Abstract
Background
The present organ shortage has led to increased use of kidneys from expanded-criteria donors, but the prognosis is disappointing due to poor graft quality. As a promising kidney protector, the Klotho gene’s role in predicting short-term prognosis has not been assessed.
Material/Methods
We retrospectively analyzed data from 41 recipients and 25 donors. Multiple clinical variables were compared between different subgroups of donors or their corresponding recipients. The area under the receiver operating characteristic curve (AUROC) was used to evaluate the distinguishing ability. Dynamic changes in serum Klotho, FGF-23, and urinary NGAL were assessed.
Results
Serum Klotho level was significantly lower in donors age ≥50 years (p=0.017), and there was a moderate negative correlation between serum Klotho expression and age (r=−0.464, p=0.019). Moreover, detection of Klotho mRNA and immunohistochemical analysis in kidneys revealed the same trend as in serum. Furthermore, for older donors (age ≥50 years), serum Klotho level had a strong negative correlation with recipient eGFR 1 month post-transplant (r=−0.686, p=0.007), which was proved to be a good predictor for estimating graft function by ROC analysis. Additionally, during the post-transplant follow-up, serum Klotho levels increased slightly after a temporary decline, while serum FGF-23 and urinary NGAL decreased significantly and then stayed low thereafter.
Conclusions
Klotho level, which decreases with age, may be a potential predictor of short-term renal function, especially for grafts from older donors.
MeSH Keywords: Aging, Biological Markers, Kidney Transplantation, Tissue Donors
Background
Expansion of the organ pool from use of expanded-criteria donor organs is a potential answer to the current organ shortage, which has long restricted the development of transplantation. However, the general prognosis of organs from older donor tends to be worse than those from younger ones. In renal transplantation, kidneys donated by older donors have higher incidence of delayed graft function (DGF) and calcineurin inhibitors (CNI) nephrotoxicity, which reduces long-term graft survival [1]. To optimize the quality of renal grafts, shortening the cold ischemia time (CIT), using machine perfusion, and other novel preservation methods are of great importance [2]. To prevent immune and nonimmune damage, timely monitoring and interventions after surgery help improve graft outcome [3,4].
As a famous anti-aging gene encoding a single-pass transmembrane protein, the Klotho gene was first identified in 1997 [5]. The kidneys are the main source and principal target of Klotho, which mediate Klotho’s effect [6]. Both secretory form and membrane form of Klotho exert prominent influences by regulating phosphate excretion and synthesis of active vitamin D in the kidneys, as well as having anti-aging activity [7]. According to recent reports, the secretory form has been shown to contribute far more biologically than the membrane one [8]. Moreover, Erben found that Klotho has a renal-protective effect via the fibroblast growth factor-23 (FGF-23)/Klotho signaling axis [9]. Emerging evidence has revealed that Klotho not only functions as a renoprotective factor, but also is closely associated with the degree of renal injury [10,11]. The kidneys of older people are more vulnerable to acute kidney injury (AKI), which occurs during organ procurement and severely restricts recipient prognosis [12]. The published literature on the role of Klotho in predicting renal transplant outcomes is sparse. Thus, we conducted the present study to assess the value of Klotho in renal transplantation and the correlation between Klotho expression and patient prognosis.
Material and Method
Study population and data collection
We enrolled 50 recipients and their corresponding donors (25) who underwent renal transplantation at Guangdong Second Provincial General Hospital between November 2015 and March 2017. Eight recipients were excluded for missing data or samples, and 1 patient was eliminated due to postoperative death. Finally, there were 41 recipients and 25 donors monitored and analyzed in this study. All recipients provided written informed consent. The study was approved by the Medical Ethics Committee of Guangdong Second Provincial General Hospital and was conducted in accordance with the principles of the Declaration of Helsinki. None of the transplant donors were from a vulnerable population, and all donors or next of kin provided written informed consent that was freely given. All organ donations and transplantations were approved by the Institutional Review Board of Guangdong Second Provincial General Hospital under the guidelines of National Health and Family Planning Commission and the current regulations of the Chinese government.
Classification and immunosuppressive regimen
We divided donors into an older subgroup (age ≥50 years) and a younger subgroup (age <50 years). Consequently, their recipients were categorized into 2 subgroups according to their corresponding donor division. A standard maintenance immunosuppressive regimen of cyclosporine (CsA)/tacrolimus, mycophenolate mofetil (MMF), and prednisone was administered to transplanted recipients.
Data collection and laboratory tests
Demographic and clinical variables were recorded, including donor sex, age, and cause of death, as well as recipient sex, age, body mass index (BMI), dialysis duration, human leucocyte antigen (HLA) mismatch, occurrence of DGF, episode of rejection, immunosuppressant use, and CIT. Donors’ blood and urine samples were collected 24 h prior to organ procurement. We also collected recipients’ blood and urine samples for laboratory tests. All samples were centrifuged at 1500 g and 4°C within 4 h of collection. The supernatants were stored in aliquots at −80°C for detecting serum levels of creatinine, calcium, phosphate, uric acid (UA), Klotho, and FGF-23, as well as urinary levels of neutrophil gelatinase-associated lipocalin (NGAL). Estimated glomerular filtration rates (eGFR, MDRD) of recipients were also calculated. Serum Klotho (Immuno Biological Laboratories Co., Tokyo, Japan, 27998), intact FGF-23 (R&D Systems, Minneapolis, MN, USA, DY2604-05), and urinary NGAL (R&D Systems, Minneapolis, MN, USA, DLCN20) were measured with enzyme-linked immunoadsorbent assay (ELISA) kits. Serum creatinine, calcium, phosphate, and UA were determined with a Johnson VITROS 5600 clinical analyzer (Johnson & Johnson Family of Companies, New Brunswick, NJ, USA).
Histological change and gene expression of Klotho in renal allograft
Pre-transplant renal biopsies were collected and immediately fixed in 4% buffered formalin, then dehydrated in ethanol and embedded in paraffin. The paraffin-embedded kidney sections (5 μm) were stained with a rabbit polyclonal anti-Klotho antibody (1: 250 dilution, Abcam, Hong Kong, ab98111), deparaffinized, and incubated with a goat polyclonal antibody to rabbit immunoglobulin G (MultiVision Detection system). Negative controls were stained with isotype control antibody. Klotho expression was scanned in an imaging system (FV1200, Olympus, Tokyo, Japan).
Total RNA was extracted from zero-time renal biopsies using Trizol (Sigma, T9424) according to the manufacturers’ instruction. The following specific primers were used for RNA amplification and quantification: forward primer 5′-GAACCTGCCTGCCCTTTCTC-3′ and reverse primer 5′-AATCTCCAGAGCCGAAAATGG-3′ for human Klotho; Forward primer 5′-GATTCCACCCATGGCAAATT-3′ and reverse primer 5′-TCTCGCTCCTGGAAGATGGT-3′ for glyceraldehyde-3 phosphate dehydrogenase (GAPDH). Real-time PCR for Klotho was conducted using the SYBR Premix Ex Taq™ II Kit (Takara Bio, Kusatsu, Shiga, Japan, RR820L) and PrimeScript™ RT reagent kit (Takara Bio, Kusatsu, Shiga, Japan, RR037A) in a C1000 Thermal cycler (Bio-Rad Laboratories, California, USA) and the ABI Prism 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Results were normalized with GAPDH using 2−ΔΔCt method.
Statistical analysis
All data were analyzed using SPSS 22.0 (IBM Co., NY, USA) and GraphPad Prism 7 (GraphPad, San Diego, CA). Measurement data with normal distribution are presented as χ̄±SD and median (25th to 75th percentile) for those with non-normal distribution. Categorical variables are expressed as percentages. Differences in data with normal distribution and non-normal distribution between groups were evaluated by t test and Mann-Whitney U test, respectively. Categorical data were compared using the chi-square test. Correlations between variables were assessed by Pearson correlation analysis. The area under the receiver operating characteristic (ROC) curve was used for measuring the ability of donor serum Klotho to predict graft function. P values less than 0.05 were considered to be statistically significant.
Results
Baseline characteristics of the donor
A schematic depiction of our study protocol is provided in Figure 1. There were 25 donors (male: female=18: 7) included in our study (Table 1). The causes of death were stroke (36.0%), trauma (52.0%), and others (12.0%). The median serum creatinine, FGF-23, and mean urinary NGAL were 98.0 (75.0–140.0) umol/L, 269.9 (109.2–1014.0) pg/ml, and 21.9±13.0 ng/ml, respectively. There were 9 donors in the older subgroup and 16 donors in the younger subgroup. No statistical differences were seen in serum creatinine, FGF-23, or urinary NGAL between the 2 subgroups.
Figure 1.
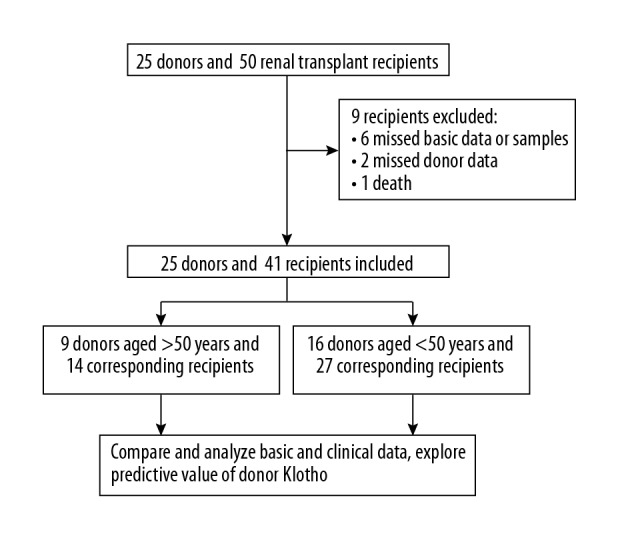
Study profile.
Table 1.
Comparison of donor characteristics of the 2 subgroups.
| Parameter | All subjects | Age ≥50 | Age <50 | p Value |
|---|---|---|---|---|
| Sex (M/F) | 18/7 | 7/2 | 11/5 | 1.000 |
| Age (years) | 38.2±14.4 | 52.2±7.3 | 30.3±11.0 | 0.000 |
| Cause of death | 0.530 | |||
| Stroke, n (%) | 9 (36.0) | 4 (44.4) | 5 (31.3) | |
| Trauma, n (%) | 13 (52.0) | 5 (55.6) | 8 (50.0) | |
| Others, n (%) | 3 (12.0) | 0 (0) | 3 (18.7) | |
| Serum creatinine (umol/L) | 98.0 (75.0–140.0) | 97.0 (76.5–189.0) | 110.5 (70.5–136.8) | 0.865 |
| Serum Klotho (pg/ml) | 954.5 (673.7–1589.8) | 658.4 (394.5–1040.1) | 1120.8 (804.9–1725.0) | 0.017 |
| Serum FGF-23 (pg/ml) | 269.9 (109.2–1014.0) | 241.1 (42.1–926.6) | 427.2 (164.5–1064.6) | 0.282 |
| Urinary NGAL (ng/ml) | 21.9±13.0 | 20.2±12.5 | 22.8±13.6 | 0.651 |
FGF-23 – fibroblast growth factor-23; NGAL – neutrophil gelatinase-associated lipocalin.
Klotho expression decreased in donors aged ≥50 years
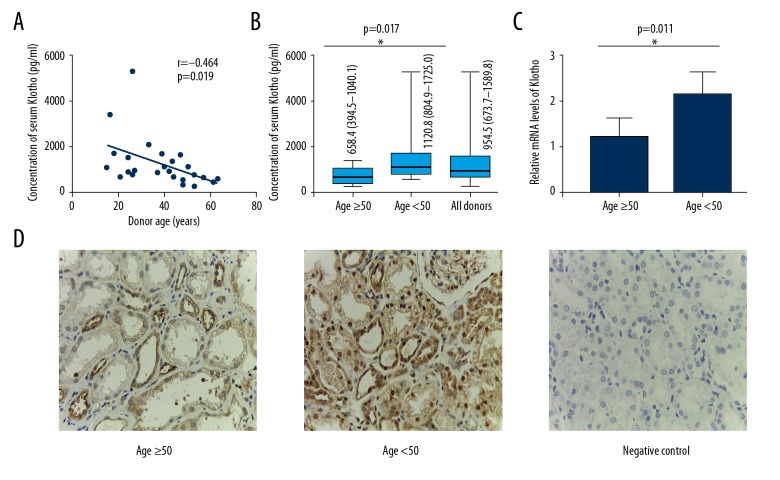
The median serum Klotho level was 954.5 (673.7–1589.8) pg/ml at organ procurement. A moderate negative correlation between donor serum Klotho level and age (r=−0.464, p=0.019, Figure 2A) was found. Serum Klotho in the older subgroup was significantly lower than in the younger subgroup (p=0.017, Figure 2B). Furthermore, we detected and compared Klotho RNA transcription in renal biopsies between the 2 subgroups. Relative mRNA level in the kidneys showed the same trend as serum Klotho (p=0.011, Figure 2C). We also confirmed these results by immunohistochemical analysis (Figure 2D).
Figure 2.
Klotho changes in donors. (A) Correlation between donor serum Klotho level and age. (B) Comparisons of serum Klotho levels between donors aged ≥50 years and <50 years (n=25). (C) Comparison of Klotho expression in zero-time renal biopsies between donors aged ≥50 years and those <50 years. (D) Representative images of immunohistochemical staining for Klotho in pretransplant renal biopsies in both subgroups as well as negative control. r – Pearson correlation coefficient; * p<0.05, ** p<0.01, *** p<0.001.
Baseline characteristics and clinical variables of recipients
We analyzed recipients’ clinical variables at 1 month (M1), 3 months (M3), and 1 year (Y1) post-transplantation (Table 2). No significant differences were found between the 2 subgroups of recipients except for age and DGF rate (p=0.008 and p=0.034, respectively). The median serum creatinine levels at M1 and M3 were significantly higher in recipients of the older subgroup than those in the younger subgroup (M1: 149.0 (120.8–203.8) umol/L vs. 110.0 (81.0–133.0) umol/L, p=0.000; M3: 193.0 (121.3–188.5) umol/L vs. 99.0 (84.0–140.0) umol/L, p=0.004). Similar results were found in eGFR at the same time (M1: 44.2±15.5 ml/min/1.73 m2 vs. 62.1±16.5 ml/min/1.73 m2, p=0.002; M3: 50.6±17.5 ml/min/1.73 m2 vs. 66.9±21.5 ml/min/1.73 m2, p=0.019). We found no significant difference in serum creatinine or eGFR at Y1.
Table 2.
Comparison of recipient characteristics of the 2 subgroups.
| Parameter | All subjects | Donor age ≥50 | Donor age <50 | p value |
|---|---|---|---|---|
| Sex (M/F) | 25/16 | 11/3 | 14/13 | 0.096 |
| Age (years) | 39.2±10.1 | 44.9±10.7 | 36.3±8.6 | 0.008 |
| BMI (kg/m2) | 21.4 (19.1–23.7) | 22.5 (19.9–23.9) | 20.0 (17.9–22.8) | 0.226 |
| Dialysis duration (months) | 12.0 (5.5–51.5) | 11.5 (5.8–29.5) | 16.0 (5.0–63.0) | 0.441 |
| CIT (minutes) | 617.2±228.8 | 556.6±188.1 | 648.7±244.6 | 0.226 |
| HLA mismatch (A, B, DR) | 64.0 (3.0–5.0) | 4.0 (3.0–4.0) | 4.0 (3.0–5.0) | 0.639 |
| DGF, n (%) | 3 (7.3) | 3 (21.4) | 0 (0) | 0.034 |
| Rejection, n (%) | 6 (14.6) | 2 (14.3) | 4 (14.8) | 0.964 |
| Immunosuppressant | 0.355 | |||
| Cyclosporin, n (%) | 11 (26.8) | 5 (35.7) | 6 (22.2) | – |
| Tacrolimus, n (%) | 30 (73.2) | 9 (64.3) | 21 (77.8) | – |
| M1 | ||||
| Serum creatinine (umol/L) | 120.0 (94.5–156.5) | 149.0 (120.8–203.8) | 110.0 (81.0–133.0) | 0.000 |
| eGFR (ml/min/1.73 m2) | 56.0±18.1 | 44.2±15.5 | 62.1±16.5 | 0.002 |
| Serum calcium (mmol/L) | 2.4 (2.3–2.5) | 2.3 (2.3–2.5) | 2.4 (2.3–2.6) | 0.141 |
| Serum phosphate (mmol/L) | 1.1 (0.9–1.3) | 1.2 (1.0–1.8) | 1.0 (0.9–1.3) | 0.102 |
| Serum UA (umol/L) | 387.3±85.4 | 403.7±101.5 | 378.7±76.5 | 0.381 |
| Serum Klotho (pg/ml) | 379.4±135.4 | 343.3±138.6 | 398.1±132.4 | 0.223 |
| Serum FGF-23 (pg/ml) | 593.4 (172.5–2401.9) | 541.5 (172.6–3478.6) | 593.4 (165.3–1620.7) | 0.805 |
| Urinary NGAL (ng/ml) | 6.9 (2.1–13.3) | 12.2 (2.1–19.4) | 4.6 (2.0–11.3) | 0.178 |
| M3 | ||||
| Serum creatinine (umol/L) | 121.0 (95.5–142.5) | 193.0 (121.3–188.5) | 99.0 (84.0–140.0) | 0.004 |
| eGFR (ml/min/1.73 m2) | 61.3±21.4 | 50.6±17.5 | 66.9±21.5 | 0.019 |
| Serum calcium (mmol/L) | 2.5±0.1 | 2.5±0.2 | 2.5±0.1 | 0.738 |
| Serum phosphate (mmol/L) | 1.2±0.3 | 1.3±0.3 | 1.2±0.3 | 0.206 |
| Serum UA (umol/L) | 401.5±109.5 | 409.7±116.0 | 397.2±108.0 | 0.733 |
| Serum Klotho (pg/ml) | 542.1 (370.3–731.5) | 444.9 (329.9–611.8) | 621.7 (423.9–872.9) | 0.054 |
| Serum FGF-23 (pg/ml) | 325.1 (134.6–901.0) | 280.6 (113.8–1804.4) | 357.8 (140.8–771.6) | 0.934 |
| Urinary NGAL (ng/ml) | 5.8 (2.2–14.0) | 8.8 (2.2–24.3) | 4.4 (2.1–10.5) | 0.458 |
| Y1 | ||||
| Serum creatinine (umol/L) | 130.0 (91.5–153.5) | 122.0 (90.8–168.8) | 133.0 (93.0–164.0) | 0.536 |
| eGFR (ml/min/1.73 m2) | 57.7±25.7 | 59.0±25.9 | 57.0±26.0 | 0.822 |
| Serum calcium (mmol/L) | 2.4±0.1 | 2.4±0.1 | 2.4±0.2 | 0.932 |
| Serum phosphate (mmol/L) | 1.2 (1.0–1.3) | 1.2 (1.1–1.4) | 1.2 (1.0–1.2) | 0.156 |
| Serum UA (umol/L) | 405.6±110.1 | 407.3±99.1 | 404.7±117.2 | 0.944 |
| Serum Klotho (pg/ml) | 594.6 (417.4–860.0) | 525.7 (423.4–903.7) | 594.6 (415.6–863.7) | 0.869 |
| Serum FGF-23 (pg/ml) | 103.2 (56.8–169.3) | 152.1 (87.3–293.5) | 73.8 (48.6–148.7) | 0.061 |
| Urinary NGAL (ng/ml) | 8.6 (3.6–22.2) | 6.2 (3.0–49.3) | 8.6 (3.6–14.1) | 0.912 |
BMI – body mass index; CIT – cold ischemia time; DGF – delayed graft function; eGFR – estimated glomerular filtration rate; FGF-23 – fibroblast growth factor-23; HLA – human leucocyte antigen; NGAL – neutrophil gelatinase-associated lipocalin; UA – uric acid.
A strong negative correlation was seen between serum Klotho of older donors and eGFR of the corresponding recipients at M1 (r=−0.686, p=0.007, Table 3). No correlations were found at other time points or in the younger subgroup. ROC analysis showed that serum Klotho in the older subgroup was a predictor for graft insufficiency at M1 (p=0.035, Table 4). The area under the curve (AUC) was 0.837 (95%CI: 0.612–1.000). At the optimal cutoff value of 727.4 pg/ml, sensitivity and specificity were 0.714 and 0.857, respectively.
Table 3.
Correlation coefficients between donor serum Klotho and graft function in the 2 subgroups.
| eGFR at various time points | Donor age ≥50 Log Klotho (pg/ml) | Donor age <50 Log Klotho (pg/ml) | ||
|---|---|---|---|---|
| r | p | r | p | |
| M1 | −0.686 | 0.007 | −0.100 | 0.620 |
| M3 | −0.456 | 0.101 | 0.097 | 0.632 |
| Y1 | −0.050 | 0.865 | −0.024 | 0.907 |
eGFR (ml/min/1.73 m2), estimated glomerular filtration rate.
Table 4.
ROC analysis of donor Klotho in diagnosis of graft insufficiency at 1 month post-transplant in both subgroups.
| Donor age ≥50 | Donor age <50 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Cutoff (pg/ml) | Sensitivity | Specificity | p Value | AUC (95% CI) | Cutoff (pg/ml) | Sensitivity | Specificity | p Value | |
| Donor Klotho | 0.837 (0.612–1.000) | 727.4 | 0.714 | 0.857 | 0.035 | 0.668 (0.474–0.862) | 1120.8 | 0.800 | 0.636 | 0.248 |
AUC – the area under curve; CI – confidence interval.
Dynamic changes of recipient serum Klotho, FGF-23, and urinary NGAL
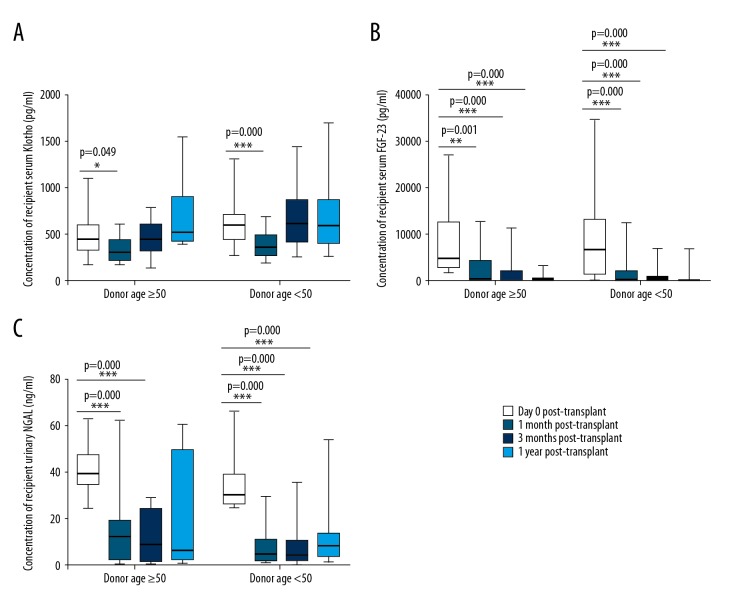
During the 1-year follow-up period, we monitored the dynamic changes in serum Klotho, FGF-23, and urinary NGAL in each subgroup. Dynamic changes of recipient serum Klotho, FGF-23, and urinary NGAL at day 0 (D0), M1, M3, and Y1 are shown in Figure 3. Serum Klotho levels increased slightly after a temporary decline after transplantation. Serum FGF-23 and urinary NGAL decreased significantly after renal transplant in both subgroups and remained low thereafter.
Figure 3.
Box-plot graphs for recipients’ serum Klotho (A), FGF-23 (B), and urinary NGAL (C) at D0, M1, M3, and Y1 post-transplant. FGF-23 – fibroblast growth factor-23; NGAL – neutrophil gelatinase-associated lipocalin; * p<0.05, ** p<0.01, *** p<0.001.
Discussion
The present study is the first to demonstrate that Klotho expression is negatively correlated with donor’s age and is associated with short-term outcomes of renal recipients, but for patient receiving a kidney from a donor aged ≥50 years, donor serum Klotho was negatively correlated with renal function at M1.
It has been proved that Klotho deficiency leads to premature aging syndrome in mice [5]. This anti-aging property is mediated by its soluble form via regulating the insulin signaling pathway and preventing oxidative stress, fibrosis, and vascular calcium deposits [13]. As the main source of Klotho, the kidneys are the principal organ mediating Klotho’s effect. During organ procurement, ischemia kidney injury inevitably occurs and changes Klotho expression. Many previous studies have found that renal ischemia/reperfusion injury has obvious relevance to Klotho production. Castellano et al. confirmed that a remarkable reduction in Klotho was observed during 24-h ischemia/reperfusion injury in an experimental swine model [14]. A cross-sectional case-control study conducted by Seibert showed serum Klotho level in AKI patients was much higher than that in healthy adults [15]. Notably, Klotho restoration attenuated renal damage from IRI-induced AKI and promoted its recovery [16,17]. In accordance with previous research, we found a mild elevation in donor serum Klotho when compared with healthy subjects, but the difference was not statistically significant (data not shown here).
In our study, a large portion of donors aged ≥50 years presented abnormal renal function or had a history of hypertension. Therefore, we chose 50 years old, which is the lower age limit of expanded criteria donors (ECD), as the cutoff value to divide donors into older and younger subgroups. Theoretically, older donors’ kidneys are susceptible to AKI and has less compensatory capacity due to aging [18]. Our results suggest that younger kidneys possess more Klotho storage and better response than older kidneys when subjected to ischemia injury during procurement. Moreover, the finding of a strong correlation between serum Klotho levels of older donors (age ≥50 years) and short-term renal function of their corresponding recipients are in line with the idea that Klotho serves as a reno-protector in AKI [16,19] . ROC analysis indicated that donor serum Klotho level is a good predictor of renal function at M1. In other words, for donors aged ≥50 years, serum Klotho level can be used to roughly estimate short-term renal function.
During the 1-year follow-up period, we monitored the dynamic changes in serum Klotho levels, FGF-23, and urinary NGAL in each subgroup. Due to hemodilution and less accumulation of injury, serum Klotho levels in both subgroups of patients declined slightly at the early post-transplant stage. However, serum Klotho levels started to rise again after a short period of time. One explanation for this phenomenon is that kidneys are inevitably damaged by immune and nonimmune factors, including acute rejection, DGF, and post-transplant CNI toxicity [20]. FGF-23, a bone-derived hormone, primarily acts on the kidneys by maintaining phosphate homeostasis via the FGF-23/Klotho signaling axis, which requires membrane Klotho as an obligate co-receptor, while the secreted Klotho functions as a endocrine factor independent of FGF-23 [21]. In our study, a sharp decline of serum FGF-23 levels was observed in both subgroups. We also found that urinary NGAL was useful in evaluating renal allograft injury at the same time. NGAL has been shown to be a novel biomarker for evaluating AKI in the field of renal transplantation [22]. The similar degrees of acute renal damage might be responsible for the nonsignificant difference found in urinary NGAL between the 2 subgroups. Additionally, for recipients of younger donors’ kidneys, their relatively higher serum Klotho and lower urinary NGAL at M1 and M3 exhibited advanced Klotho accumulation and better repair capacity, and the significantly higher eGFR and lower creatinine at the same time point support this. The stable serum calcium and phosphate levels in renal recipients during the follow-up period, to a limited extent, profited from sufficient membrane Klotho in each group protecting against calcium and phosphate disorders.
There are several limitations of our study. First, the small sample size limited the statistical power to detect differences and made it difficult to draw definite conclusions. Second, since our donors were mainly brain- or cardiac-dead donors, the results might be unsuitable for generalizing to other centers where living donor transplants are more frequently performed. Third, the retrospective and single-center features were accompanied by higher risk of causing bias. Finally, owing to lack of procedural biopsies, data on Klotho expression and renal injuries were unavailable for measurement during the follow-up period. Despite these limitations, our study shows the predictive and renoprotective utility of Klotho in renal transplantation, suggesting that monitoring and restoring Klotho levels can contribute to improvement of renal graft function.
Conclusions
The present study is the first to show that there is a moderate negative correlation between donors’ serum Klotho expression and their age. Donor serum Klotho level is negatively correlated with recipient’s renal function at 1 month post-transplant when the donor is older than 50 years old. Klotho is likely to exhibit renoprotective effects against ischemia renal injury during organ procurement as well as graft damage following transplantation. Our work may provide a novel and meaningful indicator for use in clinical practice. A multicenter study with more subjects should be performed to further confirm the value of Klotho in renal transplantation, and well-designed animal studies are needed to clarify the renoprotective mechanism of Klotho.
Footnotes
Source of support: This study was supported by the Science and Technology Program of Guangzhou, China (201707010366)
References
- 1.Le Meur Y. What immunosuppression should be used for old-to-old recipients? Transplant Rev (Orlando) 2015;29(4):231–36. doi: 10.1016/j.trre.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Denecke C, Biebl M, Pratschke J. Optimizing clinical utilization and allocation of older kidneys. Curr Opin Organ Transplant. 2015;20(4):431–37. doi: 10.1097/MOT.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 3.Nankivell BJ, Kuypers DR. Diagnosis and prevention of chronic kidney allograft loss. Lancet. 2011;378(9800):1428–37. doi: 10.1016/S0140-6736(11)60699-5. [DOI] [PubMed] [Google Scholar]
- 4.Musso CG, Giordani MC, Imperiali N. Aging kidney transplantation. Rev Invest Clin. 2016;68(2):68–74. [PubMed] [Google Scholar]
- 5.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25(10):2169–75. doi: 10.1681/ASN.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Ann Rev Physiol. 2013;75:503–33. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sopjani M, Rinnerthaler M, Kruja J, Dermaku-Sopjani M. Intracellular signaling of the aging suppressor protein Klotho. Curr Mol Med. 2015;15(1):27–37. doi: 10.2174/1566524015666150114111258. [DOI] [PubMed] [Google Scholar]
- 9.Erben RG, Andrukhova O. FGF23-Klotho signaling axis in the kidney. Bone. 2017;100:62–68. doi: 10.1016/j.bone.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Seo MY, Yang J, Lee JY, et al. Renal Klotho expression in patients with acute kidney injury is associated with the severity of the injury. Korean J Intern Med. 2015;30(4):489–95. doi: 10.3904/kjim.2015.30.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol. 2012;8(7):423–29. doi: 10.1038/nrneph.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung KP, Kasimsetty SG, McKay DB. Innate immunity in donor procurement. Curr Opin Organ Transplant. 2013;18(2):154–60. doi: 10.1097/MOT.0b013e32835e2b0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew DA, Katz R, Kritchevsky S, et al. Association between soluble Klotho and change in kidney function: The health aging and body composition study. J Am Soc Nephrol. 2017;28(6):1859–66. doi: 10.1681/ASN.2016080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano G, Intini A, Stasi A, et al. Complement modulation of anti-aging factor Klotho in ischemia/reperfusion injury and delayed graft function. Am J Transplant. 2016;16(1):325–33. doi: 10.1111/ajt.13415. [DOI] [PubMed] [Google Scholar]
- 15.Seibert E, Radler D, Ulrich C, et al. Serum klotho levels in acute kidney injury. Clin Nephrol. 2017;87(4):173–79. doi: 10.5414/CN108970. [DOI] [PubMed] [Google Scholar]
- 16.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–51. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Shi M, Gillings N, et al. Recombinant alpha-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017;91(5):1104–14. doi: 10.1016/j.kint.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Rahman EM, Okusa MD. Effects of aging on renal function and regenerative capacity. Nephron Clin Pract. 2014;127(1–4):15–20. doi: 10.1159/000363708. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Liu L, Lin W, et al. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017;91(1):144–56. doi: 10.1016/j.kint.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Cassidy H, Slyne J, O’Kelly P, et al. Urinary biomarkers of chronic allograft nephropathy. Proteomics Clin Appl. 2015;9(5–6):574–85. doi: 10.1002/prca.201400200. [DOI] [PubMed] [Google Scholar]
- 21.Kuro OM. The FGF23 and Klotho system beyond mineral metabolism. Clin Exp Nephrol. 2017;21(Suppl 1):64–69. doi: 10.1007/s10157-016-1357-6. [DOI] [PubMed] [Google Scholar]
- 22.Cappuccilli M, Capelli I, Comai G, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of allograft function after renal transplantation: Evaluation of the current status and future insights. Artif Organs. 2018;42(1):8–14. doi: 10.1111/aor.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]