Abstract
Background.
There are complex associations between immune function and mental illness, yet studies in the perinatal period focus primarily on individual inflammatory markers and depressive symptoms only, cross-sectionally. We sought to examine associations between both depressive and anxious symptoms and immune activation longitudinally across the peripartum.
Methods.
We measured mood (Beck Depression Inventory, BDI-1A) and anxiety (State-Trait Anxiety Inventory, STATE) and levels of 23 cytokines at 5 points in pregnancy and postpartum in 51 women. Within subject cytokine trajectories over time by depressive and anxious symptom grouping were assessed using linear mixed effects models with random intercept and slope. We also undertook an exploratory cluster analysis based on third trimester cytokine values.
Results.
Based on categorical BDI scores, IL-6 (p < 0.001), IL-15 (p = 0.047), GCSF (p = 0.003), and CCL3 (p < .001) were significantly different across time, with IL-6 (p < 0.001), IL-15 (p = 0.003), and CCL3 (p < 0.001) higher at the third trimester visit in more depressed subjects. Based on categorical STATE scores, GM-CSF significantly decreased across pregnancy for the less anxious group (p =0.016), but not for the more anxious, and CCL3 (p = 0.017), CXCL8 (p = 0.011), and IL-6 (p < 0.001) were higher at the third trimester visit for more anxious subjects. In exploratory cluster analysis based on cytokine level, there were no differences in mood or anxiety scores, but significant differences by race/ethnicity and overweight/obesity status. Women with higher pro-inflammatory cytokine values are more likely to be Hispanics (69.2% vs. 21.4%, p = 0.015), but less likely to be African American (23.1% vs. 60.7%, p=0.015) or overweight/obese (25% vs. 69.2%, p = 0.016) compared to women with lower pro-inflammatory cytokine values.
Conclusion.
We identified a pro-inflammatory burst at the third trimester, indicative of innate immune activation, in women with higher levels of both depressive and anxious symptoms, as well as differences in pro-inflammatory changes across time. We also found significant differences in cytokine levels by race, ethnicity, and overweight/obesity status. These results point the way toward future longitudinal work that considers race/ethnicity, timing, and weight status, and evaluates perinatal mood and anxiety disorders in the context of changing immune functioning across the peripartum.
Keywords: Depression, pregnancy, anxiety, cytokine, immunity, inflammation
1. Introduction
Perinatal depressive and anxious symptoms affect up to 15% of women in high-income countries and 20–40% of women in the developing world (Gaynes et al., 2005; World Health Organization, 2009); they can be debilitating and result in adverse outcomes for both mother and child (Skogen & Overland, 2012; Bianco-Miotto et al., 2017). Research on the pathophysiology of perinatal depression and anxiety has focused on hormonal imbalance, the role of monoamines, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Gold and Chrousos, 2002; Kammerer et al., 2003), and contributions of the immune system (Osborne & Monk, 2013; Sherer et al., 2017). Research on immune responses in normal pregnancy initially focused on pregnancy as a state of immune suppression, but our work among others now supports a more complex model, where innate immune barriers are enhanced but specific adaptive immunity shows reduced effectiveness (Kraus et al., 2012; Holtan et al., 2015). Studies on immune dysregulation in perinatal depression and anxiety are mostly cross-sectional and reflect little standardization of either immune markers or mood and anxiety measures. Two recent studies have considered a broad range of cytokines using a single score of inflammation, but neither could address change over time in either mood or cytokines (Edvinsson et al., 2017; Brann et al., 2017).
To build on this substantial work, we evaluated how changes in 23 different cytokines over five time points across the peripartum related to subjects’ depressive and anxious symptoms. We hypothesized that perinatal women might represent an “inflammatory subset” of individuals with depressive and anxious symptoms (Miller et al., 2009), and that we would therefore see higher levels of proinflammatory cytokines across the perinatal period in women with more depressive and anxious symptoms. Because this was an exploratory, hypothesis-generating study, we chose to measure 23 different cytokines in order to capture not only the pro-inflammatory shifts we expected but any other unexpected changes as well.
2. Material and methods
2.1. General Study Procedures.
The Viral Immunity in Pregnancy Study (2006–2009) followed women prospectively from early pregnancy until 6 months postpartum to examine systemic immunologic activity and enhanced susceptibility to viral pathogens. Women were recruited from a general community obstetrics practice at Mt. Sinai Medical Center, where rates of mental illness are equivalent to those in the general population. Women who had underlying medical conditions and⁄or had received therapies that might affect their immunologic responses were excluded. At enrollment (T1) (median gestational age = 14.5 weeks, SD=3.1, range from 8 to 20 weeks), 55 mL of peripheral blood and baseline information on previous pregnancies, medical history, and demographics were collected. Women were followed up for four subsequent 55-mL blood draws at approximately 26 (SD+1) (T2) and 35 (SD=0.8) (T3) weeks’ gestation and 6 weeks (PP1) and 24 weeks (PP2) postpartum; updated medical, pregnancy, and delivery histories were obtained at each visit. Participants completed the Beck Depression Inventory (BDI) 1A and the State-Trait Anxiety Inventory (STAI) Version Y at each visit. (We report here on the STATE score of the STAI and will refer to it as STATE throughout.) The study was approved by the Program for the Protection of Human Subjects Institutional Review Board at the Mount Sinai School of Medicine. (See Kraus et al., 2010; Kraus et al., 2012 for more details on study procedures.)
2.2. Cytokine Analysis.
Blood was collected in Vacutainer serum tubes (BD, Franklin Lakes, NJ, USA). Serum was separated within 6 hours of collection, stored at −80°C, and thawed on ice immediately prior to analysis, with no sample thawed more than once. All cytokines, chemokines, and growth factors were analyzed by a bead-based ELISA method by Milliplex xMAP technology (Millipore, Billerica, MA) using a Luminex 200 (Luminex Corporation, Austin, TX). Samples were run in duplicate with QCs on each plate. Data were analyzed using Milliplex Analyst. All visits of each subject were analyzed simultaneously on the same plate, eliminating any variability among assays. Hormones (cortisol, estradiol, and progesterone) were analyzed in serum by ELISA by Lenetix Medical Screening Laboratories (now BioReference Laboratories); we were unable to account for diurnal changes in these measurements or in cytokines.
2.3. Statistical Analysis.
Demographic, pregnancy and medical history characteristics of the sample were summarized using frequencies, means, and standard deviations. The presence of at least mild depressive symptoms was defined by a BDI score of >9 (Beck and Steer, 1987) and the presence of at least moderate anxious symptoms was defined by STATE score ≥35 (Spielberger et al. 1983). We chose cut-off scores rather than continuous scoring as this method enabled us to compare women with clinically significant levels of symptomatology to those without; the BDI cut-off score was based on literature standards (Beck et al., 1961; Aalto et al. 2012) while that for the STATE was based on the mean score for women in the general population (Knight et al. 1983; Santagelo et al. 2016). Data on cytokines were transformed with natural logarithms due to the skewed nature of the variables. Generalized linear mixed effects models with random intercept for woman and random slope for visit were used to model change in log-transformed cytokines over time. The random effects were assumed to be normally distributed with respective variances and a covariance. The model included fixed effects for time (4 indicator variables for each visit compared to the first visit, T1), presence of depressive symptoms or anxious symptoms (1 indicator variable), and their interaction, assuming different cytokine trajectories by concurrent depressive or anxious symptom status. This model was further expanded to include other relevant predictors, such as race (black vs. other), marital status (married vs. not), and cortisol level. We considered additional covariates, including pre-pregnancy body mass index (BMI) (analyzed as BMI>25 vs. BMI≤25, referred to throughout as “overweight/obesity status”), income, education level, employment status, asthma, estradiol and progesterone levels, and systemic infection (by self-report, presence of an infection requiring prescription medication), but found that including these factors did not alter our findings. We were not able to consider autoimmune disease, pregnancy morbidities, gestational weight gain, or immune medication use as covariates, as these factors were present in very small numbers in our sample. From these models, we were able to estimate change in cytokine levels over time, comparing each visit to all earlier visits, within depressive and anxious symptom groups. We were also able to compare cytokine levels between groups at each time point.
We used K-means partition cluster analysis to subdivide the study participants into two groups based on their 3rd trimester log-transformed cytokine values, using Euclidean distance as the similarity measure. Participant demographic, clinical, and pregnancy history as well as depressive and anxious symptom scores were compared across the two groups using t-test (for age) and Fisher’s exact test.
All tests were two-sided and were run at 0.05 statistical significance level. No adjustment was made for multiple comparisons, as this was an exploratory study and we did not want to miss potential associations that would be worthy of future study. The analyses were carried out using STATA 14 software program (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
3. Results
3.1. Patient Characteristics and Symptoms Across the Peripartum.
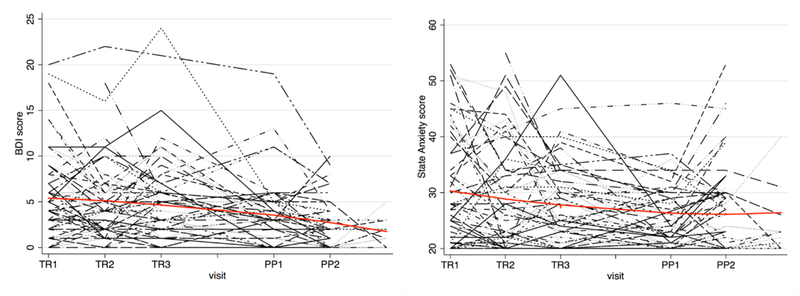
Fifty-seven women enrolled in the parent study; of these, 51 had cytokine data and were included as the current study sample. Baseline demographic tables are based on the 48 women who had complete demographic data. Table 1 displays demographic variables. Because our study sample was drawn from a non-clinical population, we hypothesized that rates of depressive and anxious symptoms would be low. We found that 14% exhibited at least mild depressive symptoms (>9 on the BDI1A) at study entry, and there were no significant differences between the more depressed and less depressed groups on basic demographic characteristics. Beck score at entry ranged from 0 to 20, with a mean of 5.4 and a median of 4; these values trended down slightly across the study (decrease of 0.57 points per visit (95% CI: 0.34–0.81, p < 0.001, Figure 1). Twenty-nine percent displayed at least moderate anxious symptoms (≥ 35 on STATE Anxiety score) at study entry, with STATE scores ranging from 20 to 53, a mean of 30.7, and a median of 28. These values also trended down across the study, with a decrease of 0.81 points per visit (95% CI: 0.3−1.3), p = 0.001 (Figure 1). Most women had either anxious or depressive symptoms only; subjects who had both ranged from 35% at T1 to 5% at PP2 (data not shown). A greater proportion of subjects was married or partnered in the less anxious group than in the more anxious group (85% vs. 29%, p <0.001).
Table 1.
Baseline Demographics by Patients’ Emotional State (N=49)
| Characteristic | Total N = 49 | More depressed N = 12 (24%) | Less depressed N = 37 (76%) | P | More anxious N = 28 (57%) | Less anxious N = 21 (43%) | P |
|---|---|---|---|---|---|---|---|
| Maternal Age* | 26.3 ± 5.2 | 25.5 ± 5.1 | 26.3 ± 5.2 | 0.6550 | 26.0 ± 5.5 | 26.3 ± 4.8 | 0.8519 |
| Race* | 0.7250 | 0.3648 | |||||
| Black | 22 (46%) | 7 (58%) | 15 (42%) | 14 (50%) | 8 (40%) | ||
| Hispanic | 24 (50%) | 5 (42%) | 19 (53%) | 12 (43%) | 12 (60%) | ||
| Other | 2 (4%) | 0 | 2 (6%) | 2 (7%) | 0 | ||
| Pre-pregnancy BMI ≥ 25* | 26 (57%) | 7 (58%) | 19 (56%) | 0.8829 | 16 (64%) | 10 (48%) | 0.2643 |
| Asthma | 13 (27%) | 3 (25%) | 10 (27%) | 0.8901 | 9 (32%) | 4 (19%) | 0.3477 |
| PE/GPH/PIH* | 9 (19%) | 2 (17%) | 7 (19%) | 1.0000 | 7 (25%) | 2 (10%) | 0.2707 |
| Low birth weight | 6 (12%) | 0 | 6 (16%) | 0.3142 | 5 (18%) | 1 (5%) | 0.2192 |
| Pre-Term Birth | 8 (16%) | 2 (17%) | 6 (16%) | 1.0000 | 6 (21%) | 2 (10%) | 0.4384 |
| Medicaid* | 44 (92%) | 12 (100%) | 32 (89%) | 0.5597 | 25 (89%) | 19 (95%) | 0.6309 |
| Edu ≤ HS* | 21 (44%) | 6 (50%) | 15 (42%) | 0.6143 | 13 (46%) | 8 (40%) | 0.6580 |
| Married/Partner* | 33 (69%) | 7 (58%) | 26 (72%) | 0.3687 | 15 (54%) | 18 (90%) | 0.0108 |
| Systemic Infection during pregnancy | 9 (18%) | 2 (17%) | 7 (19%) | 1.000 | 7 (25%) | 2 (10%) | 0.2670 |
| Median serum cortisol (μg/dL) | 13.8 | 11.1 | 0.765 | 10.1 | 12.2 | 0.798 |
Missing values exist in demographic variables.
Figure 1.
Beck Depression Inventory and STATE Anxiety Scores Across the Study
3.2. Association between Cytokines and Depressive Symptoms.
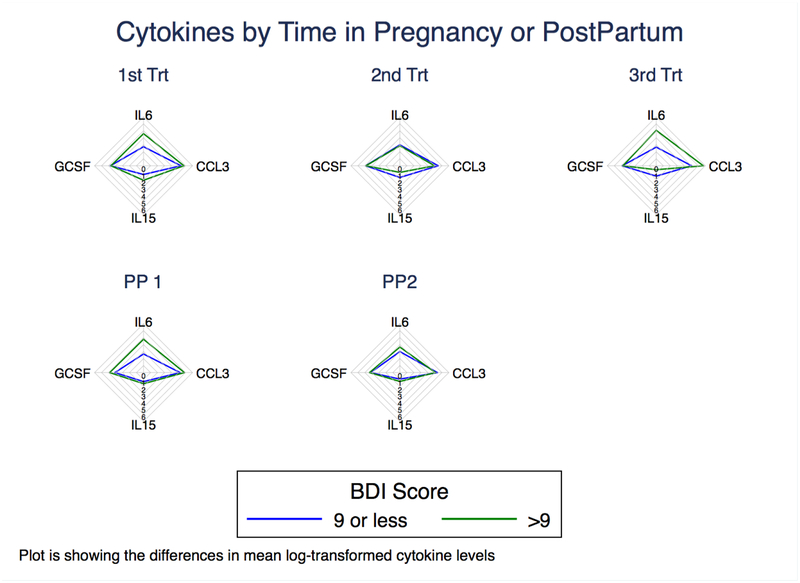
Our findings on changes in cytokine levels across time in the population as a whole have been presented previously (Kraus et al., 2010; Kraus et al., 2012). We hypothesized that women with higher depressive symptoms would show elevated levels of pro-inflammatory cytokines across the perinatal period when compared to less depressed women. We log-transformed mean cytokine levels, which were plotted against categorical BDI scores (≤9 and >9) in longitudinal analysis at each of the five time points (T1, T2, T3, PP1, PP2), using generalized linear mixed effects models. From these analyses, four pro-inflammatory cytokines and chemokines showed significant associations with depressive symptoms (see Figures 2 and 3).
Figure 2.
Radar Plot of Cytokines Across Time by Depression Status
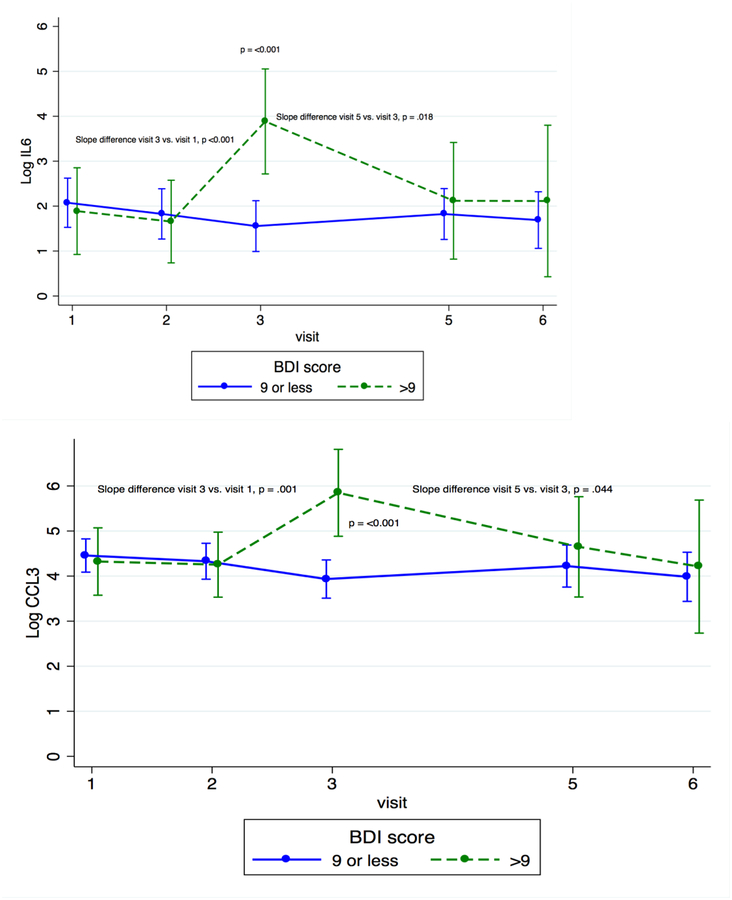
Figure 3.
Cytokine Trajectories Across Time by Depression Status
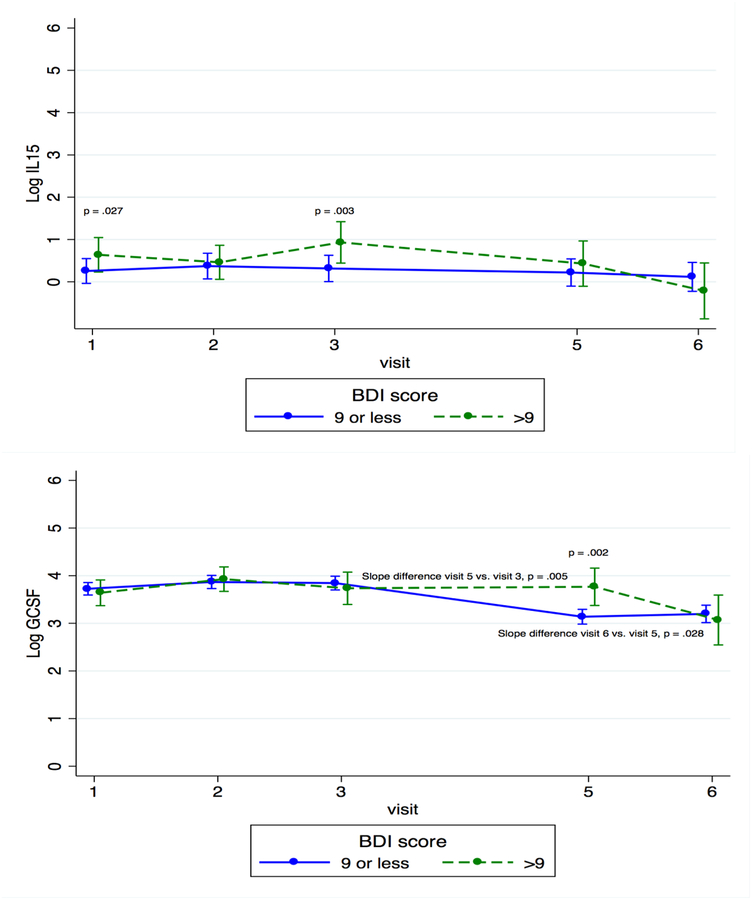
When looking at individual time points, we found that concentrations of IL-6, a cytokine that is integral in the acute phase response (Eder et al., 2009) and B and T cell activation (Unver & McAllister, 2018), and CCL3, a chemokine that is involved in acute inflammatory responses and activation (Baba & Mukaido, 2018), were significantly higher among the more depressed women at T3 than among the less depressed women (IL-6: β = 2.330, p < 0.001; CCL3: β = 1.914, p < 0.001). IL-15, which induces proliferation of innate immune cells, including NK cells (Guo et al., 2017), was significantly higher in the more depressed women than in the less depressed women at both T1 and T3 (β = 0.384, p = 0.027 and β = 0.616, p=0.003, respectively). Granulocyte colony-stimulating factor (G-CSF), an innate immune cell growth factor (Eftekhar et al, 2018), was significantly higher for the more depressed women than for the less depressed women at PP1 (β = 0.629, p = 0.002).
To evaluate the kinetics of individual cytokines, we compared the slope of cytokine change across time between more and less depressed women. In less depressed women, concentrations of IL-6 and CCL3 decreased across pregnancy (T1 to T3) and rebounded slightly at PP1; more depressed women had the opposite pattern, with IL-6 and CCL3 levels increasing across pregnancy and then decreasing slightly at PP1 (for difference of slope between groups, for IL-6, p < 0.001 from T1 to T3 and p = 0.018 from T3 to PP1; for CCL3, p = 0.001 from T1 to T3 and p = 0.044 from T3 to PP1). IL-15 decreased for both groups from T1 to the last postpartum visit (PP2), but the decline was greater for those in the more depressed group (p = 0.047 for difference in slopes). G-CSF increased across pregnancy for both groups, but postpartum patterns were significantly different. For the less depressed group, G-CSF decreased from T3 to PP1 and then increased from PP1 to PP2; for the more depressed group, it increased from T3 to PP1 and decreased from PP1 to PP2 (p = 0.005 for difference in slope between groups from T3 to PP1, p = 0.028 for difference in slope between groups from PP1 to PP2) (see Figure 3). These data show that women with more depressive symptoms had increased levels of four pro-inflammatory markers at several time points across the perinatal period, especially in the third trimester, than did less depressed women, and that the slope of change for these cytokines also differed between the two groups of women.
3.3. Association between Cytokines and Anxious Symptoms.
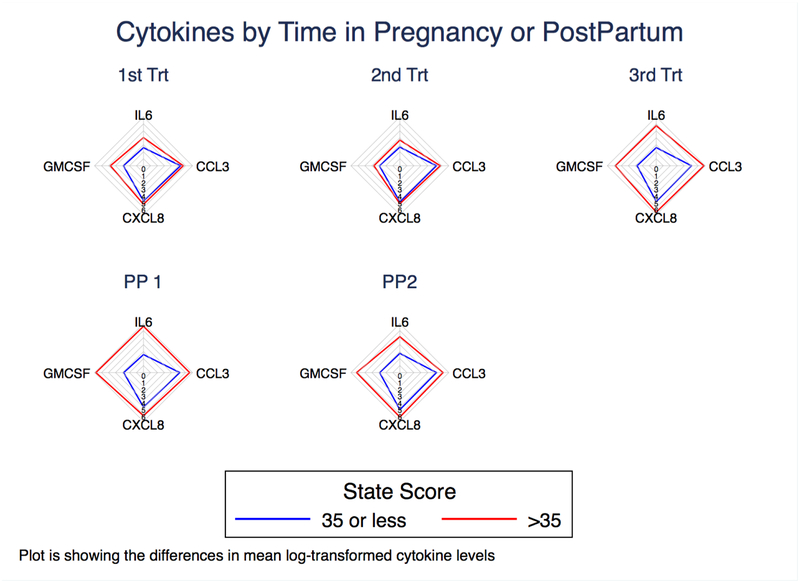
We hypothesized that perinatal women with anxious symptoms would also show elevated levels of pro-inflammatory cytokines and chemokines in late pregnancy and postpartum. Using the generalized linear effects models described above, we plotted log-transformed mean cytokine concentrations against categorical STATE scores (<35 and ≥35) and found that four pro-inflammatory markers showed a significant association with anxious symptoms (see Figures 4 and 5).
Figure 4.
Radar Plot of Cytokines Across Time by Anxiety Status
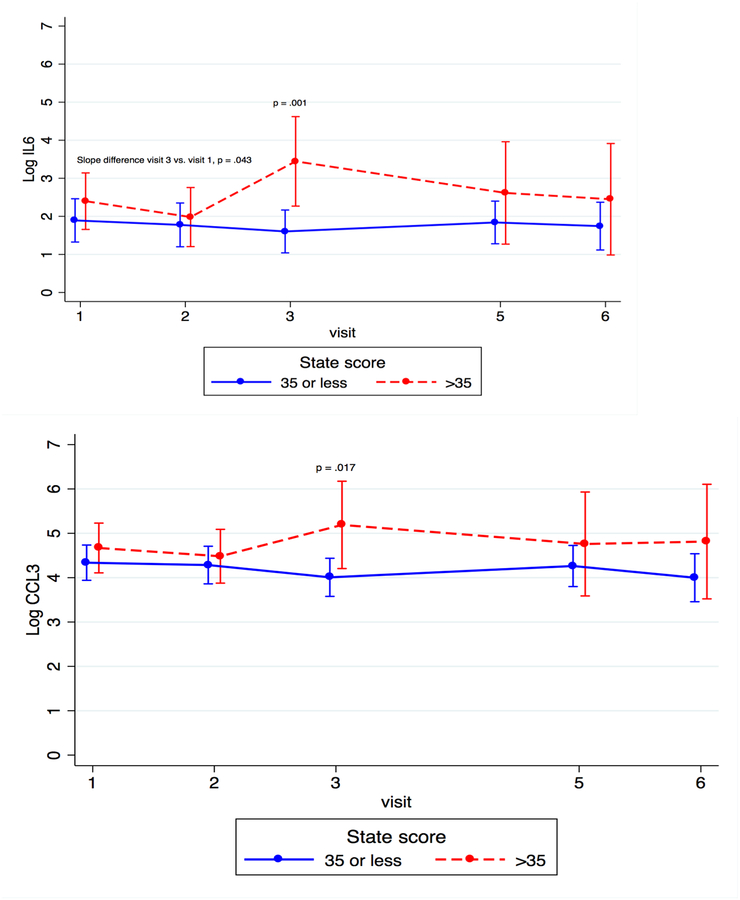
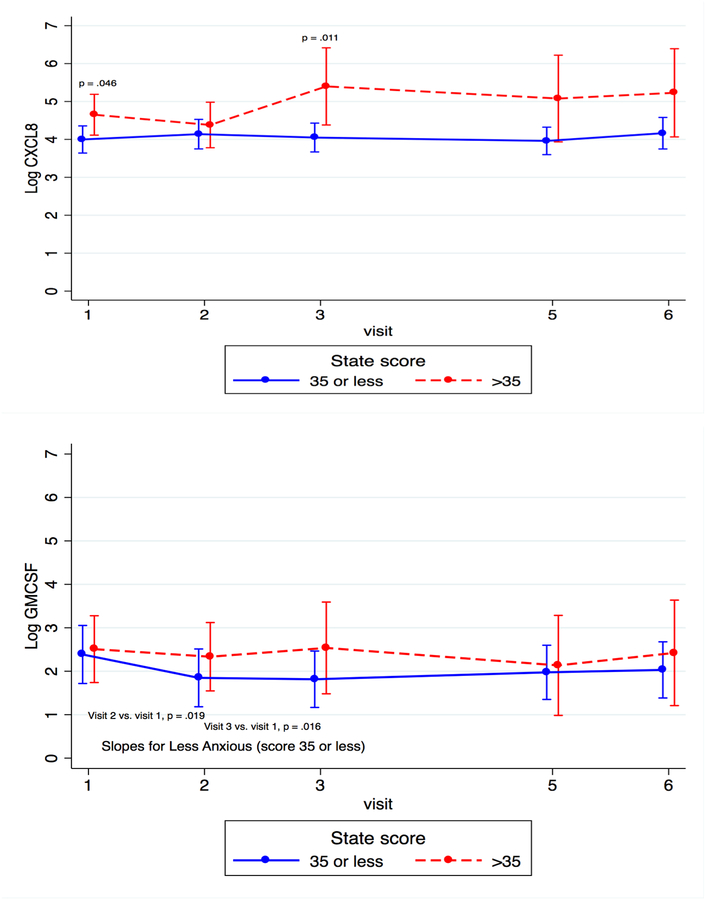
Figure 5.
Cytokine Trajectory Across Time by Anxiety Status
When looking at individual time points, we found that concentrations of IL-6 and CCL3 were significantly higher for the more anxious compared to the less anxious group at T3 (β = 1.84, p < 0.001 and β = 1.18, p =0.017, respectively), and levels of C-X-C motif ligand 8 (CXCL8), a chemokine that promotes the migration of neutrophils (Ha et al., 2017), were significantly higher for the more anxious compared to the less anxious group at both T1 and T3 (β = 0.652, p = 0.046 and β = 1.35, p = 0.011, respectively).
In examining the kinetics of individual cytokines, we found that the slope of change of IL-6 from T1 to T3 differed between more anxious and less anxious women (β = −0.291, p = 0.043). Granulocyte macrophage colony-stimulating factor (GM-CSF), a growth factor that stimulates the development of macrophages and dendritic cells (Becher et al., 2016), significantly decreased across pregnancy (β = −0.570, p = 0.016 for T3 vs. T1 and β = −0.538, p = 0.019 for T2 vs. T1) for the less anxious group, but not for the more anxious women (see Figure 5). These data show that women with more anxious symptoms had increased levels of three pro-inflammatory markers at several time points across the perinatal period, especially in the third trimester, when compared to less anxious women, and that the slope of change for these cytokines and one additional marker also differed between the two groups of women.
3.4. Cluster Analysis.
We examined our data using an exploratory cluster analysis that generated two groups by cytokine level at the T3 visit (N = 43), the time at which there were most substantial differences in cytokine values by anxiety and depression status. Group 1 (N = 13) had higher pro-inflammatory cytokine values (but lower values of IL-4, IL-7, and IL-10) and Group 2 (N = 30) had lower pro-inflammatory cytokine values. The BDI and STATE scores between the two groups were not statistically significantly different. The two groups were significantly different by race and ethnicity (Group 1, with higher cytokine values, had fewer African-American subjects (23.1% vs. 60.7%) and more Hispanic subjects (69.2% vs. 21.4%), p = 0.015), and by overweight/obesity status (Group 1 had fewer subjects with pre-pregnancy BMI above 25, 25% vs. 69.2%, p = 0.016). These data show that cytokine values may differ along lines of race, ethnicity, and weight status, but that when clustering all 23 cytokines together we did not find differences by mood or anxiety status.
4. Discussion and Conclusions
The aim of this hypothesis-generating study was to shed light on the potential role of inflammation in mood and anxiety symptoms across the peripartum. We found increases in concentrations of proinflammatory markers at several time points, especially in the third trimester; two of these markers (IL-6 and CCL-3) were elevated for both more anxious and more depressed subjects. We also saw differences in the slope of change of pro-inflammatory cytokines between groups. An exploratory cluster analysis revealed significant demographic differences, with the group with higher pro-inflammatory cytokines having fewer overweight/obese subjects, fewer African Americans, and more Hispanics, but no differences in mood or anxiety, indicating that clustering different types of cytokines together may not be a useful way to understand how immune characteristics may be related to symptoms. We were surprised that overweight/obesity did not correlate with higher pro-inflammatory cytokines. As the work of Christian et al. has shown, however, the relationship between both overweight/obesity and depressive symptoms and inflammation may differ across ethnic groups and by childhood trauma status (Christian et al., 2018; Mitchell et al., 2018), and we were unable to account for trauma differences. Differences in baseline cytokines have also been noted in a variety of other fields by weight status and by ethnicity (Bastard et al, 2006; Chen et al., 2014; Somogyi et al., 2016; Tucker et al., 2017), and these facts coupled with our data argue for careful characterization of both weight status and race and ethnicity in future work on the role of immune dysregulation in perinatal psychiatric illness. It was also surprising that including systemic infection in the model did not change our results; this is likely due to the small number of subjects, as well as the nature of the variable (self-report, based on the subject’s recollection of any infection that required prescription medication).
Our results for depressive symptoms are consistent with a growing body of literature supporting a role for increased inflammation in perinatal depression (Cassidy-Bushrow et al. 2002, Christian et al. 2012, Karlsson et al. 2017, Osborne & Monk 2013, Leff-Gelman et al., 2016, Sherer et al., 2017), though they are inconsistent with a recent study that identified decreases in three proinflammatory cytokines in women with antenatal depression (Edvinsson et al. 2017). The literature for anxiety is more sparse. Our results are consistent with those of Maes et al. (2000), who found elevations in IL-6 and IL-1ra for more anxious subjects at 1 day postpartum, but inconsistent with those of Karlsson et al. (2017), who found increases in anti-inflammatory Th2 cytokines and no relationship to pro-inflammatory cytokines in anxious women. Of note, Karlsson’s findings were obtained at a single time point in the second trimester, whereas our data show substantial differences at the third trimester and in the early postpartum.
There are limitations to this study. It is a posthoc analysis, and we did not have a choice about which psychiatric measures to use. The BDI and STATE are self-report measures and not designed for the perinatal period (though they are validated), and we may have missed some findings by using these measures categorically instead of continuously. Our small sample size was not powered to detect differences in psychiatric symptoms, and we were unable to account for circadian differences in cytokine levels. Moreover, our population was not particularly depressed or anxious, and these results might not be applicable to a more ill population.
Despite these limitations, our results can point the way for future studies. Our cluster analysis displayed no useful patterns of relationship of cytokines to psychiatric symptoms, but indicated demographic differences that suggest that future work on the immune contribution to perinatal mood and anxiety symptoms may want to evaluate and potentially factor in race, ethnicity, and weight status. In addition, we identified pro-inflammatory elevations in the third trimester that may indicate increased activation of the innate immune response and the acute phase response in late pregnancy; this is a particular contribution in anxiety where the literature is sparse. The differences we identified across time also point to the importance of longitudinal rather than cross-sectional work in this area – an approach that has already yielded considerable information on other immune-related morbidities of pregnancy (such as preeclampsia and preterm birth). Future studies in this area – in more psychiatrically ill women – might use more sophisticated techniques to examine immune functioning, such as stimulation of immune cells; explorations of peripheral immune cell phenotype (including natural killer cells, dendritic cells, monocytes and macrophages, among others); consideration of pattern recognition receptors and damage-associated molecular patterns (including, for example Toll-like receptors); and measurements of placental immune activity and its relationship to perinatal mood and anxiety symptoms. Future work might also consider the interaction between the immune system and the endocrine system across pregnancy. Considerable prior research (Bloch et al., 2000) has shown that women who develop perinatal mood and anxiety disorders are vulnerable to fluctuations in sex hormones, but the exact mechanism of that vulnerability is uncertain. Work that connects sex hormone fluctuations in early pregnancy, which may differ between healthy and psychiatrically ill women (Osborne et al., 2017), with later immune system changes, as indicated by our data here, may help to illuminate a mechanism that is responsible for significant morbidity in two vulnerable populations, mothers and children.
Highlights.
Recent evidence indicates complex associations between immune functioning and depression.
Evidence is sparse in the perinatal period, and rarely includes anxiety, which is more prevalent than depression in pregnancy.
The Viral Immunity in Pregnancy Study measured mood, anxiety, and 23 cytokines at 5 points in pregnancy and postpartum in 51 women.
We found increases in levels and slope of pro-inflammatory markers across the peripartum for subjects with more depressive and anxious symptoms.
Results lend preliminary support to an increased activation of the innate immune response in late pregnancy in psychiatrically ill women.
Acknowledgments
Dr. Osborne was supported in this work by NIH T32 MH015144. The parent study (Viral Immunity in Pregnancy) was supported by NIH N01-AI-50028 and U19 A1062623. We would like to acknowledge support for the statistical analysis from Institute for Clinical and Translational Research at Johns Hopkins University, the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079. Dr. Osborne would like to thank Dr. Sabra Klein and her lab at the Johns Hopkins Bloomberg School of Public Health for assisting with concepts and revisions to this manuscript.
Role of the Funding Source
The Viral Immunity in Pregnancy Study was supported by NIH N1-AI-50028 and U19 A106263. Dr. Osborne was supported in these analyses by NIH T32 MH015144.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The corresponding author confirms, on behalf of all authors, that there are no financial conflicts of interest to disclose.
REFERENCES
- Aalto AM, Elovaino M, Kivimaki M, Uutela A, Pirkola S, 2012. The Beck Depression Inventory and General Health Questionnaire as measures of depression in the general population: A validation study using the Composite International Diagnostic Interview as the gold standard. Psychiatry Research 197: 163–171. [DOI] [PubMed] [Google Scholar]
- Baba T, Mukaida N, 2014. Role of macrophage inflammatory protein (MIP)-1α/CCL3 in leukemogenesis. Mol Cell Oncol. 15;1(1):e29899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard J-P, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B, 2006. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw, 17(1): 4–12. [PubMed] [Google Scholar]
- Becher B, Tugues S, Greter M, 2016. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity. 15;45(5):963–973. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CG, Mendelson M, Mock J, Erbaugh J, 1961. An inventory for measuring depression. Archives of General Psychiatry 4:53–63. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, 1993. Manual for the Beck Depression Inventory, 1993 edition San Antonio (TX): The Psychological Corporation. [Google Scholar]
- Bianco-Miatto T, Craig JM, Gasser YP, van Dijk SJ, Ozanne SE, 2017. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis. 8(5):513–519. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157:924–30. [DOI] [PubMed] [Google Scholar]
- Bränn E, Papadopoulos F, Fransson E, White R, Edvinsson Å, Hellgren C, Kamali-Moghaddam M, Boström A, Schiöth HB, Sundström-Poromaa I, Skalkidou A, 2017. Inflammatory markers in late pregnancy in association with postpartum depression-A nested case-control study. Psychoneuroendocrinology 79:146–159. [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN, 2012. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J. Reprod. Immunol 94: 202–209. [DOI] [PubMed] [Google Scholar]
- Chen S-J, Liu Y-L, Sytwu H-K, 2012. Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clin. Dev. Immunol 2012;258391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Guo M, Gao Y, Chen F, Guo J, Liu T, Wu D, Jiang X, 2014. A comparative study on the levels of serum cytokines and cortisol among post-traumatic stress disorder patients of Li and Han ethnicities in Hainan. Chin Med J (Engl). 127(15):2771–4. [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams JD, 2012. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav. Immun 23: 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Kowalsky JM, Mitchell AM, Porter K, 2018. Associations of postpartum sleep, stress, and depressive symptoms with LPS-stimulated cytokine production among African American and White women. J Neuroimmunol. 15;316:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Cicuttini F, Li J, Jones G, 2009. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin Investig Drugs 18(10):1457–66.. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Hermann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Eder K, Baffy N, Falus A, Fulop AK, 2009. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 58(11):727–36. [DOI] [PubMed] [Google Scholar]
- Eftekhar M, Naghshineh E, Khani P, 2018. Role of granulocyte colony-stimulating factor in human reproduction. J Res Med Sci. 29;23:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson Å, Bränn E, Hellgren C, Freyhult E, White R, Kamali-Moghaddam M, Olivier J, Bergquist J, Boström AE, Schiöth HB, Skalkidou A, Cunningham JL, Sundström-Poromaa I, 2017. Lower inflammatory markers in women with antenatal depression brings the M1/M2 balance into focus from a new direction. Psychoneuroendocrinology 28;80:15–25. [DOI] [PubMed] [Google Scholar]
- Francisco-Cruz A, Aguilar-Santelises M, Ramos-Espinosa O, Mata-Espinosa D, Marquina-Castillo B, Barrios-Payan J, Hernandez-Pando R, 2014. Granulocyte—macrophage colony-stimulating factor: not just another haematopoietic growth factor. Med Oncol 31:774. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC, 2005. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess. (Summ) 119, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP, 2002. Organization of the stress system and its dysregulation in melancholid and atypical depression: high vs. low CRH/NE states. Mol. Psychiatry 7, 254–275. [DOI] [PubMed] [Google Scholar]
- Guo Y, Luan L, Patil NK, Sherwood ER, 2017. Immunobiology of the IL-15/IL-15Rα complex as an antitumor and antiviral agent. Cytokine Growth Factor Rev. 38:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H, Debnath B, Neamati N, 2017. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 7(6):1543–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtan SG, Chen Y, Kaimal R, Creedon DJ, Enninga EAI, Nevala WK, Markovic SN, 2015. Growth modeling of the maternal cytokine milieu throughout normal pregnancy: Macrophage-derived chemokine decreases as inflammation/counterregulation increases. J Immunol Res. 2015:952571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J, 2009. Associations of depression with C-Reactive Protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Taylor A, Glover V, 2006. The HPA axis and perinatal depression: a hypothesis. Arch. Womens Ment. Health 9, 187–196. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Nousiainen N, Scheinen NM, Maksimow M, Salmi M, Lehto SM, Tolvanen M, Likkarinen H, Karlsson H, 2017. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy – the FinnBrain Birth Cohort Study. Arch Womens Mental Health. 20:39–48. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, 1989. The biology of interleukin-6. Blood 74(1):1–10. [PubMed] [Google Scholar]
- Knight RG, Wall-Manning H, Spears GF, 1983. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression Scale. British Journal of Clinical Psychology 22: 245–249. [DOI] [PubMed] [Google Scholar]
- Kraus TA, Sperling RS, Engel SM, Lo Y, Kellerman L, Singh T, Loubeau M, Ge Y, Garrido JL, Rodríguez-García M, Moran TM, 2010. Peripheral blood cytokine profiling during pregnancy and postpartum periods. Am. J. Reprod. Immunol 64, 411–426. [DOI] [PubMed] [Google Scholar]
- Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, Escribese MM, Garrido JL, Singh T, Loubeau M, Moran TM, 2012. Characterizing the pregnancy immune phenotype: Results of the viral imunity and pregnancy (VIP) study. J. Clin. Immunol 32, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff-Gelman P, Mancilla-Herrera I, Flores-Ramos M, Cruz-Fuentes C, Reyes-Grajeda JP, Garcia-Cuetara M.d.P., Bugnot-Perez MD, Pulido-Ascencio DE, 2016. The immune system and the role of inflammation in perinatal depression. Neurosci. Bull 32(4):398–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Maes M, 2012. Mechanistic explanations how cellmediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev 36 (2), 764–785. [DOI] [PubMed] [Google Scholar]
- Maes M, Anderson G, Kubera M, Berk M, 2014. Targeting classical IL-6 signalling or Il-6 trans-signalling in depression? Expert Opin Ther Targets 18(5):495–512. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Porter K, Christian LM, 2018. Examination of the role of obesity in the association between childhood trauma and inflammation during pregnancy. Health Psychol. 37(2):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression: the fourth inflammatory morbidity of pregnancy? Theory and literature review. Psychoneuroendocrinology 38:1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL, 2017. Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology 79:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Sperling RS, Moran THM, Kraus TA, 2012. The influence of pregnancy of systemic immunity. Immunol. Res 54(1–3):254–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Lowry CA, Rook GAW, 2010. Inflammation, sanitation, and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry 67 (12), 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo G, Sacco R, Siciliano M, Bisecco A, Muzzo G, Docimo R, De Stefano M, Bonavita S, Lavorgna L, Tedeschi G, Trojano L, Gallo A, 2016. Anxiety in Multiple Sclerosis: psychometric properties of the State-Trait Anxiety Inventory. Acta Neurol Scand 134: 458–466. [DOI] [PubMed] [Google Scholar]
- Sawai K, Matsuzaki N, Okada T, Shimoya K, Koyama M, Azuma C, Saji F, Murata Y, 1997. Human decidual cell biosynthesis of leukemia inhibitory factor: regulation by decidual cytokines and steroid hormones. Biol Reprod. 56(5):1274–80. [DOI] [PubMed] [Google Scholar]
- Sherer ML, Posillico CK, Schwarz JM, 2017. The psychoneuroimmunology of pregnancy. Front Neuroendocrinol 27 pii: S0091–3022(17)30066–3. [DOI] [PubMed] [Google Scholar]
- Skogen JC, Overland S, 2012. The fetal origins of adult disease: A narrative review of the epidemiological literature. JRSM Short Rep. 3(8):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi AA, Sia AT, Tan E-C, Coller JK, Hutchinson MR, Barratt DR, 2016. Ethnicity-dependent influence of innate immune genetic markers on morphine PCA requirements and adverse effects in postoperative pain. Pain 157: 2458–2466. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA, 1983. Manual for the State-Trait Anxiety Inventory: Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Tucker P, Pfefferbaum B, Nitiema P, Khan Q, Aggarwal R, Walling EE, 2017. Possible link of Interleukin-6 and Interleukin-2 with psychiatric diagnosis, ethnicity, disaster or BMI. Cytokine 96: 247–252. [DOI] [PubMed] [Google Scholar]
- Unver N, McAllister F, 2018. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 18 pii: S1359–6101(18)30045–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2009. Mental health aspects of women’s reproductive health: a global review of the literature. Geneva: World Health Organization. [Google Scholar]