Abstract
This editorial refers to ‘CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy’†, by D. Mosqueira et al., on page 3879.
Hypertrophic cardiomyopathy (HCM) is the most prevalent inherited cardiac disease. Characterized by left ventricular wall thickening, severe HCM cases are associated with left ventricular outflow tract obstruction, diastolic dysfunction, and sudden cardiac death in adolescents and young adults.1 It is disappointing that after being studied for more than six decades, no effective and targeted HCM pharmacotherapies currently exist.2 Thus, there is a crucial need to expedite therapeutic discovery to improve clinical outcomes, especially in terms of early disease management, to reduce sudden cardiac death in young individuals.
In most HCM cases, disease-causing mutations can be isolated by studying the family pedigrees. Decades of research studying familial HCM cases have revealed >1400 mutations, with the majority of mutations located in genes encoding proteins in the sarcomere.3 Theoretically, we should be able to identify HCM patients early through family history and genetic testing. Targeted treatment can then be developed based on the mutation in question to manage the disease at its early stages. However, disease modelling and drug discovery in HCM continue to be hindered by a lack of appropriate experimental animal or cell models. For example, the majority of HCM mutations are found in the MYH7 gene coding for β-myosin heavy chain,2,3 the dominant isoform in human heart. However, mutation studies using rodent models can be misinterpreted as this is not their major cardiac myosin heavy chain isoform.4 This pitfall can now be addressed using a human-based cell/tissue platform, which was made possible by Shinya Yamanaka’s seminal discovery of human induced pluripotent stem cells (iPSCs).5
This new technology enables researchers to differentiate cells into virtually any cell types of interest for disease modelling,6–8 drug discovery and screening,9,10 and regenerative medicine.11,12 However, several challenges must be met before we can fully unleash the potential of iPSCs. First, the differences in genetic background among the iPSC lines may conceal the true phenotype induced by a single mutation. In this regard, the introduction of genome editing technology, such as clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9, allows researchers to generate isogenic iPSC lines and study the precise effect of an HCM mutation while avoiding the genetic confounding factors.13 Secondly, we currently lack the technological wherewithal to describe an HCM phenotype in cells comprehensively in an unbiased manner. The development of high-resolution tools and high-throughput quantitative assays is particularly needed to detect the diverse molecular, functional, and morphological features present in iPSC-derived cardiomyocytes (iPSC-CMs).7
Perhaps the biggest question is whether this platform can reliably mirror the true phenotype in HCM patients. It has been hotly debated whether these foetal-like cells derived from iPSCs can reflect molecular or functional alteration in an adult disease. This immaturity can be partially attributed to our current inability to simulate the complex 3-D organ structure that is essential for cardiomyocyte maturation and function.14 Moreover, it is not yet possible for the cell-based system to imitate the structural and geometrical defects in HCM hearts. Concerted efforts from bioengineering are required to improve the current 2-D system or construct the optimal 3-D tissue platform for HCM research.
In the current issue of the journal, Mosqueira and colleagues describe an integrative approach to study HCM using a pluripotent stem cell platform.15 The investigators introduced a c.C9123T-MYH7 mutation both heterozygously and homozygously into two iPSC lines and one embryonic stem cell (ESC) line to create three isogenic experimental sets. Using high-throughput flow cytometry and high-content imaging, they were able to detect an enlarged cell size, increased brain natriuretic peptide (BNP) expression, bi-/multinucleation, and sarcomeric disarray in heterozygotes across all three isogenic sets. In terms of cellular function, the investigators detected an elevated maximal respiration in both heterozygous and homozygous mutation lines from two isogenic sets, without changes in mitochondrial content. They were also able to simulate the arrhythmia observed in c.C9123T-MYH7 HCM patients. Using human engineered heart tissues (hEHTs), they revealed hypocontractility and negative clinotropy in the presence of the MYH7 mutation. Utilizing various high-volume and high-throughput phenotypic assays, they examined the effectiveness of several small molecules for this HCM mutation. Overall, this study is a prime example of how HCM can be comprehensively modelled in a relatively high-throughput fashion using both 2-D cell-based and 3-D hEHT-based platforms.
However, Mosqueira and colleagues also underscored some limitations in their work. First, it was a huge task to integrate all the findings effectively after a comprehensive characterization. Owing to the descriptive nature of their work, it remains to be explained how one mutation in MHY7 can simultaneously affect several aspects of the cardiomyocyte function. Secondly, they highlighted the challenges in deciding on 2-D cell-based vs. 3-D tissue systems, as these two platforms showed different results in the transcriptomic analysis. Thirdly, cardiomyocyte maturation remains an important issue as in the control lines because the 3-D tissue platform did not demonstrate a positive force–frequency relationship, an important response observed in an adult myocardium.16 It would be valuable to see how their platform could benefit from a more recent protocol for inducing cardiac maturation.17
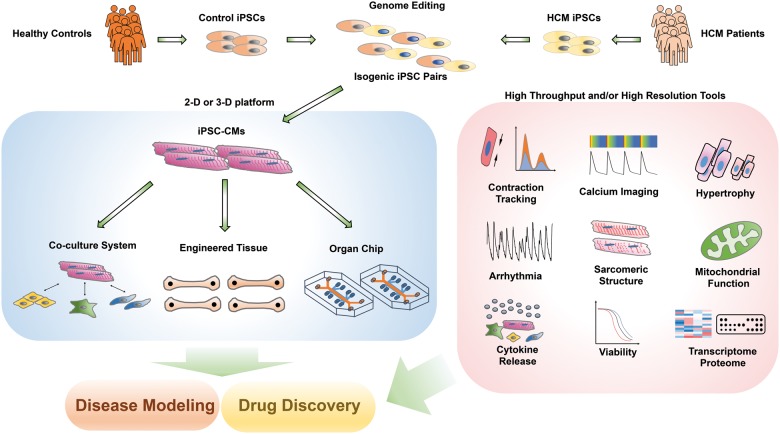
In summary, Mosqueira et al. provided an intriguing glimpse of the potential for comprehensive HCM disease modelling and drug testing using patient-specific iPSCs, genome editing, and tissue engineering. While more research is needed to improve the robustness and translational relevance of the current system, this study represents a possible direction to advance our current HCM modelling and drug discovery paradigm (Take home figure).
Take home figure.
HCM disease modelling and drug discovery using the iPSC platform. Genome editing enables the generation of isogenic cell pairs that can limit the variations in genetic background. Cardiomyocytes and/or other stromal cell types in the heart can be differentiated from iPSCs, which can be engineered into various tissues or ‘organ chips’ for integrative disease modelling and drug screening purposes.
Funding
This publication was supported in part by research grants from the National Institutes of Health (NIH) R01 HL130020, R01 HL128170, and R01 HL126527, the American Heart Association (AHA) 17MERIT3361009, and Burroughs Welcome Fund 1015009 (J.C.W.).
Conflict of interest: none declared.
Footnotes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
† doi: 10.1093/eurheartj/ehy249.
References
- 1. Marian AJ, Braunwald E.. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 2017;121:749–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H, O’Mahony C, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol Ç, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Hasdai D, Ponikowski P, Achenbach S, Alfonso F, Basso C, Cardim NM, Gimeno JR, Heymans S, Holm PJ, Keren A, Kirchhof P, Kolh P, Lionis C, Muneretto C, Priori S, Salvador MJ, Wolpert C, Zamorano JL, Frick M, Aliyev F, Komissarova S, Mairesse G, Smajić E, Velchev V, Antoniades L, Linhart A, Bundgaard H, Heliö T, Leenhardt A, Katus HA, Efthymiadis G, Sepp R, Thor Gunnarsson G, Carasso S, Kerimkulova A, Kamzola G, Skouri H, Eldirsi G, Kavoliuniene A, Felice T, Michels M, Hermann Haugaa K, Lenarczyk R, Brito D, Apetrei E, Bokheria L, Lovic D, Hatala R, Garcia Pavía P, Eriksson M, Noble S, Srbinovska E, Özdemir M, Nesukay E, Sekhri N.. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Maron MS, Semsarian C.. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol 2012;60:705–715. [DOI] [PubMed] [Google Scholar]
- 4. Lowey S, Bretton V, Gulick J, Robbins J, Trybus KM.. Transgenic mouse α- and β-cardiac myosins containing the R403Q mutation show isoform-dependent transient kinetic differences. J Biol Chem 2013;288:14780–14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 6. Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC.. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 2012;4:130ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC.. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013;12:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebert AD, Kodo K, Liang P, Wu H, Huber BC, Riegler J, Churko J, Lee J, Almeida P de, Lan F, Diecke S, Burridge PW, Gold JD, Mochly-Rosen D, Wu JC.. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci Transl Med 2014;6:255ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burridge PW, Li YF, Matsa E, Wu H, Ong S-G, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC.. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmström A, Matsa E, Zhang Y, Kumar A, Fan AC, Álamo JC Del, Wu SM, Moslehi JJ, Mercola M, Wu JC.. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med 2017;9:eaaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neofytou E, O’Brien CG, Couture LA, Wu JC.. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest 2015;125:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata K, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M.. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 2017;376:1038–1046. [DOI] [PubMed] [Google Scholar]
- 13. Seeger T, Porteus M, Wu JC.. Genome editing in cardiovascular biology. Circ Res 2017;120:778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu C, Oikonomopoulos A, Sayed N, Wu JC.. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development 2018;145:dev156166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosqueira D, Mannhardt I, Bhagwan J, Lis-Slimak K, Katili P, Scott E, Hassan M, Prondzynski M, Harmer SC, Tinker A, Smith JGW, Carrier L, Williams PM, Gaffney D, Eschenhagen T, Hansen A, Denning C.. CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur Heart J 2018;39:3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasenfuss G, Holubarsch C, Hermann H-P, Astheimer K, Pleske B, Just H.. Influence of the force–frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J 1994;15:164–170. [DOI] [PubMed] [Google Scholar]
- 17. Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G.. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018;556:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]