Abstract
Due to the cyclic function of the human heart, pressure and flow in the circulation are pulsatile rather than continuous. Addressing pulsatile haemodynamics starts with the most convenient measurement, brachial pulse pressure, which is widely available, related to development and treatment of heart failure (HF), but often confounded in patients with established HF. The next level of analysis consists of central (rather than brachial) pressures and, more importantly, of wave reflections. The latter are closely related to left ventricular late systolic afterload, ventricular remodelling, diastolic dysfunction, exercise capacity, and, in the long-term, the risk of new-onset HF. Wave reflection may also represent a suitable therapeutic target. Treatments for HF with preserved and reduced ejection fraction, based on a reduction of wave reflection, are emerging. A full understanding of ventricular-arterial coupling, however, requires dedicated analysis of time-resolved pressure and flow signals, which can be readily accomplished with contemporary non-invasive imaging and modelling techniques. This review provides a summary of our current understanding of pulsatile haemodynamics in HF.
Keywords: Pulsatile haemodynamics, Pulse pressure, Afterload, Ventricular-arterial coupling
Introduction
Heart failure (HF) is an important clinical problem in developed countries, with high rates of hospitalization and mortality.1 An increased brachial systolic blood pressure (bSBP) and brachial diastolic blood pressure (bDBP), starting at levels as low as 115 and 75 mmHg, respectively, predict incident HF across all adult age groups.2 Consequently, reduction of incident HF was the most pronounced benefit of intensive BP lowering in the recently published SPRINT trial.3
Brachial systolic blood pressure and bDBP, introduced over a century ago, are among the most widely performed measurements in clinical medicine. Despite their wide use in daily practice, the complex relationship between the pump (i.e. the heart) and the arterial circulation cannot be fully understood from two isolated pressure points at the brachial artery for the following reasons: (i) physiologically, due to the pulsatile characteristics of the pump, BP is a curve rather than two extremes (SBP, DBP), with a defined amount of pressure [pulse pressure (PP)] fluctuating around a mean value [mean arterial pressure (MAP)]; the curve contains features that provide insights into arterial function.4 (ii) Systolic blood pressure and PP increase from the ascending aorta to peripheral measurement sites, a phenomenon called pressure amplification, which is related to the mechanical properties of the arterial system. Therefore, brachial BPs do not necessarily reflect the pressures ‘seen’ on the heart. (iii) Most importantly, BP originates from the interaction of cardiac and arterial function and is the result of the flow generated by the heart and the afterload imposed by the arterial tree. Therefore, patients with identical BP may have substantially different afterload patterns due to differences in the blood flow generated by the left ventricle (LV). Therefore, LV afterload (arterial load) cannot be estimated without knowledge of both pressure and flow.
Broadly, arterial load has two components: steady (or ‘resistive’) load and pulsatile load. ‘Steady’ load [total peripheral resistance (TPR), largely determined by systemic microvascular resistance], along with cardiac output (CO), determines MAP (MAP = CO × TPR), whereas pulsatile afterload is influenced by multiple arterial properties (aortic geometry and stiffness, timing, and magnitude of arterial wave reflections). This review focuses on the pulsatile component of cardiac afterload, its assessment, prognostic value, and therapeutic consequences.
Non-invasive assessment and physiological background of the measurements
Pulse pressure
Brachial PP (bPP) is a widely used pulsatile haemodynamic index. It can be easily computed SBP minus DBP and is thus readily available. It is the result of left ventricular mechanical work interacting with the arterial tree and, as such, depends on stroke volume (SV) and forward flow on the one hand, and on aortic stiffness, size, and wave reflections on the other. When the LV chamber pump function is preserved and significant aortic valve disease is absent, a high PP is generally considered indicative of increased pulsatile afterload. In heart failure with reduced ejection fraction (HFrEF), PP is directly related to measures of LV function, such as EF, SV, CO, left ventricular (LV) dp/dt, and LV longitudinal axis shortening.5 In other words, in HFrEF, a lower PP is often a consequence of a worse LV function. This needs to be taken into account when interpreting studies that assess the prognostic value of PP.
Due to its simplicity of measurement, several epidemiological studies have investigated the prognostic role of bPP, most of them demonstrating that a high bPP is associated with a poor prognosis. According to European Guidelines on Hypertension,6 a bPP value ≥60 mmHg in elderly individuals with stiffer arteries reflects asymptomatic damage of the large arteries. As the BP wave travels from central aorta to peripheral sites (e.g. brachial artery), MAP drops only by 2 mmHg, whereas SBP and PP can increase markedly4 [SBP and PP amplification (PPA)], particularly in younger adults. The PPA ratio (peripheral PP/central PP) is determined by complex interactions between many factors, including LV contractility and ejection duration, heart rate, arterial stiffness, arterial calibre (and taper), the timing and amplitude of wave reflections, and arteriolar tone (TPR). Central PP cannot be calculated from bPP by a simple formula but requires dedicated instruments that record the time-resolved waveforms at the carotid site or in more peripheral locations. In the absence of obstructive atherosclerotic carotid disease, the carotid pressure waveform is considered a reasonable ‘direct’ surrogate of the aortic pressure waveform, whereas more peripheral waveform recordings (brachial or radial) require mathematical algorithms to estimate the aortic pressure waveform. In all instances, the obtained aortic pressure waveform needs to be calibrated with peripheral mean and diastolic pressures, which unlike systolic pressure demonstrate little variation between the aorta and peripheral sites.7 The technical details are beyond the scope of this manuscript and can be found in dedicated reviews.8
Wave reflections in the arterial tree
Left ventricular contraction generates a forward-travelling wave (incident or forward wave). The wave travels at a given speed [pulse wave velocity (PWV), ∼5 to 15 m/s in humans] along the wall of the aorta and more distal conduit arteries and is partially reflected at sites of impedance mismatch (branching points, lumen diameter tapering, and change in local stiffness).9 Innumerable reflections from distributed sites are transmitted back toward the heart, interacting to form a ‘net’ reflected wave. In young adults, aortic PWV is low, and the bulk of reflected waves arrive at the aortic root during diastole. With advancing age, PWV increases, and reflected waves arrive at the heart during mid-to-late systole.10,11 In these conditions, wave reflections exert important unfavourable effects,4 including (i) an increase in mid-to-late systolic load (relative to early systolic load); (ii) an increase in aortic SBP, although the degree of pressure augmentation vs. flow reduction depends on LV function; (iii) a decrease in DBP, including the area under the pressure waveform (pressure–time integral) in diastole, which is a key determinant of coronary blood flow. Importantly, reflected waves also re-reflect at the heart, contributing to an increase in the amplitude of the forward pressure wave, above and beyond the influence of the aortic root load and flow requirements.12
Methods to analyse waveforms
Pulse waveform analysis (PWA): The reflected wave causes a visible notch (inflection point) and an increase (i.e. augmentation) in late systolic pressure (Figures 1 and 2). Augmented pressure (AP), expressed in mmHg, is the increase in BP following the inflection point and is partially related to the effects of wave reflection on the aortic BP curve. Augmentation index (AIx) is the ratio between augmented pressure and pulse pressure (AIx = AP/cPP), typically expressed as a percentage. Both AIx and AP are higher with increasing age, lower heart rate (a relatively longer systolic period enables reflected waves to exert greater pressure augmentation during systole), smaller body height (shorter travel distance), female sex, and are lower following food ingestion and following exercise.15 Pulse waveform analysis-derived indexes are dependent not only on the magnitude but also on the timing of wave reflection. To overcome this potential limitation and focus on the amount of wave reflection only, wave separation analysis (WSA) can be used, which requires simultaneously acquired pressure and flow waves at the same location to separate the pressure wave into its forward (Pf) and backward (Pb) components.16Reflection magnitude (RM) is the ratio of amplitudes of Pb/Pf. A more recent development is wave intensity analysis (WIA),17 in which BP and flow velocity measured at the same arterial site are considered and a separation into forward and backward-travelling wavefronts can be achieved (Figure 2). Waves can originate either from the proximal (forward-travelling) or distal (backward-travelling) end of the circulation and can be either a compression (‘pushing’) or decompression (‘sucking’) wave. A compression wave will accelerate or decelerate blood flow depending on its origin: if it arises proximal to the site of measurement, it will increase pressure and accelerate flow, but compression waves of distal origin will increase pressure and decelerate blood flow.18 Wave intensity analysis is a useful approach that complements WSA, but it overemphasizes high-frequency components of the pulse (i.e. rapid changes in pressure and flow waves) and thus tends to under-represent reflected waves (which are rich in low-frequency content). A key advantage of WIA may be related to the study of cardiac-derived compression and suction waves (rather than wave reflection per se): the early systolic S-compression wave peak is related to the maximum derivative of left ventricular pressure increase in early systole, while the D-late systolic forward-travelling suction wave peak is related to the time constant of pressure decay in late systole/early diastole.19 Both may, therefore, provide insights into ventricular function14 and LV-arterial coupling. Wave power analysis, a recently proposed technique based on volume flow rather than flow velocity, has important advantages over WIA and requires further study in humans.20
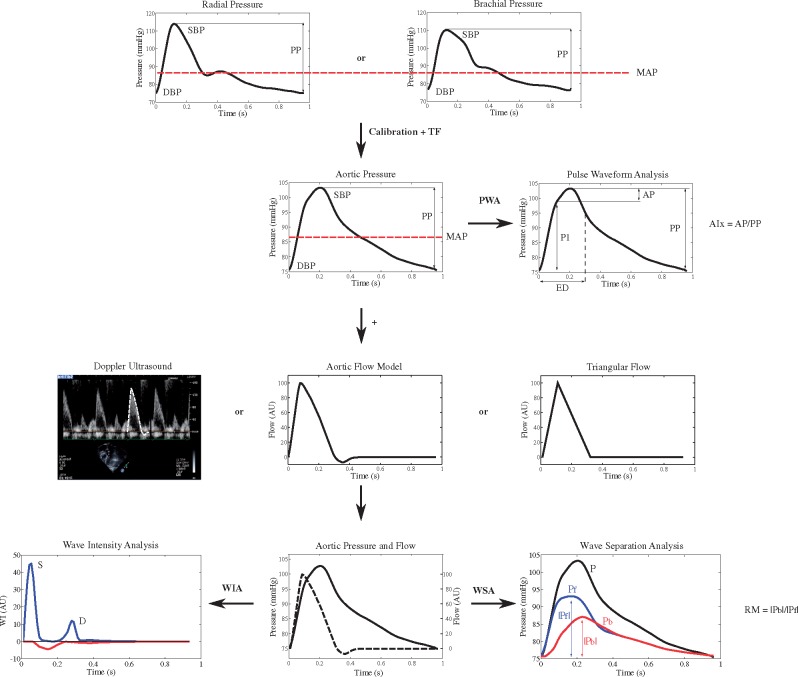
Figure 1.
Assessment of pulsatile haemodynamics—overview. Top line: recording of signal-averaged radial or brachial pressure waveforms with tonometry or brachial cuff. Second line: following calibration with brachial pressures, aortic waveforms are calculated with a transfer function (TF). Pulse waveform analysis, based on pressure signals alone, yields measures of the first (P1) and second (P2) systolic peaks for computation of augmented pressure and augmentation index (AP, AIx). Third line: flow waveforms are obtained, either with Doppler recording of LV outflow (which equals aortic inflow), or as model-derived flow or triangular flow as a proxy. Bottom line: combined and time-aligned analysis of a pressure–flow pair is used for wave separation analysis, wave intensity analysis, and other analytical approaches (Courtesy of Bernhard Hametner, modified from Parragh et al.13 and from Hametner et al.14). DBP, diastolic blood pressure; ED, ejection duration; MAP, mean arterial pressure; PP, pulse pressure; PWA, pulse waveform analysis, RM, reflection magnitude; SBP, systolic blood pressure; WIA, wave intensity analysis; WSA, wave separation analysis.
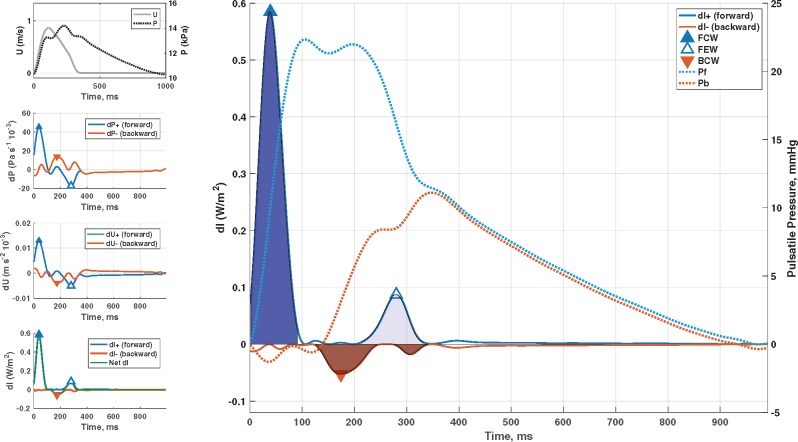
Figure 2.
Wave separation analysis vs. wave intensity analysis. Identification of forward-travelling and backward travelling waves in the proximal aorta using wave intensity analysis. The method is based on the assessment of changes in pressure (P) and flow velocity (left panels), which can be multiplied to compute instantaneous wave intensity (left bottom panel). The right panel shows forward and backward wave intensity curves, which can be analysed to identify the timing and magnitude of key wave fronts: early systolic forward compression wave (dark blue), late systolic forward suction wave (light blue), and backward compression wave (red). The cumulative sum of forward and backward pressure changes (dP, used to computed wave intensity) are superimposed on the right y-axis. These curves (Pf, Pb) are equivalent to forward and backward waves obtained via classic wave separation analysis. It can be seen that wave intensity tends to markedly under-represent reflected waves, because it emphasizes rapid changes in pressure and flow (high frequencies), whereas reflected waves are enriched in lower frequencies. BCW, backward compression wave; FCW, forward compression wave; FEW, forward expansion suction wave.
Pulse wave velocity, characteristic impedance, total arterial compliance
Pulse wave velocity is the travel distance divided by transit time of the pulse between two recording sites. Carotid-femoral (cf) PWV is currently considered the gold standard metric of aortic stiffness.21 Pulse wave velocity is not a direct measure of ventricular afterload, but is informative of arterial wall properties, and has important prognostic implications.4,21,22 Proximal aortic impedance (Zc) is the slope of the pressure–flow relation in the absence of wave reflections and represents the pulsatile load imposed by the proximal aorta. It is highly dependent on proximal aortic size and also dependent on its stiffness. Total arterial compliance (TAC) represents the lumped compliance provided by the arterial tree. In the systemic circulation, it is largely determined by conduit arteries (including the aorta and more distal muscular conduit arteries).
Practical recording and devices
See Supplementary material online for details.
The relationship between cardiac and arterial function
Myocardial vs. ventricular afterload
Left ventricular afterload can be defined as the hydraulic load imposed by the systemic circulation (i.e. relationship between pressure and flow as discussed above), whereas myocardial afterload is best defined as the myocardial wall stress (MWS) required to generate fibre shortening.23–26 Myocardial afterload does not only depend on arterial load but also on the time-varying LV geometry during ejection, which in turn affects the relationship between MWS and LV chamber pressure. The time-varying LV geometry during ejection is dependent on: (i) LV volume at the beginning of LV contraction (i.e. end-diastolic volume), which in turn is determined by chronic LV remodelling and preload and (ii) the interaction between myocardial contraction, LV geometry, and arterial load throughout ejection.
In accordance with Laplace’s law of the heart, MWS is lower for any given LV pressure, as the ratio of LV chamber volume to LV wall volume decreases. This is true not only in end-diastole or end-systole but throughout ejection (Figure 3). Among normotensive and hypertensive adults with a normal LV ejection fraction (EF), peak MWS typically occurs in early systole, when quasi-diastolic geometry coexists with systolic pressure.27–29 This is followed by a marked change in the relationship between LV pressure and MWS during mid-systole, which determines a lower MWS for any given LV (and aortic) pressure (Figure 3).28 This phenomenon appears ideal to protect cardiomyocytes against excessive load in mid-to-late systole28,30 and depends on the dynamic reduction of LV chamber size relative to wall volume, and its magnitude is highly variable between individuals.28 Subjects with lower ejection fraction,28 concentric remodelling,28 or those who demonstrate poor early systolic contraction (and ejection)31 demonstrate less pronounced shifts in the pressure–stress relation.
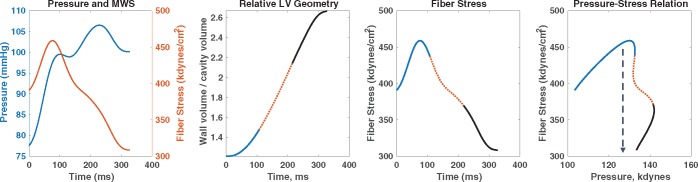
Figure 3.
Time-resolved myocardial wall stress. The first panel shows the ejection-phase aortic pressure and myocardial wall stress (MWS) profiles. The second panel shows the time-resolved relative myocardial geometry (ratio of wall volume to cavity volume) that correlates with wall stress via the Laplace law; the first, second, and last thirds of systole are shown in blue, dotted red, and black lines, respectively. The third panel shows the ejection-phase myocardial wall stress, and the fourth panel shows pressure–MWS relation. It can be seen that myocardial wall stress peaks in early systole and subsequently decreases, even in the context of increasing pressure. This is due to a mid-systolic shift in the pressure–stress relation (dashed arrow) which favours lower MWS for any given pressure. This shift is due to the geometric reconfiguration of the LV (decreased cavity volume relative to LV wall volume) and is impaired in the presence of reductions in LV ejection fraction, concentric geometric remodelling, and reduced early systolic ejection (reduced early-phase ejection fraction). LV, left ventricular.
Therefore, there is an important interaction between myocardial geometry, the myocardial contraction pattern, and the effect of wave reflections on LV hydraulic load (Figure 4). Wave reflections tend to increase mid-to-late systolic LV load and MWS,27,28 but the time course of LV contraction impacts the degree to which cardiomyocytes are ‘exposed’ to the ill effects of wave reflections in mid-to-late systole, a period in which there appears to be particular vulnerability to the deleterious effects of increased afterload.30,32–35 Time-varying MWS, therefore, provides highly relevant integrated information about myocardial-ventricular-arterial coupling.4,27–29,36
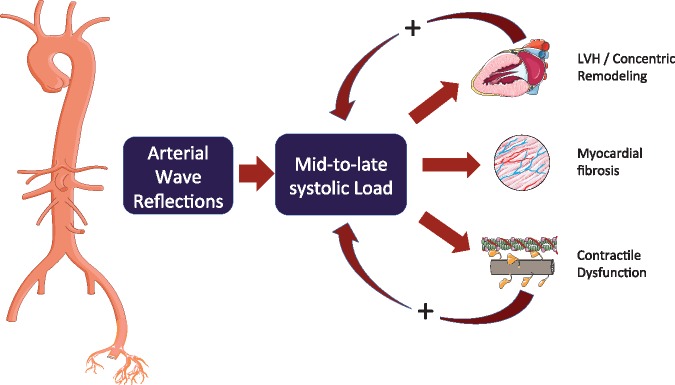
Figure 4.
Wave reflections increase late systolic left ventricle load, which favours left ventricular remodelling and myocardial dysfunction. However, the effect of wave reflection on myocardial load is modulated by contraction pattern and the time course of myocardial wall stress. Left ventricles in which the mid-systolic shift in the pressure–stress relation is impaired (due to a reduced ejection fraction, concentric geometric remodelling, and/or reduced early systolic ejection) fail to protect cardiomyocytes against the load induced by wave reflections in late systole, a period of vulnerability to load. This may represent a vicious cycle that favours development and furthers progression of heart failure. Modified from Chirinos.26 LVH, left ventricular hypertrophy.
In rotationally symmetric ventricles, time-resolved ejection-phase MWS can be estimated non-invasively with a combination of arterial tonometry and either Doppler echocardiography28 or cardiac magnetic resonance imaging (MRI),37 using the method described by Arts et al.38 Myocardial wall stress can be used to more directly infer late systolic myocardial load. Prominent late systolic MWS is associated with impaired LV relaxation29 and left atrial dysfunction.37 Furthermore, peak systolic MWS is closely and linearly related to invasively measured myocardial oxygen consumption (MVO2)39 and therefore can be used to assess the mechanical efficiency of the cardiovascular (CV) system, with fewer assumptions than in the pressure–volume plane.
Differences in the effects of arterial load in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction
When LV pump function is preserved, the reflected wave typically induces a late systolic pressure peak in the pressure waveform, augmenting aortic pressure in mid-to-late systole. These features are prominent in patients with heart failure with preserved ejection fraction (HFpEF)40–43 and may be useful in the diagnostic workup of the condition41: measures of pulsatile arterial function (including brachial PP, but favouring central haemodynamics) were as good as tissue Doppler echocardiography in separating patients with HFpEF from those without the condition in a population of patients with exertional dyspnoea. When LV pump function is reduced, however, wave reflection may exert more pronounced effects to decrease flow, with no apparent alteration in the appearance of the pressure waveform (when the latter is analysed in isolation). In patients with severe LV systolic dysfunction (LVEF ≤30%), wave reflections truncate flow, reduce SV, and induce a shortening of ejection duration.13,44,45 In addition, forward waves are also altered: in patients with severely reduced EF (mean value 27.8%), WIA derived ratio of first to second systolic peak is reduced,46 as compared to individuals with normal EF, and divided patients with HFrEF from controls with normal EF with an area under the curve of 0.879.14
Disadvantages of effective arterial elastance
Given the value of the pressure–volume plane to study LV chamber function and energetics, an ‘extension’ of the pressure–volume approach to assess arterial load and ventricular-arterial coupling was proposed, primarily to understand the determinants of SV.47–50 In this proposed paradigm, arterial load is quantified as an ‘effective arterial elastance’ (EA), which is computed as the ratio of end-systolic pressure to SV.
However, arterial load is time-varying, complex, and cannot be expressed as a single number.9,25EA fails to capture key features of pulsatile load and ventricular-arterial coupling, particularly time-varying phenomena during ejection51 and the LV loading sequence (early vs. late systolic load), which as reviewed above, is an important determinant of maladaptive remodelling, hypertrophy, diastolic dysfunction, atrial dysfunction, and HF risk.9,29,33,40,52–55 In addition, the assumption that EA is a lumped parameter of resistive and pulsatile arterial load, is in fact incorrect. 25,56–58EA is not a true elastance (i.e. the inverse of a compliance) and is almost exclusively dependent on vascular resistance (a microvascular, rather than a conduit artery property)57 and heart rate.57,58EA bears an almost perfect relationship to the product of TPR and heart rate, but demonstrates weak, inconsistent and in some cases, erratic/paradoxical relationships with gold standard measures of pulsatile load.58 Importantly, EA is not related to aortic wall stiffness58; therefore, an increase in EA should not be interpreted as arterial ‘stiffening’ and, by extension, and parallel increase in EA and EES should not be interpreted as a state of ‘ventricular-arterial stiffening’.
The inability of EA to properly capture pulsatile arterial load is explained by the multiple simplifying assumptions made during its original derivation (as previously discussed in detail),24,25,57,58 which translate into important limitations to the application of this approach to characterize physiologic abnormalities and obtain useful clinical inferences. For example, in a recent study, measures of wave reflection, but not EA or TPR, were significantly correlated with the invasively measured time constant of isovolumic relaxation, the gold standard index of diastolic relaxation.59 Similarly, a recent study demonstrated that EA did not predict incident HF in the Multiethnic Study of Atherosclerosis (MESA) cohort,60 whereas wave reflections (RM) and late systolic load were strong predictors.61,62 The use of EA to assess pulsatile or ‘global’ arterial load is therefore strongly discouraged.
Clinical impact of pulsatile arterial load
Arterial load and left ventricular hypertrophy
Left ventricular hypertrophy (LVH) is an important marker of asymptomatic organ damage in hypertension6 and an important intermediate step from hypertension to HF.63 Animal models have shown that an increase in aortic stiffness without any change in TPR leads to LVH.64 Moreover, late systolic loading resulted in much more prominent hypertrophy than early systolic loading in rats.53 Humans with isolated systolic hypertension, a condition related to increased aortic stiffness, exhibit higher left ventricular mass (LVM) than those with systolic–diastolic hypertension. Moreover, LVM is more strongly related to PP than to MAP, underlining the importance of pulsatile phenomena.65 This relationship is stronger for cPP,66,67 in particular when measured over 24-h.68 The relationship between LVM and arterial stiffness/wave reflections is apparent even in adolescents and young adults.69 In a recent study including >4000 adults from the general population, the contribution of steady state load (TPR) and pulsatile haemdynamics (TAC, Pf, Pb) on LVM and geometry was investigated.70 In multivariable models, systemic vascular resistance (SVR), TAC, and Pb were directly, and Pf was inversely associated with LVM, with wave reflection (Pb) demonstrating the strongest relationship, and SVR demonstrating a relatively weak relationship. In a longitudinal study in a family-based population sample, progression to LV concentric remodelling pattern over 4.7 years was independently associated with higher baseline cfPWV.71 Moreover, in women, higher cPP at baseline predicted the longitudinal increase in LVM. Reductions in LVM, which have proven prognostic benefit, are more closely associated with reductions in wave reflection than with reductions in brachial BP.54,72 When different drugs (angiotensin converting enzyme (ACE)-inhibitor-diuretic combination vs. a beta-blocker) were compared, those favourably affecting pulsatile haemodynamics (reducing cSBP and cPP) were superior in reducing LVM,73 whereas both therapeutic regimens did not differ regarding steady state haemodynamics (CO and TPR).
Arterial load and exercise capacity
Consistent with the important role of pulsatile arterial load on the myocardium, pulsatile arterial properties have been shown to be associated with exercise capacity, as discussed in the Supplementary material online.
Arterial load and risk of incident heart failure
In the Framingham study, bPP (and bSBP) were stronger predictors than DBP for congestive HF (CHF)74 in middle-aged men and women.75 In 5690 participants from the MESA study, RM was strongly and independently predictive of new-onset HF.62 In particular, RM compared favourably to other risk factors for CHF as per various measures of model performance, reclassification, and discrimination and predicted CHF even in patients with normal BP. Along the same lines of evidence, independently of the absolute level of peak BP, late systolic hypertension was strongly associated with incident HF.61 In the same population, and in contrast, SVR, TAC, and EA did not predict HF, indicating the importance of the loading sequence.60 In the Framingham Heart study, after adjustments for standard risk factors including MAP, cfPWV was independently associated with incident clinical HF76 after a follow-up of 10.1 years. Moreover, greater cfPWV was associated with both HFpEF and HFrEF, although the findings did not achieve statistical significance, in part due to a modest number of HF events. In 2602 patients with chronic kidney disease (mean glomerular filtration rate (GFR) 45 mL/min/1.73 m2), after a mean follow-up of 3.5 years, cfPWV as well as bSBP, cSBP, and PP predicted hospitalized HF, with cfPWV showing the best relationship.77 In another community-based cohort of 2290 older adults (mean age 74 years),78 cfPWV was associated with overall HF and HFrEF only in unadjusted analysis and, with respect to overall HF, only in partially, but not in fully adjusted models. Finally, in asymptomatic patients at risk for HF, worsening of arterial stiffness (increase in brachial-ankle PWV) within 5 years was associated with increased risk of incident HF.79 In summary, available evidence supports a relationship between arterial stiffness and particularly, measures of wave reflection/late systolic load, and the risk of incident HF in the community.
Prognostic value of pulsatile haemodynamics in established heart failure
Due to the ease of assessment, most of the evidence available is related to bPP (Supplementary material online, Table S1). In advanced HFrEF, a lower bPP often is associated with a worse prognosis. In these patients, a low bPP is due to a poor LV function. In patients with less severe HFrEF, which can be indicated by higher bPP or higher SBP, the relationship may become direct (i.e. a higher bPP being associated with a worse prognosis). In these patients, PP is more reflective of arterial stiffness and increased pulsatile afterload. In HFpEF, the relationship between bPP and outcomes tends to be direct. In some studies, however, particularly in acute HFpEF, patients with the lowest bPPs also demonstrate a worse prognosis. These patients may have pronounced concentric remodelling with lower SVs, despite a preserved EF (which does not prove preserved myocardial contractility in HFpEF80).
Given the important confounding effect of LV function on PP, direct estimations of arterial load are likely to be more informative. One single-centre study demonstrated the adverse prognostic value of wave reflections in patients with acute decompensated HF.81 Similarly, PWV as a more direct measure of arterial stiffness seems to be directly related to prognosis (HF hospitalization, CV, and all-cause mortality) in HFrEF and HFpEF.82,83
Therapeutic implications
Role of pulse pressure in heart failure with preserved ejection fraction studies
No proven effective pharmacologic treatment is currently available to reduce morbidity or mortality in patients with HFpEF (Supplementary material online, Table S2). Given the important pathophysiological role of impaired pulsatile haemodynamics in HFpEF, it is worth assessing haemodynamic characteristics of study populations in various trials, and the effects of interventions on pulsatile haemodynamics. Unfortunately, current evidence is largely limited to bPP. In two recent Phase II trials, ALDO-DHF84 and in PARAMOUNT,85 a substantial decrease in bPP was achieved in the active intervention arm (Spironolactone or Sacubitril-Valsartan, respectively), associated with an improvement in filling pressures or natriuretic peptides. In clinical endpoint trials, baseline bPP has generally been <60 mmHg, the cutoff defined by European Hypertension Guidelines,6 suggesting that enrolled populations exhibited a relative paucity of pulsatile haemodynamic abnormalities demonstrated in other HFpEF studies. Moreover, in most clinical endpoint trials in HFpEF, bPP was not substantially reduced. For instance in the largest study (I-PRESERVE86), which showed a neutral outcome, bPP was lowered by only 1.7 mmHg by Irbesartan (and unchanged with placebo).
Wave reflections as a potential therapeutic target
In HFrEF, standard pharmacologic therapy may substantially reduce arterial load and wave reflections in some patients, although the response is variable and not readily judged by standard clinical parameters. A recent preliminary randomized study of arterial pressure waveform-guided therapy for HFrEF (aimed at reducing AIx) demonstrated that this strategy resulted in a greater improvement in peak oxygen consumption compared to standard care.87 Drugs more often used in the active treatment group were aldosterone antagonists, hydralazine, and nitrates. Higher wave reflections at baseline and their larger decrease during treatment were associated with functional improvement.88
Not all vasodilators are equally effective at reducing wave reflections. In HFrEF, nitroprusside has been shown to reduce wave reflections at rest and during exercise.89 Oral nitric oxide donors also reduce wave reflection in the acute setting. However, despite the well-documented acute effect on nitroglycerine and other organic nitrates on wave reflection,90 the combination of isosorbide dinitrate and hydralazine administered chronically (24 weeks) did not reduce wave reflection in patients with HFpEF in a recent study.91 This may be due to tolerance associated with long-term use.92
There is increasing interest in the role of inorganic nitrate and nitrite as potential therapeutic agents in HF. These agents harness the endogenous nitrate–nitrite–NO pathway, in which inorganic nitrate (derived from dietary ingestion or from the oxidation of endogenous NO) undergoes a regulated two-step reduction process to NO (nitrate→nitrite→NO). In addition to the well-known hypoxia/acidosis-dependent microvascular reduction of nitrite to NO (which favours microvascular vasodilation during exercise), a normoxia-dependent reduction pathway that operates in the wall of conduit muscular systemic arteries has recently been described.93 Normoxia-dependent activation accounts for the high selectivity of inorganic nitrate and nitrite for conduit muscular arteries, and the recently described effect of exogenously administered inorganic nitrate/nitrite on arterial wave reflection.41,93–95 Among patients with HFpEF, exogenous inorganic nitrate has been shown to reduce late systolic LV load by wave reflections, and to shift the reflected wave into diastole, during which it boosts coronary perfusion pressure, improving the myocardial oxygen supply–demand ratio.41,94 Unlike organic nitrates, these effects were achieved without significantly reducing MAP or cerebrovascular resistance and without increasing pulsatile power penetration into the cerebrovascular circulation.94,96
Several Phase IIa studies have suggested a therapeutic potential of inorganic nitrate/nitrite in HFpEF41,94,95,97 and non-ischaemic HFrEF.98–100 So far, mainly an improvement in exercise haemodynamics101 and exercise capacity42 has been shown. The main agents being investigated are inhaled sodium nitrite and potassium nitrate. Inhaled sodium nitrite has a very short half (<40 min), and its intermittent administration results in pronounced circulating nitrite level fluctuations, which are unlikely to exert sustained therapeutic effects throughout the day. This issue may underlie the negative results in the INDIE trial [NCT02742129], which studied inhaled inorganic nitrite in HFpEF. Inorganic nitrates, on the other hand, have a much longer half-life, which allows for dosing with milder circulating level fluctuations. The KNO3CK OUT HFpEF [NCT02840799] with potassium nitrate will provide further insights into the role of this approach and pharmacologic differences between these agents in HFpEF. The effects of soluble guanylate cyclase stimulators/activators on pulsatile load and wave reflections are critical areas of future research.
Outlook
Non-invasive techniques are now available to comprehensively characterize arterial pulsatile haemodynamics in the clinic (Figure 5). The availability of contemporary modelling techniques, the ongoing shift towards personalized medicine, and the emergence of drugs that may favourably target pulsatile LV load independently of blood pressure, provide a framework for the clinical translation of arterial haemodynamics into therapeutic approaches. However, it is essential that mechanistic studies continue and that future trials incorporate deeper phenotyping of arterial haemodynamics (which can now be done with minimal patient burden even in multicentre trials), in order to truly advance our clinical approach to the treatment of HF using these concepts. In particular, despite its popularity, AIx appears to be inferior to WSA-based parameters as RM62 or Pb.88 Therefore, WSA should be more broadly applied in future studies. Finally, several haemodynamic principles and analytic techniques (such as wave separation, wave intensity, and wave power analyses) can be applied in sites other than the aorta,69,102 which can provide important insights into cerebrovascular and coronary haemodynamics in patients with HF.
Figure 5.
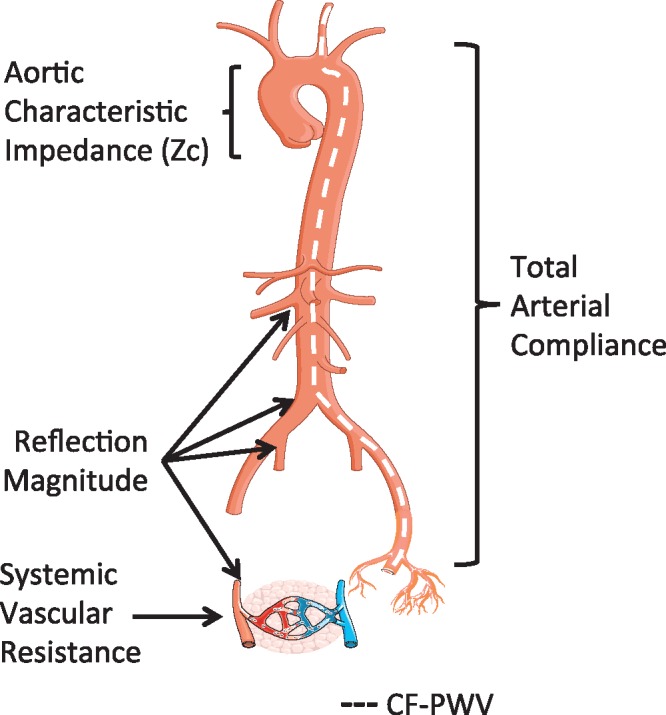
Anatomic origin of arterial properties that impact left ventricular afterload. Although arterial load results from complex interaction between various arterial segments, in general, specific loading patterns can be attributed to anatomic sites. cf-PWV, a measure of large artery wall stiffness, is also shown, although this is not a measure of LV load per se. Modified from Chirinos and Segers23. cf-PWV, carotid-femoral pulse wave velocity.
Conflict of interest: T.W. received a research grant for a multicentre study from i.e.m., Stolberg, Germany. J.A.C. has received consulting honoraria from Bristol-Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft, Ironwood Pharmaceuticals, Sanifit, Pfizer, Bayer, Vital Labs, and Merck. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda-Denshi, Bristol-Myers Squibb, Microsoft, and device loans from AtCor Medical and Uscom. J.A.C. is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of heart failure and preserved ejection fraction.
Supplementary Material
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H.. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM, Group SR. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. J Am Med Assoc 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parragh S, Hametner B, Bachler M, Kellermair J, Eber B, Wassertheurer S, Weber T.. Determinants and covariates of central pressures and wave reflections in systolic heart failure. Int J Cardiol 2015;190:308–314. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA.. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 7. Papaioannou TG, Karageorgopoulou TD, Sergentanis TN, Protogerou AD, Psaltopoulou T, Sharman JE, Weber T, Blacher J, Daskalopoulou SS, Wassertheurer S, Khir AW, Vlachopoulos C, Stergiopulos N, Stefanadis C, Nichols WW, Tousoulis D.. Accuracy of commercial devices and methods for noninvasive estimation of aortic systolic blood pressure a systematic review and meta-analysis of invasive validation studies. J Hypertens 2016;34:1237–1248. [DOI] [PubMed] [Google Scholar]
- 8. McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB.. Central blood pressure: current evidence and clinical importance. Eur Heart J 2014;35:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nichols WW, O’Rourke MF, Vlachopoulos C.. McDonald’s Blood Flow in Arteries. London: Hodder Arnold; 2011. [Google Scholar]
- 10. Kelly R, Hayward C, Avolio A, O’Rourke M.. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 1989;80:1652–1659. [DOI] [PubMed] [Google Scholar]
- 11. Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 2005;18:3–10S. [DOI] [PubMed] [Google Scholar]
- 12. Phan TS, Li JK, Segers P, Chirinos JA.. Misinterpretation of the determinants of elevated forward wave amplitude inflates the role of the proximal aorta. J Am Heart Assoc 2016;5:e003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parragh S, Hametner B, Bachler M, Weber T, Eber B, Wassertheurer S.. Non-invasive wave reflection quantification in patients with reduced ejection fraction. Physiol Meas 2015;36:179–190. [DOI] [PubMed] [Google Scholar]
- 14. Hametner B, Parragh S, Weber T, Wassertheurer S.. Wave intensity of aortic root pressure as diagnostic marker of left ventricular systolic dysfunction. PLoS One 2017;12:e0179938.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Rourke MF, Pauca A, Jiang XJ.. Pulse wave analysis. Br J Clin Pharmacol 2001;51:507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westerhof N, Sipkema P, Bos GCVD, Elzinga G.. Forward and backward waves in the arterial system. Cardiovasc Res 1972;6:648–656. [DOI] [PubMed] [Google Scholar]
- 17. Parker KH, Jones CJ.. Forward and backward running waves in the arteries: analysis using the method of characteristics. J Biomech Eng 1990;112:322–326. [DOI] [PubMed] [Google Scholar]
- 18. Manisty CH, Zambanini A, Parker KH, Davies JE, Francis DP, Mayet J, Mc GTSA, Hughes AD; Anglo-Scandinavian Cardiac Outcome Trial Investigators. Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure: an Anglo-Scandinavian Cardiac Outcome Trial substudy. Hypertension 2009;54:724–730. [DOI] [PubMed] [Google Scholar]
- 19. Ohte N, Narita H, Sugawara M, Niki K, Okada T, Harada A, Hayano J, Kimura G.. Clinical usefulness of carotid arterial wave intensity in assessing left ventricular systolic and early diastolic performance. Heart Vessels 2003;18:107–111. [DOI] [PubMed] [Google Scholar]
- 20. Mynard JP, Smolich JJ.. Novel wave power analysis linking pressure-flow waves, wave potential, and the forward and backward components of hydraulic power. Am J Physiol Heart Circ Physiol 2016;310:H1026–H1038. [DOI] [PubMed] [Google Scholar]
- 21. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H.. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 22. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB.. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chirinos JA, Segers P.. Noninvasive evaluation of left ventricular afterload: Part 1: Pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension 2010;56:555–562. [DOI] [PubMed] [Google Scholar]
- 24. Chirinos JA. Deep phenotyping of systemic arterial hemodynamics in HFpEF (Part 1): Physiologic and technical considerations. J Cardiovasc Transl Res 2017;10:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chirinos JA, Segers P.. Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension 2010;56:563–570. [DOI] [PubMed] [Google Scholar]
- 26. Chirinos JA. Deep phenotyping of systemic arterial hemodynamics in HFpEF (Part 2): Clinical and therapeutic considerations. J Cardiovasc Transl Res 2017;10:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chirinos JA, Segers P, Gillebert TC, Gupta AK, De Buyzere ML, De Bacquer D, St John-Sutton M, Rietzschel ER, Asklepios I.. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension 2012;60:64–70. [DOI] [PubMed] [Google Scholar]
- 28. Chirinos JA, Segers P, Gupta AK, Swillens A, Rietzschel ER, De Buyzere ML, Kirkpatrick JN, Gillebert TC, Wang Y, Keane MG, Townsend R, Ferrari VA, Wiegers SE, St John Sutton M.. Time-varying myocardial stress and systolic pressure-stress relationship: role in myocardial-arterial coupling in hypertension. Circulation 2009;119:2798–2807. [DOI] [PubMed] [Google Scholar]
- 29. Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC, Asklepios I.. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension 2013;61:296–303. [DOI] [PubMed] [Google Scholar]
- 30. Shah SJ, Wasserstrom JA.. Increased arterial wave reflection magnitude: a novel form of stage B heart failure? J Am Coll Cardiol 2012;60:2178–2181. [DOI] [PubMed] [Google Scholar]
- 31. Gu H, Li Y, Fok H, Simpson J, Kentish JC, Shah AM, Chowienczyk PJ.. Reduced first-phase ejection fraction and sustained myocardial wall stress in hypertensive patients with diastolic dysfunction: a manifestation of impaired shortening deactivation that links systolic to diastolic dysfunction and preserves systolic ejection fraction. Hypertension 2017;69:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eichhorn EJ, Willard JE, Alvarez L, Kim AS, Glamann DB, Risser RC, Grayburn PA.. Are contraction and relaxation coupled in patients with and without congestive heart failure? Circulation 1992;85:2132–2139. [DOI] [PubMed] [Google Scholar]
- 33. Gillebert TC, Lew WY.. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol 1991;261:H805–H813. [DOI] [PubMed] [Google Scholar]
- 34. Hori M, Inoue M, Kitakaze M, Tsujioka K, Ishida Y, Fukunami M, Nakajima S, Kitabatake A, Abe H.. Loading sequence is a major determinant of afterload-dependent relaxation in intact canine heart. Am J Physiol 1985;249:H747–H754. [DOI] [PubMed] [Google Scholar]
- 35. Yano M, Kohno M, Kobayashi S, Obayashi M, Seki K, Ohkusa T, Miura T, Fujii T, Matsuzaki M.. Influence of timing and magnitude of arterial wave reflection on left ventricular relaxation. Am J Physiol Heart Circ Physiol 2001;280:H1846–H1852. [DOI] [PubMed] [Google Scholar]
- 36. Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, Gottdiener J, Haluska B, Ofili E, Segers P, Senior R, Tapp RJ, Zamorano JL.. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). Eur Heart J Cardiovasc Imaging 2015;16:577–605. [DOI] [PubMed] [Google Scholar]
- 37. Chirinos JA, Phan TS, Syed AA, Hashmath Z, Oldland HG, Koppula MR, Tariq A, Javaid K, Miller R, Varakantam S, Dunde A, Neetha V, Akers SR.. Late systolic myocardial loading is associated with left atrial dysfunction in hypertension. Circ Cardiovasc Imaging 2017;10:e006023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arts T, Bovendeerd PH, Prinzen FW, Reneman RS.. Relation between left ventricular cavity pressure and volume and systolic fiber stress and strain in the wall. Biophys J 1991;59:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strauer BE. Myocardial oxygen consumption in chronic heart disease: role of wall stress, hypertrophy and coronary reserve. Am J Cardiol 1979;44:730–740. [DOI] [PubMed] [Google Scholar]
- 40. Weber T, O’Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B.. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens 2008;21:1194–1202. [DOI] [PubMed] [Google Scholar]
- 41. Weber T, Wassertheurer S, O’Rourke MF, Haiden A, Zweiker R, Rammer M, Hametner B, Eber B.. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol 2013;61:1874–1883. [DOI] [PubMed] [Google Scholar]
- 42. Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA.. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 2015;131:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weber T, Auer J, O'Rourke MF, Punzengruber C, Kvas E, Eber B.. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart 2006;92:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paglia A, Sasso L, Pirozzi F, Iannuzzi A, Carlomagno A, Abete P, Petretta M, Bonaduce D.. Arterial wave reflections and ventricular-vascular interaction in patients with left ventricular systolic dysfunction. Int Heart J 2014;55:526–532. [DOI] [PubMed] [Google Scholar]
- 45. Denardo SJ, Nandyala R, Freeman GL, Pierce GL, Nichols WW.. Pulse wave analysis of the aortic pressure waveform in severe left ventricular systolic dysfunction. Circ Heart Fail 2010;3:149–156. [DOI] [PubMed] [Google Scholar]
- 46. Curtis SL, Zambanini A, Mayet J, Mc GTSA, Foale R, Parker KH, Hughes AD.. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am J Physiol Heart Circ Physiol 2007;293:H557–H562. [DOI] [PubMed] [Google Scholar]
- 47. Sunagawa K, Maughan WL, Burkhoff D, Sagawa K.. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983;245:H773–H780. [DOI] [PubMed] [Google Scholar]
- 48. Chirinos JA. Ventricular-arterial coupling: invasive and non-invasive assessment. Artery Res 2013;7:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sunagawa K, Maughan WL, Friesinger G, Guzman P, Chang MS, Sagawa K.. Effects of coronary arterial pressure on left ventricular end-systolic pressure-volume relation of isolated canine heart. Circ Res 1982;50:727–734. [DOI] [PubMed] [Google Scholar]
- 50. Sunagawa K, Sagawa K, Maughan WL.. Ventricular interaction with the loading system. Ann Biomed Eng 1984;12:163–189. [DOI] [PubMed] [Google Scholar]
- 51. Chirinos JA. Ventricular-arterial coupling: invasive and non-invasive assessment. Artery Res 2013;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fukuta H, Ohte N, Wakami K, Asada K, Goto T, Mukai S, Tani T, Kimura G.. Impact of arterial load on left ventricular diastolic function in patients undergoing cardiac catheterization for coronary artery disease. Circ J 2010;74:1900–1905. [DOI] [PubMed] [Google Scholar]
- 53. Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M.. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation 1996;94:3362–3368. [DOI] [PubMed] [Google Scholar]
- 54. Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'rourke MF.. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens 2008;26:1017–1024. [DOI] [PubMed] [Google Scholar]
- 55. Quail MA, Short R, Pandya B, Steeden JA, Khushnood A, Taylor AM, Segers P, Muthurangu V.. Abnormal wave reflections and left ventricular hypertrophy late after coarctation of the aorta repair. Hypertension 2017;69:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maughan WL, Sunagawa K, Burkhoff D, Sagawa K.. Effect of arterial impedance changes on the end-systolic pressure-volume relation. Circ Res 1984;54:595–602. [DOI] [PubMed] [Google Scholar]
- 57. Segers P, Stergiopulos N, Westerhof N.. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol 2002;282:H1041–H1046. [DOI] [PubMed] [Google Scholar]
- 58. Chirinos JA, Rietzschel ER, Shiva-Kumar P, De Buyzere ML, Zamani P, Claessens T, Geraci S, Konda P, De Bacquer D, Akers SR, Gillebert TC, Segers P.. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension 2014;64:1022–1031. [DOI] [PubMed] [Google Scholar]
- 59. Goto T, Ohte N, Fukuta H, Wakami K, Tani T, Kimura G.. Relationship between effective arterial elastance, total vascular resistance, and augmentation index at the ascending aorta and left ventricular diastolic function in older women. Circ J 2013;77:123–129. [DOI] [PubMed] [Google Scholar]
- 60. Zamani P, Lilly SM, Segers P, Jacobs DR Jr, Bluemke DA, Duprez DA, Chirinos JA.. Pulsatile load components, resistive load and incident heart failure: the Multi-Ethnic Study of Atherosclerosis (MESA). J Card Fail 2016;22:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chirinos JA, Segers P, Duprez DA, Brumback L, Bluemke DA, Zamani P, Kronmal R, Vaidya D, Ouyang P, Townsend RR, Jacobs DR Jr.. Late systolic central hypertension as a predictor of incident heart failure: the Multi-ethnic Study of Atherosclerosis. J Am Heart Assoc 2015;4:e001335.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P.. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol 2012;60:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Messerli FH, Rimoldi SF, Bangalore S.. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail 2017;5:543–551. [DOI] [PubMed] [Google Scholar]
- 64. Ioannou CV, Morel DR, Katsamouris AN, Katranitsa S, Startchik I, Kalangos A, Westerhof N, Stergiopulos N.. Left ventricular hypertrophy induced by reduced aortic compliance. J Vasc Res 2009;46:417–425. [DOI] [PubMed] [Google Scholar]
- 65. Pini R, Cavallini MC, Bencini F, Silvestrini G, Tonon E, De Alfieri W, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ.. Cardiovascular remodeling is greater in isolated systolic hypertension than in diastolic hypertension in older adults: the Insufficienza Cardiaca negli Anziani Residenti (ICARE) a Dicomano Study. J Am Coll Cardiol 2002;40:1283–1289. [DOI] [PubMed] [Google Scholar]
- 66. Sharman JE, Fang ZY, Haluska B, Stowasser M, Prins JB, Marwick TH.. Left ventricular mass in patients with type 2 diabetes is independently associated with central but not peripheral pulse pressure. Diabetes Care 2005;28:937–939. [DOI] [PubMed] [Google Scholar]
- 67. Norton GR, Majane OH, Maseko MJ, Libhaber C, Redelinghuys M, Kruger D, Veller M, Sareli P, Woodiwiss AJ.. Brachial blood pressure-independent relations between radial late systolic shoulder-derived aortic pressures and target organ changes. Hypertension 2012;59:885–892. [DOI] [PubMed] [Google Scholar]
- 68. Weber T, Wassertheurer S, Schmidt-Trucksass A, Rodilla E, Ablasser C, Jankowski P, Lorenza Muiesan M, Giannattasio C, Mang C, Wilkinson I, Kellermair J, Hametner B, Pascual JM, Zweiker R, Czarnecka D, Paini A, Salvetti M, Maloberti A, McEniery C.. Relationship between 24-hour ambulatory central systolic blood pressure and left ventricular mass: a prospective multicenter study. Hypertension 2017;70:1157–1164. [DOI] [PubMed] [Google Scholar]
- 69. Urbina EM, Dolan LM, McCoy CE, Khoury PR, Daniels SR, Kimball TR.. Relationship between elevated arterial stiffness and increased left ventricular mass in adolescents and young adults. J Pediatr 2011;158:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zamani P, Bluemke DA, Jacobs DR Jr., Duprez DA, Kronmal R, Lilly SM, Ferrari VA, Townsend RR, Lima JA, Budoff M, Segers P, Hannan P, Chirinos JA.. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: the Multi-Ethnic Study of Atherosclerosis. Hypertension 2015;65:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cauwenberghs N, Knez J, D’Hooge J, Thijs L, Yang W-Y, Wei F-F, Zhang Z-Y, Staessen JA, Kuznetsova T.. Longitudinal changes in LV structure and diastolic function in relation to arterial properties in general population. JACC Cardiovasc Imaging 2017;10:1307–1316. [DOI] [PubMed] [Google Scholar]
- 72. Hashimoto J, Imai Y, Orourke M.. Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens 2007;20:378–384. [DOI] [PubMed] [Google Scholar]
- 73. de Luca N, Asmar RG, London GM, O’Rourke MF, Safar ME. and Investigators RP. Selective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjects. J Hypertens 2004;22:1623–1630. [DOI] [PubMed] [Google Scholar]
- 74. Haider AW, Larson MG, Franklin SS, Levy D, Framingham Heart S.. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med 2003;138:10–16. [DOI] [PubMed] [Google Scholar]
- 75. Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH.. Increased pulse pressure and risk of heart failure in the elderly. J Am Med Assoc 1999;281:634–639. [DOI] [PubMed] [Google Scholar]
- 76. Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS.. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc 2015;4:e002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR; CRIC Study Investigators. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail 2014;7:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pandey A, Khan H, Newman AB, Lakatta EG, Forman DE, Butler J, Berry JD.. Arterial stiffness and risk of overall heart failure, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction: the health ABC study (Health, Aging, and Body Composition). Hypertension 2017;69:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aisu H, Saito M, Inaba S, Morofuji T, Takahashi K, Sumimoto T, Okura T, Higaki J.. Association of worsening arterial stiffness with incident heart failure in asymptomatic patients with cardiovascular risk factors. Hypertens Res 2017;40:173–180. [DOI] [PubMed] [Google Scholar]
- 80. Brutsaert DL, De Keulenaer GW.. Diastolic heart failure: a myth. Curr Opin Cardiol 2006;21:240–248. [DOI] [PubMed] [Google Scholar]
- 81. Sung SH, Yu WC, Cheng HM, Chuang SY, Wang KL, Huang CM, Chen CH.. Pulsatile hemodynamics and clinical outcomes in acute heart failure. Am J Hypertens 2011;24:775–782. [DOI] [PubMed] [Google Scholar]
- 82. Regnault V, Lagrange J, Pizard A, Safar ME, Fay R, Pitt B, Challande P, Rossignol P, Zannad F, Lacolley P.. Opposite predictive value of pulse pressure and aortic pulse wave velocity on heart failure with reduced left ventricular ejection fraction: insights from an Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) substudy. Hypertension 2014;63:105–111. [DOI] [PubMed] [Google Scholar]
- 83. Meguro T, Nagatomo Y, Nagae A, Seki C, Kondou N, Shibata M, Oda Y.. Elevated arterial stiffness evaluated by brachial-ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J 2009;73:673–680. [DOI] [PubMed] [Google Scholar]
- 84. Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo DHFI.. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. J Am Med Assoc 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- 85. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- 86. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 87. Borlaug BA, Olson TP, Abdelmoneim SS, Melenovsky V, Sorrell VL, Noonan K, Lin G, Redfield MM.. A randomized pilot study of aortic waveform guided therapy in chronic heart failure. J Am Heart Assoc 2014;3:e000745.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wohlfahrt P, Melenovsky V, Redfield MM, Olson TP, Lin G, Abdelmoneim SS, Hametner B, Wassertheurer S, Borlaug BA.. Aortic waveform analysis to individualize treatment in heart failure. Circ Heart Fail 2017;10:e003516.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brin KP, Yin FC.. Effect of nitroprusside on wave reflections in patients with heart failure. Ann Biomed Eng 1984;12:135–150. [DOI] [PubMed] [Google Scholar]
- 90. Pauca AL, Kon ND, O'Rourke MF.. Benefit of glyceryl trinitrate on arterial stiffness is directly due to effects on peripheral arteries. Heart 2005;91:1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zamani P, Akers S, Soto-Calderon H, Beraun M, Koppula MR, Varakantam S, Rawat D, Shiva-Kumar P, Haines PG, Chittams J, Townsend RR, Witschey WR, Segers P, Chirinos JA.. Isosorbide dinitrate, with or without hydralazine, does not reduce wave reflections, left ventricular hypertrophy, or myocardial fibrosis in patients with heart failure with preserved ejection fraction. J Am Heart Assoc 2017;6:e004262.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Omar SA, Artime E, Webb AJ.. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide 2012;26:229–240. [DOI] [PubMed] [Google Scholar]
- 93. Omar SA, Fok H, Tilgner KD, Nair A, Hunt J, Jiang B, Taylor P, Chowienczyk P, Webb AJ.. Paradoxical normoxia-dependent selective actions of inorganic nitrite in human muscular conduit arteries and related selective actions on central blood pressures. Circulation 2015;131:381–389;discussion 389. [DOI] [PubMed] [Google Scholar]
- 94. Chirinos JA, Londono-Hoyos F, Zamani P, Beraun M, Haines P, Vasim I, Varakantam S, Phan TS, Cappola TP, Margulies KB, Townsend RR, Segers P.. Effects of organic and inorganic nitrate on aortic and carotid haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail 2017;19:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP, Borlaug BA.. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Londono-Hoyos F, Zamani P, Beraun M, Vasim I, Segers P, Chirinos JA.. Effect of organic and inorganic nitrates on cerebrovascular pulsatile power transmission in patients with heart failure and preserved ejection fraction. Physiol Meas 2018;39:044001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW.. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail 2016;4:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramamurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR.. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: a double-blind, placebo-controlled, randomized trial. Circ Heart Fail 2015;8:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chirinos JA. The nitrate-nitrite-NO pathway as a novel therapeutic target in heart failure with reduced ejection fraction. J Card Fail 2018;24:74–77. [DOI] [PubMed] [Google Scholar]
- 100. Kerley CP, O’Neill JO, Reddy Bijjam V, Blaine C, James PE, Cormican L.. Dietary nitrate increases exercise tolerance in patients with non-ischemic, dilated cardiomyopathy-a double-blind, randomized, placebo-controlled, crossover trial. J Heart Lung Transplant 2016;35:922–926. [DOI] [PubMed] [Google Scholar]
- 101. Borlaug BA, Koepp KE, Melenovsky V.. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2015;66:1672–1682. [DOI] [PubMed] [Google Scholar]
- 102. Broyd CJ, Hernandez-Perez F, Segovia J, Echavarria-Pinto M, Quiros-Carretero A, Salas C, Gonzalo N, Jimenez-Quevedo P, Nombela-Franco L, Salinas P, Nunez-Gil I, Del Trigo M, Goicolea J, Alonso-Pulpon L, Fernandez-Ortiz A, Parker K, Hughes A, Mayet J, Davies J, Escaned J.. Identification of capillary rarefaction using intracoronary wave intensity analysis with resultant prognostic implications for cardiac allograft patients. Eur Heart J 2018;39:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.