Abstract
Background
We assessed body composition, adiposity, cardiovascular risk, and cognitive functions in healthy young adult females and investigated the possible correlation between neurocognitive decline, adiposity, and cardiovascular risk markers.
Material/Methods
This cross-sectional study was conducted on 83 healthy, young adult, Saudi women (age 19–23 years). Subjects were classified into group (A) with 19 non-obese subjects and negative family history (FH) of cardiovascular diseases (CVD), group (B) with 38 non-obese subjects with a positive FH of CVD, and group (C) with 18 obese subjects with positive FH of CVD. Body composition was analyzed by bioelectrical impedance analysis. Cognitive functions were evaluated using the Cambridge Neuropsychological Automated Battery (CANTAB). The blood samples were tested for lipoprotein(a) [Lp(a)] and high-sensitivity C-reactive Protein (hs-CRP).
Results
There was significantly prolonged Attention-Switching Task (AST) latency in obese subjects with negative family history of CVD (p=0.014) and those with positive family history of CVD (p=0.026) compared to controls, but the difference in AST Percent Correct Trials, Intra-Extra Dimensional Set Shift (IED) Total Errors, and Simple Reaction Time (SRT) was not significant. Simple response time had a weak but significant inverse correlation with BMI (r=−0.227, p<0.05). BMI was correlated positively with Lp(a) and hs-CRP, while BF% was correlated with hs-CRP only. No correlation was observed between the CANTAB tests, Lp(a), and hs-CRP.
Conclusions
Cardiovascular risk increases with higher adiposity and the presence of a positive family history of cardiovascular disease. Neurocognitive function may decline with higher adiposity; however, no relationship was observed between neurocognitive functions and cardiovascular risk markers.
MeSH Keywords: Apoprotein(a), C-Reactive Protein, Cardiology, Obesity
Background
Cardiovascular disease (CVD) is a major cause of disability and death worldwide. According to the World Health Organization (WHO), CVD accounted for 17.5 million deaths in 2012 globally, and was the leading cause of non-communicable disease deaths [1]. It has also been estimated that in 2014, CVDs were responsible for approximately 46% of total deaths in the Kingdom of Saudi Arabia [2]. Simultaneously, in the last decade, obesity has also become a critical problem in Saudi Arabia, as it has been estimated that nearly 33.6% of Saudi adults are classified as overweight and 33.8% are obese [3]. A recent alarming epidemiological study found that the rate of childhood obesity among primary school children was 20.2%, with 12.4% being overweight, which poses a significant threat and health burden to society [4]. It is now widely accepted that obesity is one of the major risk factors contributing to the emergence of CVD [5,6]. Additionally, it has been reported by several studies that obesity and cardiovascular diseases risk factors influence cognitive functions [7]. Cognitive functions include all the abilities and processes of perception, attention, memory, and executive functions [8]. Individuals with cardiovascular disease risk factors have been observed to have poor cognitive functions. In a large population-based cohort, a worse overall cardiovascular risk profile was associated with poorer cognitive function in young adults aged 35–44 years [9].
Moreover, hypertension is a condition known to be a major risk factor for acquiring cardiovascular disease and has been observed to have a role in cognitive impairment. High systolic blood pressure has been correlated with cognitive decline, dysfunction, and deterioration [10]. Elevated diastolic blood pressure was also associated with decreased cognitive function testing that measures immediate recall [11]. Similarly, other studies have shown that memory and the non-memory type cognitive function scores of individuals with hypertension were worse than in normotensive individuals [12].
Obesity was also frequently found to be associated with a decline in mental ability. Increased risk of dementia and Alzheimer disease has been associated with greater midlife body mass index [13]. Furthermore, it has been reported that in diet-induced obesity, hippocampal-dependent memory and nonhippocampal-dependent memory were impaired [14]. Nevertheless, findings on the relationship between adiposity and the risk of developing cognitive impairment have been controversial.
To the best of our knowledge, there have been few studies which show an integrated approach encompassing adiposity, cardiovascular risk factors, and cognitive impairment. Therefore, this study was designed to provide an integrated approach to studying the relationship among neurocognitive functions, cardiovascular risks, and adiposity indices, and to determine if there is cognitive impairment in subjects with increased adiposity and increased cardiovascular risk in a sample of young Saudi women.
Material and Methods
This cross-sectional study was conducted on healthy young Saudi women aged 19–23 years from November 2015 to April 2016 through a non-probability quota sampling technique at the Departments of Physiology and Medicine, College of Medicine, King Khalid University Hospital, Riyadh, Saudi Arabia. The study was approved by the College of Medicine Institutional Review Board (IRB). We recruited 83 subjects, out of whom 76 fulfilled the selection criteria and were then divided into 3 groups: group A included 19 subjects who were non-obese and had no family history of cardiovascular diseases (CVD), group B included 38 non-obese subjects with a positive family history of CVDs and group C included 19 obese/overweight subjects with a positive CVD family history. The subjects were classified according to BMI criteria as normal (BMI<25 kg/m2) and overweight to obese (BMI ≥25 kg/m2) [15]. Positive family history was defined as a CVDs or hypertension in a first-degree family relative at age <65 years for females and <55 years for males [16,17].
Subjects with cognitive impairment, anxiety, depression, family history of mental illnesses, liver or renal dysfunction, diabetes, CVD, or pregnancy were excluded. We also excluded the overweight subjects with a negative CVD family history, as they did not fit any of the classification criteria.
Anthropometric and body composition analysis
All participants underwent body composition analysis in the morning following an overnight fast and wearing light clothes. Body composition, including (body fat%, fat mass, free fat mass trunk fat%, trunk fat mass, trunk fat free mass, predicted muscle mass, total body water, and basal metabolic rate), was analyzed by bioelectrical impedance analysis (BIA) using a commercially available body analyzer (TANITA, USA). The subjects were asked to take off their shoes and then stand over the machine’s electrodes. Data were recorded in 1–2 min. Other different body composition indices were performed, such as BMI, waist-to-hip ratio, and body surface area. BMI was calculated via BIA. Waist circumference was measured in centimeters at the level of the highest point of the iliac crest. Hip circumference was measured at the widest area over the hips in centimeters. The body surface area was calculated using the Mosteller formula: (SQR [body weight (kg)×Height (cm)/3600]) [18,19].
Cognitive functions assessment
Cognitive functions were evaluated using the Fatigue Severity Scale (FSS) [20], Mini-Mental State Examination (MMSE) [21], and Cambridge Neuropsychological Test (CANTAB). The following tests were selected from the CANTAB to assess cognitive functions:
1. Intra-Extra Dimensional set shift (IED)
IED is a test that assesses the shifting and flexibility of attention in the fronto-striatal areas of the brain and takes about 7 min. There are 2 dimensions that are used in this test: color-filled shapes and white lines. The simple stimuli are the color-filled shapes and the compound stimuli are both the color-filled shapes and the white lines. This test starts with 2 simple stimuli appearing in the screen and the subject has to learn the correct stimuli and respond by touching it. Feedback teaches the subject the correct stimuli. After 6 correct responses, the stimuli and/or the rule changes [21]. The IED test assesses the errors, and the number of trials and stages completed [21].
2. Simple Reaction Time (SRT)
This test measures simple reaction time through delivery of a known stimulus to a known location in order to elicit a known response and takes about 6 min to complete. First, we assured that the subjects were sitting comfortably, and then we instructed them to press a button on a press pad as soon as they see a square on the screen. This test was used to assess general alertness, motor speed, correct responses, and errors of commission and omission [21]. A study has shown that as the age increases, the motor output slows, thus increasing the SRT latencies [22].
3. Attention-Switching Task (AST)
This task assesses the subject’s ability to switch attention between the direction of an arrow and its location on the screen, and it also assesses the ability to ignore the task-irrelevant information that interferes with or distracts from an event and lasts for about 8 min. The test displays an arrow which can appear and point to either side of the screen (right or left). On each trial, a cue appears at the top of the screen indicating if the subject has to press the right or left button according to either the side in which the arrow has appeared or the direction to which the arrow was pointing [21]. It measures the response latencies and error scores that reflect the subject’s attention-switching ability.
Laboratory assays
Blood samples were collected after an overnight fast, centrifuged, and serum was separated. It was stored at −80°C until assayed. The samples were tested for lipoprotein(a) and high-sensitivity C-reactive protein (hs-CRP) by standard sandwich ELISA, using kits from Elabnscience, Wuhan, China.
Statistical analysis
The data are expressed as mean ±SD or median (IQR). Multiple group comparisons were done by ANOVA for normally distributed data and by Kruskal-Wallis test for skewed data. We used the Kolmogorov test to see whether the data were normally distributed. We used the post hoc Bonferroni test for each group comparison, and Spearman’s rank order and Pearson correlations were also determined. Data were analyzed using SPSS (IBM Corp, released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). A p value of ≤0.05 was considered to be statistically significant.
Results
We compared the different variables between Group A (non-obese and have no family history CVD), Group B (non-obese and have a positive family history of CVD), and Group C (overweight/obese subjects and have a positive family history of CVD) by one-way analysis of variance (ANOVA). We compared anthropometric and demographic data of all subjects and subgroups, as shown in Table 1. SBP (p=0.005), DBP(p=0.003), BMI (p<0.001), BF% (p<0.001), FM (p<0.001), TF% (p<0.001), TFM (p<0.001), Lp(a) (p=0.021), and hs-CRP (p=0.036) were significantly higher in high BMI subjects with a positive cardiovascular disease family history.
Table 1.
Comparison of anthropometric, demographic and body composition variables between Group A, B and C.
| Variables | Group A | Group B | Group C | P-value |
|---|---|---|---|---|
| Age (years) | 20.79±0.92 | 21.21±1.16 | 21.05±1.21 | 0.384* |
| SBP (mmHg) | 101.89±8.56 | 101.59±8.41 | 108.71±6.9 | 0.005* |
| DBP (mmHg) | 72±9.72 | 72.14±7.81 | 79.90±9.27 | 0.003* |
| BMI | 21.38±3.37 | 21.15±2.9 | 28.16±3.6 | <0.001* |
| WHR | 0.72±0.05 | 0.70±0.04 | 0.73±0.07 | 0.224* |
| BF% | 29.25±6.67 | 27.3±5.73 | 37.94±5.22 | <0.001* |
| FM (kg) | 17.15 (10) | 15.10 (7) | 25.55 (7) | <0.001** |
| TF% | 28.75 (11) | 24.10 (13) | 33.70 (6) | <0.001** |
| TFM (kg) | 8.40 (6) | 7.16 (6) | 13.45 (17) | <0.001** |
| TFFM (kg) | 20.11±3.66 | 21.13±2.49 | 22.19±4.11 | 0.145* |
| TBW (Liters) | 27.22±2.94 | 28.07±2.73 | 29.88±4.59 | 0.040* |
| Lp(a) | 19.7 (24.03) | 23.7 (20.9) | 40.50 (43.03) | 0.021** |
| hsCRP | 1.23 (1.08) | 1.28 (1.10) | 1.79 (1.02) | 0.036** |
By one way analysis of variance (ANOVA);
by Kruskal-Wallis test.
Data is expressed as Mean ±SD and median (Interquartile range). Group A – Normal BMI subjects & Negative family history of CVD; Group B – Normal BMI subjects & Positive family history of CVD; Group C – High BMI subjects & Positive family history of CVD.
Figure 1 shows the comparison of screening tests of MMSE and FSS among Groups A, B, and C. The differences among the 3 groups were non-significant. This indicates that the 3 groups were matched for their fatigue and mental state alertness by standardized and validated screening tools such as the Fatigue Severity Scale (FSS) and Mini-Mental State Exam (MMSE).
Figure 1.
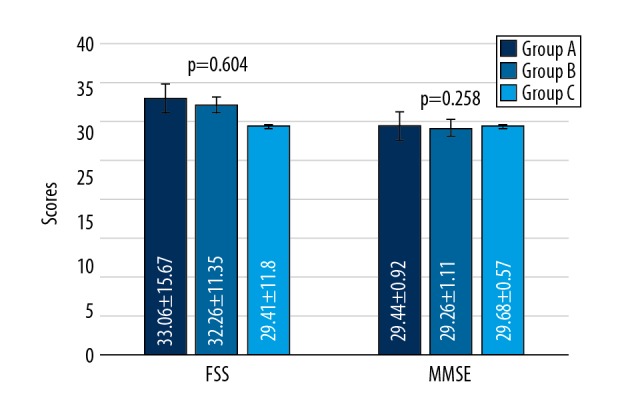
Comparison of screening MMSE and FSS between Group A, B, and C. Data compared by one-way analysis of variance (ANOVA). Values are expressed as mean ±SD.
Table 2 shows results of the neurocognitive assessment in all subjects and different subgroup comparisons. The neurocognitive assessment by CANTAB analysis showed significantly prolonged AST latency in overweight/obese women with a positive family history of CVD (p=0.010), which indicates impairment in the top-down cognitive process involving the prefrontal cortex, but no significant difference was observed between normal subjects with either negative or positive cardiovascular disease family history. Post hoc Bonferroni analysis showed significantly prolonged AST latency in obese subjects with negative family history of CVD (p=0.014) and those with positive family history of CVD (p=0.026) compared to control subjects. Differences in AST Percent Correct Trials, IED Total Errors, IED Stages Completed, SRT Mean Correct Latency, and SRT Percent Correct Trials were non-significant. We performed Pearson’s correlation analysis to determine the relationship between body parameters and cardiovascular risk markers (Table 3). We observed a significant positive correlation between SBP and BMI (r=0.440, p<0.01), BF% (r=0.314, p<0.01), FM (r=0.369, p<0.01), and TF% (r=0.305, p<0.01). Serum levels of hs-CRP were positively correlated with BMI (r=0.394, p<0.01), BF% (r=0.489, p<0.01), FM (r=0.444, p<0.01), and TF (r=0.500, p<0.01). The correlations for Lp(a) were non-significant. We also determined the relationship between the body parameters and the CANTAB tests of neurocognitive assessment results (Table 4), showing that simple response time had a weak but significant inverse correlation with BMI (r=-0.227, p<0.05), indicating that when BMI increases, the SRT decreases.
Table 2.
Comparison of screening and Neurocognitive assessment among Groups A, B and C.
| Variables | Group A | Group B | Group C | P-value |
|---|---|---|---|---|
| Attention switching ability | ||||
| AST Mean Correct Latency (ms) | 538.17 (148) | 642.73 (180) | 642.27 (176) | 0.010** |
| AST Percent Correct Trials (%) | 95.07±3.2 | 95.50±3.88 | 95.20±3.6 | 0.900* |
| Shifting and flexibility of attention | ||||
| IED Total Errors (adjusted) (numbers) | 14.0 (8) | 12.0 (9) | 11.50 (9) | 0.607** |
| IED Stages Completed (items) | 8.79±0.63 | 8.95±0.33 | 8.91±0.43 | 0.460* |
| Simple reaction time | ||||
| SRT Mean Correct Latency (ms) | 269.92 (62) | 283.85 (82) | 290.08 (45) | 0.393** |
| SRT Percent Correct Trials (%) | 98.37±1.77 | 98.32±3.22 | 98.36±2.15 | 0.998* |
By one way analysis of variance (ANOVA);
by Kruskal-Wallis test.
Data is expressed as Mean ±SD and median (interquartile range). Group A – Normal BMI subjects & Negative family history of CVD; Group B – Normal BMI subjects & Positive family history of CVD; Group C – High BMI subject & Positive family history of CVD
Table 3.
Correlation analysis between body demographics, body composition analysis and cardiovascular risk markers in all subjects.
| SBP | DBP | BMI | WHR | BMR (KJ) | BF% | FM | TF% | hsCRP | LP(a) | |
|---|---|---|---|---|---|---|---|---|---|---|
| SBP | 1 | .604** | .440** | −.109 | .075 | .314** | .369** | .305** | .002 | .031 |
| DBP | 1 | .305** | −.053 | −.090 | .168 | .265* | .219 | .049 | .117 | |
| BMI | 1 | .231* | .081 | .888** | .930** | .783** | .394** | .225* | ||
| WHR | 1 | .196 | .213 | .232* | .153 | .197 | −.008 | |||
| BMR | 1 | .190 | .182 | .163 | .138 | −.149 | ||||
| BF% | 1 | .956** | .925** | .489** | .169 | |||||
| FM | 1 | .885** | .444** | .165 | ||||||
| TF% | 1 | .500** | .166 | |||||||
| hsCRP | 1 | .024 | ||||||||
| Lp(a) | 1 |
Data analysis by Pearson’s correlation test.
Correlation is significant at the 0.05 level (2-tailed);
correlation is significant at the 0.01 level (2-tailed).
Table 4.
Correlation between body demographics and CANTAB tests.
| SBP | DBP | BMI | WHR | BF% | TF% | AST MCL | AST PCT | IED TE | IED SC | SRT MCL | SRT PCT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | 1.000 | .600** | .389** | −.082 | .255* | .247* | .031 | −.038 | .161 | −.019 | .056 | .024 |
| DBP | 1.000 | .248* | −.048 | .161 | .206 | .036 | .092 | .028 | −.125 | .047 | .012 | |
| BMI | 1.000 | .206 | .871** | .739** | .023 | −.144 | .029 | .001 | .114 | −.227* | ||
| WHR | 1.000 | .211 | .199 | −.166 | −.109 | .076 | −.102 | −.041 | −.119 | |||
| BF% | 1.000 | .909** | .037 | −.067 | −.012 | .108 | .084 | −.178 | ||||
| TF% | 1.000 | −.058 | −.006 | −.012 | .122 | −.004 | −.170 | |||||
| AST MCL | 1.000 | .003 | −.167 | .181 | .408** | .125 | ||||||
| AST PCT | 1.000 | −.160 | .112 | −.056 | .387** | |||||||
| IED TE | 1.000 | −.383** | .105 | −.147 | ||||||||
| IED SC | 1.000 | .028 | .018 | |||||||||
| SRT MCL | 1.000 | .078 | ||||||||||
| SRT PCT | 1.000 |
Data analysis by Spearman’s correlation test.
Correlation is significant at the 0.05 level (2-tailed);
correlation is significant at the 0.01 level (2-tailed).
Discussion
This study offers an insight into an integrated approach to observe the relationship between adiposity, cardiovascular risk, and neurocognitive assessment. A new observation of the present study is the significant relationships between neurocognitive function, levels of non-traditional cardiovascular risk markers in the circulation, and adiposity indices. Therefore, this study serves a theoretical purpose by contributing new findings to the literature, as well as confirming previous studies. In our study, group C subjects had significantly higher systolic and diastolic blood pressure compared to groups A and B, in agreement with the study by Mungreiphy et al. [22], who found elevated systolic and diastolic blood pressure in subjects with high BMI. Another confirmatory study showed that elevated BMI increases the risk of developing hypertension [23]. We also observed that the circulating levels of hs-CRP and Lp(a) in overweight individuals with family history of CVD were high, which is in line with the study Habib et al. [24], which concluded that higher levels of circulating hs-CRP and Lp(a) profoundly increases the risk of cardiovascular disease in obese individuals. Also, Michele et al. demonstrated that high serum level of lipoprotein(a) is a predictor of coronary artery disease [25]. A study by Gupta et al. titled ‘Emerging risk factors for cardiovascular diseases: Indian context’ [26] reported that persons with a positive family history for cardiovascular disease were more likely to have higher levels of serum hs-CRP and lipoprotein(a).
Furthermore, in terms of the outcomes of the neurocognitive assessment carried out on the subjects of this study, it was observed that subjects in group C had a significantly higher mean AST latency, which essentially indicates they exhibited a decline in their ability to switch attention. This result is in agreement with John et al., showing that BMI is significantly and inversely associated with attention-switching test score [27]. An inverse relationship between the BMI and the SRT% of correct trials was detected, which fundamentally means that as the BMI increases, the likelihood of making an error in the SRT test is higher. The latter 2 findings are in line with the study conducted by Wang et al. [28], which concluded that a higher adiposity is associated with neurocognitive decline. Further, a study conducted by Stanek et al. found an association between obesity and a frontal-subcortical pathology [29].
One study concluded that there is a significant correlation between anthropometric measures and total body fat and CVD risk factors, and another study showed that the CVD risk depends on the number and age of the first-degree relative suffering from cardiovascular diseases [30,31]. As for the neurocognitive assessment performed in the current study, it was concluded that neurocognitive function might decline with higher adiposity, similar to the finding of Jason et al., who concluded that obesity increases the risk of developing mild cognitive impairment [32]. Kaela et al. showed that attention/mental flexibility of obese adolescents was significantly worse than in healthy-weight adolescents [33], but there was insufficient evidence to prove the existence of a link between neurocognitive function and circulating levels of hs-CRP and lipoprotein(a). The limitations of the present study are the small sample size and the cross-sectional study design.
Conclusions
Cardiovascular risk increases with higher adiposity and positive family history of cardiovascular disease. Neurocognitive function may decline with higher adiposity; however, no relationship was observed between neurocognitive functions and the cardiovascular risk markers. Finally, we recommend long-term, large-scale, prospective trials to confirm the relationship between hs-CRP, Lp(a), and neurocognitive functions. Moreover, patients with high risk of cardiovascular diseases should be screened for neurocognitive functions.
Acknowledgement
We thank nurse Jane for her cooperation and support, and everyone who volunteered in our study.
Abbreviations
- CVD
cardiovascular disease
- FH
family history
- EX
exercise
- SBP
systolic blood pressure (mmHg)
- DBP
diastolic blood pressure (mmHg)
- BMI
body mass index
- WHR
waist-hip ratio
- BMR
basal metabolic rate (kilo joules)
- BF%
body fat%
- FM
fat mass (kg)
- TF%
trunk fat%
- TFFM
trunk fat free mass (kg)
- TFM
trunk fat mass (kg)
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- AST MCL
Attention Switching Task Mean Correct Latency
- AST PCT
Attention Switching Task Percent Correct Trials
- IED TE
Intra-Extra Dimensional Set Shift Total Errors (adjusted)
- IED SC
Intra-Extra Dimensional Set Shift Stages Completed
- SRT MCL
Simple Reaction Time Mean Correct Latency
- SRT PCT
Simple Reaction Time Percent Correct Trials
- NS
non-significant
- vs.
versus
Footnotes
Source of support: Deanship of Scientific Research (Grant Number: RGP-1438-048) King Saud University, Riyadh, Saudi Arabia
References
- 1.World Health Organization. Obesity and overweight. 2006. http://www.who.int/mediacentre/factsheets/fs311/en/print.html.
- 2.Aljefree N, Ahmed F. Prevalence of cardiovascular disease and associated risk factors among adult population in the gulf region: A systematic review. Advances in Public Health. 2015;2015 235101. [Google Scholar]
- 3.Habib SS. Body mass index and body fat percentage in assessment of obesity prevalence in saudi adults. Biomed Environ Sci. 2013;26(2):94–99. doi: 10.3967/0895-3988.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shehri JA. Childhood obesity prevalence among primary schoolboys at Al-Iskan sector, Holy Makkah, Saudi Arabia. Int J Med Sci. 2014;3(2):150–55. [Google Scholar]
- 5.Lopez-Lopez J, Lopez-Jaramillo P, Camacho PA, et al. The link between fetal programming, inflammation, muscular strength, and blood pressure. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/710613. 710613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–85. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 7.Joosten H, van Eersel ME, Gansevoort RT, et al. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke J. 2013;44(6):1543–49. doi: 10.1161/STROKEAHA.111.000496. [DOI] [PubMed] [Google Scholar]
- 8.Wall KJ, Isaacs ML, Copland DA, Cumming TB. Assessing cognition after stroke. Who misses out? A systematic review. Int J Stroke. 2015;10(5):665–71. doi: 10.1111/ijs.12506. [DOI] [PubMed] [Google Scholar]
- 9.Joosten H, van Eersel ME, Gansevoort RT, et al. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke J. 2013;44(6):1543–49. doi: 10.1161/STROKEAHA.111.000496. [DOI] [PubMed] [Google Scholar]
- 10.Böhm M, Schumacher H, Leong D, et al. Systolic blood pressure variation and mean heart rate is associated withcognitive dysfunction in patients with high cardiovascular risk. 2015;65(3):651–61. doi: 10.1161/HYPERTENSIONAHA.114.04568. [DOI] [PubMed] [Google Scholar]
- 11.Power MC, Tchetgen EJ, Sparrow D, et al. Blood pressure and cognition: Factors that may account for their inconsistent association. Epidemiology. 2013;24(6):886–93. doi: 10.1097/EDE.0b013e3182a7121c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T, Xiang J, Bai J, et al. The association of duration of hypertension and changes in cognitive function in hypertension patients. Zhonghua Nei Ke Za Zhi. 2014;53(4):278–82. [in Chinese] [PubMed] [Google Scholar]
- 13.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia. Arch Neurol. 2009;66:336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farr SA, Yamada KA, Butterfield DA, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149(5):2628–36. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 16.Cardiovascular disease risk factors – Family history | World Heart Federation [Internet] 2016. World-heart-federation.org.
- 17.Scheuner M, Setodji C, Pankow J, et al. General cardiovascular risk profile identifies advanced coronary artery calcium and is improved by family history: The multiethnic study of atherosclerosis. Circulation: Cardiovascular Genetics. 2009;3(1):97–105. doi: 10.1161/CIRCGENETICS.109.894527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55(4):515–24. doi: 10.1016/j.metabol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Choi J, Kim H. Determination of body surface area and formulas to estimate body surface area using the alginate method. J Physiol Anthropol. 2008;27(2):71–82. doi: 10.2114/jpa2.27.71. [DOI] [PubMed] [Google Scholar]
- 20.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–23. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 21.Authority IHP: Standardised Mini-Mental State Examination (SMMSE) [Internet] Australian Government Department of Health; [Google Scholar]
- 22.ADD Testing (Attention Deficit Disorder) – Cambridge Cognition [Internet] 2016. Cambridgecognition.com.
- 23.Woods D, Wyma J, Yund E, et al. Factors influencing the latency of simple reaction time. Front Hum Neurosci. 2015;9:131. doi: 10.3389/fnhum.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mungreiphy N, Kapoor S, Sinha R. Association between BMI, blood pressure, and age: Study among Tangkhul Naga Tribal Males of Northeast India. Journal of Anthropology. 2011;2011:1–6. [Google Scholar]
- 25.Dua S, Bhuker M, Sharma P, et al. Body mass index relates to blood pressure among adults. N Am J Med Sci. 2014;6(2):89–95. doi: 10.4103/1947-2714.127751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habib SS, Al Masri A. Relationship of high sensitivity C-reactive protein with presence and severity of coronary artery disease. Pak J Med Sci. 2013;29(6):1425–29. doi: 10.12669/pjms.296.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaguarnera M, Vacante M, Russo C, et al. Lipoprotein(a) in cardiovascular diseases. BioMed Res Int. 2013;2013:1. doi: 10.1155/2013/650989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Bhise M, Gaurav K, Gudapati R. Emerging risk factors for cardiovascular diseases: Indian context. Indian J Endocrinol Metab. 2013;17(5):806–14. doi: 10.4103/2230-8210.117212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunstad J, Paul R, Cohen R, et al. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Chan J, Ren L, Yan J. Obesity reduces cognitive and motor functions across the lifespan. Neural Plasticity. 2016;2016:1–13. doi: 10.1155/2016/2473081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanek K, Strain G, Devlin M, et al. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27(2):141–51. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreira T, Staiano A, Harrington D, et al. Anthropometric correlates of total body fat, abdominal adiposity, and cardiovascular disease risk factors in a biracial sample of men and women. Mayo Clinic Proc. 2012;87(5):452–60. doi: 10.1016/j.mayocp.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michael R, Kolber C. Family history of cardiovascular disease. Canadian Family Physician. 2014;60(11):1016. [PMC free article] [PubMed] [Google Scholar]


