Abstract
Background
Implementation of the newly approved high-sensitivity cardiac troponin (hs-cTn) in the United States presents a challenge for clinical practice. Sex-specific cut-offs, clinical protocols, and workflows will likely require modifications before implementation.
Methods
We conducted a cross-sectional survey of international physicians and laboratorians already utilizing hs-cTn for the evaluation of acute myocardial infarction (AMI).
Results
Twenty-two of 54 (41%) eligible participants completed the survey, representing nine countries and 18 hospitals. All reported successful hs-cTn implementation and diagnostic utility (mean 8.6+1.2 out of 10 for best implementation). The major perceived benefit was more rapid evaluation of AMI (14/19, 74%) and the most frequently cited limitation was an increase in the number of measurable hs-cTn values that required further evaluation (8/18, 44%). Institutions using the hs-cTnI assay favored sex-specific cut-offs (5/6, 83%) while institutions employing the hs-cTnT assay favored a combined cut-off (12/12, 100%). Timing of serial hs-cTn measurements varied, with 0–3 hours (8/17, 47%) most frequent, followed by 0–2 hours (4/17, 24%), 0–1 hour (3/17, 18%), and other (2/17, 12%).
Conclusions
Our survey of hs-cTn implementation at international institutions reveals satisfaction with new assays but reflects important variations in clinical practice. The use of sex-specific vs combined cut-offs and timing of serial hs-cTn measurements varies across institutions and are subjects that US centers must define without consensus from international practices.
Keywords: High-sensitivity cardiac troponin, acute myocardial infarction, clinical implementation
INTRODUCTION
High-sensitivity cardiac troponin (hs-cTn) has been a key tool for diagnosis and exclusion of acute myocardial infarction (AMI) for nearly a decade outside the United States (US).1 In contrast, US institutions have used less sensitive “contemporary” cTn assays. On January 19, 2017, the US Federal Drug Administration (FDA) approved the first hs-cTn assay.
The strengths of hs-cTn assays are precise measurement of lower levels of troponin than contemporary assays and earlier identification of myocardial injury2,3—affording rapid rule-out and rule-in of AMI by accelerated diagnostic protocols.4–6 As defined by the International Federation for Clinical Chemistry (IFCC), a “high sensitivity” cTn assay exhibits ≤10% coefficient of variation, or imprecision, at the cut-off for the reference population and has the capacity to measure troponins less than the cut-off in at least 50% of apparently healthy individuals.7 In contrast, contemporary assays exhibit analytical imprecision >10% at the cut-off, and at best, can quantify troponins in up to 35% of healthy individuals.7 For hs-cTn assays, sex-specific cut-offs have also been proposed by the IFCC due to reported differences at each respective cut-off according to sex,8 although data are conflicting on whether or not sex-specific cut-offs result in improved clinical outcomes.9,10 Two hs-cTn assays, hs-cTnT (Roche Diagnostics, Rotkreuz, Switzerland) and hs-cTnI (Abbott Diagnostics, Chicago, IL), have been widely employed clinically outside the US.
Despite perceived benefits, the clinical implementation of hs-cTn presents several challenges. Sex-specific cut-offs,11,12 various clinical protocols and workflows,13,14 and different approaches to educate providers may present significant impediments to the adoption of hs-cTn. Strategies for defining and/or overcoming potential challenges are not well described. We sought to characterize practices associated with successful hs-cTn implementation outside the US by surveying physicians and laboratorians from institutions that have implemented hs-cTn for the evaluation of AMI.
METHODS
We conducted a cross-sectional survey of physicians and laboratorians at institutions outside the US using hs-cTn for standard patient care. We included (1) authors of PubMed-indexed studies involving hs-cTn for the evaluation of AMI and (2) physicians and laboratory scientists identified by these authors as having a critical role in the implementation of hs-cTn at their institution. We excluded physicians and laboratorians who are from the US, those who were associated with institutions that did not clinically use hs-cTn, or were not involved with the implementation of hs-cTn. The survey was hosted by Qualtrics (Qualtrics, Provo, UT) online survey platform. We distributed email invitations with a web link to the online survey to eligible participants from July to August 2017. We made up to four attempts over two months to recruit eligible participants.
The survey assessed the clinical implementation process of hs-cTn including the major topics of: (1) clinical use of hs-cTn, (2) effects of implementation, (3) challenges in implementation, and (4) leadership and education (Appendix). Survey questions were developed based on expert opinion and literature review. The survey was pilot tested on three subjects. Minor revisions were made based on the pilot testing and pilot responses were included in the final analysis.
Our primary outcome was participants’ overall rating of the success of hs-cTn clinical implementation, ranging from 0 for “not at all successful” to 10 for “extremely successful.” For open-ended questions, we identified themes in the responses which were coded and analyzed quantitatively.
We calculated proportions for each outcome. Continuous variables were expressed as mean ± standard deviation (SD) and analyzed by Student’s 2-sample t-test; categorical variables were analyzed by Chi square. Differences were considered significant if p <0.05. The JMP statistical package (JMP 13.0.0, SAS Institute Inc., Cary, NC) was used for analysis. This study was deemed not to be human subjects research and was exempt from review by our institutional review board.
RESULTS
Of the 62 potential participants screened for eligibility, eight were excluded: six for not using hs-cTn clinically and two for not being involved in hs-cTn implementation. Of the 54 participants who met inclusion criteria, 22 (41%, 22/54) completed the survey (Table 1). The participants represent nine countries, 18 institutions, and five departments (Table 1). Participants either use hs-cTnT (59%, 13/22) or hs-cTnI (41%, 9/22) assays. All participants reported successful hs-cTn implementation and diagnostic utility (8.6 ±1.2 out of 10 for best implementation).
TABLE 1.
Characteristics of Survey Participants
| Country/Institutions | Department | No. of Annual ED Visits* | No. of Hospital Beds† | Hs-cTn Assay | Sex-Specific vs. Combined Cut Offs | Timing of Serial Troponins | Further Cardiac Testing‡ |
|---|---|---|---|---|---|---|---|
| Australia | |||||||
| University of Western Australia | Lab | >90 | ≥10 | I | S | 2 hr | Y |
| Canada | |||||||
| Saskatchewan Health Region/Royal University Hospital | Lab | 60–90 | 5–10 | T | C | 3 hr | Y |
| University of Calgary | Card | >90 | ≥10 | T | C | Other | - |
| Hamilton Health Sciences | Lab | >90 | ≥10 | I | C | 3 hr | - |
| Germany | |||||||
| Kerckhoff Heart and Thorax Center | Card | <30 | 2–5 | T | C | 3 hr | Y |
| Netherlands | |||||||
| Isala | IM | 30–60 | ≥10 | T | C | 3 hr | Y |
| Leiden University Medical Center | Card | - | 5–10 | T | C | 1 hr | Y |
| New Zealand | |||||||
| Canterbury District Health Board | Lab | 60–90 | 5–10 | I | S | 3 hr | Y |
| Christchurch Hospital | EM | >90 | 5–10 | I | S | 2 hr | Y |
| University of Otago | Chem | 30–60 | 5–10 | I | S | - | - |
| Canterbury District Health Board | Chem | >90 | 5–10 | I | S | 2 hr | Y |
| Christchurch Hospital | Card | <30 | 5–10 | I | S | 2 hr | Y |
| Singapore | |||||||
| National Heart Centre Singapore | Card | >90 | ≥10 | T | C | 2 hr | N |
| Sweden | |||||||
| University of Gothenburg | Chem | 30–60 | ≥10 | T | C | Other | Y |
| Karolinska University Hospital | EM | >90 | ≥10 | T | C | 1 hr | N |
| Switzerland | |||||||
| University Hospital Basel | Card | >90 | 2–5 | T | C | 1 hr | Y |
| Cantonal Hospital Baselland | IM | <30 | 2–5 | T | C | 3 hr | Y |
| Cantonal Hospital Baselland | IM | 30–60 | 2–5 | T | C | 3 hr | Y |
| United Kingdom | |||||||
| North Bristol NHS Trust UK | EM | 60–90 | 5–10 | T | C | 2 hr | N |
| University of Manchester | EM | >90 | 2–5 | T | C | 3 hr | Y |
| Royal Infirmary of Edinburgh, Scotland | Card | 60–90 | 2–5 | I | S | Other | Y |
| Royal Infirmary of Edinburgh, Scotland | Card | >90 | 5–10 | I | S | 3 hr | N |
In thousands;
In hundreds;
Utilization of further cardiac testing in the emergency department rule out protocol;
ED = Emergency department; Lab = Laboratory Medicine; Card = Cardiology; Chem = Clinical Chemistry; EM = Emergency Medicine; IM = Internal Medicine; T = hs-cTnT, Roche Diagnostics; I = hs-cTnI, Abbott Diagnostics; S = Sex-specific cut offs; C = Combined cut off. Y = Yes; N = No.
Clinical Use of hs-cTn
Institutions employing the hs-cTnI assay favored sex-specific cut-offs (5/6, 83%) while institutions using the hs-cTnT assay favored a combined cut-off (12/12, 100%) (Table 1).
Timing of serial hs-cTn measurements varied, with 0 to 3 hours (8/17, 47%) being the most frequent, followed by 0 to 2 hours (4/17, 24%), 0 to 1 hour (3/17, 18%), and other (2/17, 12%) (Table 1). The two institutions that use an “other” timing interval included “physician-dependent, but many using 2-hour intervals” and “3 [hour] only if first [hs-cTn] is >5 ng/l.”
Nearly all institutions (16/18, 89%) integrate hs-cTn in a protocol for exclusion of AMI. The most frequent criteria used are ≥2 normal hs-cTn values (10/15, 67%) with approximately half also including a clinical risk score (8/15, 53%) and a normal electrocardiogram (7/15, 47%).
In the protocols for exclusion of AMI in the emergency department, the majority (53%, 8/15) use additional cardiac testing at the discretion of the attending physician; 27% (4/15) require no additional cardiac testing, and 20% (3/15) use additional cardiac functional testing as directed by protocol (Table 1).
Effects of hs-cTn Implementation
Approximately three-fourths of participants (74%) identified more rapid rule-in and rule-out of AMI as a positive impact of hs-cTn implementation with comments such as: “Rapid rule out… Reduction in [length of stay] for low risk patients (12 hours to 6 hours)” and “… we implemented the 0/1 hour rule out protocol. Consequently, patients could be faster discharged or admitted... the duration of patients in the emergency department is reduced.” (Appendix Table 1). Other positive impacts were improved diagnostic accuracy (37%, 7/19) and simplified evaluation of AMI (32%, 6/19) resulting in fewer admissions, less additional cardiac testing, and lower costs. (Appendix Table 1).
Increased measurable hs-cTn values were the most commonly identified negative impact of implementation (8/18, 44%): “Increased awareness of myocardial necrosis without type 1 myocardial infarction without a clear evidence base for investigation or management in these patients” (Appendix Table 2). Seven participants (39%, 7/18) reported no negative impacts of hs-cTn implementation. Less than one third of participants (28%, 5/18) reported increased downstream testing or challenges in clinical interpretation: “Over testing/over diagnosis in patients without chest pain;” “Confusion among [emergency department] doctors on how to deal with borderline positive values.” Increased cardiology consultation was noted by two participants and increased hospitalizations by one participant.
Challenges in hs-cTn Implementation
The most frequent challenge which was identified by 35% (7/20) of participants was in education: “getting everyone on the same page” and “to make sure that everyone got the message and we didn’t miss anyone on the switch to the new assay.” Other challenges included physician buy in (20%, 4/19): “a small group of ER physicians… did not want numerical highly sensitive troponin tests that required clinical interpretation.” Participants also reported difficulty with over-diagnosis of AMI (15%, 3/19) and the development of new protocols (10%, 2/19).
Leadership and Education
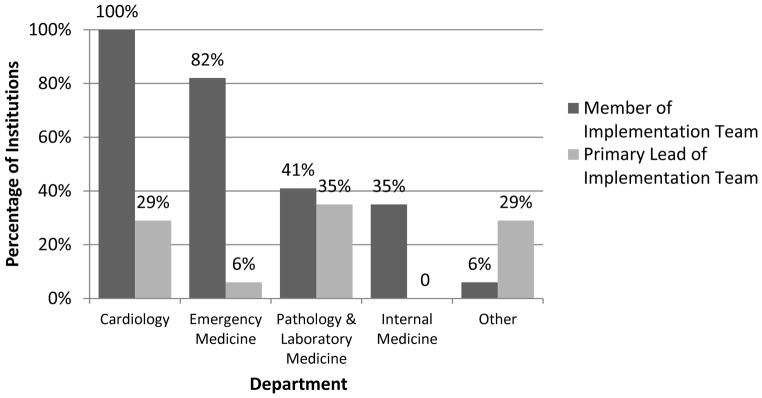
A multidisciplinary team led hs-cTn implementation at most institutions (Figure 1). Primary leadership was most often pathology and laboratory medicine (35%, 6/17), cardiology (29%, 5/17), and other (29%, 5/17) [3 joint efforts, 1 unknown].
Figure 1. Implementation Leadership Team by Department.
A multidisciplinary team led hs-cTn implementation at most institutions.
Education for other health care providers on hs-cTn relied on multiple educational tools. Used by over 60% of participants, most common strategies were announcements at departmental meetings, educational lectures, emails, and informative letters. Less than a quarter of participants used methods such as electronic medical record messages, online resources, posters, or other strategies.
DISCUSSION
Our results in a cross-sectional survey of more than 20 international physicians and laboratorians who use hs-cTn for the evaluation of AMI reveals satisfaction with the new assays and reflects important variations in clinical practice. To our knowledge, this is the first report that compares practices for hs-cTn use across multiple institutions, countries, and popular testing platforms. Whereas existing literature outlines the clinical algorithms available,13,14 our evaluation reveals the frequency of use of different clinical practices and workflows with the potential to serve as a guide for other institutions in the process of hs-cTn implementation.
Our study group, although small, is a heterogeneous cohort representing diverse countries spanning North America to Australasia, a breadth of departments, institutions ranging from small to large, and a fairly even distribution of the hs-cTnI and hs-cTnT assays (Table 1). These attributes help broaden the applicability of our findings to other users of hs-cTn.
An area of current institutional variability concerning use of hs-cTn is whether or not to incorporate sex-specific versus combined cut-offs. The IFCC recommends sex-specific cut-offs based on the distinct cut-off values for men and women.8 Some studies have also demonstrated improved diagnosis of AMI with use of sex-specific cut-offs.15 However, data on whether or not use of sex-specific cutoffs results in improved clinical outcomes, rather than merely diagnostic reclassification, are contradictory.9,10 Our study reveals that outside the US, institutions which employ hs-cTnI favor sex-specific cut-offs (83%) and those which utilize hs-cTnT favor a combined cut-off (100%). This trend is likely a result of the hs-cTnT manufacturer, Roche, releasing only a combined cut-off for institutions outside the US. Facilities using hs-cTnT are already experienced with the combined cut-off and may be reluctant to transition to the sex-specific cut-offs proposed by more recent literature.8 Additionally, the difference in use of sex-specific cut-offs by type of assay may be a result of reports showing that sex-specific cut-offs had a negligible impact on AMI diagnosis with hs-cTnT, but a larger impact on diagnosis with hs-TnI.13 Institutions in the US will need to consider whether or not to incorporate sex-specific cut-offs without consensus from international practices. One strategy may be to follow the trend towards a combined cut-off since only hs-cTnT is currently available in the US. However, we caution against this approach since the difference between the sex-specific cut-off values in US populations (hs-cTnT, 8 ng/L) is larger than in populations outside the US (hs-cTnT, 2.5 ng/L),12 thereby potentially amplifying the diagnostic impact of sex-specific cut-offs potentially larger in the US. Additionally, Roche has received FDA approval for hs-cTnT sex-specific cut-offs in the US. Based on these key differences from the international population, we propose further studies to determine optimal cut-off values from US reference populations which can more appropriately guide US institutions in the adoption of sex-specific cut-offs.
In addition to defining cut-offs, clinical protocols for the evaluation of AMI may also require modification. Institutions may transition to shorter timing intervals for serial troponin measurements since hs-cTn can more precisely measure lower levels of troponin and identify myocardial injury earlier.2,3 We found that the majority of institutions use 0 to 3 hour serial testing, with others applying both 0 to 1 hour and 0 to 2 hour (Table 1). These methods are in line with the class I recommendations from the European Society of Cardiology for 0 to 1 hour and 0 to 3 hour timing algorithms.16 A recent comprehensive review from Twerenbold, et al. provides an overview of the six well-validated triage protocols using hs-cTn which include one 0 to 3 hour protocol, two 0 to 2 hour protocols, two 0 to 1 hour protocols, and one single hs-cTn measurement protocol.13 Our study provides new information on how frequently the different timing intervals are used among multiple institutions and countries. Currently in the US, the American Heart Association and American College of Cardiology recommend serial testing with contemporary cTn ranging from 0 to 3 hours to 0 to 6 hours.17 The US may need to carry out similar multicenter studies to validate new timing intervals and protocols in this country.
There is a paucity of data on the need for clinical risk scores or additional cardiac testing with hs-cTn based protocols for AMI. Prior studies showed similar negative predictive values and sensitivity in protocols that use solely troponins versus troponin combined with a clinical risk score;6,18 however, we found that a majority (58%) of institutions use a clinical risk score as part of their hs-cTn protocol. Only two of the six hs-cTn protocols (the 0 to 3 hour European Society of Cardiology protocol and the 0 to 2 hour accelerated diagnostic protocol) that Twerenbold, et al describe use a clinical risk score as part of the evaluation protocol.13 Additionally, almost three-fourths of our surveyed institutions use additional cardiac testing, either at the discretion of the attending physician or as directed by the protocol, to rule out AMI with hs-cTn.
All participants reported successful hs-cTn implementation and diagnostic utility (mean 8.6 ± 1.2 out of 10 for best implementation) with the major perceived benefit more rapid evaluation of AMI (74%). This is in accordance with a large multicenter study which showed that median time to discharge from the emergency department decreased by 79 minutes.19 Nearly a third of participants also reported that hs-cTn resulted in a simplified evaluation of AMI including fewer admissions, with the remainder having no comment. Nonetheless, this finding extends the data from a prior single center study in Sweden in which admissions for chest pain were reduced by 36% during the first four years after hs-cTn implementation.20 However, post-market surveillance studies in the US are needed to better determine if AMI evaluation has been simplified by incorporating hs-cTn into routine practice.
The most frequent response under the question of limitations was increased measureable hs-cTn values which included more diagnoses of type II MI as well as measureable hs-cTn values both above and below the cut-off without associated acute pathology. This perceived limitation may in fact be an underappreciated potential benefit. High sensitivity cTn is a new important marker of subclinical disease with recent studies showing a strong association between detectable levels of hs-cTn and adverse outcomes.21,22 These findings suggest that troponin assays are now an improved prognostic tool.
Few data exist on how to implement hs-cTn. This study shows that a multidisciplinary team usually led implementation, highlighting the importance of including all key stakeholders. By doing so, each department or division has the opportunity to present concerns and priorities regarding the impact of hs-cTn on their specialty and to facilitate the education of colleagues. Educational efforts were multimodal including lectures, emails, and presentations likely to maximize the audience reached and to provide repetition. Other institutions can adopt this framework as an example for their own implementation.
A number of limitations of our study require consideration. First, our study is subject to the limitations inherent in survey methods including sampling bias, nonresponse bias, and measurement errors. However, we attempted to minimize these biases by identifying >50 potential participants from the literature, making four attempts over two months to recruit eligible participants, and creating well-constructed, clear survey questions. Additionally, a survey method was necessary in order to collect clinicians’ experiences with hs-cTn. Second, our sample size is small with 22 participants. However, our 41% response rate is satisfactory, and participants represented diverse institutions, departments, and countries. Third, since we identified participants via PubMed-indexed studies, we may have missed clinicians who are associated with community hospitals and therefore our results may not reflect community use of hs-cTn. Lastly, the efficacy of education programs for transitioning to hs-cTn has not been studied and was not evaluated in this survey.
CONCLUSIONS
Our survey of hs-cTn implementation at international institutions reveals satisfaction with new assays, but points to important variations in clinical practice including differences in the timing and frequency of testing as well as use of risk scores. Education was multidisciplinary and involved common stakeholders including the clinical laboratory, cardiology, and emergency medicine. The use and values of sex-specific vs. combined cut-offs remains controversial, including within the international community. This is further compounded by substantial differences in sex-specific cut-off for the US population. To this end, facilities in the US will have to decide on the optimal cut-off values without consensus from the international community. Further studies will be needed as hs-cTn is adopted across the US to refine best practices.
Acknowledgments
Source of Funding: This study was supported in part by the National Center for Advancing Translational Sciences, NIH, grant number UL1 TR001860 and linked award KL2 TR001859 (J.E.L.), Harold S. Geneen Charitable Trust Awards Program (J.E.L.), the Rosenfeld Heart Foundation (J.E.L.), and the National Heart, Lung, and Blood Institute (NHLBI) through grant #5K08HL130546 (B.E.M.).
We thank survey participants for their responses. We also thank Carmen Wiley, PhD, from Roche Diagnostics for her technical assistance.
APPENDIX
Appendix Table 1.
Positive Impacts of High Sensitivity Cardiac Troponin Implementation
| Faster Rule Out/In of AMI (74%, 14/19) |
|
| Improved Diagnostic Accuracy (37%, 7/19) |
|
| Simplified Evaluation of AMI (32%, 6/19) |
|
AMI, acute myocardial infarction; cTn, cardiac troponin; ED, emergency department; MI, myocardial infarction; NSTEMI, non-ST segment myocardial infarction.
Appendix Table 2.
Negative Impacts of High Sensitivity Cardiac Troponin Implementation
| Increased Detection of hs-cTn (44%, 8/18) |
|
| Challenge in Clinical Interpretation (28%, 5/18) |
|
| Increased Downstream Testing (28%, 5/18) |
|
AMI, acute myocardial infarction; hs-cTn, high sensitivity-cardiac troponin; ED, emergency department; MI, myocardial infarction; NSTEMI, non-ST segment myocardial infarction.
References
- 1.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-segment Elevation. Rev Esp Cardiol (Engl Ed) 2015;68(12):1125. doi: 10.1016/j.rec.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 3.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 4.Pickering JW, Greenslade JH, Cullen L, et al. Assessment of the European Society of Cardiology 0-Hour/1-Hour Algorithm to Rule-Out and Rule-In Acute Myocardial Infarction. Circulation. 2016;134(20):1532–1541. doi: 10.1161/CIRCULATIONAHA.116.022677. [DOI] [PubMed] [Google Scholar]
- 5.Wildi K, Nelles B, Twerenbold R, et al. Safety and efficacy of the 0 h/3 h protocol for rapid rule out of myocardial infarction. Am Heart J. 2016;181:16–25. doi: 10.1016/j.ahj.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091–2098. doi: 10.1016/j.jacc.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Apple FS, Collinson PO Biomarkers ITFoCAoC. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58(1):54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 8.Apple FS, Jaffe AS, Collinson P, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem. 2015;48(4–5):201–203. doi: 10.1016/j.clinbiochem.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Shah AS, Griffiths M, Lee KK, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller-Hennessen M, Lindahl B, Giannitsis E, et al. Diagnostic and prognostic implications using age- and gender-specific cut-offs for high-sensitivity cardiac troponin T - Sub-analysis from the TRAPID-AMI study. Int J Cardiol. 2016;209:26–33. doi: 10.1016/j.ijcard.2016.01.213. [DOI] [PubMed] [Google Scholar]
- 11.Cullen LA, Mills NL. Point: The Use of Sex-Specific Cutpoints for High-Sensitivity Cardiac Troponin Assays. Clin Chem. 2017;63(1):261–263. doi: 10.1373/clinchem.2016.254672. [DOI] [PubMed] [Google Scholar]
- 12.Giannitsis E. Counterpoint: Potential Concerns Regarding the Use of Sex-Specific Cutpoints for High-Sensitivity Troponin Assays. Clin Chem. 2017;63(1):264–266. doi: 10.1373/clinchem.2016.254680. [DOI] [PubMed] [Google Scholar]
- 13.Twerenbold R, Boeddinghaus J, Nestelberger T, et al. Clinical Use of High-Sensitivity Cardiac Troponin in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol. 2017;70(8):996–1012. doi: 10.1016/j.jacc.2017.07.718. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA. Clinician’s Guide to Early Rule-Out Strategies With High-Sensitivity Cardiac Troponin. Circulation. 2017;135(17):1612–1616. doi: 10.1161/CIRCULATIONAHA.117.026717. [DOI] [PubMed] [Google Scholar]
- 15.Apple FS, Jaffe AS, Sharkey S, et al. Best Practices for Monitoring Cardiac Troponin in Detecting Myocardial Injury. Clin Chem. 2017;63(1):37–44. doi: 10.1373/clinchem.2016.257428. [DOI] [PubMed] [Google Scholar]
- 16.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 17.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Reichlin T, Cullen L, Parsonage WA, et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med. 2015;128(4):369–379. e364. doi: 10.1016/j.amjmed.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Twerenbold R, Jaeger C, Rubini Gimenez M, et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J. 2016;37(44):3324–3332. doi: 10.1093/eurheartj/ehw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandstein N, Ljung R, Lundback M, Johansson M, Holzmann MJ. Trends in admissions for chest pain after the introduction of high-sensitivity cardiac troponin T. Int J Cardiol. 2017;240:1–7. doi: 10.1016/j.ijcard.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Melki D, Lugnegard J, Alfredsson J, et al. Implications of Introducing High-Sensitivity Cardiac Troponin T Into Clinical Practice: Data From the SWEDEHEART Registry. J Am Coll Cardiol. 2015;65(16):1655–1664. doi: 10.1016/j.jacc.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Roos A, Bandstein N, Lundback M, Hammarsten O, Ljung R, Holzmann MJ. Stable High-Sensitivity Cardiac Troponin T Levels and Outcomes in Patients With Chest Pain. J Am Coll Cardiol. 2017;70(18):2226–2236. doi: 10.1016/j.jacc.2017.08.064. [DOI] [PubMed] [Google Scholar]