significantly higher than that of HS (55.7%) (P=0.007). The specificity and positive predictive value of MRI in the prediction of cervical involvement are significantly higher than those of HS (P=0.000 and P=0.029, respectively). The negative predictive value of HS in the prediction of cervical involvement is high, but the sensitivity, specificity, accuracy, and positive predictive value are low. In contrast, the specificity and negative predictive value of MRI are high, which indicates that MRI is highly accurate in the exclusion of cervical involvement. MRI can lead to a more accurate preoperative staging for patients undergoing surgical treatment and can be a reference for clinicians during the selection of surgical procedures.
Age, tumor size, differentiation, and depth of myometrial invasion are not associated with the difference between MRI and HS for cervical assessment when the relationship between the clinicopathological characteristics and the diagnostic deviation is analyzed. However, tumor location may affect the deviation of HS-directed assessment. A lower location of the tumors corresponds to a higher deviation (P=0.06). In clinical practice, examiners easily mistake lower uterine tumors that descend into the cervix for cervical involvement. When determining the extent of cervical involvement, HS uses the anatomical internal cervical os as a marker, while pathological diagnosis uses the histological internal cervical os as a marker. Additionally, in this study, cervical involvement, as determined by direct observation under HS, was not confirmed by biopsy in most patients. Patients who had a biopsy were not subjected to direct observation but instead underwent conventional fractional curettage, which, to some extent, reduced the diagnostic accuracy.
This study has some limitations. It was a retrospective study with a limited number of subjects, and the constituent ratio of cases at each stage was not ideal. In a study by Sorosky, among the patients who were initially diagnosed with endometrial carcinoma, 72% had stage I disease, 12% had stage II disease, 13% had stage III disease, and 3% had stage IV disease [14]. In our study, only 4.5% of the patients had stage II disease, which is lower than that reported by Sorosky, and this may have affected our results. Our patients in the present study had the hysteroscopy without anesthesia, and the cost was nearly equal to that of MRI.
In the comparison of MRI vs. hysteroscopy, we want to give some advice on the examination of cervical extension, rather than determining which is the best. Sometimes, the combination of MRI and hysteroscopy maybe a good choice, because each of them has advantages.
Conclusions
MRI is better than HS for cervical assessment. The negative predictive values of both MRI and HS are high and unsatisfactory. Therefore, a more reliable diagnostic method is needed.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Koh WJ, Abu-Rustum NR, Bean S, et al. Uterine neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(2):170–99. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 2.Denny L, Quinn M. FIGO cancer report 2015. Int J Gynaecol Obstet. 2015;131(Suppl 2):S75. doi: 10.1016/j.ijgo.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Masroor I, Rashid S, Afzal S, et al. Diagnostic accuracy of pelvic MRI for determination of the cervical involvement in endometrial cancer. J Coll Physicians Surg Pak. 2018;28(4):262–65. doi: 10.29271/jcpsp.2018.04.262. [DOI] [PubMed] [Google Scholar]
- 5.Seki H, Takano T, Sakai K. Value of dynamic MR imaging in assessing endometrial carcinoma involvement of the cervix. Am J Roentgenol. 2000;175:171–76. doi: 10.2214/ajr.175.1.1750171. [DOI] [PubMed] [Google Scholar]
- 6.Murakami T, Kurachi H, Nakamura H, et al. Cervical invasion of endometrial carcinoma – evaluation by parasagittal MR imaging. Acta Radiol. 1995;36:248–53. [PubMed] [Google Scholar]
- 7.Ørtoft G, Dueholm M, Mathiesen O, et al. Preoperative staging of endometrial cancer using TVS, MRI, and hysteroscopy. Acta Obstet Gynecol Scand. 2013;92(5):536–45. doi: 10.1111/aogs.12103. [DOI] [PubMed] [Google Scholar]
- 8.Kietlinska Z, Stelmachow J, Antczak-Judycka A, et al. Fractional curettage, hysteroscopy and imaging techniques: transvaginal sonography (TVS), magnetic resonance imaging (MRI) and computed tomography (CT) in the diagnosis of cervical canal involvement in cases of endometrialcarcinoma. Eur J Gynaecol Oncol. 1998;19:561–64. [PubMed] [Google Scholar]
- 9.Lo KW, Cheung TH, Yim SF, Chung TK. Preoperative hysteroscopic assessment of cervical invasion by endometrial carcinoma: A retrospective study. Gynecol Oncol. 2001;82:279–82. doi: 10.1006/gyno.2001.6270. [DOI] [PubMed] [Google Scholar]
- 10.Luomaranta A, Leminen A, Loukovaara M. Magnetic resonance imaging in the assessment of high-risk features of endometrial carcinoma: A meta-analysis. Int J Gynecol Cancer. 2015;25(5):837–42. doi: 10.1097/IGC.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 11.Duncan KA, Drinkwater KJ, Frost C, et al. Staging cancer of the uterus: A national audit of MRI accuracy. Clin Radiol. 2012;67:523–30. doi: 10.1016/j.crad.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Avila ML, Ruiz R, Cortaberria JR, et al. Assessment of cervical involvement in endometrial carcinoma by hysteroscopy and directed biopsy. Int J Gynecol Cancer. 2008;18(1):128–31. doi: 10.1111/j.1525-1438.2007.00950.x. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Yang X, Yang Y, et al. Value of hysteroscopy and dilatation and curettage in diagnosis of endometrial cancer. Zhonghua Fu Chan Ke Za Zhi. 2015;50(2):120–24. [PubMed] [Google Scholar]
- 14.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120(2 Pt 1):383–97. doi: 10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]


