1. Introduction
The development of methodologies for efficient asymmetric synthesis is one of the most important areas of synthetic organic chemistry.1 The syntheses of biologically relevant natural and unnatural organic molecules in optically pure form are of central interest in medicinal chemistry and related disciplines. Variations in the stereochemistry of molecular probes for a target enzyme or receptor sites very often display dramatic differences in their binding properties and biological activities. For meaningful biological studies it is important, if not mandatory, to synthesize such agents in enantiomerically pure form. Recent advances in molecular biology and modern instrumentation techniques have led to a better understanding of many complex human diseases at the molecular level. Concurrent with these remarkable achievements have come new challenges and opportunities for asymmetric synthesis. Thus, from the design of enzyme inhibitors to the synthesis of receptor agonists or antagonists and bioactive natural products, asymmetric synthesis is of fundamental significance in biology and medicine.
The advances in asymmetric synthesis have now reached the point that many organic molecules can be prepared with near complete enantioselectivity. This technology is particularly sophisticated in the generation of new stereogenic centers in the presence of existing chiral centers. A number of asymmetric catalysts or so called ‘abiological catalysts’ are approaching an efficiency and selectivity comparable to enzymes such as in the asymmetric hydrogenation of dehydroamino acids utilizing chiral bisphosphine–rhodium complexes,2 asymmetric isomerization of allylic amines with rhodium(I)–BINAP complexes,3 asymmetric epoxidation of allylic alcohols,4 asymmetric epoxidation of unfunctionalized olefins,5 asymmetric reductions with chiral oxazaborolidenes6 and asymmetric dihydroxylation reactions.7 The advantage of abiological catalysts, however, is the availability of either enantiomer of the catalyst which enables one to synthesize either enantiomer of the target molecule. Today there is enormous emphasis on the design and development of efficient chiral catalysts for enantioselective synthesis and this field has become one of the most intense areas of organic chemical research.
In recent years, C2-symmetric chiral bis(oxazoline) ligand–metal complexes have received a great deal of attention through their use in various catalytic process.1c The bis(oxazoline) ligands are structurally related to C2-symmetric semicorrins pioneered by Pfaltz and co-workers.8a–c The inception of bis(oxazoline) ligands, however, added a new dimension in terms of flexibility in ligand design, convenient synthesis and availability of ligands in both enantiomeric forms. Since the early 1990s, many impressive enantioselective carbon–carbon bond forming reactions, aziridination reactions, hydrosilylations, oxidations and reductions have been recorded using bis(oxazoline)–metal complexes. The present review is intended to focus on the recent developments of bis(oxazoline) ligand–metal catalyzed asymmetric reactions and their applications in organic synthesis. The authors do not intend to provide an exhaustive review of this area since earlier developments have been reviewed by Pfaltz8a–c and Bolm.9 Applications of mono- and tris(oxazoline) ligand–metal complex catalyzed reactions are not included in this review.
2. C2-Symmetric bis(oxazoline) ligands
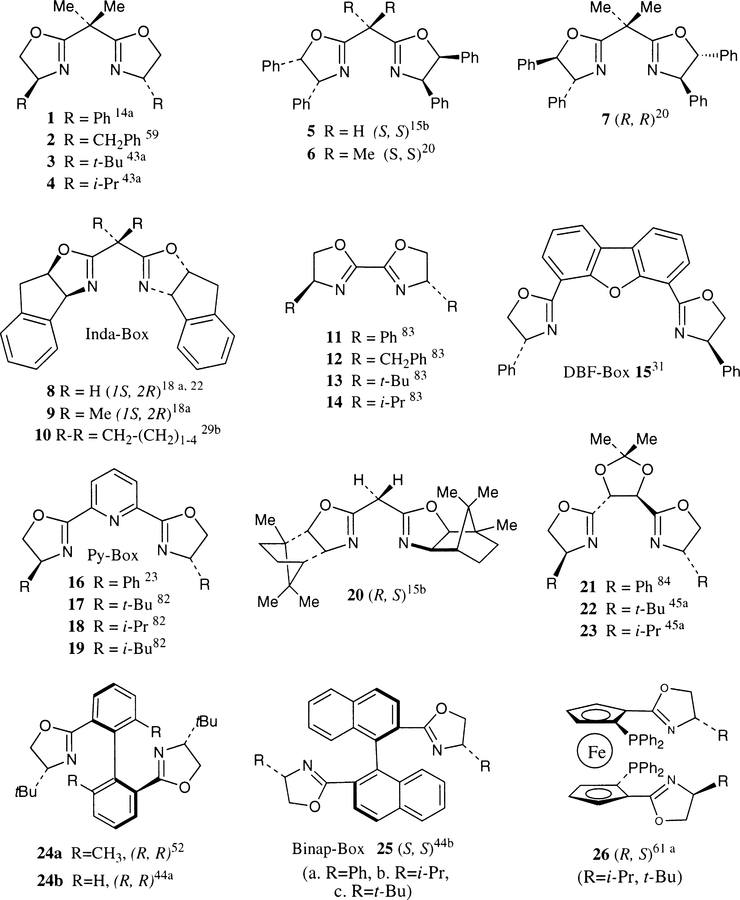
Chiral bis(oxazoline) ligands with a great deal of structural diversity have been introduced since 1989. Representative structural features of these novel ligands are shown in Fig. 1. In general, bis(oxazoline) ligands 1–10 with a one carbon spacer between the oxazoline rings are most frequently utilized. These ligands form a six membered metal chelate and the substituents on the ring are close to the metal center. These ligands were designed and applied for catalytic asymmetric allylic substitution (Pfaltz 199559), allylic oxidation (Pfaltz 1995,78 Andrus 199579), aziridination of olefins (Evans 199375), and imines (Jacobsen 199576), cyclopropanation (Masamune 1990,15 Pfaltz 1991,13 Evans 1991,43 Shibasaki 199653), Diels–Alder reaction (Corey 1991,14 Evans 1993,26 Ghosh 1996,22 Davies 1996,29 Desimoni 1996,30 Kanemasa and Curran 199731), hetero Diels–Alder reaction (Jorgensen 1995,32 Ghosh 199633), free radical addition (Porter 1995,66 Sibi 199668), Mukaiyama aldol reaction (Evans 199655), and nucleophilic addition reactions to aldehydes (Corey 199373) and imines (Denmark 199472). In a particular asymmetric process, both the choice of substituents on the ligand and the metal are critical to optimum enantioselectivity. This will be discussed in detail in the appropriate context. Ligands 11–14 which can form a five membered chelate were synthesized for hydrosilylation (Helmchen 199183) and transfer hydrogenation (Pfaltz 199113) reactions.
Fig. 1.
C2-Symmetric bis(oxazoline) ligands
Conformationally constrained ligand 20 was introduced by Masamune and co-workers for asymmetric cyclopropanation reactions.15 Ligands 21–23 containing a two carbon spacer which form a seven membered metal chelate have been designed for cyclopropanation reactions (Andersson 199645). Dibenzofurandiyl ligand 15 was utilized in the preparation of cationic aqua complexes for enantioselective Diels–Alder reactions.31 Tridentate bis(oxazolinyl) pyridine ligands 16–19 (py-box ligands) were designed for hydrosilylation reactions (Nishiyama 198982). Conceptually intriguing, ligands 24 and 25 were designed for catalytic cyclopropanation reactions (Corey 1995,52 Hayashi 199644b). In these ligands, two oxazoline rings are held in a nine-membered metal chelation. Finally, an interesting C2-symmetric bis(oxazolinyl) ferrocene ligand 26 has been designed for palladium catalyzed asymmetric allylic substitution reactions (Ikeda 199661a).
3. Bis(oxazoline)–metal complexes
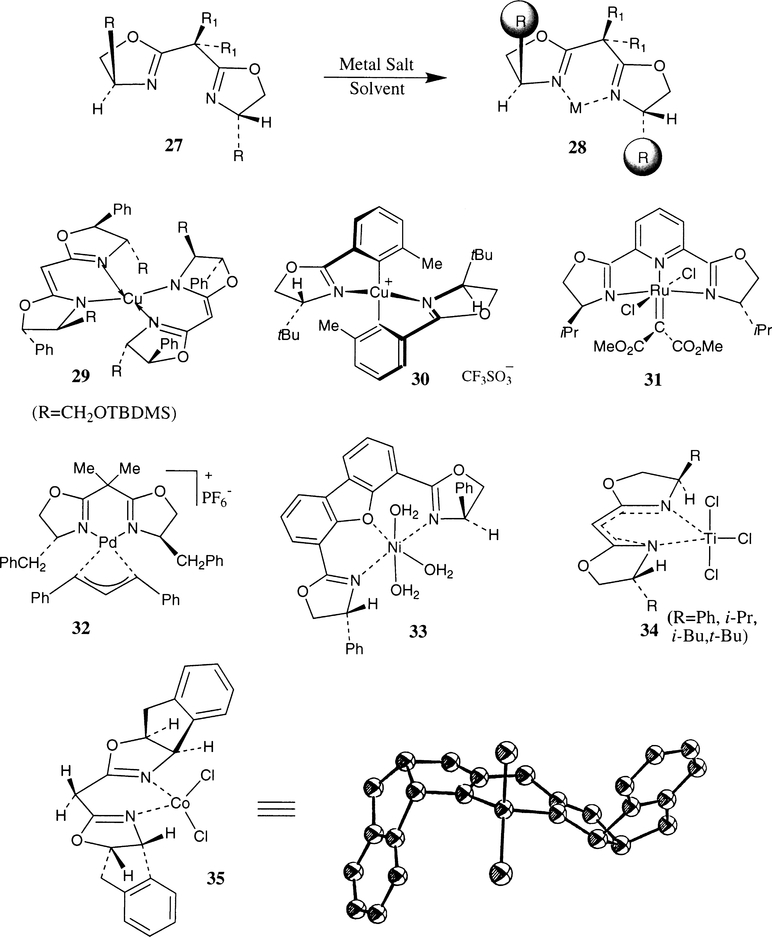
Chiral bis(oxazoline) ligand–metal complexes are efficient catalysts in numerous asymmetric reactions. The ligand–metal complexes are prepared in situ by mixing the corresponding metal salt and the bis(oxazoline) ligands prior to their use as chiral catalysts. The formation of monomeric or dimericcomplexes depends upon the reaction conditions, reactivity of the metal ions and the ligand structure. As shown in Fig. 2, a 1:1 mixture of bis(oxazoline) ligand 27 and the metal ion of choice is assumed to form a chelated metal complex 28. A number of features of the chiral bis(oxazoline) derived catalysts are noteworthy. The presence of a C2-symmetric axis in the bis(oxazoline) ligands minimizes the number of possible transition states in a particular reaction.10 The metal chelate is conformationally constrained and the chiral centers are located in close proximity to donor nitrogens thereby imposing a strong directing effect on the catalytic site. The chirality in the bis(oxazoline) ligand is derived from a wide variety of readily available optically active natural and unnatural aminoacids or acylic and cyclic aminoalcohols thus allowing optimization of ligand substituents with respect to a specific asymmetric process. The variation of the metal chelate ring size also provides an important handle to optimize the necessary ligand geometry at the metal center.
Fig. 2.
Bis(oxazoline)–metal complexes
Structural studies of the ligand–metal complexes often provide information regarding possible transition-state assembly. Already, a number of ligand–metal complex structures have been elucidated on the basis of X-ray diffraction analysis. Lehn et al. have determined the structure of bis(oxazoline)–Cu(II) complex 29 with a view to incorporating such ligands into an oligomeric species to form helicates with asymmetric induction.21 This ligand–metal complex is very similar to a semicorrin–Cu complex determined by Pfaltz et al.13 Various cyclopropanation catalysts prepared by Masamune et al. possess similar structures.15 Corey et al. developed bis(oxazoline)–Cu(I) complex 30 as an efficient cyclopropanation catalyst.52 The complex structure is stable and monomeric in nature and the structure was resolved by X-ray analysis. Nishiyama et al. have demonstrated that the carbene moiety of the py-box–Ru complex 31 can be transferred to styrene.46a Pfaltz et al. have proposed a mechanism for palladium catalyzed allylic substitution reactions based on catalyst structure 32.59 Cationic aqua complex 33 was recently developed by Kanemasa and Curran as an air stable catalyst for Diels–Alder reactions.31 The catalyst complex structures 31–33 were determined by X-ray studies.
Singh has reported the preparation of various titanium(IV) bis(oxazoline) complexes by treatment of a variety of bis(oxazoline) ligands with titanium(IV) reagents (TiX4, X=Cl, NEt2, iOPr) in toluene.24 The complexes 34 were studied spectroscopically and the structure of the box–Ti complexes have been proposed to adopt a trigonal bipyramidal geometry in which two nitrogen atoms of the box-ligand occupy the equatorial site. Cryoscopic determination revealed that the box–Ti complexes are monomeric in nitrobenzene. Attempted crystallization of the complexes resulted in the decomposition of the complexes. The authors have prepared ligand–metal complex 35 whose structure was determined by X-ray studies.25
4. Synthesis of bis(oxazoline) ligands
The syntheses of various racemic bis(oxazoline) derivatives were recorded in early 1970s. Witte and Seeliger prepared a number of 5-methyl substituted bis(oxazoline) derivatives with aromatic and cyclohexane scaffolding between the oxazoline rings.11 As outlined in Scheme 1, the synthesis of ligand 36 was carried out by condensation of the corresponding nitriles and the aminoalcohol in the presence of a substoichiometric amount of metal salt. Metal salts such as ZnCl2, ZnBr2, ZnI2, ZnSO4, Zn(OAc)2, Mn(OAc)2, Ni(OAc)2, Co(OAc)2 and Cd(OAc)2 were employed and found to be effective catalysts for the formation of bis(oxazoline) derivatives. In 1976, Butula and Karlovic prepared bis(oxazoline) derivatives in optically active and racemic form and investigated their use in catalytic hydrogenation and metal hydride reductions.12 As shown in Scheme 1, various bis(oxazoline) derivatives 39 were prepared by condensation of aminoalcohols with diethyl carboxylates to form the bis(hydroxy)amide derivatives 37. Treatment of these bis-amides with SOCl2 afforded the bis-chloride derivatives 38 which, upon exposure to base, furnished the bis(oxazoline) derivatives 39 in very good yield.
Scheme 1.
Since the late 1980s, numerous bis(oxazoline) ligands have been synthesized for use in metalcatalyzed asymmetric synthesis. The majority of the syntheses of bis(oxazoline) ligands followed a general synthetic route in which oxalic acid or the substituted malonic acids were first condensed with the corresponding optically active 1,2-aminoalcohol to form the bis(hydroxy)amide derivatives. The hydroxyl groups in the bis(hydroxy)amide were then activated and the resulting intermediate was cyclized to provide the bis(oxazoline) ligands. Activating agents such as SOCl2 (Corey,14a Pfaltz13), methanesulfonic acid for certain tertiary alcohols (Corey14b), Me2SnCl2 (Masamune15), ZnCl2 (Bolm16), diethylamino-sulfurtrifluoride (DAST) reagent (Knight17), triflic acid and BF3·OEt2 (Davies18) were employed. Denmark et al. have reported a series of 4-substituted bis(oxazoline) derivatives in which the hydroxyl groups were activated either with SOCl2 or by formation of the bis-mesylate followed by cyclization by treatment with aqueous or alcoholic base.19
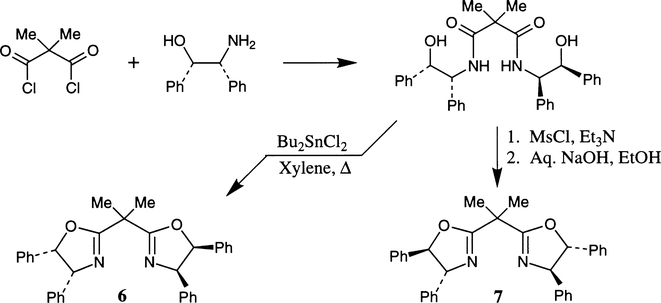
Desimoni et al. reported the synthesis of cis- and trans-4,5-disubstituted chiral bis(oxazoline) derivatives 6 and 7 from the same optically active 1,2-disubstituted aminoalcohol.20 As depicted in Scheme 2, the bis(hydroxy)amide was subjected to two different cyclization conditions to effect either retention or inversion of configuration at the C-5 position. Exposure of the bis(hydroxy)amide under the Masamune protocol (Bu2SnCl2, reflux) furnished the cis-1,2-disubstituted bis(oxazoline) 6 in 75% yield.15 On the other hand, formation of the bis-mesylate followed by treatment with base afforded the trans-1,2-disubstituted bis(oxazoline) 7 in 86% yield.
Scheme 2.
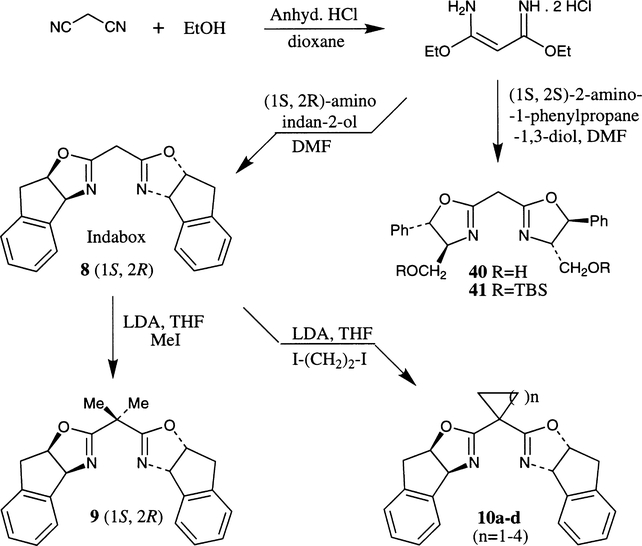
A convenient synthesis of bis(oxazoline) derivative 41 was described by Lehn and co-workers.21 As shown in Scheme 3, treatment of malononitrile with anhydrous HCl in ethanol afforded the corresponding imidate salt. Condensation of the imidate salt with optically active aminoalcohol furnished the bis(oxazoline) 40. Protection of the hydroxyl group under standard reaction conditions afforded the TBS–ether 41. Following this procedure, the authors have prepared conformationally constrained (1S,2R)-inda-box 8 and ent-8 in multigram quantities in 60–65% yield.22 Alkylation of inda-box 8 with lithium diisopropylamide and methyl iodide furnished the inda-box 9. On the other hand, alkylation with appropriate diiodoalkane provided convenient access to various spirocyclic inda-box 10.29b
Scheme 3.
Other constrained bis(oxazoline) ligands 9, 43 and 44 were prepared by Davies et al. by using a Rittertype reaction.18a As shown in Scheme 4, reaction of optically active 1S,2R-indandiol and dinitriles in the presence of trifluoromethanesulfonic acid afforded various bis(oxazoline) derivatives 9 and 43–44. The use of malonitrile with indanediol afforded the bis(oxazoline) 8 in 60% yield and the corresponding mono(oxazoline) in 10% yield. Alternatively, use of dimethyl malonitrile afforded bis(oxazoline) 9 in 30% yield and the major product was the mono(oxazoline) derivative 42.
Scheme 4.
The syntheses of various tridentate bis(oxazolinyl) pyridine ligands 16–19 were reported by Nishiyama and co-workers.23 As outlined in Scheme 5, pyridine-2,6-dicarboxylic acid chloride was first converted to the corresponding bis(hydroxy)amide 45. Treatment of bis(hydroxy)amide 45 with SOCl2 afforded the corresponding chloroamide which was then cyclized with aqueous NaOH. Recently, Davies reported the cyclization of bis(hydroxy)amide by treatment with BF3·OEt2 at 120°C to form various py-box ligands in good yields.18b Ligands ent-16 and ent-19 were obtained in 62% and 75% yields respectively. This protocol is also convenient for the synthesis of constrained py-box ligands derived from cyclic cisaminoalcohol with retention of configuration. For example, py-box ligand 46 was prepared in 70% yield.
Scheme 5.
Structurally intriguing bis(oxazoline) ligand 15, constructed on a dibenzofuran platform, was synthesized by Kanemasa and Curran.31 As shown in Scheme 6, dibenzofuran was converted to dicarboxylic acid 47 by lithiation with nBuLi and followed by carboxylation of the resulting dianion with dry ice. Formation of the acid chloride with SOCl2 and subsequent reaction with (R)-2-amino-2-phenylethanol provided the bis-amide 48. Treatment of 48 with SOCl2 followed by aqueous NaOH solution afforded (R,R)- 4,6-dibenzofurandiyl-2,2″-bis(4-phenyloxazoline) (DBF-box) 15 in 28% overall yield.
Scheme 6.
5. Carbon–carbon bond forming reactions
Since the early 1990s, bis(oxazoline)–metal complexes have been increasingly utilized in catalytic asymmetric carbon–carbon bond forming reactions. Undoubtedly, this area has become one of the most active areas of research. As a result, numerous methodologies have been developed for enantioselective carbon–carbon bond formation and, in some areas, the level of enantioselectivity and catalytic efficiency is approaching that of enzymatic reactions.2–7 The versatility of bis(oxazoline) ligands has been already documented in the following catalytic asymmetric carbon–carbon bond forming reactions.
5.1. Diels–Alder reactions
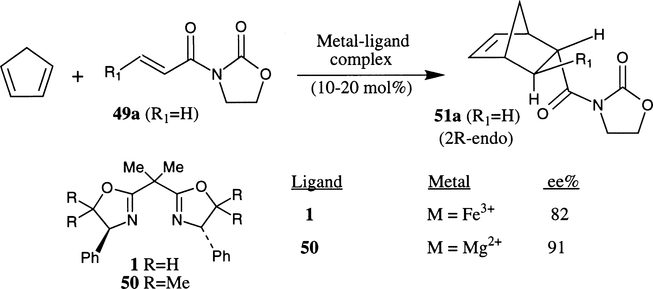
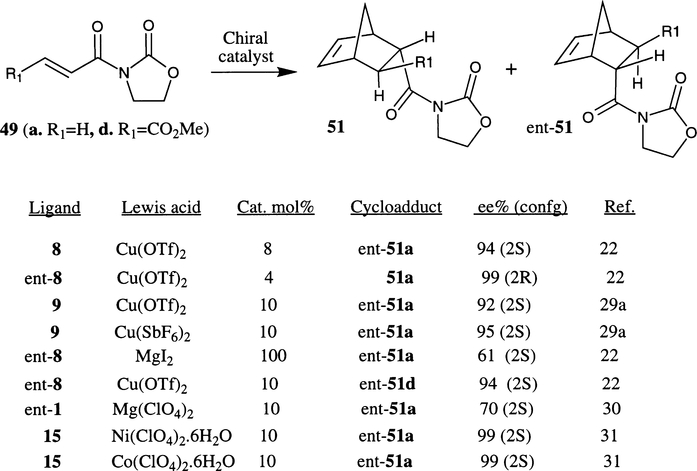
In 1991, Corey et al. first demonstrated the remarkable potential of bis(oxazoline)–metal complexes in enantioselective Diels–Alder reactions.14a Various ligand–metal complexes were prepared by stirring anhydrous FeCl2 or FeI2 with phenyl bis(oxazoline) ligand (phe-box) 1 in CH3CN followed by treatment with an appropriate amount of iodine at 23°C. As shown in Scheme 7, the reaction of the 10 mol% active complex (ligand 1·FeI3) with N-acryloyl oxazolidinone 49a and cyclopentadiene afforded the endo cycloadduct 51a selectively (endo:exo ratio 96:4) in 95% yield and endo enantioselectivity was assessed to be 82% ee (2R configuration). The stereochemical outcome of the 1·FeX2+ (X=Cl, I)-complex catalyzed reaction was rationalized by assuming the s-cis conformation of the dienophile 49 which chelates on the octahedral metal center at the a1–e1 site of the model A prior to reaction with cyclopentadiene from the Si-face of the dienophile (Fig. 3).
Scheme 7.
Fig. 3.
Subsequently, Corey and Ishihara designed a catalytic system in which the reaction was expected to proceed via a transition state with a tetrahedral geometry rather than an octahedral geometry for the metal complex as proposed for the iron system.14b In this context, metal–ligand complexes of weak Lewis acid such as MgX2 have been demonstrated to be efficient catalysts for the Diels–Alder cycloaddition process. Conformationally rigid bis(oxazoline) ligand 50 was prepared and the use of 20 mol% of Mg–bis(oxazoline) complex (with ligand 50) led to the cycloadduct with very high endo:exo selectivity (98:2) in 91% ee (2R) for the endo isomer. The Mg complex prepared either from MgI2 and a co-catalyst of one equivalent of I2 or two equivalents of AgSbF6 afforded the same level of enantioselectivity (91%). The reaction is believed to proceed through a tetrahedral metal complex such as B in which endo-Si-face attack provided the 2R-endo isomer as shown in Fig. 3.
Evans et al. have demonstrated that the ligand–metal complexes derived from bis(oxazoline) ligand (bu-box) 3 and mild Lewis acid such as Cu(OTf)2 are also very efficient chiral catalysts for the Diels–Alder reaction with cyclopentadiene and substituted acrylimide derivatives.26a Among various ligands examined, the bu-box 3 ligand consistently provided a very high level of endo/exo selectivity as well as endo enantioselectivity (ent-51, 90–98% ee with 5–10 mol% catalyst) and isolated yield (82–92%) with a number of substituted dienophiles (Scheme 8). The Cu(II) complexes of phe-box 1 and ipro-box 4 are not effective catalysts as they have shown considerably lower enantioselectivity (30 and 58% ee respectively). In the bu-box–3·Cu(OTf)2 system, the asymmetric induction is postulated to arise from square-planar geometry C to account for >98% endo enantioselectivity with 2S-configuration with dienophile 49a.
Scheme 8.
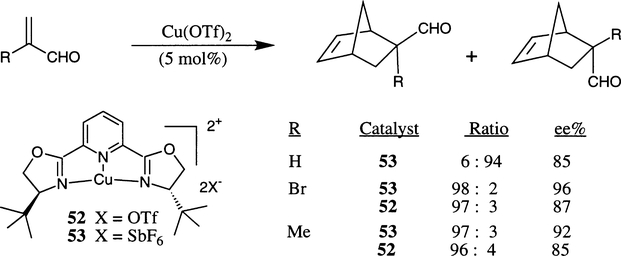
The Cu(II)–py-box complexes have also been shown to be efficient chiral catalysts for cycloaddition of substituted acrolein derivatives with cyclopentadiene as well as β-substituted acrylimide with a variety of dienes.26b,c Dienes such as 1,3-cyclohexadiene and isoprene also undergo cycloaddition with acrylimide derivatives at a high level of enantioselection (Scheme 9). An intriguing feature of the Cu(II)–bis(oxazoline) complexes is that the Cu(II)-derived chiral Lewis acids have shown pronounced counterion effects. The py-box·Cu(SbF6)2 complex 53 has shown superior results to py-box–Cu(OTf)2 52 in Diels–Alder reactions with acrolein derivatives. The bu-box–Cu(SbF6)2 55 complex (as shown in Scheme 10) has also exhibited similar results.
Scheme 9.
Scheme 10.
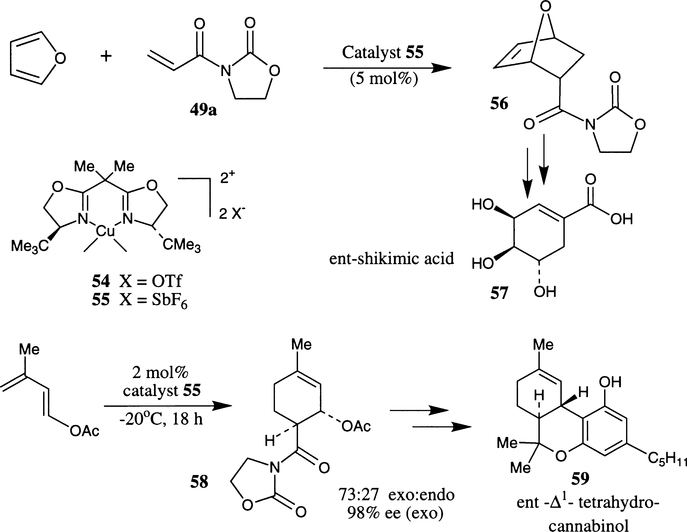
The usefulness of Cu(II)–bis(oxazoline) complexes has been further demonstrated in enantioselective Diels–Alder reactions of N-acryloyl oxazolidinone 49a with furan and 1-acetoxy-3-methyl butadiene as dienes.27a,b The corresponding cycloadducts 56 and 58 were obtained in 97% and 98% ee respectively.27a,b These cycloadducts were converted to ent-shikimic acid 57 and ent-Δ1-tetrahydrocannabinol 59 (Scheme 10) using standard synthetic sequences.
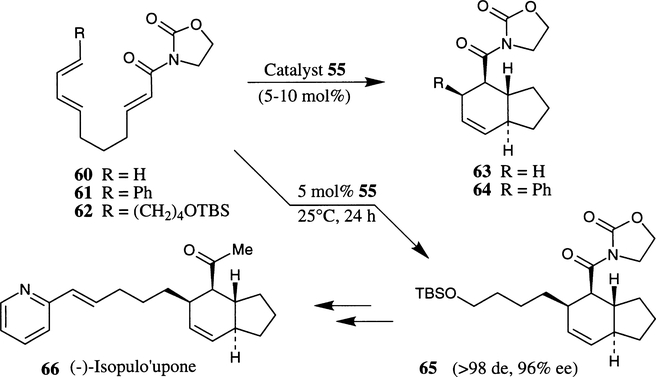
The Cu(II)–bis(oxazoline) complex 55 was also shown to be very effective in enantioselective intramolecular Diels–Alder cycloaddition processes.28 For example, an intramolecular Diels–Alder reaction of 60 with 10 mol% bu-box–3·Cu(OTf)2 complex 54 at room temperature for 5 days afforded the cycloadduct in less than 20% yield. However, cycloaddition of 60 with hexafluoroantimonate complex 55 provided 63 in 89% yield and 86% ee after 24 hours reaction time. Substituted trienimide 61 with 5 mol% 55 furnished 64 as a single diastereomer within 5 h at 25°C in 86% yield and 92% ee (Scheme 11). Similarly, trienimide 62 afforded a single cycloadduct 65 in 96% ee. Cycloadduct 65 has been converted to (−)-isopulo’upone 66 in a number of synthetic steps.
Scheme 11.
The Diels–Alder reaction of cyclopentadiene and substituted α,β-unsaturated N-oxazolidinones in the presence of ligand–metal complexes derived from conformationally constrained inda-box ligands 9 and 10 were investigated by Ghosh et al. and Davies et al. independently.22,29 As shown in Scheme 12, Cu(OTf)2 and inda-box complexes have shown excellent enantioselectivity (92–99% ee) with Nacrolyloxazolidinone 49 compared to phe-box ligand 1 (30% ee) as reported by Evans and co-workers.26a Substitutions in the bis(oxazoline) scaffolds have exhibited interesting effects in enantioselectivity. For example, 8·Cu(OTf)2 and ent-8·Cu(OTf)2 complexes (8 mol% catalyst) have provided 94% ee and 99% ee (4 mol% catalyst) respectively in the Diels–Alder reaction with dienophile 49a.22 In comparison, 9·Cu(OTf)2 complex afforded the corresponding cycloadduct in 92% ee.29a Consistent with Evans’ finding, the use of a catalyst derived from Cu(SbF6)2 furnished slightly higher enantioselectivity (95% ee) with ligand 9.26,29a
Scheme 12.
The bis(oxazoline)–Cu(II) complexes derived from 8 or ent-8 are also very effective in the cycloaddition of N-crotonyl and monoethyl fumaroyl oxazolidinones. The corresponding cycloadducts were obtained in excellent endo diastereoselectivities, endo-enantioselectivities (ent-51d, 94% ee) and isolated yields.22 The authors have further demonstrated that the Mg–bis(oxazoline) complexes consistently effect dramatic reversal in stereoselectivity (up to 61% ee) with respect to Cu(II)–ligand catalyzed reactions. As shown in Fig. 4, the reversal of enantioselectivity is attributed to a Corey–Ishihara type model in which the Mg(II) complex assumes a tetrahedral geometry B rather than a square planar geometry A that is most probable for Cu(II).14b
Fig. 4.
Desimoni et al. reported that the cycloaddition of N-acryloyl oxazolidinone with cyclopentadiene in the presence of Mg(ClO4)2–bis(oxazoline) complexes affords the adduct in 68–70% ee of the 2S isomer.30 On the other hand, addition of two moles of water to the catalyst caused reversal in the stereoselection leading to 59–65% ee in the 2R enantiomer. The reversal in stereoselection was explained by invoking different transition state geometries of the catalyst. In the absence of water molecules, Mg–bis(oxazoline) adopts a tetrahedral geometry and leads to the 2S enantiomer; but the addition of water increases the coordination number so that the catalyst adopts an octahedral geometry thus resulting in the 2R enantiomer. Kanemasa et al. recently reported cationic aqua complexes derived from DBF-box 15 and various metal perchlorate hydrates.31 Metal–ligand complexes of Co(ClO4)·6H2O and Ni(ClO4)·6H2O afforded excellent endo selectivities (>98%) and virtually complete endo enantioselectivities (>99% ee). Even with 2 mol% catalyst ent-51a was formed in 96% ee.
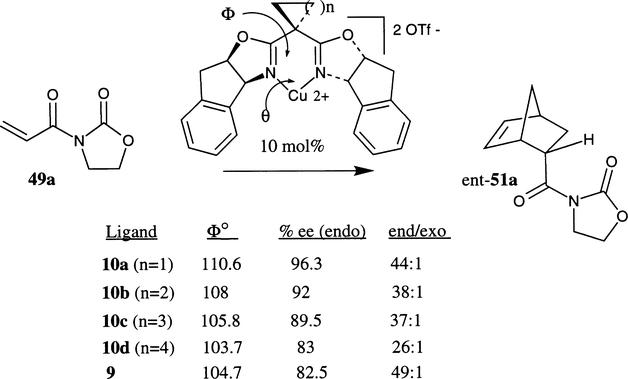
The influence of the bite angle in enantioselectivity has been studied by Davies et al. using spirocyclic bis(oxazoline) ligands (Scheme 13).29b The synthesis of such ligands is outlined in Scheme 3. In various spiro bis(oxazoline)–Cu(II) complex catalyzed Diels–Alder reactions, it has been shown that a larger bite angle in the ligand affords higher endo/exo selectivities as well as endo enantioselectivities of the cycloadduct ent-51a. Spiro inda-box 10a (cyclopropane substituent, Φ=110.6°) derived copper(II) complex has provided substantially higher enantioselectivity in the cycloadduct (ent-51a, 96.3% ee) compared to substituted bis(oxazoline) 9 (Φ=104.7°) and Cu(OTf)2 catalyzed reaction which has afforded the cycloadduct (ent-51a) in 82% ee.
Scheme 13.
5.2. Hetero Diels–Alder/ene reactions
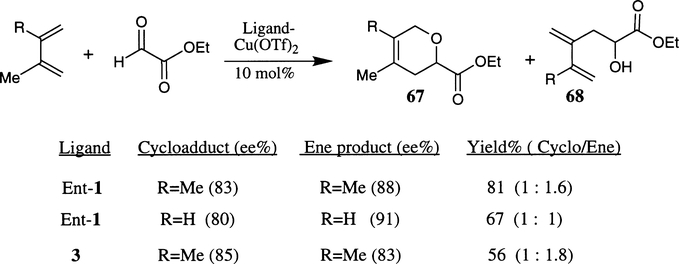
Jorgensen reported that Cu(II)–bis(oxazoline) complex catalyzed the hetero Diels–Alder reaction of alkyl glyoxalate esters and 1,3-dienes provided both the hetero Diels–Alder cycloadduct 67 (60–95% ee) as well as the ene product 68 (83–94% ee) in high enantioselectivities and generally good overall yields (Scheme 14).32 For example, reaction of 2-methyl-1,3-butadiene and ethyl glyoxalate in the presence of 10 mol% ent−1·Cu(OTf)2 complex afforded a 1:1 mixture of the hetero Diels–Alder cycloadduct and the ene product in 80% and 91% ee, respectively. The reaction with 2,3-dimethyl-1,3-butadiene, on the other hand, provided a mixture ratio of 1:1.6 and high enantioselectivities. Catalyst complex 3·Cu(OTf)2 also provided similar enantioselectivity but the combined yields were considerably lower. In general, the ene reaction product was obtained as a major product.
Scheme 14.
Furthermore, reaction of 1,3-cyclohexadiene with 2 mol% ligand ent-1–Cu(II) complex afforded the bicyclic endo product 69 exclusively in 60% ee in 72% yield (Scheme 15). However, a catalyst derived from bu-box 3 in CH3NO2 solvent afforded the cycloadduct 69 in 97% ee.32b It should be noted that the complexes of ent-1·Cu(OTf) and 3·Cu(OTf)2 resulted in the same endo cycloadduct enantioselectively. Saponification followed by acidification with aqueous HCl provided the enantiopure (>99.8 ee) rearrangement product 70, which is a useful synthon for natural product synthesis.
Scheme 15.
The authors have demonstrated that the chiral bis(oxazoline)–metal complexes are effective catalysts for hetero Diels–Alder reactions of glyoxalate esters and Danishefsky’s diene to provide the dihydropy-ranone derivatives enantioselectively (Scheme 16).33a Both the Mukaiyama aldol product 71 as well as the hetero Diels–Alder product 72 were isolated after the reaction. However, treatment of the mixture with trifluoroacetic acid provided only the hetero Diels–Alder product enantioselectively in good yields. Of the various metal–bis(oxazoline) complexes investigated, ent-8·Cu(OTf)2 complex has provided the optimum results with ethyl glyoxalate. Dihydropyranone 72a (R=CO2Et) was obtained in 72% ee. Interestingly, phe-box 1 and bu-box 3 derived catalysts have shown considerably lower enantioselectivity (44% and 17% ee, respectively). Furthermore, the authors have demonstrated that the chelating, bidentate aldehydes such as dithiane carboxaldehyde and benzyloxy acetaldehyde are very effective in providing the corresponding dihydropyranones 72b (R=1,3-dithiane) and 72c (R=CH2OBn) in 81 and 85% ee, respectively,33b Corey et al. and Keck et al. have also developed convenient enantioselective protocol for the synthesis of similar dihydropyranones.34,35
Scheme 16.
The synthetic utility of the dihydropyranone building blocks has been amply established by Danishefsky and coworkers.36 Dihydropyranones 72a–c serve as potential key intermediates for a variety of biologically important molecules.37 As outlined in Scheme 17, the authors have converted 72c via 73 to the C3–C14 segment 74 of antitumor macrolide laulimalide 75 utilizing a Ferrier rearrangement and asymmetric conjugate addition as the key steps to set the C-9 and C-11 stereochemistry.33b The above dihydropyranones have also been employed in the synthesis of high-affinity nonpeptidal ligands for the HIV-1 protease substrate binding site.38
Scheme 17.
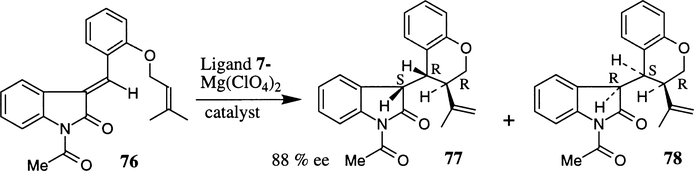
Competitive hetero Diels–Alder and an intramolecular ene reaction was investigated (Scheme 18) using a bis(oxazoline)–Mg(ClO4)2 complex with (E)-N-acetyl-3-arylideneindolin-2-one derivatives 76.39 The ligand–metal complexes of both cis and trans-1,2-disubstituted as well as mono-substituted bis(oxazoline) ligands were examined. It was found that the catalyst derived from cis-disubstituted bis(oxazoline) ligand 6 was less chemo- and enantioselective than the trans-ligand 7. The ligand–metal complex of transbis(oxazoline) 7 and Mg(ClO4)2 afforded the intramolecular ene product 77 as the major product at room temperature and the enantioselectivity was determined to be 88% ee. The cis-bis(oxazoline) 6-derived catalyst also provided 77 as the major product; however the enantioselectivity was substantially lower (51% ee).
Scheme 18.
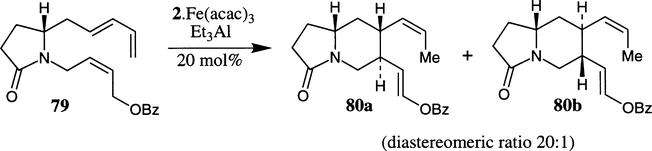
Takacs et al. reported a Fe–bis(oxazoline) complex mediated ene-carbocyclization of trienes to the corresponding cyclic lactam with high diastereoselectivity and isolated yield.40 As depicted in Scheme 19, treatment of (±)-lactam 79 with 20 mol% Fe–bis(oxazoline) complex derived from 0.2 equivalent of Fe(acac)3, 3 equivalents of Et3Al and 1 equivalent of bis(oxazoline) 2 afforded the cyclized product 80a diastereoselectively (selectivity 80a:80b >20:1). However, the cyclization product 80a was obtained as a racemic mixture. Using this carbocyclization strategy, the alkaloid (−)-protoemetinol has been synthesized.40b
Scheme 19.
5.3. 1,3-Dipolar cycloaddition
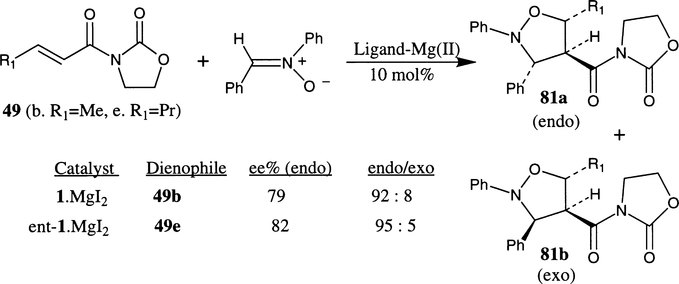
Complexes derived from magnesium(II) and chiral bis(oxazoline) ligands have been shown (Scheme 20) to catalyze the 1,3-dipolar cycloaddition of nitrone with an α,β-unsaturated oxazolidinone with high endo selectivity as well as endo enantioselectivity.41 The chiral catalysts were prepared from MgI2, I2 and ligands 1 and 3. Reaction of crotonoyl oxazolidinone 49b (R1=Me) and benzylidenephenylamine N-oxide in the presence of phe-box 1 derived catalyst (10 mol%) at room temperature afforded endo cycloadduct 81a selectively (92:8 ratio) in 72% yield after 24 hours and endo enantioselectivity was shown to be 79%. The reaction of hexenoyloxazolidinone 49e (R1=EtCH2) with the ent-1 ligand-derived catalyst is significantly slower than the alkene 49b. After 14 days >95% conversion to product 81 was achieved. The cycloadduction reaction has exhibited excellent endo/exo selectivity as well as good endo-enantioselectivity (82% ee).
Scheme 20.
5.4. [2+2] Photochemical cycloaddition
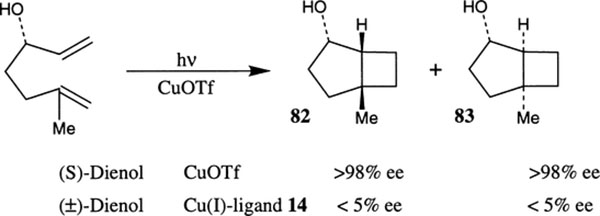
Intramolecular [2+2] photocycloaddition of dienes has been investigated with chiral bis(oxazoline) and CuOTf derived catalysts (Scheme 21).42 Optically active (S)-hydroxy 1,6-diene undergoes smooth photocycloaddition in the presence of CuOTf to provide the bicyclo[3.2.0] heptane derivatives 82 and 83 in >98% ee. The same reaction was also investigated with racemic dienol in the presence of 14·CuOTf complex. Interestingly, however, the reaction time is considerably increased and the enantioselectivity of the cycloadducts was shown to be less than 5%. Furthermore, it has been shown that if the metal/ligand ratio exceeds two, no cycloaddition reaction takes place. The low enantioselectivity is due to low reactivity of the chiral copper complex compared to copper coordinated to solvent molecules. One possible explanation put forward for the low reactivity of the chiral-Cu(I) complex was that a charge transfer process between the chiral ligand and the copper occurred after excitation instead of between the copper ion and the reacting olefin.42
Scheme 21.
5.5. Cyclopropanation reactions
5.5.1. Intermolecular cyclopropanations
Pfaltz et al. have first demonstrated that the C2-symmetric chiral semicorrin–Cu(I) complexes are excellent catalysts for enantioselective cyclopropanation of olefins with α-diazo esters.8,13 Depending upon the substituents on the semicorrin and the choice of diazo compound, high enantioselectivities (92 to 97% ee) were observed. One major drawback of the semicorrin ligands is that the preparation of the ligands involves multistep synthesis and only one enantiomer is generally available.8b However, the C2-symmetric bis(oxazoline) ligands can be prepared in both enantiomeric forms and the synthesis is straightforward. Enantioselective cyclopropanation reactions with various metal salts and chiral bis(oxazoline) ligand derived catalysts were independently investigated by a number of research groups.13,15,43,46,52,53 As a result of their efforts, Cu–bis(oxazoline) and Ru–py-box complexes have emerged as excellent catalysts for enantioselective cyclopropanation of olefins.
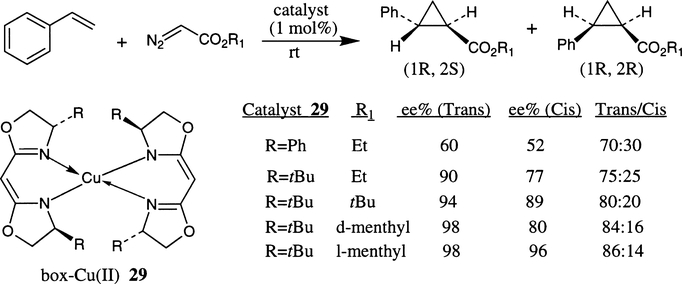
In 1990, Masamune et al. demonstrated the catalytic potential of bis(oxazoline)–copper(II) complexes 29 in enantioselective cyclopropanation of a variety of olefins.15 The bis(oxazoline) ligands were designed based on the semicorrin platform pioneered by Pfaltz and co-workers.8b The bis(oxazoline)–Cu(II) complexes 29 were prepared by reaction of 1 equivalent of CuCl2 with 2 equivalents of lithio-bis(oxazoline) derivative obtained after deprotonation of the corresponding bis(oxazoline) ligand with nBuLi in THF. The active Cu(I)-catalysts were obtained by reduction of the complexes with phenylhydrazine prior to the cyclopropanation reaction. As shown in Scheme 22, cyclopropanation of a model olefin, styrene, with ethyl diazoacetate proceeded at room temperature with excellent enantioselectivity for the trans-isomer using the complex 29 (R=tBu) as a catalyst. The trans/cis diastereoselectivity, as well as enantioselectivity were further improved when (−)-menthyl diazoacetate was used. An excellent level of enantioselectivity was shown with mono-substituted, trans-disubstituted and terminal-disubstituted olefins.
Scheme 22.
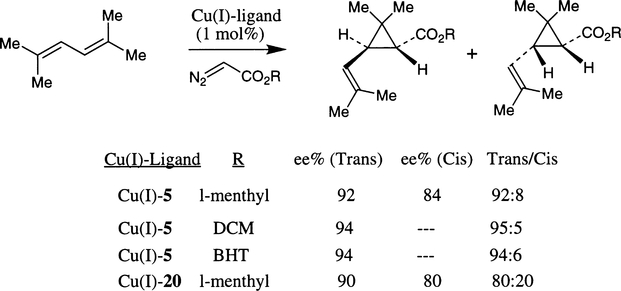
For trisubstituted and unsymmetrical cis-1,2-disubstituted olefins, catalysts derived from ligands 5 and 20 provided excellent results and the utility of this methodology was demonstrated through the synthesis of trans-chrysanthemic acid (Scheme 23).15b The sterically hindered diazoacetates, such as 2,6-di-tert-butyl-p-tolyl (BHT) and dicyclohexylmethyl (DCM) diazoacetate have exhibited the best results. However, the DCM-ester is more convenient to use since it can be removed under standard hydrolytic conditions unlike the BHT-ester.
Scheme 23.
Evans and co-workers have shown that the bis(oxazoline) ligands and Cu(OTf) complexes are excellent catalysts for enantioselective cyclopropanation of mono- and 1,1-disubstituted olefins.43 Unlike the Masamune conditions, the active catalyst was prepared by treatment with neutral bis(oxazoline) ligands and CuOTf. The cyclopropanation was carried out at ambient temperature and almost complete enantioselectivity (>99% ee) was observed with the bu-box–3 derived catalyst. Cyclopropanation of 1,1-disubstituted olefins with ethyl diazoacetate also proceeded with almost complete enantioselectivity.43a
The ligand–metal complex of CuOTf and bis(oxazoline) ligands 24b and 25 on biphenyl and binaphthyl backbones, respectively were also shown to catalyze the intermolecular cyclopropanation reactions (Scheme 24).44a,b The reactions of α-diazoesters with styrene afforded the corresponding cyclopropane in moderate to high ee. The ligand 25 is more effective in providing better enantioselectivities than ligand 24b. The use of 2 mol% catalyst derived from ligand 25 afforded the cyclopropane derivatives up to 97% ee.44b Bis(oxazoline) ligands 21–23 on a tartaric acid backbone have been synthesized and their complexes with Cu(OTf) have also furnished good to excellent enantioselectivity in the cyclopropanation of styrene.17,45 In general, the bulkier diazoesters have shown improved enantioselectivities as well as cis/trans diastereoselectivities.
Scheme 24.
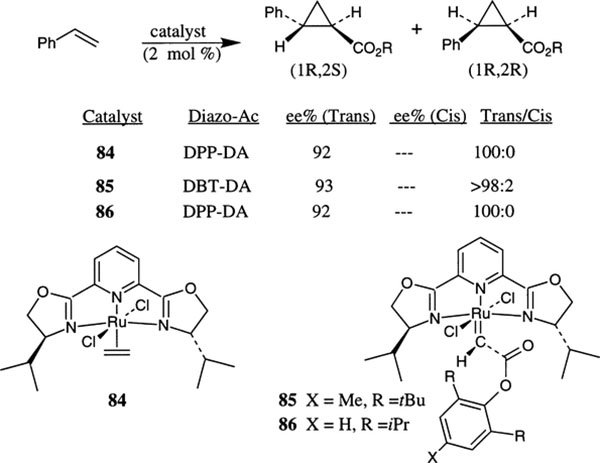
Nishiyama et al. have shown that chiral ruthenium(II)–py-box complexes are excellent catalysts for the enantioselective asymmetric cyclopropanation of styrene with several diazoacetates.46 The py-box ligand 18-derived catalyst was prepared by reaction of 18 with the RuCl2(p-cymene) complex in the presence of an ethylene atmosphere (Scheme 25). The resulting complex 84 was purified by silica gel chromatography at 0°C using CH2Cl2 and MeOH as the solvent system. The reaction of 84 with 2,6-ditert-butyltolyl diazoacetate (DBT-DA) and 2,6-di-isopropylphenyl diazoacetate (DPP-DA) in benzene at 50°C provided the stable complexed 85 and 86, respectively. The reaction of 85 or 86 with styrene under stoichiometric conditions proceeded at 60°C affording the trans-isomer exclusively in 55% and 97% ee, respectively (80–82% yield). The cyclopropanation reaction under catalytic conditions (2 mol% catalyst) provided 92–93% ee with styrene. The Ru–py-box complexes 84 and 86 are excellent catalysts for enantioselective cyclopropanation of styrene and a variety of other olefins. For example, 1-heptene, 1,1-diphenylethylene and 4-methyl-1,3-pentadienes provided the corresponding cyclopropanes in 99%, 68% and 98% ee, respectively. Isolation of the carbene complex 86 and its ability to catalyze cyclopropanation reactions with diazoacetate provided mechanistic evidence that the reaction proceeds through the carbene via asymmetric carbene transfer to olefin.
Scheme 25.
Unlike Ru–py-box complexes, the Cu–py-box complexes have shown very poor enantioselectivity (maximum of 35% ee for the trans-cyclopropanation product) in the cyclopropanation of styrene with ethyl and tert-butyl diazoesters.47 Although the cis/trans diastereoselectivity is very poor, the reaction yields were excellent. The py-box–copper complex has also been shown to catalyze the [4+2] cycloaddition process but no enantioselection was observed.47
Cyclopropanation of both chiral and achiral enol ethers was examined with the 3·CuOTf-derived complex (Scheme 26).48 Treatment of a glucose derived enol ether 87 with diazomethyl acetate in the presence of 3·CuOTf complex at 20°C afforded the cyclopropanation product 88 (74% yield) with excellent cis/trans diastereomeric ratio but moderate trans-enantioselectivity. The use of bulky t-butyl diazoacetate gave similar results but in reduced yield (32%). The mannose derived enol ether 89 furnished 90 with a similar level of trans-diastereoselectivity. The use of Cu(acac)2 as a catalyst gave a 83:17 mixture of trans:cis diastereomer but very poor enantioselectivity for the trans isomer. The achiral enol ethers (tert-butyl, isopropyl and trimethylsilyl) afforded the corresponding cyclopropane derivatives in moderate yields (39–54%) and high trans:cis selectivity (>97:3) but the trans-enantioselectivity was poor (32–49% ee).
Scheme 26.
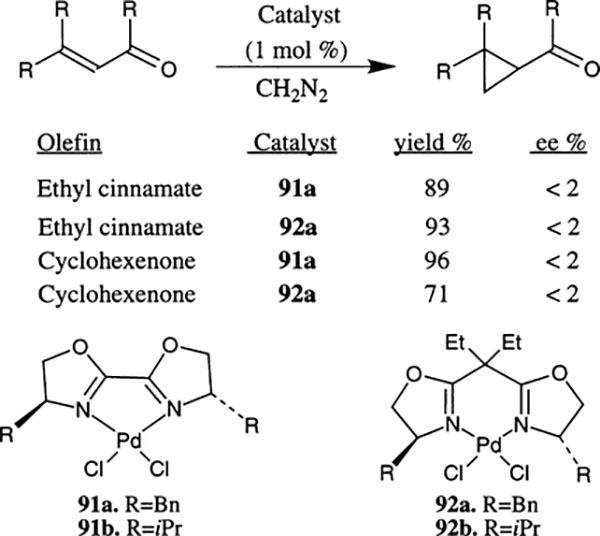
Recently, Denmark et al. reported the chiral bis(oxazoline)–Pd(II)-catalyzed cyclopropanation of electron-deficient olefins with diazomethane (Scheme 27).49 The reaction of a number of α,β-unsaturated carbonyl compounds with 2–4 equivalents of diazomethane at 0°C in the presence of 1 mol% Pdbox complexes 91 derived from ligands 12 and 14 afforded excellent yields of the corresponding cyclopropane derivatives. However, in all cases, the product was obtained in a racemic form. The Pdbox complexes derived from substituted methylene box-ligands 92 also provided an excellent yield of racemic cyclopropanes. In a control experiment it has been demonstrated that diazomethane alone did not provide cyclopropane at 0°C after several hours. The lack of enantioselectivity is possibly due to partial or complete ligand dissociation from the palladium, taking place prior to the cyclopropanation reaction.
Scheme 27.
5.5.2. Intramolecular cyclopropanations
Koskinen and Hassila investigated intramolecular cyclopropanation of allyl diazomalonate esters catalyzed by 93·CuOTf-derived complexes (Scheme 28).50 The reaction proceeded at 65°C affording the corresponding cyclopropanolactone derivative 94 in 72–73% yield. However, the enantioselectivity of the cyclopropanation products was very low (~32% ee). Control studies have shown that below 65°C, the carbene complex decomposed very slowly and increasing amounts of side products were formed. Higher reaction temperatures (85°C) resulted in the completion of the reaction in 3 hours but the enantioselectivity dropped to 15% ee (R=tBu).
Scheme 28.
Pfaltz et al. have examined the intramolecular cyclopropanation of various diazoketones with ligand–metal complexes of 93b (R=tBu) and CuOTf as well as aza-semicorrin derived complex 95 (Scheme 29).51 The bis(oxazoline) derived catalysts exhibited considerably lower ee (77% ee) compared to the aza-semicorrin derived catalyst 95 (93% ee) in the process. Intramolecular cyclopropanation of a number of substituted diazoketones has also been examined. However, observed enantioselectivities were much lower than with unsubstituted diazoketones.
Scheme 29.
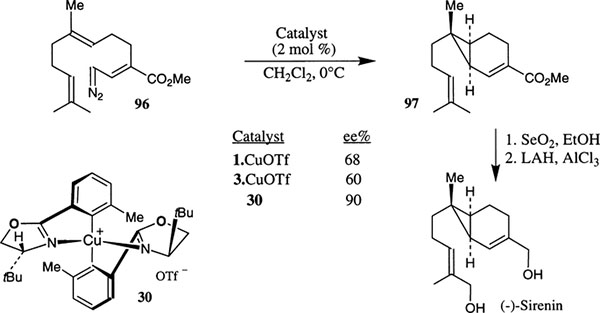
During the enantioselective synthesis of the chemotactic factor (−)-sirenin, Corey et al. developed a highly effective chiral catalyst for the enantioselective intramolecular cyclopropanation of the diazo ester 96 (Scheme 30).52 The catalyst was prepared from optically active biaryl bis(oxazoline) ligand 24b and CuOTf. Unlike other Cu–bis(oxazoline) complexes, the ligand–metal complex 30 is stable in air as well as in solution. The monomeric catalyst structure was determined by X-ray crystallographic studies and the N–Cu–N angle was determined to be 134° in the solid state. The unusual stability of the complex is attributed to the 134° N–Cu–N angle in a 9-membered chelate. Evidently, the monomeric triflate ion pair is the active component of the catalyst. As shown, treatment of diazo ester 96 with 2 mol% of catalyst 30 in CH2Cl2 at 0°C for 3 h afforded the bicyclic ester 97 in 90% ee. Enantiomerically pure 97 was obtained in 77% yield after purification on a preparative Chiracel OD column. A variety of other known bis(oxazoline)–Cu complexes were examined for this cycloaddition process however, much lower enantioselectivity was observed (4–68%). The bicyclic cycloadduct 97 was converted into optically active (−)-sirenin in a two step sequence.
Scheme 30.
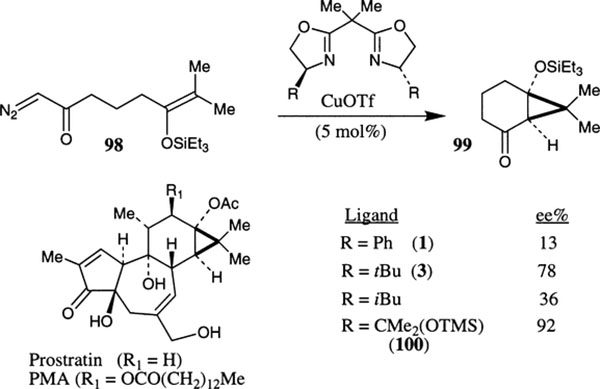
Shibasaki and co-workers have constructed the CD ring skeleton of prostratin via intramolecular cyclopropanation of silyl enol ether 98 using Cu(I)–bis(oxazoline) complexes (Scheme 31).53 The catalysts derived from CuOTf and phe-box 1 and bu-box 3 ligands afforded the cyclopropane derivative 99 in 13% and 78% ee, respectively. The bis(oxazoline) ligand 100 derived from L-serine furnished the cycloadduct in 92% ee and a 70% isolated yield.
Scheme 31.
An interesting enantioselective C–H insertion reaction of diazoesters has been investigated in which a high throughput catalyst screening was utilized (Scheme 32).54 In this screening process, ninety six potential catalytic systems with numerous metal salts and a variety of ligands including chiral bis(oxazoline) ligands ent-1, 5 and 18 were examined. The best diastereoselectivity and yields were obtained with a catalyst derived from CuOTf and ent-1. Also, a comparable level of diastereoselectivity was observed with ent-1·AgSbF6-derived complex which is unique and unprecedented in insertion reactions. Influence of the solvent on diastereoselectivity has also been studied and THF was found to be a better solvent than CHCl3.
Scheme 32.
5.6. Mukaiyama aldol reactions
Mukaiyama aldol reactions of silylketene acetals and benzyloxy acetaldehyde catalyzed by chiral bis(oxazoline)–Cu(II)-derived complexes have been investigated by Evans and co-workers.55a Among various bis(oxazoline)–Cu(II) complexes (54, 55, 101 and 102) examined, py-box·Cu(II) hexafluoroantimonate 102 afforded the Muakaiyama aldol products with an exceptionally high level of enantioselectivity and isolated yields. As shown in Scheme 33, reaction of ketene acetals derived from tbutyl thioacetate and benzyloxyacetaldehyde with 5 mol% catalyst derived from bis(oxazoline)–Cu(II) complex 54 furnished the corresponding aldol product in 91% ee (R-isomer). The same reaction in the presence of metal–ligand complex 102 resulted in the reversal of enantioselectivity affording the aldol product in 99% ee. The reaction of ketene acetals and pyruvate esters in the presence of a 10 mol% catalyst 54 also furnished the corresponding aldol product in excellent enantiomeric excess (98%).55b Reaction of Chan’s diene56 proceeded with 0.5 mol% of the catalyst providing the corresponding aldol product which was reduced stereoselectively with Me4N[B(OAc)3H]57 to yield the anti-diol as the major product (anti:syn ratio 15:1, major anti-ester 97% ee).
Scheme 33.
5.7. Mukaiyama–Michael addition reactions
Bernardi and co-workers have examined bis(oxazoline)–Cu(II) complex promoted Mukaiyama–Michael addition of propionate silyl ketene acetals to carbomethoxy cyclopentenone (Scheme 34).58 In the presence of stoichiometric quantities of bis(oxazoline)–Cu(II) complexes, the reaction provided a moderate level of enantioselectivity (11–66% ee). A pronounced counterion effect was observed in the reaction. Among various ligand–metal complexes investigated, the catalyst derived from Cu(OTf)2 and ent-1 ligand has shown the optimum level of asymmetric induction in the process (66% ee for product 103a). The use of Cu(SbF6)2 and ent-1 ligand resulted in reversal of enantioselectivity (60% ee for product 103b). The use of a catalytic amount of chiral ligand caused a drop in ee as well as product yield.
Scheme 34.
5.8. Allylic substitution reactions
Pfaltz and co-workers have demonstrated that Pd–bis(oxazoline) complexes are excellent catalysts for allylic substitution reactions (Scheme 35).13,59 The reaction of racemic 1,3-diphenyl-2-propenyl acetate with dimethyl malonate was studied in the presence of 1 to 2 mol% palladium–bis(oxazoline) complexes derived from a number of bis(oxazoline) ligands including ent-2, ent-12 and ent-41. Ligand ent-41 has shown excellent enantioselectivity of 97% with near quantitative isolated yield (97%) of substitution product 104.
Scheme 35.
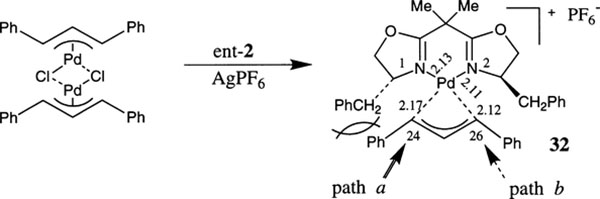
To rationalize the stereochemical outcome, enantiomerically pure (S,E)-1-(4-tolyl)-3-phenylprop-2-enyl acetate 105 was subjected to allylation reaction with dimethyl malonate. Consistent with other Pdcatalyzed allylic alkylations, it was shown that the ent-2-derived catalyst, the reaction proceeded with syn displacement of the acetate providing the syn-substitution product 106a as the major isomer (ratio 93:7).60 Furthermore, Pfaltz and co-workers have prepared and isolated the actual complex 32 which is involved in the catalysis (Fig. 5).59 It was demonstrated that both the complex 32 and the catalyst prepared in situ from {Pd(η3-C3H5)Cl}2 and ent-2 ligand, have provided the same enantioselectivity. A rationale for the preferential attack of the nucleophile on one of the allylic termini was provided based on the X-ray crystal structure data and NMR studies of the complex 32. As was observed in the crystal structure, the Pd–C(24) bond is significantly longer than the Pd–C(26) bond. This is likely due to steric repulsion between the allylic C(24)–Ph-group and the adjacent benzyl side chain of the bis(oxazoline) ring. Further evidence of weakening of the C(24)–Pd bond was provided by 13C-NMR in which C(24) resonance was shifted to higher frequency. Thus, consistent with the product absolute configuration, it is apparent that the nucleophilic attack proceeds through path a wherein the C(24)–Pd bond is longer and more reactive.
Fig. 5.
Palladium catalyzed asymmetric alkylations have also been studied with a series of chiral bis(oxazolinyl) ferrocence ligands 26 (R=iPr, tBu).61,62 With dimethyl malonate as the nucleophile, excellent enantioselectivity was observed (up to 99% ee) for racemic (E)-1,3-diphenylprop-2-enyl-1-acetate. The best enantioselectivity of 63% ee was obtained with racemic (E)-1,3-methylprop-2-enyl-1-acetate. The substituent on the bis(oxazoline) ligands 26 (R=iPr, tBu) has shown influence on the product enantios-electivity. The bulky t-butyl substituent exhibited enantioselectivity of 99% compared to the isopropyl substituent which has shown 96% ee.
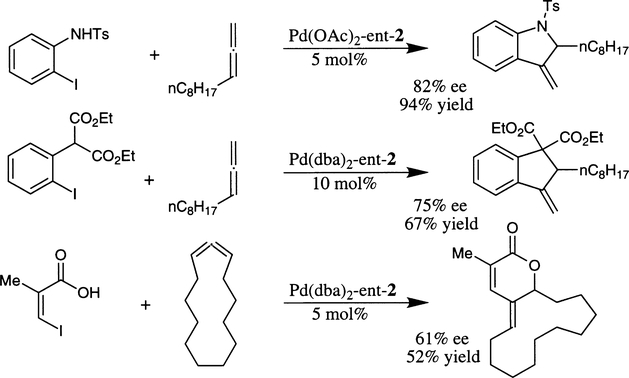
Larock and co-workers have investigated palladium–bis(oxazoline) complex catalyzed intramolecular enantioselective allylic substitution reactions of structurally complex systems (Scheme 36).63 Hetero and carboannulation of a number of allenes with a variety of aryl or vinyl iodides afforded the product with enantioselectivity up to 82% and excellent isolated yields. Bis(oxazoline) ligands that form sixmembered chelates with palladium tend to provide higher ee than five-membered chelates. Among the various bis(oxazoline) ligands examined, ent-2 ligand provided the optimum results. The observed regioselectivity of the reaction was very high and even better than their earlier reported procedure using PPh3 as the ligand.64
Scheme 36.
Currently, the absolute configurations of the annulation products are undetermined. However, based on the possible metal complex in the transition states (Fig. 6), it has been hypothesized that the steric interaction between the benzyl side chain of the ent-2 ligand and the terminal alkyl substituents of the π-allylpalladium intermediates lead to the observed enantioselection.
Fig. 6.
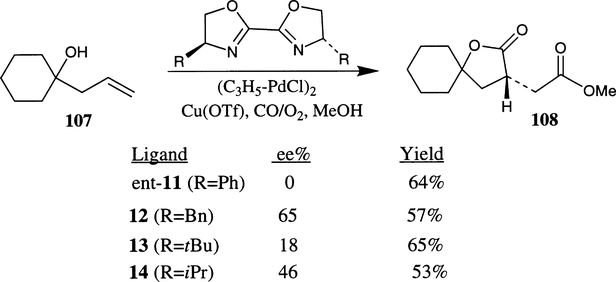
Palladium catalyzed asymmetric intra and intermolecular bis(alkoxycarbonylation) of homoallylic alcohols was investigated by Ukaji and co-workers.65 The Cu(I) and Pd(II)-catalyzed reaction of homoallylic alcohols under atmospheric pressure of carbon monoxide and oxygen provided optically active γ-butyrolactones 108 in 0–65% ee. The reaction was carried out with 2 mol% of {Pd(η3-C3H5)Cl}2, 5 mol% of CuOTf and 8 mol% of a bis(oxazoline) ligand. Among the various bis(oxazoline) ligands examined, bn-box 12 provided the best enantioselectivity (65% ee). Other bis(oxazoline) ligands such as ent-11 (R=Ph,), 13 (R=tBu) and 14 (R=iPr) are less effective (Scheme 37). The influence of solvent polarity was examined and it was found that a 1:1 mixture of THF and MeOH afforded maximum enantioselectivity (65%). The reaction has also been examined with other phosphine ligands such as (+)-DIOP, (+)-BINAP and diethyl tartrate as the ligand, however, the corresponding lactones were obtained in <10% ee.
Scheme 37.
The stereochemical outcome of the formation of lactone 108 can be explained by assuming that CuOTf reacts with the Pd-complex A to set up cationic transition states B and C in which palladium is expected to coordinate strongly with the olefinic π-system (Fig. 7). Between these transition states, the reaction is likely to proceed through transition state B since the steric effects between the substituent in the homoallylic alcohol and bis(oxazoline) side chain would disfavor transition state C.
Fig. 7.
5.9. Allylation and addition reactions
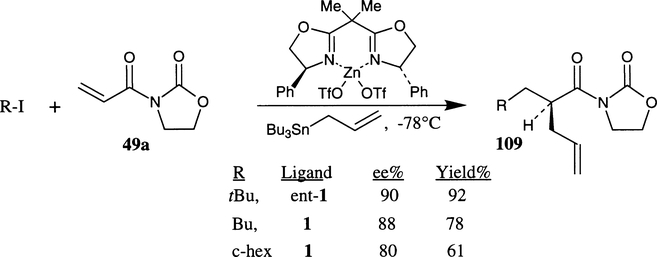
Chiral bis(oxazoline)–Zn(II) complex promoted enantioselective free radical carbon–carbon bond forming reactions have been recently demonstrated by Porter and co-workers (Scheme 38).66 The reaction of N-acryloyl-N-oxazolidinone 49a with cyclohexyl or tert-butyl iodide and allyltributylstannane at −78°C in the presence of a stoichiometric amount of ligand–metal complex derived from phe-box 1 and Zn(OTf)2 afforded the α-allylation product 109 enantioselectively (34–90% ee) with good to excellent isolated yields (55–92%). The reactions were typically initiated with triethylborane, a low- temperature radical initiator. The reaction proceeds by activation of the alkene by chiral Lewis acid towards nucleophilic addition of tert-butyl or cyclohexyl radical followed by stereoselective addition and fragmentation reaction of the intermediate radical with the allyltributylstannane. The phe-box ligand 1 has shown best enantioselectivities and isolated yields.
Scheme 38.
The stereochemical outcome of the α-allylation product can be rationalized by a catalyst complex model similar to that proposed by Corey and Ishihara for the enantioselective Diels–Alder reaction (Fig. 8).14b In this model, the s-cis conformation of the α-amidyl radical is preferred for steric reasons.67 The preferred Si-face attack leads to the observed product stereochemistry.
Fig. 8.
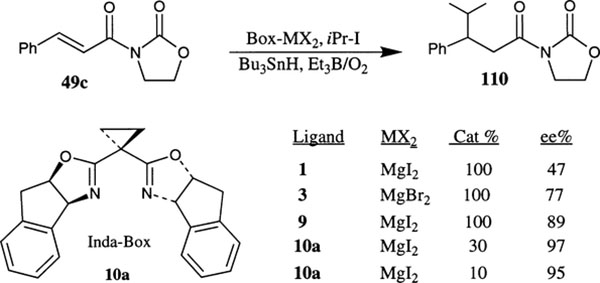
Chiral bis(oxazoline) derived Lewis acid promoted free radical conjugate additions to β-substituted α,β-unsaturated-N-oxazolidinone derivatives have also been investigated by Porter and Sibi and coworkers.68a Stoichiometric amounts of metal–ligand complexes derived from a number of box-ligands and a variety of Lewis acids including MgBr2, MgI2, Mg(OTf)2, Mg(ClO4) and Zn(OTf)2 were examined and the observed enantioselectivity of the product 110 ranged from 37–82% (Scheme 39). A dramatic improvement in enantioselectivity (up to 97% ee) was achieved under stoichiometric conditions when the aminoindanol derived conformationally constrained inda-box ligands 9 and 10 were utilized.68b As demonstrated by Sibi et al., a substoichiometric amount of catalyst (5–30 mol%) derived from MgI2 and inda-box 10a at −78°C retained an excellent level of enantioselectivity (90–97%ee) and isolated yields (83–95%).68b The reaction with 30 mol% catalyst at room temperature also provided an excellent level of enantioselectivity (93% ee) and the product was obtained in 87% isolated yield.
Scheme 39.
The allylation of aldehydes promoted by chiral bis(oxazoline)–metal complex has been investigated by Cozzi and co-workers.69 The reaction of aliphatic and aromatic aldehydes with allyl tributyltin in the presence of a variety of metal–bis(oxazoline) complexes were examined (Scheme 40). Among the metal salts, zinc halides and Zn(OTf)2 have shown the optimum results with chiral bis(oxazoline) ligands 5 and 93c. The observed enantioselectivities of the allylated product 111 were in the range of 40–46% ee.
Scheme 40.
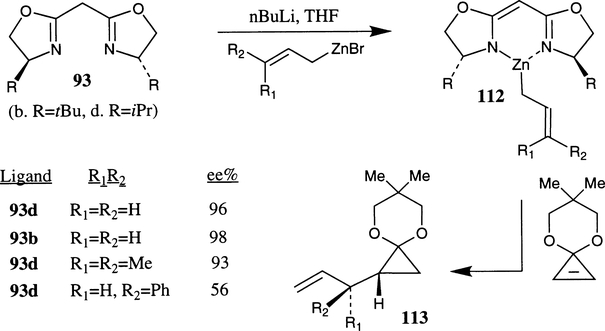
Nakamura and co-workers have investigated chiral bis(oxazoline) promoted enantioselective allylzincation of cyclopropene acetals (Scheme 41).70a Chiral allylzinc reagents 112 (R1=R2=H) were prepared by reaction of allylzinc bromide (obtained from activated zinc and allyl bromide) and lithiated chiral bis(oxazoline) derived from bis(oxazoline) and nBuLi in THF. The chiral allylzinc reagent readily reacted with cyclopropene acetals at room temperature to provide the allylcyclopropane derivative 113 enantioselectively. Chiral ligands with isopropyl and tert-butyl side chains were the most effective, providing up to 98% ee in the corresponding carbometalation product.
Scheme 41.
As shown in Scheme 42, the chiral allylzinc reagent 112 also reacted with cyclic aldimines 114 and 116 to afford the allylated secondary amines 115 and 117 enantioselectively (88–99% ee).70b Reactions of acyclic E-benzaldehyde N-phenylimine with allylzinc reagent derived from ligand 93d (R=iPr) furnished the allylation product in 95% yield, but enantioselectivity was very poor (6%). In the case of cyclic imines, the bis(oxazoline) ligand 93d derived reagent afforded the highest enantioselectivity whereas sterically bulkier bis(oxazoline) 93b (R=tBu) exhibited slightly lower enantioselectivity. The phe-box 1 derived catalyst has shown considerably lower ee (59%) with dihydroisoquinoline 114.
Scheme 42.
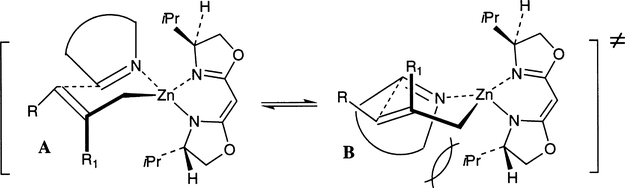
The stereochemical course of allylation of imines can be rationalized on the basis of a hypothetical chair-like transition state model A in which steric interactions between the imine substituents and the substituent at C4 of the bis(oxazoline) ligand are at a minimum (Fig. 9). The reaction of Z-aldimine will proceed through transition state model A over B because of steric interaction between the isopropyl side chain and the substituent on the aldimine. The model is consistent with the observed stereoselectivities of amines 115 and 117.
Fig. 9.
Allylation of the oximes of α-ketoesters has been investigated by Hanessian and Yang using allylz-inc–bis(oxazoline) complex 118 derived from phe-box ligand 93c (Scheme 43).71 The oxime ethers derived from α-ketoesters were reacted with allylzinc reagent at −78°C in THF to afford the corresponding allyl glycine derivatives in very high ee. The bulky tert-butyl ester provided considerably higher ee (93%) than the isopropyl ester (67%). The reagents derived from other substituted bis(oxazoline) ligands such as ent-93a (R=Bn) or ent-93b (R=tBu) have shown essentially no asymmetric induction. Allylzinc reagents obtained from methylallyl and 2-phenylallyl bromides also furnished the corresponding products with 92% and 87% ee, respectively. Interestingly, prenylzinc reagent afforded the γ-addition product in 94% ee and 62% isolated yield. To demonstrate the synthetic utility, the oxime ethers were converted to the corresponding BOC-derivatives 119 by a standard reaction sequence as shown in Scheme 43.
Scheme 43.
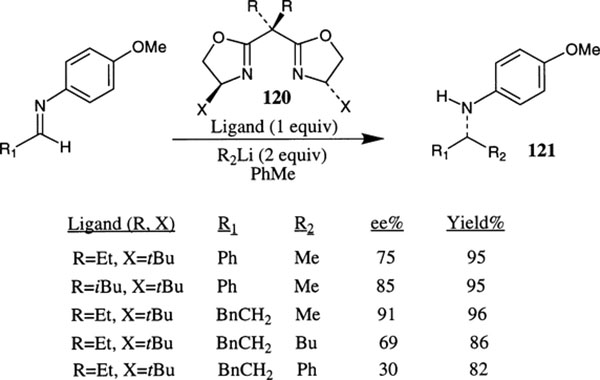
Denmark et al. have shown enantioselective additions of organolithium reagents to imines in the presence of C2-symmetric chiral bis(oxazoline) ligands to give the amines 121 with high enantiomeric excess (up to 91% ee, Scheme 44).72 The addition of MeLi to benzaldehyde N-anisyl imine is extremely slow at −78°C, providing only 6% yield after 4 h. The presence of a stoichiometric amount of bis(oxazoline) ligand 120 substantially accelerated the addition reaction furnishing the amine 121 in high enantiomeric excess and isolated yields (82–96%). The use of a catalytic amount (10–20 mol%) of chiral bis(oxazoline) was also shown to provide the corresponding amine in excellent yield but the product enantioselectivity was slightly lower (Scheme 44). Among various box ligands examined, tertbutyl substituted ligands have shown the best results. For enolizable imines such as the imine derived from phenylpropionaldehyde, the product enantioselectivity was lower for addition of nBuLi and PhLi compared to MeLi addition. However, stoichiometric and substoichiometric amount of addition of (−)-sparteine provided comparable results to MeLi additions (up to 91% ee for nBuLi addition).
Scheme 44.
5.10. Hydrocyanation of aldehydes
Corey and Wang have developed a conceptually intriguing protocol for the enantioselective synthesis of cyanohydrin trimethylsilyl ethers from various aliphatic and aromatic aldehydes.73 The procedure utilizes two chiral bis(oxazoline) ligands, one to activate the aldehyde carbonyl through metal chelation and the other bis(oxazoline) to deliver the cyanide enantioselectively to the aldehyde as shown in Fig. 10. The metal–ligand complex 122 was prepared by reaction of bis(oxazoline) 93c with nBuLi in THF and benzenesulfonyl cyanide to provide the corresponding cyanide which was reacted with an equivalent amount of nBuMgCl in ether. The ether was removed under reduced pressure to provide 122 which was subsequently dissolved in a mixture of CH2Cl2 and propionitrile for hydrocyanation of aldehydes.
Fig. 10.
The reaction of TMSCN and cyclohexane carboxaldehyde in the presence of 20 mol% catalyst complex provided the cyanohydrin TMS-ether 123 (R=cyclohexyl) in 65% ee (Scheme 45). The same reaction in the presence of 12 mol% of bis(oxazoline) 93c as a co-catalyst enhanced the enantioselectivity to 94% ee. The reaction in the presence of 12 mol% ent-93c ligand, however, afforded the cyanohydrin TMS-ether 123 in 38% ee thus demonstrating the mismatch influence of co-catalyst in the reaction. In general, a very high level of enantioselectivity was observed for aliphatic and non-conjugated aldehydes. The ees were lower for aromatic and α,β-unsaturated aldehydes.
Scheme 45.
A py-box–AlCl3 complex has been shown to catalyze trimethylsilylcyanation of aldehydes at room temperature to the corresponding trimethylsilyloxy cyanides in good yield.74 At room temperature, benzaldehyde gave trimethylsilyloxy mandelonitrile in 44% ee and at lower temperature (5–10°C) the ee is increased to 90%. The above catalysts have been shown to effect trimethylsilylcyanation of a variety of bidentate aldehydes to the nitrile derivatives in high chemical yield but no ees were reported.
6. Aziridination reactions
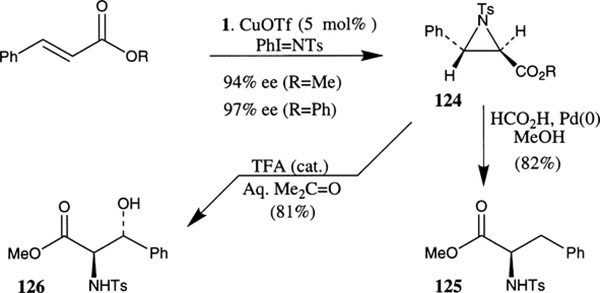
Evans and co-workers have demonstrated that the CuOTf–bis(oxazoline) complexes are efficient catalysts for aziridination of olefins (Scheme 46).75 Aryl substituted olefins are the most effective substrates for aziridination. For cinnamate esters, the corresponding N-tosylaziridine 124 was obtained in excellent enantiomeric excess (up to 97% ee in benzene). Among various bis(oxazoline) ligands examined, phebox 1 has exhibited superior results over the sterically demanding bu-box 3 for aziridination of cinnamate esters. The significant solvent effect on enantioselectivity is noteworthy. For example, methyl cinnamate in acetonitrile has shown 70% ee (21% yield) compared to 94% ee in benzene (63% yield). The synthetic utility of the aziridines 124 have been demonstrated by their conversion to phenyl alanine 125 and the β-hydroxy-α-amino ester derivative 126.
Scheme 46.
The Cu(I)–bis(oxazoline) complex catalyzed enantioselective aziridination of imines has been reported by Jacobsen and co-workers (Scheme 47).76 The reaction of N-benzylidene aniline and ethyl diazoacetate in the presence of copper hexafluorophosphate and bis(oxazoline) ligands afforded an enantiomerically enriched cis/trans mixture of N-aryl aziridine derivatives 127 along with racemic pyrrolidine derivative 128. In general, the yields of the aziridination products were poor (10–37%) and the observed enantioselectivities for the cis isomer were in the range of 11–67% ee and for the trans isomer the ees were even lower (2–35% ee). The phe-box ligand 1 afforded the best results in terms of enantioselectivity, cis/trans diastereoselectivity and isolated yields. Interestingly, achiral bis(oxazoline) gave the best yield (65%) and best cis/trans ratio (10:1).
Scheme 47.
7. Oxidations
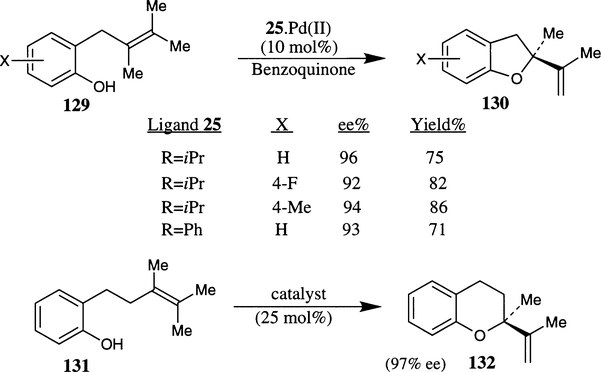
Recently, a highly enantioselective Wacker-type cyclization of o-allylphenol has been reported utilizing palladium(II)–bis(oxazoline) complex as the catalyst (Scheme 48).77 Of various Pd(II) salts, chiral bis(oxazoline) and co-oxidants investigated, for cyclization of 2-(2,3-dimethyl-2-butenyl)-phenol 129 and other substituted derivatives, the combination of Pd(trifluoroacetate)2, (S,S)-binap-box 25 and pbenzoquinone respectively has provided the best results. The reaction proceeded in the presence of 10 mol% of catalyst complex at 60°C for 24 hours to provide the corresponding (S)-(−)-methyl-2-isopropenyl-2,3-dihydrobenzofuran 130 in 96% ee and 75% isolated yield. When the reaction was carried out at 35°C for 3 days, the product was obtained in 97% ee and 72% yield. The phenyl substituted ligand 25a (R=Ph) afforded the product in 93% ee and comparable yield. The cyclization of 2-(2,3-dimethyl-2-pentenyl)-phenol 131 also afforded benzodihydropyran 132 in 97% ee and 67% yield in the presence of a 25 mol% 25a·Pd(II) complex. Bis(oxazoline) ligands 4 and 14 were not so effective, providing the unsubstituted benzofuran product in 35% ee (6% yield) and 18% ee (64% yield) respectively.
Scheme 48.
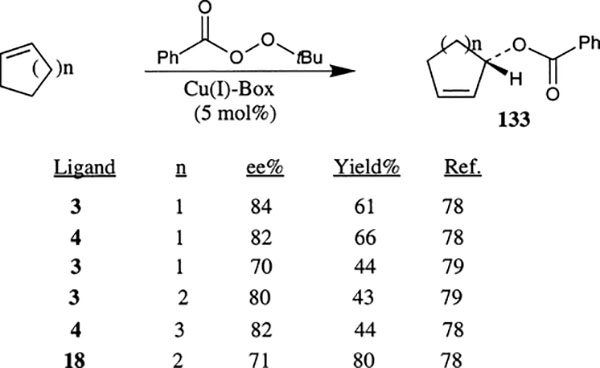
Enantioselective allylic oxidation of cycloalkenes such as cyclopentene, cyclohexene and cycloheptene with tert-butyl perbenzoate was investigated by Pfaltz and co-workers with a variety of catalysts derived from bis(oxazoline) ligands and copper(I) triflate complexes (Scheme 49).78 The ligand–Cu(I) complexes derived from ligands 1, 3 and 4 have shown comparable results. The best enantioselectivities for cyclopentene, cyclohexene and cycloheptene were 84%, 80% and 82%, respectively with 5 mol% catalyst. The 2-benzoate derivatives 133 were isolated in moderate to good yields. The catalyst derived from py-box ligand 18 has also shown comparable enantioselectivity.
Scheme 49.
Andrus et al. have also independently investigated the allylic oxidation of cyclic and acyclic alkenes with Cu(I)–bis(oxazoline) complexes.79 Cyclopentene and cyclohexene have provided comparable ees as Pfaltz and co-workers.78 The reactions were typically run with 5 mol% catalyst at −20°C for five days. The acyclic olefins at low temperature exhibited no optical activity. However, at 55°C for two days, allylbenzene and 1-octene afforded 36% ee and 30% ee, respectively. The proposed model in Fig. 11 accounts for the observed stereoinduction.79 The mechanism involves coordination of an allyl radical intermediate to the Cu–ligand carboxylate complex followed by transfer of benzoate to the allyl system as proposed by Beckwith and Zavitsas.80 The Cu(III) intermediate is assumed to have a distorted square planar geometry placing the benzoate and the allyl groups slightly above and below the bis(oxazoline) plane to minimize steric interaction with tert-butyl groups on the ligand.
Fig. 11.
The Ru–bis(oxazoline) complex catalyzed enantioselective epoxidation of trans-stilbene in the presence of sodium periodate was investigated by Waegell and co-workers (Scheme 50).81 The complexes derived from RuCl3 and bis(oxazoline) ligands including 18 and 135 afforded very low yield (2–7%) of stilbene epoxide and the enantioselectivity was below 5% ee. The Ru–ligand complex derived from pyridyl oxazoline ligand 136 furnished high yield of epoxide (up to 80% yield), but an enantiomeric excess of only 18%.
Scheme 50.
8. Hydrosilylations/reductions
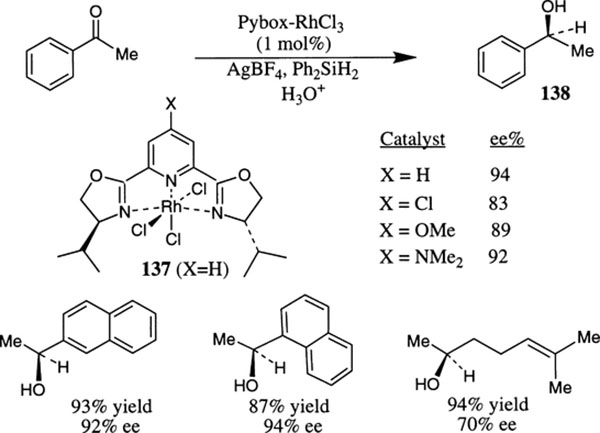
Enantioselective hydrosilylation of ketones using py-box ligands and rhodium complexes was first reported by Nishiyama and co-workers (Scheme 51).82 Reaction of py-box 18 and RhCl3 in ethanol furnished the py-box–RhCl3 complex 137 (X=H) whose X-ray structure reveals that the rhodium complex adopts a distorted octahedral geometry. Ligand–metal complexes derived from RhCl3 and py-box ligands 16–18 did not promote hydrosilylation of acetophenone. However, in the presence of Lewis acid or silver ions, the complex becomes activated for the hydrosilylation reaction. The reaction proceeded smoothly providing the (S)-phenylethanol after hydrolysis. Hydrosilylation of acetophenone in the presence of 1 mol% Rh–complex 137 (X=H), 1.6 equivalents of Ph2SiH2 and 2 mol% of AgBF4 afforded the (S)-phenylethanol in 94% ee. The py-box ligand 16 derived complex has provided 91% ee under the same reaction conditions. Ligand 17 gave 83% ee in the presence of AgOTf. Among various ligands examined for the hydrosilylation reaction, the py-box ligand 18 furnished the best results.
Scheme 51.
The influence of the substituent on the pyridine nucleus of py-box 18 has been examined with various 4-substituted py-box ligands.82b It has been established that for reduction of acetophenone, the electron withdrawing substituent on the ligand increases the reaction rate and the electron releasing substituent reduces the reaction rate. The py-box ligands bearing 4-OMe and 4-NMe2 substituents gave higher enantioselectivities (89% and 92% ee respectively) than the ligand bearing chloride at the 4-position of the pyridine nucleus (83% and 94% ee respectively). Reduction of a number of different ketones also proceeded with high ee and isolated yield with catalyst 137.
Despite the steric bulk associated with chiral rhodium complex 137, reduction of 4-tertbutylcyclohexanone 139 proceeded via preferential axial attack providing a mixture (67:33) of trans and cis alcohols 140 and 141 in 92% combined yield. As shown in Scheme 52, various 2-substituted cyclohexanones were reduced utilizing the py-box–Rh catalyzed hydrosilylation reaction. While the cis/trans diastereoselectivity was very poor, each alcohol exhibited high enantiomeric purity (89–99% ee) which indicates that the Re/Si-face selection by the catalyst predominates greatly rather than the attack from the axial or equatorial side.82c,d
Scheme 52.
Hydrosilylation of acetophenone was further investigated by using a bipy-box ligand on the bipyridine backbone (Scheme 53).82c Reduction of acetophenone with Rh(cyclooctene)2Cl–bipy-box ligand complex 143 afforded the alcohol in low ee (67%) and a substantial amount (32–39%) of silyl enol ether was formed as a byproduct. The reduction in the presence of a bipy-box–RhCl3 complex 143 and 2 equivalents of additional bipy-box ligand 142, however, afforded mainly the phenylethanol and very little enol ether. The product enantioselectivity also considerably improved (up to 90% ee).
Scheme 53.
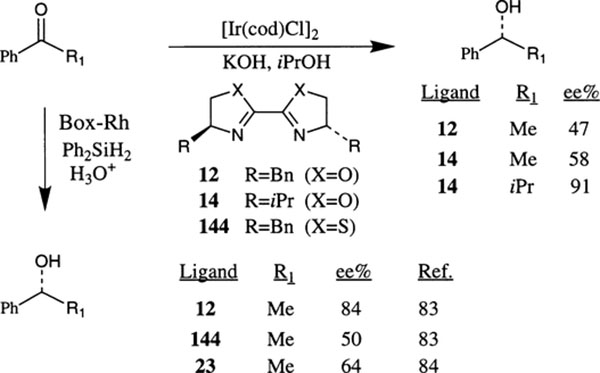
Pfaltz et al. reported the iridium–bis(oxazoline) complex catalyzed transfer hydrogenation of ketones (Scheme 54).13 The active catalysts were typically prepared in situ from 0.5 mol% of [Ir(cycloocta-1,5-diene)Cl]2 and 1.3 mol% of bis(oxazoline) ligands 12–14. The reduction of a number of aromatic ketones proceeded in isopropanol at refluxing temperature providing an excellent yield of the alcohol and the enantioselectivity of alcohol ranged from 47–91% ee. Independently, Helmchen et al. investigated the Rh-box complex catalyzed hydrosilylation of acetophenone using bis(oxazoline) ligands 11–14 and bithazoline ligands 144.83 Hydrosilylation of acetophenone at 0°C in CCl4 using 0.5 mol% [Rh(cyclooctadiene)Cl] and 5 mol% of ligand 12 afforded phenylethanol in 84% ee and 59% chemical yield after removal of silyl ether. With 2.5 mol% of ligand 12, when the reaction was carried out at room temperature, the ee dropped to 15%. The corresponding bis-thiazoline ligand 144 in toluene provided the optically active alcohol with 50% ee. Hydrosilylation of acetophenone by rhodium complexes derived from novel bis(oxazoline) ligands 21–23 have shown enantioselectivity up to 65%.84
Scheme 54.
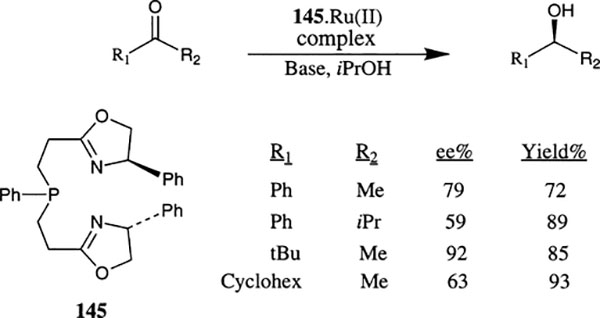
Transfer hydrogenation of ketones utilizing ruthenium(II) complexes of a tridentate ligand 145 have been investigated by Zhang and co-workers (Scheme 55).85 Reduction of a number of aryl, alkyl and dialkyl ketones proceeded in excellent yields and enantioselectivity up to 92% ee was observed for reduction of tert-butylmethyl ketone at room temperature. Reduction of aryl ketones typically required heating of the reaction mixture to 80°C for high conversion and high enantioselectivity. The aliphatic ketones were found to undergo reduction smoothly at room temperature. The isopropyl substituted ligand has shown considerably lower enantioselectivity.
Scheme 55.
9. Miscellaneous reactions
The enantioselective synthesis of (α-chloroalkyl)boronates from (dichloromethyl)boronates catalyzed by chiral bis(oxazoline)–metal complexes has been reported by Jadav and Man (Scheme 56).86 Among various box-ligands and metal triflates examined, the catalyst derived from Yb(OTf)3 and ent-1 ligand was found to induce high enantioselectivity. Reaction of (dichloromethyl)boronate 146 with nBuLi in the presence of a stoichiometric amount of ent-1·Yb(OTf)3 complex afforded the (α-chloropentyl)boronate 148 in 71% ee. The use of 0.3 equivalent of Yb(OTf)3 and a large excess of chiral ligand (5 equivalents) provided the boronate 148 in 88% ee. When the amount of ligand was reduced to 0.5 equivalents, the ee dropped to 55%. The reaction is likely to proceed through the butyl–borate complex 147 in which the 1,2-butyl group migration leads to (α-chloropentyl)boronate and a LiCl byproduct. It was postulated that the excess of ligand is necessary since LiCl, that forms during the reaction, competes with Yb(OTf)3 for the phe-box-ligand. The catalysts derived from Cu(OTf)2 and Zn(OTf)2 afforded considerably lower enantioselectivity (35–45% ee).
Scheme 56.
.
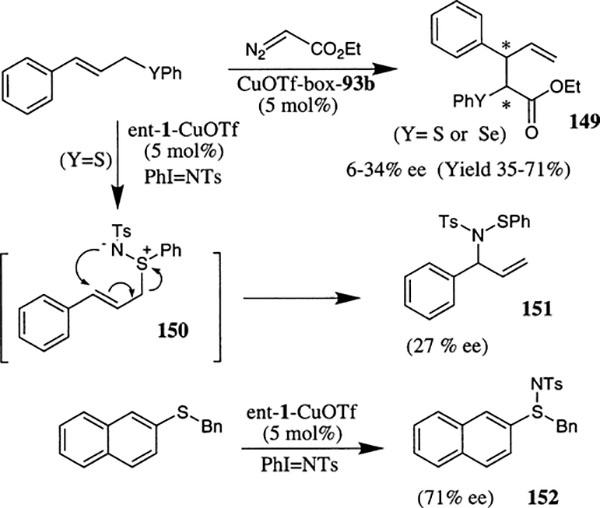
Uemura et al. have shown that Cu(I)–bis(oxazoline) complexes catalyzed the addition of a carbenoid to chalcogen atoms to form the intermediate chalcogen ylide which undergoes 2,3-sigmatropic rearrangement (Scheme 57).87,88 The resulting products 149 were obtained with enantiomeric excess values up to 34%. The reactions of aryl cinnamyl chalcogenides with the carbenoid derived from ethyl diazoacetate were examined in the presence of 5 mol% of 93b·CuOTf complex. Introduction of electron withdrawing substituents such as NO2 or CF3 at the ortho position of the substrate showed a slight increase in enantioselectivity and electron releasing groups such as OMe at the ortho position of aryl ring inhibited the reaction completely under the reaction conditions. The diastereomeric mixture ratio of 149 ranged from 1:1 to 2:1 at best and yields were moderate.
Scheme 57.
The reaction of allylic or benzylic sulfides with an equivalent amount of PhI=NTs in the presence of 5 mol% ent-1·CuOTf-derived complex was also investigated.88 The reaction of 1-naphthyl benzylsulfide provided 71% ee and 75% yield of the corresponding sulfimide 152. The reaction with cinnamyl phenylsulfide provided no aziridination product. Instead, the corresponding allylic sulfimide intermediate 150 was formed which underwent 2,3-sigmatropic rearrangement to provide allylic amide 151 in 27% ee and 40% yield.
Brookhart and Wagner have synthesized optically active isotactic poly(4-tert-butylstyrene-alt–CO) utilizing chiral Pd(II)-catalysts 153 derived from bu-box 3 (Scheme 58).89 Copolymerization of p-tertbutyl styrene (TBS) and carbon monoxide in the presence of 0.1 mol% chiral catalyst 153 afforded the alternating co-polymer 154 with a highly isotactic microstructure and excellent optical purity. The stereoregularity of the polymer is >98% and the polymer exhibits high molar rotation [ϕ]589 −536 (c 0.5, CH2Cl2).
Scheme 58.
10. Conclusion
This review reports recent applications of C2-symmetric chiral bis(oxazoline) ligands in catalytic asymmetric synthesis. From enantioselective carbon–carbon bond forming reactions to asymmetric transfer hydrogenation and hydrosilylation reactions, the versatility of these ligands has been demonstrated in numerous catalytic asymmetric syntheses. Since their introduction in 1989, many new and novel C2-symmetric bis(oxazoline) ligands encompassing a diverse array of structural features have been designed and synthesized. With the inception of bis(oxazoline) ligands, opportunities have come to explore various metal salts that can serve as mild Lewis acids in a particular asymmetric process. In this context, the potential use of a number of metal salts and their counterions has been documented. While the ingenuity and creativity of the researchers involved has led to unprecedented success in bis(oxazoline)–metal catalyzed asymmetric synthesis, there remain many new opportunities yet to be explored. We hope that this review will stimulate further research with bis(oxazoline)–metal catalyzed reactions in other areas of organic synthesis.
Acknowledgements
Financial support of our work by the National Institutes of Health (GM 53386) is gratefully acknowledged. We thank Merck Research Laboratories, Sepracor Inc. and BASF, Germany for providing us with optically active aminoindan-2-ol used for our studies. The authors also thank Professors David Crich and George Gould for helpful discussions.
Footnotes
Dedicated to Professor E. J. Corey with deep respect and sincere appreciation.
References
- 1.For recent reviews, see: (a) Chiral Auxiliaries and Ligands in Asymmetric Synthesis; Seyden-Penne J, Ed.; Wiley Interscience: New York, 1995; [Google Scholar]; (b) Stereoselective Synthesis; Nogradi M, Ed.; VCH Publishers: New York, 1995; [Google Scholar]; (c) Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers; : New York, 1993. and references cited therein. [Google Scholar]
- 2.Takaya H,; Ohta T; Noyori R Asymmetric Hydrogenation In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; 1–39. [Google Scholar]
- 3.Akutagawa S; Tani K Asymmetric isomerization of Allylamines In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; 41–61. [Google Scholar]
- 4. (a).Sharpless KB Tetrahedron 1994, 50, 4235; [Google Scholar]; (b) Johnson RA; Sharpless KB Catalytic Asymmetric Epoxidation of Allylic Alcohols In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; 103–158. [Google Scholar]
- 5.Jacobsen EN Asymmetric Catalytic Epoxidation of Unfunctionalized Olefins In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; 159–202. [Google Scholar]
- 6.(a) Corey EJ; Bakshi RK; Shibata S J. Am. Chem. Soc 1987, 109, 5551; [Google Scholar]; (b) Corey EJ; Bakshi RK; Shibata S; Chen C-P; Singh VK J. Am. Chem. Soc 1987, 109, 7927; [Google Scholar]; (c) Wallbaum S; Martens J Tetrahedron: Asymmetry 1992, 3, 1475 and references cited therein. [Google Scholar]
- 7.(a) Kolb HC; VanNieuwenhze MS; Sharpless KB Chem. Rev 1994, 94, 2483; [Google Scholar]; (b) Johnson RA; Sharpless KB Catalytic Asymmetric Dihydroxylation In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; 227–272; [Google Scholar]; (c) Lohray BB Tetrahedron: Asymmetry 1992, 3, 1317 and references cited therein. [Google Scholar]
- 8.(a) Pfaltz A Acta Chemica Scandinavica 1996, 50, 189; [Google Scholar]; (b) Pfaltz A Acc. Chem. Res 1993, 26, 339; [Google Scholar]; (c) Pfaltz A Chimia. 1990, 44, 202. [PubMed] [Google Scholar]
- 9.Bolm C Angew. Chem. Int. Ed. Engl 1991, 30, 542. [Google Scholar]
- 10.Whitesell JK Chem. Rev 1989, 89, 1581. [Google Scholar]
- 11.Witte H; Seeliger W Liebigs. Ann. Chem 1974, 996. [Google Scholar]
- 12.Butula I; Karlovic G Liebigs. Ann. Chem 1976, 1455. [Google Scholar]
- 13.Muller D; Umbricht G; Weber B; Pfaltz A Helv. Chim. Acta 1991, 74, 232. [Google Scholar]
- 14.(a) Corey EJ; Imai N; Zhang H J. Am. Chem. Soc 1991, 113, 728; [Google Scholar]; (b) Corey EJ; Ishihara K Tetrahedron Lett 1992, 33, 6807. [Google Scholar]
- 15.(a) Lowenthal RE; Abiko A; Masamune S Tetrahedron Lett 1990, 31, 6005; [Google Scholar]; (b) Lowenthal RE; Masamune S Tetrahedron Lett. 1991, 32, 7373. [Google Scholar]
- 16.Bolm C; Weickhardt K; Zehnder M; Ranff T Chem. Ber 1991, 124, 1173. [Google Scholar]
- 17.Harm AM; Knight JG; Stemp G Synlett 1996, 677. [Google Scholar]
- 18.(a) Davies IW; Senanayake CH; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett 1996, 37, 813; [Google Scholar]; (b) Davies IW; Gerena L; Lu N; Larsen RD; Reider PJ J. Org. Chem 1996, 61, 9629. [Google Scholar]
- 19.Denmark SE; Nakajima N; Nicaise OJ-C; Faucher A-M; Edwards JP J. Org. Chem 1995, 60, 4884. [Google Scholar]
- 20.Desimoni G; Faita G; Mella M Tetrahedron 1996, 52, 13649. [Google Scholar]
- 21.Hall J; Lehn JM; DeCian A; Fischer J Helv. Chim. Acta 1991, 74, 1. [Google Scholar]
- 22.Ghosh AK; Mathivanan P; Cappiello J Tetrahedron Lett. 1996, 37, 3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiyama H; Kondo M; Nakamura T; Itoh K Organometallics 1991, 10, 500. [Google Scholar]
- 24.Singh RP Synth. React. Inorg. Met.—Org. Chem 1997, 27, 155. [Google Scholar]
- 25.Ghosh AK; Wink D; Mathivanan P; Cappiello J unpublished results.
- 26.(a) Evans DA; Miller SJ; Lectka T J. Am. Chem. Soc 1993, 115, 6460; [Google Scholar]; (b) Evans DA; Murry JA; Matt PV; Norcross RD; Miller SJ Angew. Chem. Int. Ed. Engv 1995, 34, 798; [Google Scholar]; (c) Evans DA; Kozlowski MC; Tedrow JS Tetrahedron Lett. 1996, 37, 7481. [Google Scholar]
- 27.(a) Evans DA; Barnes DM Tetrahedron Lett. 1997, 38, 57; [Google Scholar]; (b) Evans DA; Shaughnessy EA; Barnes DM Tetrahedron Lett. 1997, 38, 3193. [Google Scholar]
- 28.Evans DA; Johnson JS J. Org. Chem 1997, 62, 786. [Google Scholar]
- 29.(a) Davies; Senanayake CH; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1996, 37, 1725; [Google Scholar]; (b) Davies IW; Gerena L; Castonguay L; Senanayake CH; Larsen RD; Verhoeven TR; Reider PJ Chem. Commun 1996, 1753; [Google Scholar]; (c) Davies IW; Gerena L; Cai D; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1997, 38, 1145. [Google Scholar]
- 30.Desimoni G; Faita G; Righetti PP Tetrahedron Lett. 1996, 37, 3027. [Google Scholar]
- 31.Kanemasa S; Oderaotoshi Y; Yamamoto H; Tanaka J; Wada E; Curran DP J. Org. Chem 1997, 62, 6454. [Google Scholar]
- 32.(a) Johannsen M; Jorgensen KA J. Org. Chem 1995, 60, 5757; [Google Scholar]; (b) Johannsen M; Jorgensen KA Tetrahedron 1996, 52, 7321. [Google Scholar]
- 33.(a) Ghosh AK; Mathivanan P; Cappiello J; Krishnan K Tetrahedron: Asymmetry 1996, 7, 2165; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK; Mathivanan P; Cappiello J Tetrahedron Lett 1997, 38, 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corey EJ; Cywin CL; Roper TD Tetrahedron Lett. 1992, 33, 6907. [Google Scholar]
- 35.Keck GE; Li X-Y; Krishnamurthy DJ Org. Chem 1995, 60, 5998. [Google Scholar]
- 36.(a) Danishefsky SJ Aldrichim. Acta 1986, 19, 59; [Google Scholar]; (b) Bednarski MD; Lyssikatos JP Comprehensive Organic Synthesis: Selectivity, Strategy and Efficiency in Modern Organic Chemistry, Trost BM Ed., 1991, 2, 661; [Google Scholar]; (c) Bertinato P; Sorensen EJ; Meng D; Danishefsky SJ J. Org. Chem 1996, 61, 8000 and references cited therein. [DOI] [PubMed] [Google Scholar]
- 37.Paterson I; Osborne S Tetrahedron Lett. 1990, 31, 2213 and references cited therein. [Google Scholar]
- 38.Ghosh AK; Krishnan K; Cho H; Walters E; Holland L, unpublished results.
- 39.Desimoni G; Faita G; Righetti PP; Sardone N Tetrahedron 1996, 52, 12019. [Google Scholar]
- 40.(a) Takacs JM; Weidner JJ; Takacs BE Tetrahedron Lett. 1993, 34, 6219; [Google Scholar]; (b) Takacs JM; Boito SC Tetrahedron Lett. 1995, 36, 2941. [Google Scholar]
- 41.Gothelf KV; Hazell RG; Jorgensen KA J. Org. Chem 1996, 61, 346. [Google Scholar]
- 42.Langer K; Mattay JJ Org. Chem 1995, 60, 7256. [Google Scholar]
- 43.(a) Evans DA; Woerpel KA; Hinman MM; Faul MM J. Am. Chem. Soc 1991, 113, 726; [Google Scholar]; (b) Evans DA; Woerpel KA; Scott MJ Angew. Chem. Int. Ed. Engl 1992, 31, 430. [Google Scholar]
- 44.(a) Imai Y; Zhang W; Kida T; Nakatsuji Y; Ikeda I Tetrahedron Lett. 1997, 38, 2681; [Google Scholar]; (b) Uozumi Y; Kyota H; Kishi E; Kitayama K; Hayashi T Tetrahedron: Asymmetry 1996, 7, 1603. [Google Scholar]
- 45.(a) Bedekar AV; Andersson PG Tetrahedron Lett. 1996, 37, 4073; [Google Scholar]; (b) Bedekar AV; Koroleva EB; Andersson PG J. Org. Chem 1997, 62, 2518. [DOI] [PubMed] [Google Scholar]
- 46.(a) Nishiyama H; Aoki K; Itoh H; Iwamura T; Sakata N; Kurihara O; Motoyama Y Chem. Lett 1996, 1071; [Google Scholar]; (b) Park S-B; Sakata N; Nishiyama H Chem. Eur. J 1996, 2, 303; [Google Scholar]; (c) Park S-B; Murata K; Matsumoto H; Nishiyama H Tetrahedron: Asymmetry 1995, 6, 2487; [Google Scholar]; (d) Nishiyama H; Itoh Y; Sugawara Y; Matsumoto H; Aoki Y; Itoh K Bull. Chem. Soc. Jpn 1995, 68, 1247; [Google Scholar]; (e) Nishiyama H; Itoh Y; Matsumoto H; Park S; Itoh KJ Am. Chem. Soc 1994, 116, 2223. [Google Scholar]
- 47.Gupta AD; Bhuniya D; Singh VK Tetrahedron 1994, 50, 13725. [Google Scholar]
- 48.Schumacher R; Reissig H-U Synlett 1996, 1121. [Google Scholar]
- 49.Denmark SE; Stavenger RA; Faucher A-M; Edwards JP J. Org. Chem 1997, 62, 3375. [DOI] [PubMed] [Google Scholar]
- 50.Koskinen AMP; Hassila HJ Org. Chem 1993, 58, 4479. [Google Scholar]
- 51.Pique C; Fahndrich B; Pfaltz A Synlett. 1995, 491. [Google Scholar]
- 52.Gant TG; Noe MC; Corey EJ Tetrahedron Lett. 1995, 36, 8745. [Google Scholar]
- 53.Tokunoh R; Tomiyama H; Sodeoka M; Shibasaki M Tetrahedron Lett. 1996, 37, 2449. [Google Scholar]
- 54.Burgess K; Lim H-J; Porte AM; Sulikowski GA Angew. Chem. Int. Ed. Engl 1996, 35, 220. [Google Scholar]
- 55.(a) Evans DA; Murry JA; Kozlowski MC J. Am. Chem. Soc 1996, 118, 5814; [Google Scholar]; (b) Evans DA; Kozlowski MC; Burgey CS; MacMillan DW C. J. Am. Chem. Soc 1997, 119, 7893. [Google Scholar]
- 56.Brownbridge P; Chan TH; Brook MA; Kang GJ Can. J. Chem 1983, 61, 688. [Google Scholar]
- 57.Evans DA; Chapman KT; Carreira EM J. Am. Chem. Soc 1988, 110, 3560. [Google Scholar]
- 58.Bernardi A; Colombo G; Scolastico C Tetrahedron Lett. 1996, 37, 8921. [Google Scholar]
- 59.von Matt P; Lloyd-Jones GC; Minidis ABE; Pfaltz A; Macko L; Neuburger M; Zehnder M; Ruegger H;Pregosin PS Helv. Chim. Acta 1995, 78, 265. [Google Scholar]
- 60.(a) Hayashi T Asymmetric Allylic Substitution and Grignard Cross-Coupling In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; 325–365; [Google Scholar]; (b) Godleski SA in Comprehensive Organic Synthesis; Eds. Trost BM; Fleming I Pergamon, Oxford, 1991, 4, 585–661 and references cited therein. [Google Scholar]
- 61.(a) Zhang W; Hirao T; Ikeda I Tetrahedron Lett. 1996, 37, 4545; [Google Scholar]; (b) Zhang W; Kida T; Nakatsuji Y; Ikeda I Tetrahedron Lett. 1996, 37, 7995. [Google Scholar]
- 62.Ahn KH; Cho C-W; Park J; Lee S Tetrhedron: Asymmetry 1997, 8, 1179. [Google Scholar]
- 63.Larock RC; Zenner JM J. Org. Chem 1995, 60, 482. [Google Scholar]
- 64.Larock RC; Berrios-Pena NG; Fried CA J. Org. Chem 1991, 56, 2615. [Google Scholar]
- 65.Ukaji Y; Miyamoto M; Mikuni M; Takeuchi S; Inomata K Bull. Chem. Soc. Jpn 1996, 69, 735. [Google Scholar]
- 66.Wu JH; Radinov R; Porter NA J. Am. Chem. Soc 1995, 117, 11029. [Google Scholar]
- 67.Porter NA; Giese B; Curran DP Acc. Chem. Res 1991, 24, 296. [Google Scholar]
- 68.(a) Sibi MP; Ji J; Wu J-H; Gurtler S; Porter NA J. Am. Chem. Soc 1996, 118, 9200; [Google Scholar]; (b) Sibi MP; Ji JJ Org.Chem 1997, 62, 3800. [Google Scholar]
- 69.Cozzi PG; Orioli P; Tagliavini E; Umani-Ronchi A Tetrahedron Lett. 1997, 38, 145. [Google Scholar]
- 70.(a) Nakamura M; Arai M; Nakamura E J. Am. Chem. Soc 1995, 117, 1179; [Google Scholar]; (b) Nakamura M; Hirai A; Nakamura EJ Am. Chem. Soc 1996, 118, 8489. [Google Scholar]
- 71.Hanessian S; Yang R-Y Tetrahedron Lett. 1996, 37, 8997. [Google Scholar]
- 72.Denmark SE; Nakajima N; Nicaise OJ-C J. Am. Chem. Soc 1994, 116, 8797. [Google Scholar]
- 73.Corey EJ; Wang Z Tetrahedron Lett. 1993, 34, 4001. [Google Scholar]
- 74.Iovel I; Popelis Y; Fleisher M; Lukevics E Tetrahedron: Asymmetry 1997, 8, 1279. [Google Scholar]
- 75.Evans DA; Faul MM; Bilodeau MT; Anderson BA; Barnes DM J. Am. Chem. Soc 1993, 115, 5328. [Google Scholar]
- 76.Hansen KB; Finney NS; Jacobsen EN Angew. Chem. Int. Ed. Engl 1995, 34, 676. [Google Scholar]
- 77.Uozumi Y; Kato K; Hayashi T J. Am. Chem. Soc 1997, 119, 5063. [Google Scholar]
- 78.Gokhale AS; Minidis ABE; Pfaltz A Tetrahedron Lett. 1995, 36, 1831. [Google Scholar]
- 79.Andrus MB; Argade AB; Chen X; Pamment MG Tetrahedron Lett 1995, 36, 2945. [Google Scholar]
- 80.Beckwith ALJ; Zavitsas AA J. Am. Chem. Soc 1986, 108, 8230. [Google Scholar]
- 81.Augier C; Malara L; Lazzeri V; Waegell B Tetrahedron Lett. 1995, 36, 8775. [Google Scholar]
- 82.(a) Nishiyama H; Sakaguchi H; Nakamura T; Horihata M; Kondo M; Itoh K. Organometallics 1989, 8, 846; [Google Scholar]; (b) Nishiyama H; Yamaguchi S; Kondo M; Itoh KJ Org. Chem 1992, 57, 4306; [Google Scholar]; (c) Nishiyama H; Yamaguchi S; Park S-B; Itoh K Tetrahedron: Asymmetry 1993, 4, 143; [Google Scholar]; (d) Nishiyama H; Park S-B; Itoh K Tetrahedron: Asymmetry 1992, 3, 1029. [Google Scholar]
- 83.Helmchen G; Krotz A; Ganz K-T; Hansen D Synlett 1991, 257. [Google Scholar]
- 84.Imai Y; Zhang W; Kida T; Nakatsuji Y; Ikeda I Tetrahedron: Asymmetry 1996, 7, 2453. [Google Scholar]
- 85.Jiang Y; Jiang Q; Zhu G; Zhang X Tetrahedron Lett. 1997, 38, 215. [Google Scholar]
- 86.Jadhav PK; Man H-WJ Am. Chem. Soc 1997, 119, 846. [Google Scholar]
- 87.(a) Takada H; Nishibayashi Y; Ohe K; Uemura S Chem. Commun 1996, 931; [Google Scholar]; (b) Takada H; Nishibayashi Y; Ohe K; Uemura S; Baird CP; Sparey TJ; Taylor PC J. Org. Chem 1997, 62, 6512. [Google Scholar]
- 88.Nishibayashi Y; Ohe K; Uemura S Chem. Commun 1995, 1245. [Google Scholar]
- 89.(a) Brookhart M; Wagner MI J. Am. Chem. Soc 1994, 116, 3641; [Google Scholar]; (b) Brookhart M; Wagner MI J. Am. Chem. Soc 1996, 118, 7219. [Google Scholar]