Typhoid fever, caused by Salmonella enterica serovar Typhi, is responsible for an estimated burden of approximately 17 million new episodes per year worldwide. Adequate and timely antimicrobial treatment invariably cures typhoid fever. The increasing antimicrobial resistance (AMR) of S. Typhi severely limits the treatment options. We studied whole-genome sequences (WGS) of 536 S. Typhi isolates collected in Bangladesh between 1999 and 2013 and compared those sequences with data from a recent outbreak in Pakistan and a laboratory surveillance in Nepal. The analysis suggests that multiple ancestral origins of resistance against ciprofloxacin and ceftriaxone are present in three countries. Such independent genetic events and subsequent dissemination could enhance the risk of a rapid global spread of these highly resistant clones. Given the current treatment challenges, vaccination seems to be the most appropriate short-term intervention to reduce the disease burden of typhoid fever at a time of increasing AMR.
KEYWORDS: Bangladesh, Salmonella Typhi, antibiotic resistance, genomics
ABSTRACT
Typhoid fever, caused by Salmonella enterica serovar Typhi, is a global public health concern due to increasing antimicrobial resistance (AMR). Characterization of S. Typhi genomes for AMR and the evolution of different lineages, especially in countries where typhoid fever is endemic such as Bangladesh, will help public health professionals to better design and implement appropriate preventive measures. We studied whole-genome sequences (WGS) of 536 S. Typhi isolates collected in Bangladesh during 1999 to 2013 and compared those sequences with data from a recent outbreak in Pakistan reported previously by E. J. Klemm, S. Shakoor, A. J. Page, F. N. Qamar, et al. (mBio 9:e00105-18, 2018, https://doi.org/10.1128/mBio.00105-18), and a laboratory surveillance in Nepal reported previously by C. D. Britto, Z. A. Dyson, S. Duchene, M. J. Carter, et al. [PLoS Negl. Trop. Dis. 12(4):e0006408, 2018, https://doi.org/10.1371/journal.pntd.0006408]. WGS had high sensitivity and specificity for prediction of ampicillin, chloramphenicol, co-trimoxazole, and ceftriaxone AMR phenotypes but needs further improvement for prediction of ciprofloxacin resistance. We detected a new local lineage of genotype 4.3.1 (named lineage Bd) which recently diverged into a sublineage (named Bdq) containing qnr genes associated with high-level ciprofloxacin resistance. We found a ceftriaxone-resistant isolate with the blaCTX-M-15 gene and a genotype distinct from the genotypes of extensively drug-resistant (XDR) isolates from Pakistan. This result suggests a different source and geographical origin of AMR. Genotype 4.3.1 was dominant in all three countries but formed country-specific clusters in the maximum likelihood phylogenetic tree. Thus, multiple independent genetic events leading to ciprofloxacin and ceftriaxone resistance took place in these neighboring regions of Pakistan, Nepal, and Bangladesh. These independent mutational events may enhance the risk of global spread of these highly resistant clones. A short-term global intervention plan is urgently needed.
INTRODUCTION
Typhoid fever is a life-threatening infectious disease caused by Salmonella enterica serovar Typhi. S. Typhi colonizes only humans, is transmitted through the fecal-oral route, and is endemic in tropical countries, especially in Africa and South and Southeast Asia. Worldwide, approximately 17 million people are infected every year by this pathogen (1–4). Though the mortality rate remains low (<1%), 1 in 20 to 25 cases experiences residual disability (5).
Adequate and timely antimicrobial treatment invariably cures typhoid fever. However, the increasing antimicrobial resistance (AMR) of S. Typhi limits the treatment options. In spite of suggested regional decreases in the levels of antibiotic resistance (6–8), the first cases of S. Typhi isolates showing multidrug resistance (MDR) (defined as co-occurring resistance to ampicillin [amp], chloramphenicol [chl], and co-trimoxazole [sxt]) were reported in the early 1970s (9, 10). Ciprofloxacin (cip) resistance first emerged in the early 1990s. At present, over 90% of clinical isolates from regions of endemicity show reduced susceptibility to ciprofloxacin (6, 7, 11). These events shifted the first-line and empirical treatments to other classes of antimicrobial agents, such as ceftriaxone (cro) and azithromycin. Alarmingly, reports of resistance against these agents have now been published (6, 12–18). Moreover, a recent report from Pakistan described the first large-scale outbreak of an S. Typhi clone that is extensively drug resistant (XDR; defined as MDR plus resistance to ciprofloxacin and ceftriaxone) (12).
Whole-genome sequence (WGS)-based approaches using next-generation sequencing (NGS) have become effective tools for the study of genetic diversity and prediction of resistance phenotypes (12, 19–25). Several studies have correlated WGS data with various resistance phenotypes in S. Typhi (12, 20, 25, 26). However, most of these studies involved small numbers of isolates from multiple countries, isolates from single outbreaks, or clusters of travel-related typhoid cases, which do not accurately represent the situation in countries where typhoid fever is endemic over longer periods of time (12, 20, 25–29). These shortcomings limit our overall understanding fo the dynamics of typhoid fever in regions of endemicity, especially in South Asia, where the disease burden is high.
We generated a WGS data set of 536 S. Typhi strains, which were mostly isolated from the blood of pediatric patients in Bangladesh over a period of 15 years (1999 to 2013). In this study, we explored the phenotypic and genotypic diversity of these isolates using whole-genome single nucleotide polymorphism (wgSNP) analysis, classical multilocus sequence typing (MLST), and core genome MLST (cgMLST). We also examined the phylogenetic relationships between these isolates and compared the results with two published data sets from two neighboring countries, representing a hospital-based surveillance study in Nepal and an outbreak during 2016 to 2017 in Pakistan (12, 29). Additionally, we investigated the utility of NGS data for the prediction of phenotypic resistance to multiple antibiotics. We focused on the genes involved in MDR and mutations in the DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) enzymes that lead to ciprofloxacin resistance.
RESULTS
Genotypic diversity of S. Typhi in Bangladesh.
Among the 539 strains presumptively identified as S. Typhi, 536 (99%) were confirmed to be S. Typhi by WGS-based serotyping and were analyzed further. A total of 61% (329/536) of them were from hospitalized patients. Genotype 4.3.1 was dominant (65%; 350/536), followed by genotype 3.3 (13%; 69/536), genotype 3.2.2 (11%; 61/536), and 12 other genotypes (Table 1). Classical MLST analysis revealed the presence of only three different sequence types (ST) among our isolates, namely, ST1 (n = 351), ST2 (n = 166), and ST2209 (n = 18) (Fig. 1a), all of which had similar ratios of hospitalized and outpatient cases (∼60% versus ∼40%). Overall, 99% of the ST1 strains had the 4.3.1 genotype (349/351), while all of the ST2209 strains had genotype 2.3.3 (18/18; 100%). Other genotypes were within ST2 (Fig. 1a). One isolate was nontypeable (NT) by MLST analysis. With respect to the haplotyping scheme, haplotype 58 (H58) was dominant (65%; 350/536), followed by H1 (130/536; 24%) (Table 1).
TABLE 1.
Genotyping and haplotyping results for the 536 S. Typhi isolates from Bangladesh based on WGS
| Genotype | No. of isolates |
% of total | Haplotype |
|---|---|---|---|
| 4.3.1 | 350 | 65.30 | H58 |
| 3.3 | 69 | 12.87 | H1 |
| 3.2.2 | 61 | 11.38 | H1 |
| 2 | 18 | 3.36 | NT |
| 2.3.3 | 18 | 3.36 | NT |
| 2.1.7 | 4 | 0.75 | H8 |
| 2.0.1 | 3 | 0.56 | NT |
| 2.2 | 3 | 0.56 | NT/H39 |
| 1.2.1 | 2 | 0.37 | NAa |
| 2.5 | 2 | 0.37 | H55 |
| 3 | 2 | 0.37 | H13 |
| 3.0.1 | 2 | 0.37 | H13 |
| 3.0.2 | 1 | 0.19 | H13 |
| 4.1 | 1 | 0.19 | H52/15/10/67 |
| Total | 536 | 100 |
NA, not available.
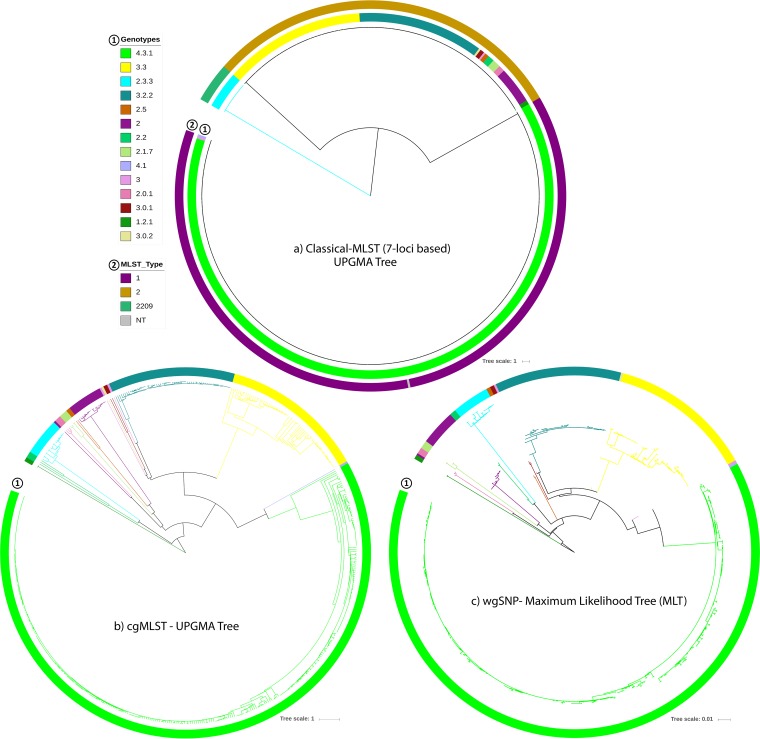
FIG 1.
Genomic diversity and phylogenetic relationships among S. Typhi isolates from Bangladesh. (a and b) UPGMA trees constructed on the basis of (a) Classical MLST (7-locus-based) results in comparison with the genotypes and (b) core-genome MLST (cgMLST) results. (c) Maximum likelihood tree (MLT) constructed on the basis of results of whole-genome SNP (wgSNP) analyses. All phylogenetic trees are colored according to genotypes.
Phylogenetic relationships and new lineages.
Genotypes 3.2.2 and 3.3 clustered together in the same classical MLST type (ST2) but formed two distinct subclades in the unweighted pair group method using average linkages (UPGMA) tree based on cgMLST analyses of 3,002 core loci (Fig. 1a and b). Like genotype 2.3.3, genotype 4.3.1 also generated its own subclade in the tree, with the notable presence of multiple subgroups (Fig. 1b). The same UPGMA tree presented a distinct population structure and a similar genotypic differentiation as observed in the maximum likelihood tree (MLT), which was based on 2,328 SNPs from our WGS data compared to the S. Typhi CT18 reference genome (Fig. 1c) (30).
Genotype 1.2.1 mapped closest to the root of the MLT; genotype 4.3.1 was the most remote (Fig. 1c). As the dominant genotype, 4.3.1 formed a large subclade, with genotype 4.1 in its primary clade. Another primary clade divided into two major subclades, genotypes 3.3 and 3.2.2, which comprised the second and third most prevalent genotypes in Bangladesh. Two small subclades of genotype 2.3.3 and genotype 2.0 were also present in the MLT, rooting with genotype 2.2 and 2.1.7, respectively.
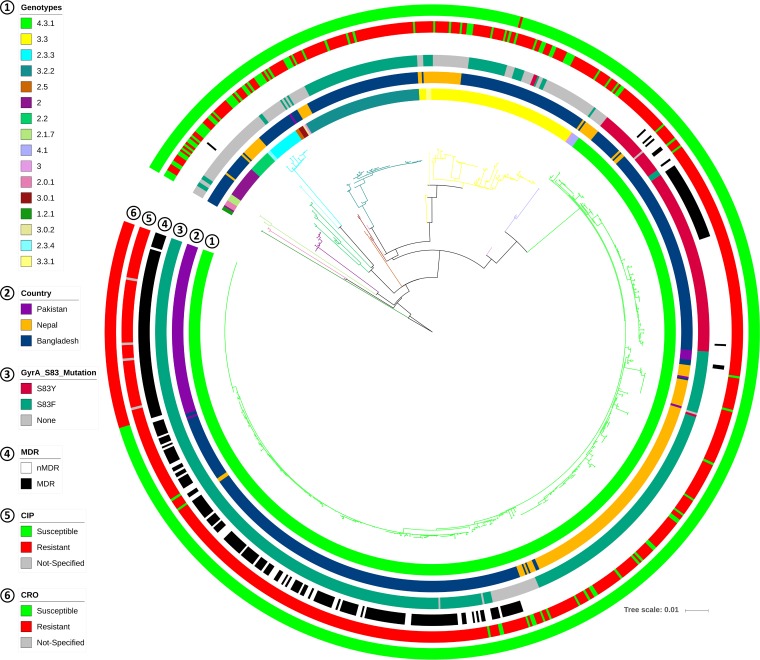
The comparative MLT created using our data and the strains from neighboring countries showed country-specific clusters inside the dominant 4.3.1 genotype and also in genotypes 3.3 and 3.2.2 (Fig. 2 and 3). This suggests different points of origin for the various lineages of S. Typhi in each country. All XDR Pakistani isolates extended into a single branch of the MLT, showing H58 lineage Ia and a minimally divergent pattern (Fig. 3; see also Fig. S1 in the supplemental material), whereas the Nepali strains were dominant in lineage II. The isolates from Bangladesh included only four isolates from lineage II but showed two distinct clusters inside genotype 4.3.1 (H58): lineage Ia (n = 223) and a previously nondescribed lineage (n = 108; Fig. 3). The latter lineage did not match the SNP definition of H58 lineage I or lineage II, suggesting a previously undetected H58 lineage (genotype 4.3.1). On the basis of the MLT, this nondescribed lineage could have had the same point of origin as lineage I but then clearly followed a different pattern of divergence and formed its own subclade inside genotype 4.3.1 (Fig. 3). This new H58 lineage can be distinguished by the SNPs at nucleotide position 561056 (C→A) and 2849843 (A→C) of the CT18 reference genome (this previously undescribed lineage is referred to as “lineage Bd” in the remainder of the article).
FIG 2.
Comparison of Bangladesh isolates with Pakistan and Nepal isolates in a wgSNP-derived MLT. No singleton was considered in the consensus SNP data. The tree is colored by genotype. Different data points, including country, presence of different gyrA-83 mutations, MDR, and cip resistance and cro resistance phenotypes are indicated (by colors) in different circles around the tree. nMDR, no multidrug resistance.
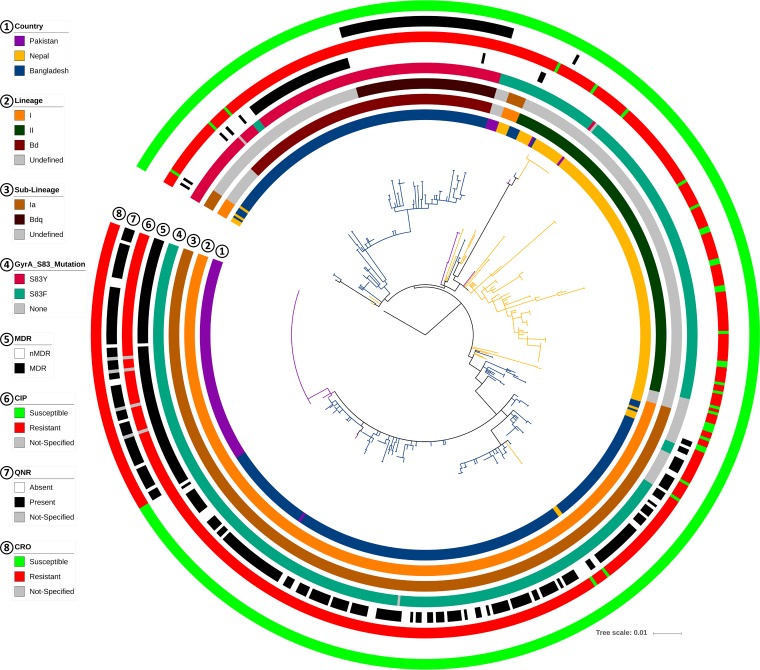
FIG 3.
Comparison of genotype 4.3.1 (H58) isolates from Bangladesh, Pakistan, and Nepal in a wgSNP-derived MLT. No singleton was considered in the consensus SNP data. The tree is colored by country. Different data points, including lineage, sublineage (details), presence of different gyrA-83 mutations, MDR, cip resistance, presence of qnr genes, and cro resistance phenotypes are indicated (by colors) in different circles around the tree.
Comparison of genotype 4.3.1 (H58) isolates from Bangladesh, Pakistan, and Nepal in a wgSNP-derived (with singleton) MLT. (a) MLT with all singletons. (b) Divergence among all XDR isolates from Pakistan. All singletons were considered in the consensus SNP data. The tree is colored by country. Different data points, such as those corresponding to lineages, sublineages (details), the presence of different gyrA-83 mutations, MDR, cip resistance, presence of qnr genes, and cro resistance phenotypes, are shown (by colors) in different circles around the tree. Download FIG S1, TIF file, 3.4 MB (3.4MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Resistance phenotypes and genotypes.
On the basis of analyses performed with five different antibiotics—ampicillin (amp), chloramphenicol (chl), co-trimoxazole (sxt), ciprofloxacin (cip), and ceftriaxone (cro)—the 536 S. Typhi isolates from Bangladesh were found to harbor 12 different phenotypic resistance profiles (phenotypes; Table 2). Isolates with the “MDR, cip-R” profile (n = 202) were most prevalent in our library, followed by “cip-R only” (n = 169) and “Susceptible to all” (n = 62). A comparison of MDR and ciprofloxacin-resistant isolates with different genotypes is presented in Table 3. The single ceftriaxone-resistant (cro-R) strain (MIC > 32 µg/ml) (susceptible to sxt, chl, and cip) in our library, isolated in 2000, displayed genotype 3.3 (haplotype H1) and contained the blaCTX-M-15 gene (ceftriaxone resistance). The other resistance genes detected are listed in Table 4, including blaTEM-1B (ampicillin resistance); catA1 (chloramphenicol resistance); dfrA7, sul1, and sul2 (co-trimoxazole resistance); and qnrS1 (ciprofloxacin resistance).
TABLE 2.
Resistance phenotypes in our library of 536 S. Typhi isolatesa
| Phenotype | No. of isolates | % of total |
|---|---|---|
| MDR, cip-R | 202 | 37.69 |
| MDR | 4 | 0.75 |
| amp-R, sxt-R, cip-R | 1 | 0.19 |
| amp-R, chl-R, cip-R | 2 | 0.37 |
| amp-R, cip-R | 53 | 9.89 |
| amp-R, cro-R | 1 | 0.19 |
| sxt-R, chl-R, cip-R | 25 | 4.66 |
| sxt-R, chl-R | 1 | 0.19 |
| chl-R, cip-R | 15 | 2.80 |
| chl-R only | 1 | 0.19 |
| cip-R only | 169 | 31.53 |
| Susceptible to all | 62 | 11.57 |
| Total | 536 | 100 |
Five different antibiotics were considered: ampicillin (amp), co-trimoxazole (sxt), chloramphenicol (chl), ciprofloxacin (cip), and ceftriaxone (cro). MDR (multidrug resistance) refers to co-occurring resistance to amp, sxt, and chl. “S” and “R” refer to susceptible and resistant phenotypes, respectively (interpretations according to EUCAST-2018).
TABLE 3.
Comparison between multidrug resistance (MDR) and ciprofloxacin resistance (cip-R) with genotypes among Bangladesh isolates
| Genotype | No. of isolates |
||||
|---|---|---|---|---|---|
| MDR |
cip-R |
Total | |||
| Yes | No | Yes | No | ||
| 1.2.1 | 0 | 2 | 0 | 2 | 2 |
| 2 | 1 | 17 | 6 | 12 | 18 |
| 2.0.1 | 0 | 3 | 3 | 0 | 3 |
| 2.1.7 | 0 | 4 | 2 | 2 | 4 |
| 2.2 | 0 | 3 | 2 | 1 | 3 |
| 2.3.3 | 0 | 18 | 8 | 10 | 18 |
| 2.5 | 0 | 2 | 0 | 2 | 2 |
| 3 | 0 | 2 | 1 | 1 | 2 |
| 3.0.1 | 0 | 2 | 1 | 1 | 2 |
| 3.0.2 | 0 | 1 | 0 | 1 | 1 |
| 3.2.2 | 0 | 61 | 52 | 9 | 61 |
| 3.3 | 0 | 69 | 52 | 17 | 69 |
| 4.1 | 0 | 1 | 1 | 0 | 1 |
| 4.3.1 | 205 | 145 | 339 | 11 | 350 |
| Total | 206 | 330 | 467 | 69 | 536 |
TABLE 4.
List of resistance genes detected in our isolatesa
| Resistance gene | Antibiotic class | No. of isolates |
% of total |
Phenotype | Matched NCBI accession no. |
|---|---|---|---|---|---|
| blaTEM-1B | Beta-lactam | 271 | 50.28 | amp-R | JF910132 |
| blaCTX-M-15 | Beta-lactam | 1 | 0.19 | cro-R | DQ302097 |
| catA1 | Phenicol | 256 | 47.50 | chl-R | V00622 |
| dfrA7 | Trimethoprim | 257 | 47.68 | tmp-R | JF806498 |
| qnrS1 | Quinolone | 55 | 10.2 | cip-R | AB187515 |
| strA | Aminoglycoside | 210 | 38.96 | str-R | AF321551 |
| strB | Aminoglycoside | 210 | 38.96 | str-R | M96392 |
| sul1 | Sulfonamide | 257 | 47.68 | sul-R | CP002151 |
| sul2 | Sulfonamide | 265 | 49.17 | sul-R | HQ840942, FJ197818, GQ421466 |
| tet(A) | Tetracycline | 51 | 9.46 | tet-R | AJ517790 |
| tet(B) | Tetracycline | 46 | 8.53 | tet-R | AF326777 |
The table columns list each gene name, the antimicrobial class that it works against, the number of isolates that contained the gene, the percentage of isolates that contained the gene, the resulting resistance phenotype, and the NCBI gene accession number.
Comparison of phenotypic and WGS-derived resistance profiles.
On the basis of the resistance genes identified for the five antimicrobial agents, a WGS resistance (WGS-res) profile was assigned and compared with the phenotypic profile of each isolate to evaluate the ability of the WGS approach to predict the resistance phenotype (Table 5). For all antimicrobial agents except ciprofloxacin, the two profiles corresponded at a level of 99% for the resistant isolates. In contrast, some susceptible isolates (n = 33) harbored resistance genes, which reduced the specificity of the method (≥91%). Three of them had truncated genes (considered inactive genes; Table 5), but the other 30 isolates had the complete coding sequences without any phenotypic resistance. This might suggest impairments (e.g., transcriptomic, translational, protein modification, etc.) in downstream steps of the resistance pathway or the presence of counteracting genes.
TABLE 5.
Evaluation of the ability of WGS-res profiles to predict S. Typhi resistance phenotypes of our isolatesa
| Antimicrobial resistance category |
Presence of gene(s) and WGS-res profile | No. of isolates with indicated phenotype |
Sensitivity (%)b | Specificity (%)c | |
|---|---|---|---|---|---|
| Resistant | Susceptible | ||||
| Ampicillin resistance | Total | 263 | 273 | ||
| blaTEM-1B | 262 | 7 | |||
| Truncated blaTEM-1B | 0 | 2 | |||
| No blaTEM-1B | 1 | 264 | |||
| WGS-res profile: resistant | 262 | 7 | 99.6 | 97.4 | |
| WGS-res profile: susceptible | 1 | 266 | |||
| Co-trimoxazole resistanced | Total | 233 | 303 | ||
| dfrA7 + sul1 + sul2 | 205e ,i | 4f ,i | |||
| dfrA7 + sul1 only | 26 | 22g | |||
| sul2 only | 1e ,i | 55h ,i | |||
| None of three | 1 | 222 | |||
| WGS-res profile: resistant | 231 | 26 | 99.1 | 91.4 | |
| WGS-res profile: susceptible | 2 | 277 | |||
| Chloramphenicol resistance | Total | 250 | 286 | ||
| catA1 | 248j | 7j | |||
| Truncated catA1 | 0 | 1j | |||
| No catA1 | 2 | 278 | |||
| WGS-res profile: resistant | 248 | 7 | 99.2 | 97.6 | |
| WGS-res profile: susceptible | 2 | 279 | |||
| Ceftriaxone resistance | Total | 1 | 535 | ||
| blaCTX-M15 | 1 | 0 | |||
| No blaCTX-M15 | 0 | 535 | |||
| WGS-res profile: resistant | 1 | 0 | 100.0 | NA | |
| WGS-res profile: susceptible | 0 | 535 | |||
Four antimicrobials were considered (ampicillin, co-trimoxazole, chloramphenicol, and ceftriaxone); resistance to these agents is caused mainly by acquisition of resistance genes.
Sensitivity data represent proportions of isolates identified as phenotypically resistant by the WGS-res profile.
Specificity data represent proportions of isolates identified as phenotypically susceptible by the WGS-res profile.
For co-trimoxazole (sxt), we considered the presence of dfrA7, plus sul1 and/or sul2 genes to exert the resistance (R) phenotype.
A total of 206 detected sul2 genes matched three different GenBank IDs: FJ197818 (n = 74), GQ421466 (n = 1), and HQ840942 (n = 131).
Of the four sul2 genes, two matched FJ197818 and two HQ840942. One sul1 gene had unreliable bases (N) in its sequence; that result was considered a sequencing error, and the complete sequence was used in calculations.
One sul1 gene had unreliable bases (N) in its sequence; that result was considered a sequencing error, and the complete sequence was used in calculations.
Only sul2 genes that matched HQ840942 had complete sequences. Genes that matched FJ197818 and GQ421466 were either truncated or mutated.
All catA1 gene sequence had one silent mutation in amino acid 195 (lysine) (CTG→TTG).
On the other hand, for isolates with resistant phenotypes but susceptible WGS res-profiles, we screened for mutations in genes with efflux pump or membrane permeability functions (see Table S1 in the supplemental material). However, no relevant patterns were detected for AMR.
List and characteristics of detected genes with efflux pump and membrane permeability activity. Download Table S1, DOCX file, 0.01 MB (13.4KB, docx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Ciprofloxacin resistance, background mutations, and genotypes.
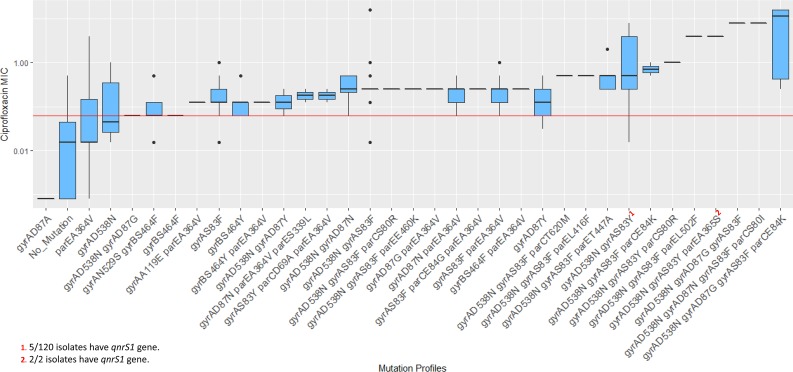
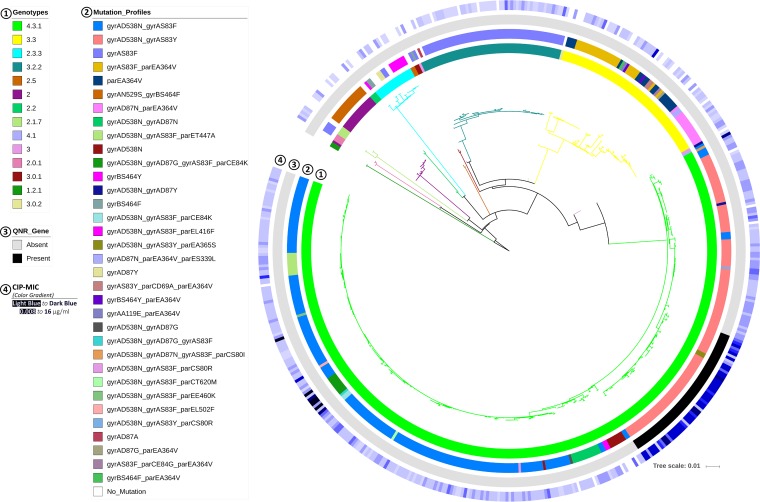
The resistance gene analysis identified the qnrS1 gene in 55 isolates (Table 4) and detected a number of different mutations (n = 24) in the gyrA and gyrB genes encoding DNA gyrase and in the topoisomerase IV enzyme parC and parE genes (Table 6, columns 1 to 3). The most prevalent mutation was gyrA D538N (n = 352), followed by gyrA S83F (n = 299), gyrA S83Y (n = 125), and parE A364V (n = 69). On the basis of mutations in gyrA/B and parC/E genes, 34 cip-mutation profiles were generated and compared with the ciprofloxacin MIC of each isolate (Table 6, columns 4 and 5) (Fig. 4 and 5). All of the profiles, apart from gyrA D538N and parE A364V, were associated with resistance (MIC > 0.06 µg/ml). Two different profiles with triple mutations (gyrAD87G gyrAS83F parCE84K for eight isolates and gyrAD87N gyrAS83F parCS80I for one isolate) had median MICs of ≥8.0 µg/ml (Fig. 5) (Table 6, columns 4 and 5). Profiles with qnr genes also had MICs of ≥1.0 µg/ml (Fig. 4 and 5). No mutations were present in 18 isolates, but 3 of them showed resistance to ciprofloxacin (MIC of 0.25 to 0.5 µg/ml; Fig. 4).
TABLE 6.
Mutations detected in DNA gyrase (gyrA and gyrB genes) and topoisomerase IV (parC and parE genes) individually, and combined mutation profiles based on them
| Gene | Mutation | No. of mutations |
Mutation combination (profile) | No. of mutation profiles |
|---|---|---|---|---|
| gyrA | D538N | 352 | gyrA-D538N, gyrA-S83F | 179 |
| S83F | 299 | gyrA-D538N, gyrA-S83Y | 120 | |
| S83Y | 125 | gyrA-S83F | 66 | |
| D87N | 30 | gyrA-S83F, parE-A364V | 26 | |
| N529S | 17 | parE-A364V | 18 | |
| D87G | 11 | gyrA-N529S, gyrB-S464F | 17 | |
| D87Y | 4 | gyrA-D87N, parE-A364V | 15 | |
| A119E | 1 | gyrA-D538N, gyrA-D87N | 12 | |
| D87A | 1 | gyrA-D538N, gyrA-S83F, parE-T447A | 9 | |
| gyrB | S464F | 21 | gyrA-D538N | 8 |
| S464Y | 10 | gyrA-D538N, gyrA-D87G, gyrA-S83F, parC-E84K | 8 | |
| parC | E84K | 10 | gyrB-S464Y | 8 |
| S80R | 2 | gyrB-S464F | 3 | |
| D69A | 2 | gyrA-D538N, gyrA-D87Y | 2 | |
| T620M | 1 | gyrB-S464Y, parE-A364V | 2 | |
| E84G | 1 | gyrA-D87Y | 2 | |
| S80I | 1 | gyrA-S83Y, parC-D69A, parE-A364V | 2 | |
| parE | A364V | 69 | gyrA-D538N, gyrA-S83F, parE-L416F | 2 |
| T447A | 9 | gyrA-D87N, parE-A364V, parE-S339L | 2 | |
| L416F | 2 | gyrA-D538N, gyrA-S83Y, parE-A365S | 2 | |
| S339L | 2 | gyrA-D538N, gyrA-S83F, parC-E84K | 2 | |
| A365S | 2 | Other combination pattern (one isolate for each) | 13 | |
| L502F | 1 | No mutation | 18 | |
| E460K | 1 | |||
| Total | 536 |
FIG 4.
Mutation profiles detected in genes associated with ciprofloxacin resistance in our isolates and correlation with ciprofloxacin MIC. The horizontal red line indicates the threshold MIC level (0.06 µg/ml) of resistance (according to EUCAST v8.0).
FIG 5.
Comparison of mutation profiles and presence of qnr genes with the level of ciprofloxacin resistance (cip MIC) and genotypes in a wgSNP-MLT. No singleton was considered in the consensus SNP data. The tree is colored by genotype. Circles around the tree are numbered and colored based on the data points shown.
Mutations in codon 83 of gyrA (S83F and S83Y) were the most prevalent among our ciprofloxacin-resistant isolates (411/467; 88%) (Table 6, columns 1 to 3). The S83Y mutation (122/411) was closely associated with genotype 4.3.1 in Bangladesh (123/125; 98%) and was present in 96% of our H58 lineage Bd isolates (104/108; Fig. 3). In contrast, 89% of our lineage Ia isolates had the S83F mutation (198/223) and exhibited lower mean (0.74 versus 1.71 µg/ml) and median (0.25 versus 0.5 µg/ml) ciprofloxacin MIC values than the lineage Bd isolates. The latter lineage also displayed more divergence than lineage I in Bangladesh (Fig. 3; mean pairwise distances, 12.8 versus 11.2). The root of lineage Bd contained isolates detected at earlier time points (1999 to 2004) and formed a noticeable subclade at the tip composed of isolates (n = 55) collected from 2006 onward (Fig. 3; see also Fig. S2 and S3). In addition, this small subclade had the universal presence of the qnr gene and MIC values of ≥1.0 µg/ml (Fig. 3). This small, qnr-specific subclade within lineage Bd can be defined by SNPs at nucleotide positions 1253109 (T→G), 2385340 (A→G), 2676540 (A→T), and 2688285 (C→T) of the CT18 reference genome (and is referred to as sublineage Bdq in the rest of this article). Comparison with other lineages in our cohort of isolates from Bangladesh revealed that sublineage Bdq had a very high median ciprofloxacin MIC (4 µg/ml; Fig. S4) and low divergence (mean pairwise distance, 8.4). Many isolates from Pakistan in lineage Ia also contained qnr genes (Fig. 3) but showed no specific divergence pattern.
Annual distribution of genotypes in a wgSNP-derived MLT, showing the mutation profiles, qnr genes, MIC, and CIP resistance phenotypes of our isolates. No singleton was considered in the consensus SNP data. The tree is colored by year, and additional information is displayed in the different circles. Download FIG S2, TIF file, 4.2 MB (4.2MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Annual distribution of dominant H58 sublineages in Bangladesh. Only sublineages Ia, Bdq, and Bd (except Bdq) are considered here. Download FIG S3, TIF file, 3.2 MB (3.2MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of the (a) ciprofloxacin MIC and (b) ceftriaxone MIC for different H58 lineages of isolates from Bangladesh. Download FIG S4, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In total, 11 ciprofloxacin-resistant isolates (11/467; 2%) did not have any other mutation in DNA gyrase and topoisomerase IV genes, leading to an estimated sensitivity of 98% for the WGS method in correctly predicting ciprofloxacin resistance. In contrast, 36 of 69 ciprofloxacin-susceptible isolates had at least one mutation (not linked to a specific genotype) in one of these four genes (specificity = 52%).
Comparison with neighboring countries.
All genotype 4.3.1 isolates from Bangladesh (this study), Nepal (surveillance in Kathmandu), and Pakistan (outbreak in Sindh) had the same gyrA D538N mutation (Fig. S5). Likewise, the parE A364V mutation was present in all genotype 3.3 isolates from Bangladesh (70/70) and Nepal (17/19) and in genotype 3.3.1 (3/3) isolates from Nepal. Genotype 2.0 isolates from Bangladesh also had the gyrA N529S mutation present (94%; 17/18). However, none of these mutations seemed to have any association with AMR (Fig. 4).
Association of specific mutations with different genotypes in a wgSNP-derived MLT. No singleton was considered in the consensus SNP data. Mutations such as gyrA S538N, gyrA N529S, and parE A364V are shown in comparison with genotypes 4.3.1, 2.0, and 3.3, respectively. All isolates from Bangladesh (our study), Pakistan, and Nepal are included. Download FIG S5, TIF file, 3.9 MB (3.9MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparisons performed with the blaCTX-M-15 gene sequence of our ceftriaxone-resistant isolate revealed 92% coverage and 99% identity with the XDR isolate (GenBank accession no. LT906492.1) from the Pakistani outbreak (Table 7). In contrast, the blaCTX-M-15 gene from Bangladesh shared complete homology with the Klebsiella pneumoniae blaCTX-M-15 gene (FJ815436.1). A detailed comparison of our sequence data with the sequences of the isolates from Pakistan and Nepal is presented in Table 7.
TABLE 7.
Comparison of the isolates from Bangladesh with isolates described in other studies from two neighboring countriesa
| Criterion | Result(s) |
||
|---|---|---|---|
| Bangladesh (present study) | Nepal (29) | Pakistan (12) | |
| Sample source | Hospital surveillance (hospitalized and outpatient services) |
Laboratory surveillance of typhoidal Salmonella |
Outbreak |
| Timeline | 1999–2013 | 2008–2016 | November 2016–March 2017 |
| No. of S. Typhi samples analyzed |
536 | 198 | 100 |
| Age limit | <18 yrs for hospitalized cases; no age limit for outpatient cases |
<14 yrs | None |
| No. of MDR or XDR isolates | MDR, 206 (38%); XDR, none | MDR, 6 (0.03%); XDR, none | MDR, 89 (89%); XDR, 87 (87%) |
| No. of isolates with ciprofloxacin resistance |
467 (87%) | 171 (86%) | 96 (96%) |
| No. of isolates with ceftriaxone resistance (cro-R) |
1 (0.2%) (caused by blaCTX-M15) | None | 88 (88%) (caused by blaCTX-M15) (another 12 cro-S isolates were selected for comparison) |
| Genotype of cro-R isolate(s) | 3.3 | NA | 4.3.1 (lineage Ia) |
| Phenotype of cro-R isolate(s) | amp-R, cro-R | NA | XDR |
|
blaCTX-M15 identity and coverage |
92% coverage and 99% identity with gene sequence from Pakistan |
NA | NA |
| No. of isolates with indicated dominant genotypes |
4.3.1 (H58), 350 (65%); 3.3 (H1), 69 (13%); 3.2.2 (H1), 61 (11%) |
4.3.1 (H58), 154 (78%); 3.3.0, 19 (10%) |
4.3.1 (H58), 99 (99%) |
| No. of isolates with indicated dominant H58 lineage(s) |
Ia, 223 (63% of H58); Bd, 108 (31% of H58) |
I, 21 (10% of H58); II 133 (67% of H58) |
Ia, 92 (92% of H58) |
| Local lineage(s) detected? | Yes; lineage Bd (108 isolates [31% of H58]) and sublineage Bdq (55 isolates [16% of H58]) |
Yes; local lineage II (no. of isolates not given) |
No; a clone of lineage Ia with possible local origin |
| AMR details of local lineage | All sublineage Bdq contain qnr genes; 88% have cip-MIC ≥1 µg/ml (median, 4 µg/ml) |
(a) Intermediate resistance to CIP; (b) no MDR; (c) contains gyrA-S83F mutations |
Not a lineage but a clone of lineage Ia; predominantly XDR |
| Time of emergence for local lineages |
See Fig. S3 | Possibly after 2008 | November 2016–present |
MDR, multidrug resistance, defined as co-occurring resistance to ampicillin, chloramphenicol, and co-trimoxazole; XDR, extensive drug resistance, defined as MDR plus resistance to ciprofloxacin and ceftriaxone.
DISCUSSION
S. Typhi multilocus sequence types and other genotypes in Bangladesh.
Genotyping and the phylogenetic inferences agreed with the genotyping framework interpretation (27) and showed genotype 4.3.1 (haplotype 58, H58) to be dominant among the isolates from Bangladesh (Table 1). This was no surprise, as this genotype possibly emerged from South Asia in the early 1990s and now dominates in regions of typhoid endemicity in the world (26, 31). The same genotype was also dominant among the isolates from Nepal and Pakistan (Table 7) (12, 29). On the other hand, classical MLST revealed only three sequence types (ST), with dominance of ST1 and ST2, which accords with global MLST report (32). There are 46 complete MLST types available for S. Typhi (33). Interestingly, the third most common MLST type in our data, ST2209, had a complete match with genotype 2.3.3 (100%; 18/18) (Fig. 1a). Isolates of this genotype from 2013 (n = 8) had the same mutation (gyrB S464Y). Five of 8 had a cip-resistant phenotype, which could indicate the beginning of new clonal dissemination (see Fig. S2 in the supplemental material).
Moreover, 99% (349/351) of all ST1 isolates from Bangladesh belonged to genotype 4.3.1 (Fig. 1a). This association was previously described in a small study involving 32 isolates (34). A phylogeographical report of S. Typhi included an estimate that divergence for genotype 4.3.1 commenced in the very late 1980s (26). However, the presence of ST1 could be detected before the 1980s (33, 35), as is likely the case for H58.
Presence of genotype-specific mutations.
Isolates with genotype 4.3.1 from all three countries shared a common but as-yet-unreported mutation, gyrA D538N (nucleotide position 2332398 of the CT18 genome; Fig. S5). This mutation is not linked to ciprofloxacin resistance (Fig. 4 and 5) but could be crucial to the structure of the DNA gyrase enzyme, considering the associated change in the isoelectronic point (pI; D→N: 2.77 → 5.41) of the amino acid due to this mutation (36). Similar associations were also observed between genotype 2.0 and gyrA N529S (hydrophobicity, 3.47 → 1.83), and genotype 3.3 and parE A364V (hydrophobicity, 0.0 → −0.78; Fig. S5) (37). These mutations could have potential as markers to trace genotypes, especially the more prevalent genotypes such as 4.3.1 and 3.3.
New H58 lineages with high-level ciprofloxacin resistance.
According to the published scheme that defines the different lineages of genotype 4.3.1 (H58) (26, 38), lineage Ia was dominant among the isolates from the recent Pakistan XDR outbreak, while most isolates from the Nepal surveillance belong to lineage II (Table 7). An undefined cluster within lineage II was also noticed among the Nepali isolates (Fig. 3), as has been described previously (12, 29). Among our isolates from Bangladesh, we found a new lineage of genotype 4.3.1 (H58), Bd (n = 108), which represented the second most dominant lineage after Ia (n = 223). This new lineage has decreased susceptibility to ciprofloxacin compared to lineage Ia (mean MIC, 1.71 versus 0.74 µg/ml). Ciprofloxacin MICs of >0.06 µg/ml are classified as resistant following the EUCAST guidelines. However, as the resistance breakpoint specified by the Clinical and Laboratory Standards Institute (CLSI) is 1 µg/ml, some strains could be classified as susceptible in countries that use the CLSI guidelines (12, 17, 29). Moreover, lineage Bd is probably of local origin, as it was absent in both neighboring countries (Fig. 3) and does not match the published SNP definition of lineage I or II (38). This local variant also had a higher pairwise distance in the SNP matrix (mean, 12.8 versus 11.2) than lineage Ia, suggesting a different pattern of divergence.
Remarkably, a sublineage of lineage Bd (Bdq; n = 55) showed increased resistance compared to other isolates from the same lineage, with median ciprofloxacin MICs of 4.0 µg/ml (mean MIC, 3.4 versus 0.4 µg/ml; Fig. S4). Sublineage Bdq predominantly carried qnr genes, in addition to gyrA mutations (Fig. 3 and 5), and showed more clonality than other lineages (mean pairwise distance, 8.4 versus 11.2 for Ia). Moreover, sublineage Bdq emerged recently, as all isolates were from 2006 onward, but became more prevalent after 2007 (Fig. S3). Therefore, antimicrobial treatment with fluoroquinolones of infections caused by sublineage Bdq may lead to failure.
A similar highly resistant lineage with triple mutations (gyrAS83F gyrAD87G parCE84G) but with no qnr genes was previously reported to cause failure of treatment with gatifloxacin in Nepal (28, 29). Our MLT also showed a small subclade (n = 8) inside lineage Ia for Bangladesh, with a triple mutation (gyrAS83F gyrAD87G parCE84K) and median ciprofloxacin MICs of 8.0 µg/ml (Fig. 4 and 5).
Notably, the number of lineage II isolates (n = 4) in Bangladesh was extremely low (Fig. 3), despite the dominance of this lineage in Nepal and India (26, 27, 29). The surveillance data from Nepal, which mostly describes the isolates from Kathmandu valley, showed a shifting pattern of H58 lineages (from lineage I to lineage II) over the years (29). Such a changing pattern is not observed in Bangladesh, probably because of relatively high prevalence and dominance of local lineages, such as the previously unreported lineage Bd. The Nepal surveillance also reported association of MDR with lineage I and of cip resistance with lineage II (29). However, no such association has been found for lineage I or lineage Bd in Bangladesh (see Data Set S1 in the supplemental material).
Summary of our samples sequenced in this study. Data points include the following: sample identifier (id), accession numbers, year of isolation, age, sex, antimicrobial susceptibility patterns (ampicillin, AMP; co-trimoxazole, SXT; chloramphenicol, CHL; ciprofloxacin, CIP; ceftriaxone, CRO), MDR (or non-MDR) status, phenotype, MLST type, genotype, H58 lineages and sublineages, quinolone resistance-determining region (QRDR) mutations (in gyrA/B and parC/E genes), and presence of resistance genes (blaTEM-1B, catA1, dfrA7, sul1, sul2, qnrS1, strA, strB, tetA, and tetB). Download Data Set S1, XLSX file, 0.1 MB (91.3KB, xlsx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
wgSNP analysis suggests regional clonality of S. Typhi in Bangladesh.
The wgSNP analyses of our isolates generated 2,328 SNPs, revealing that the S. Typhi population in Bangladesh is highly clonal. However, addition of the isolates from Nepal (n = 198) and Pakistan (n = 100) increased the number of SNPs to 3,251 but decreased the number to 627 for genotype 4.3.1 isolates only (n = 603). As the filtering criteria remain the same, the number of SNPs for all Bangladesh isolates is relatively low compared to the global or multicountry context (25–27) but is similar to country-specific data. For example, 1,850 SNPs were detected in isolates from Thailand (n = 44) and 2,187 SNPs in isolates from Nepal (n = 198) (29, 39). The wgSNP-MLT data showed distinct differentiation of all genotypes, much like the data from the cgMLST-UPGMA tree, except the latter lacked clear inferences for different H58 lineages (Fig. 1b and c and Fig. S6). Genotype 1.2.1 mapped close to the root of the MLT, suggesting that this genotype is one of the oldest circulating types. Likewise, being the most distantly related, genotype 4.3.1 could be one of the more recent genotypes circulating in Bangladesh (Fig. 1c) and neighboring countries (Fig. 2).
Comparison of all isolates from Bangladesh in a cgMLST-derived UPGMA tree. The tree is colored according to genotype. Data points such as those corresponding to MLST type, lineage, and sublineage are indicated (by colors) in different circles around the tree. Download FIG S6, TIF file, 4.2 MB (4.3MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
WGS predicts AMR phenotypes with high sensitivity.
The WGS-based resistance profiles showed >99% sensitivity and >91% specificity in describing the phenotypes (for amp, sxt, chl, and cro) of AMR isolates (Table 5). Remarkably, the dfrA7 genes (involved in trimethoprim resistance) were always detected in the presence of the sul1 gene (sulfonamide resistance) and never alone. Table 5). Similarly, sul1 was never detected in the absence of dfrA7. Two isolates had discordant results, as we did not detect the concordant resistance genes in WGS analyses (Table 5). Repeating the antimicrobial susceptibility tests (ASTs) reconfirmed the resistant phenotype. Other resistance mechanisms, e.g., efflux pumps or membrane permeability changes, may be involved (40).
Ciprofloxacin resistance in 11 isolates with no mutation in DNA gyrase or topoisomerase IV genes (and no qnr genes) can suggest the presence of other mechanisms. Indeed, MDR bacteria can increase the expression of efflux pump genes, including acrAB, acrEF and tolC (through overexpression of ramA or repression of acrR genes). This enables the bacteria to expel fluoroquinolone molecules, resulting in ciprofloxacin resistance (40–43), as well as ampicillin or chloramphenicol resistance, even in the absence of bla or catA genes (44–46). On the other hand, isolates carrying a bla gene without the resistance phenotype could be the result of mutations in the promoter regions of outer membrane protein genes, such as the ompC gene, which facilitates penetration of beta-lactams through the outer membrane (47, 48). This could be the scenario for several susceptible isolates (n = 30) in our library that have the full-length resistance gene. However, transcriptomic or proteomic approaches may be required to further explore these possibilities.
Different genotypic backgrounds of ceftriaxone resistance in Bangladesh and Pakistan.
The ceftriaxone-resistant (cro-R) strain from our library was isolated in 2000. The first report of a cro-R strain was published in 1999 (16). Interestingly, this isolate harbored the same extended-spectrum-beta-lactamase (ESBL) gene, blaCTX-M-15 (17, 49, 50), that caused the ceftriaxone-resistant phenotype in an ongoing typhoid outbreak in Pakistan (12). Other ESBL genes, including blaCMY-2 and blaCTX-M-14, have also been reported in relation with ceftriaxone resistance in other Salmonella species (51, 52) but never in S. Typhi. The sequence identity of blaCTX-M-15 between our isolate and the Pakistani isolates was 99%, with 92% coverage (Table 7). The resistance phenotype and genotype were also different from those of our isolate (Table 7). The Pakistani outbreak isolates formed a distinct cluster in the H58-specific MLT and showed high-level clonality (Fig. 3 and Fig. S1). In contrast, our ceftriaxone-resistant isolate had genotype 3.3, which suggests a different source and geographical origin. Moreover, no other ceftriaxone-resistant strains of genotype 3.3 have been reported from Bangladesh. We hypothesize that acquisition of the blaCTX-M15 gene might compromise the fitness of S. Typhi although as of now no data have been published in support of this. Also, no association with fitness has been found for ciprofloxacin resistance mutations in DNA gyrase genes (53). Therefore, the possibility of a global dissemination of these recently emerging variants cannot be excluded given the successful multicontinent spreading of its H58 ancestor (genotype 4.3.1).
This study had some limitations. The isolates from Pakistan are from a still-ongoing outbreak in Hyderabad and Karachi that started in 2016. The Nepal isolates are from a prospective surveillance in the area of Kathmandu valley and cover a period of 9 years (2008 to 2016). The collection of strains from Bangladesh was selected from a biobank of >3,000 strains recovered over a period of 15 years (1999 to 2013) from two different hospital settings in Dhaka. The majority (97%) of the isolates from Bangladesh are from children (<18 years old). Therefore, none of the collections cover the whole population in their respective countries. Also, there is no overlap of the isolate collection periods between Bangladesh and Pakistan. Country-to-country comparisons of the observed data may therefore be biased.
Conclusion.
Our study demonstrated that WGS has high sensitivity and specificity for prediction of S. Typhi resistance phenotypes. However, this genomic method still lacks sensitivity and needs fine-tuning for the detection of ciprofloxacin resistance. We detected three different mutations associated with specific genotypes that could be used to develop genotype-specific tracking tools. We report a new, local variant of genotype 4.3.1, lineage Bd, which contains a recently emerged sublineage, Bdq, that exhibits a high level of ciprofloxacin resistance. A triple mutant variant (gyrAS83F gyrAD87G parCE84K) of lineage Ia with high ciprofloxacin resistance was also detected. A similar triple mutant variant of lineage II (gyrAS83F gyrAD87G parCE84G) has been reported from Nepal and possesses the same phenotype (28, 29). Our ceftriaxone-resistant isolate contains the blaCTX-M-15 gene but has a genotype and gene sequence different from those of the same gene of XDR S. Typhi strains from the Pakistan outbreak, defining a different ancestral origin. Thus, dissemination of this isolate throughout the region from a single point is therefore less likely. However, multiple independent genetic events in neighboring countries and possible subsequent dissemination enhance the risk of the global spread of these highly resistant clones.
The data presented in this study will add to the accumulating information, from Pakistan and Nepal in particular, concerning the increasing drug resistance of S. Typhi. The emergence of XDR S. Typhi is strongly compromising effective treatment of typhoid fever. The spread of these resistant lineages and their occurrence in various Asian countries emphasize the need to inform public health professionals and sensitize the global community. Measures to implement a two-pronged approach for typhoid control need to be accelerated (54, 55). Both short-term vaccine interventions for high-risk populations and long-term water and sanitation interventions will undoubtedly be the cornerstones of a global prevention plan to address control of typhoid fever.
MATERIALS AND METHODS
Isolate collection and antimicrobial susceptibility profiles.
All S. Typhi isolates used in this study were collected from the Child Health Research Foundation (CHRF) at the Department of Microbiology, Dhaka Shishu (Children) Hospital, in Dhaka, Bangladesh. The CHRF team has been preserving invasive Salmonella isolates since 1999 and maintained a biobank of >3,500 S. Typhi isolates, largely from children (<18 years of age). All strains were isolated from the blood of patients diagnosed with typhoid fever in two different settings: hospital inpatients (hospitalized), and out-patients attending the consultation facility (56). Clinical and epidemiological data were collected for all isolates collected from hospital inpatients.
We selected 539 S. Typhi isolates for this study; data were available for those isolates with respect to the date of isolation (1999 to 2013), hospital setting, and phenotypic resistance for five different antibiotics (ampicillin, chloramphenicol, co-trimoxazole, ciprofloxacin, and ceftriaxone). Age data were available for 85% (456/536) cases; among those cases, 97% (443/456) patients were <18 years of age, while 76% (345/456) were <5 years of age. We checked the identity of the isolates by the use of standard biochemical tests and Salmonella agglutinating antisera (Thermo Scientific, MA, USA). Antimicrobial susceptibility for ampicillin (amp), co-trimoxazole (sxt), and chloramphenicol (chl) was determined using the disk diffusion method (Oxoid, Thermo Scientific, MA, USA). Broth microdilution was used to determine the MIC values for ciprofloxacin (cip) and ceftriaxone (cro; Sigma-Aldrich, MO, USA). All zone diameter and MIC data were interpreted according to EUCAST v8.0 clinical breakpoints (57). Fig. S7 in the supplemental material shows the complete workflow. All sequence data have been submitted to the European Nucleotide Archive (ENA). Data Set S1 in the supplemental material summarizes relevant details of our isolates.
Workflow of our study from the biobank of >3,000 S. Typhi isolates to the WGS data analysis of 539 isolates. Download FIG S7, TIF file, 2 MB (2.2MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DNA extraction and whole-genome sequencing.
Isolates were grown on MacConkey agar (Oxoid) overnight, and the colonies were suspended in water. The QIAamp DNA minikit (Qiagen, Hilden, Germany) was used to extract DNA from the suspension on the same day. WGS was performed using an Illumina HiSeq 4000 platform (The Oxford Genomics Centre at the Wellcome Trust Centre for Human Genetics, Oxford, United Kingdom). One Salmonella Paratyphi isolate was also sequenced so that it could be included in comparative phylogenetic analysis (as an outgroup).
Data quality check.
Sequence data quality was checked using FastQC v0.11.15 (58). We summarized all quality indicators using MultiQC v3 (59). If the summary revealed the presence of adapter sequences, they were removed using Trimmomatic v0.36 (60). KmerFinder was used to confirm the species of the strains (61, 62). Another tool, SeqSero, was used for WGS-based serotyping, to determine the Salmonella serovar of the isolates and confirm the wet-lab serotyping results (63).
WGS data analyses with BioNumerics.
Adaptor-free fastq files were imported into BioNumerics version 7.6.2 (Applied Maths NV, Sint-Martens-Latem, Belgium) and analyzed via the use of the integrated Calculation Engine. For the comparison with isolates from neighboring countries, we used recently published WGS data on 100 S. Typhi isolates from Pakistan (12) and 198 S. Typhi isolates from Nepal (29). The Pakistan isolates were mostly from an ongoing outbreak of XDR S. Typhi, in Hyderabad and Karachi, Sindh, Pakistan, between November 2016 and March 2017 (12). In contrast, the Nepal isolates were part of a hospital-based enteric fever surveillance performed during 2008 to 2016, based on one of the large referral hospitals in Kathmandu Valley, namely, Patan Academy of Health Sciences (PAHS). (29).
Details of the quality control of the WGS data, mapping against the reference genome, filtering the SNPs, allele calling for cgMLST, detecting the presence of acquired resistance genes, and SNP-based genotyping are described in Text S1.
Supplemental methods. Download Text S1, DOCX file, 0.01 MB (18.5KB, docx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Classical 7-locus MLST.
The complete sequences of seven loci (aroC, dnaN, hemD, hisD, purE, sucA, and thrA) were identified in extracted contigs. All sequences were matched with Enterobase (Achtman 7-gene MLST) (http://enterobase.warwick.ac.uk/species/index/senterica) to determine the classical MLST type of each isolate.
Phylogenetic analyses.
We used RaxML v8.2.10 to build maximum likelihood phylogenetic trees (MLT) (65) on the basis of the alignment of 2,328 SNPs from 536 S. Typhi isolates in our study, 3,251 SNPs from 834 isolates in the comparisons with neighboring countries, and 627 SNPs from all 603 H58 isolates. Lineages for all H58 isolates were determined as previously described (38). We employed the generalized time-reversible model and a Gamma distribution to model site-specific rate variation (the GTRGAMMA in RaxML). Support for the MLT phylogeny was assessed via 100 bootstrap pseudoanalyses. The S. Paratyphi A strain from Bangladesh (Sample: 311189_229186) was included as an outgroup for tree rooting. All MLT and UPGMA trees were displayed and annotated using the iTOL6 online version (66). To compute the genetic distances between different groups (e.g., countries, H58 lineages, etc.), a pairwise SNP distance matrix was generated between isolates by computing the number of SNP loci at which pairs of isolates had discordant alleles. Median distances within or between groups were computed from this distance matrix.
Statistical analyses were performed using R v3.5 (64); the same application was used to generate the line graphs and box plots.
Data availability.
All sequence data determined in work have been submitted to the European Nucleotide Archive (ENA) (study identifier [ID]: ERP109468).
Summary of detail quality parameters of whole-genome sequences in this study. Different quality parameters at the posttrimming and de novo assembly stages were added separately. Download Data Set S2, XLSX file, 0.1 MB (69.6KB, xlsx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We thank the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics (funded by Wellcome Trust grant reference 090532/Z/09/Z) for the generation of the sequencing data. We acknowledge the guidance from Maksuda Islam and technical assistance from Hafizur Rahman during the antimicrobial susceptibility tests.
This study received funding from the EU Horizon 2020 research and innovation program under grant agreement no. 643476. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
A.M.T. received a “Allocations de Recherche pour une Thèse au Sud (ARTS)” PhD scholarship from Institut de Recherche pour le Développement (IRD) and from Fondation Mérieux in France.
We declare no conflicts of interest.
Footnotes
Citation Tanmoy AM, Westeel E, De Bruyne K, Goris J, Rajoharison A, Sajib MSI, Van Belkum A, Saha SK, Komurian-Pradel F, Endtz HP. 2018. Salmonella enterica serovar Typhi in Bangladesh: exploration of genomic diversity and antimicrobial resistance. mBio 9:e02112-18. https://doi.org/10.1128/mBio.02112-18.
Contributor Information
Keith P. Klugman, Emory University.
Firdausi Qadri, International Centre for Diarrhoeal Disease Research, Bangladesh.
Peter Gerner-Smidt, Centers for Disease Control & Prevention.
REFERENCES
- 1.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull World Health Organ 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, Mintz ED. 2010. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. 2002. Typhoid fever. N Engl J Med 347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 4.Franco-Paredes C, Khan MI, Gonzalez-Diaz E, Santos-Preciado JI, Rodriguez-Morales AJ, Gotuzzo E. 2016. Enteric fever: a slow response to an old plague. PLoS Negl Trop Dis 10:e0004597. doi: 10.1371/journal.pntd.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. 2015. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das S, Samajpati S, Ray U, Roy I, Dutta S. 2017. Antimicrobial resistance and molecular subtypes of Salmonella enterica serovar Typhi isolates from Kolkata, India over a 15 years period 1998–2012. Int J Med Microbiol 307:28–36. doi: 10.1016/j.ijmm.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Zellweger RM, Basnyat B, Shrestha P, Prajapati KG, Dongol S, Sharma PK, Koirala S, Darton TC, Dolecek C, Thompson CN, Thwaites GE, Baker SG, Karkey A. 2017. A 23-year retrospective investigation of Salmonella Typhi and Salmonella Paratyphi isolated in a tertiary Kathmandu hospital. PLoS Negl Trop Dis 11:e0006051. doi: 10.1371/journal.pntd.0006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saha S, Saha S, Ruhulamin M, Hanif M, Islam M. 1997. Decreasing trend of multiresistant Salmonella typhi in Bangladesh. J Antimicrob Chemother 39:554–556. doi: 10.1093/jac/39.4.554. [DOI] [PubMed] [Google Scholar]
- 9.Olarte J, Galindo E. 1973. Salmonella typhi resistant to chloramphenicol, ampicillin, and other antimicrobial agents: strains isolated during an extensive typhoid fever epidemic in Mexico. Antimicrob Agents Chemother 4:597–601. doi: 10.1128/AAC.4.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SM, Palumbo PE, Edelson PJ. 1984. Salmonella strains resistant to multiple antibiotics: therapeutic implications. Pediatr Infect Dis 3:455–460. doi: 10.1097/00006454-198409000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Iyer RN, Jangam RR, Jacinth A, Venkatalakshmi A, Nahdi FB. 2017. Prevalence and trends in the antimicrobial susceptibility pattern of Salmonella enterica serovars Typhi and Paratyphi A among children in a pediatric tertiary care hospital in South India over a period of ten years: a retrospective study. Eur J Clin Microbiol Infect Dis 36:2399–2404. doi: 10.1007/s10096-017-3073-x. [DOI] [PubMed] [Google Scholar]
- 12.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, Wong VK, Dallman TJ, Nair S, Baker S, Shaheen G, Qureshi S, Yousafzai MT, Saleem MK, Hasan Z, Dougan G, Hasan R. 2018. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 9:e00105-18. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gul D, Potter RF, Riaz H, Ashraf ST, Wallace MA, Munir T, Ali A, Burnham C-A, Dantas G, Andleeb S. 2017. Draft genome sequence of a Salmonella enterica serovar Typhi strain resistant to fourth-generation cephalosporin and fluoroquinolone antibiotics. Genome Announcements 5:e00850-17. doi: 10.1128/genomeA.00850-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SR, Bharti S, Pratap CB, Nath G. 2017. Drug resistance pattern in the recent isolates of Salmonella Typhi with special reference to cephalosporins and azithromycin in the Gangetic plain. J Clin Diagn Res 11:DM01. doi: 10.7860/JCDR/2017/23330.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godbole GS, Day MR, Murthy S, Chattaway MA, Nair S. 2018. First report of CTX-M-15 Salmonella Typhi From England. Clin Infect Dis 66:1976–1977. doi: 10.1093/cid/ciy032. [DOI] [PubMed] [Google Scholar]
- 16.Saha SK, Talukder SY, Islam M, Saha S. 1999. A highly ceftriaxone-resistant Salmonella typhi in Bangladesh. Pediatr Infect Dis J 18:387. doi: 10.1097/00006454-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Djeghout B, Saha S, Sajib MSI, Tanmoy AM, Islam M, Kay GL, Langridge GC, Endtz HP, Wain J, Saha SK. 2018. Ceftriaxone-resistant Salmonella Typhi carries an IncI1-ST31 plasmid encoding CTX-M-15. J Med Microbiol 67:620–627. doi: 10.1099/jmm.0.000727. [DOI] [PubMed] [Google Scholar]
- 18.Choudhary A, Gopalakrishnan R, Senthur NP, Ramasubramanian V, Ghafur KA, Thirunarayan M. 2013. Antimicrobial susceptibility of Salmonella enterica serovars in a tertiary care hospital in southern India. Indian J Medical Res 137:800–802. [PMC free article] [PubMed] [Google Scholar]
- 19.Ashton PM, Owen SV, Kaindama L, Rowe WPM, Lane CR, Larkin L, Nair S, Jenkins C, de Pinna EM, Feasey NA, Hinton JCD, Dallman TJ. 2017. Public health surveillance in the UK revolutionises our understanding of the invasive Salmonella Typhimurium epidemic in Africa. Genome Med 9:92. doi: 10.1186/s13073-017-0480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day MR, Doumith M, Do Nascimento V, Nair S, Ashton PM, Jenkins C, Dallman TJ, Stevens FJ, Freedman J, Hopkins KL, Woodford N, De Pinna EM, Godbole G. 2018. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Salmonella enterica serovars Typhi and Paratyphi. J Antimicrob Chemother 73:365–372. doi: 10.1093/jac/dkx379. [DOI] [PubMed] [Google Scholar]
- 21.Anes J, Hurley D, Martins M, Fanning S. 2017. Exploring the genome and phenotype of multi-drug resistant Klebsiella pneumoniae of clinical origin. Front Microbiol 8:1913. doi: 10.3389/fmicb.2017.01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tasmin R, Hasan NA, Grim CJ, Grant AQuette, Choi SY, Alam MS, Bell R, Cavanaugh C, Balan KV, Babu US, Parveen S. 2017. Genotypic and phenotypic characterization of multidrug resistant Salmonella Typhimurium and Salmonella Kentucky strains recovered from chicken carcasses. PLoS One 12:e0176938. doi: 10.1371/journal.pone.0176938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eppinger M, Pearson T, Koenig SSK, Pearson O, Hicks N, Agrawal S, Sanjar F, Galens K, Daugherty S, Crabtree J, Hendriksen RS, Price LB, Upadhyay BP, Shakya G, Fraser CM, Ravel J, Keim PS. 2014. Genomic epidemiology of the Haitian cholera outbreak: a single introduction followed by rapid, extensive, and continued spread characterized the onset of the epidemic. mBio 5:e01721-14. doi: 10.1128/mBio.01721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moura A, Criscuolo A, Pouseele H, Maury MM, Leclercq A, Tarr C, Björkman JT, Dallman T, Reimer A, Enouf V. 2017. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat Microbiol 2:16185. doi: 10.1038/nmicrobiol.2016.185 ]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong VK, Holt KE, Okoro C, Baker S, Pickard DJ, Marks F, Page AJ, Olanipekun G, Munir H, Alter R, Fey PD, Feasey NA, Weill F-X, Le Hello S, Hart PJ, Kariuki S, Breiman RF, Gordon MA, Heyderman RS, Jacobs J, Lunguya O, Msefula C, MacLennan CA, Keddy KH, Smith AM, Onsare RS, De Pinna E, Nair S, Amos B, Dougan G, Obaro S. 2016. Molecular surveillance identifies multiple transmissions of typhoid in West Africa. PLoS Negl Trop Dis 10:e0004781. doi: 10.1371/journal.pntd.0004781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA, Kingsley RA, Thomson NR, Keane JA, Weill F-X, Edwards DJ, Hawkey J, Harris SR, Mather AE, Cain AK, Hadfield J, Hart PJ, Thieu NTV, Klemm EJ, Glinos DA, Breiman RF, Watson CH, Kariuki S, Gordon MA, Heyderman RS, Okoro C, Jacobs J, Lunguya O, Edmunds WJ, Msefula C, Chabalgoity JA, Kama M, Jenkins K, Dutta S, Marks F, Campos J, Thompson C, Obaro S, MacLennan CA, Dolecek C, Keddy KH, Smith AM, Parry CM, Karkey A, Mulholland EK, Campbell JI, Dongol S, Basnyat B, Dufour M, Bandaranayake D, et al. 2015. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 47:632–639. doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong VK, Baker S, Connor TR, Pickard D, Page AJ, Dave J, Murphy N, Holliman R, Sefton A, Millar M, Dyson ZA, Dougan G, Holt KE, International Typhoid Consortium. 2016. An extended genotyping framework for Salmonella enterica serovar Typhi, the cause of human typhoid. Nat Commun 7:12827. doi: 10.1038/ncomms12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thanh DP, Karkey A, Dongol S, Thi NH, Thompson CN, Rabaa MA, Arjyal A, Holt KE, Wong V, Thieu NTV. 2016. A novel ciprofloxacin-resistant subclade of H58 Salmonella Typhi is associated with fluoroquinolone treatment failure. Elife 5:e14003. doi: 10.7554/eLife.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britto CD, Dyson ZA, Duchene S, Carter MJ, Gurung M, Kelly DF, Murdoch DR, Ansari I, Thorson S, Shrestha S, Adhikari N, Dougan G, Holt KE, Pollard AJ. 2018. Laboratory and molecular surveillance of paediatric typhoidal Salmonella in Nepal: antimicrobial resistance and implications for vaccine policy. PLoS Negl Trop Dis 12:e0006408. doi: 10.1371/journal.pntd.0006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MT, Sebaihia M, Baker S, Basham D, Brooks K, Chillingworth T, Connerton P, Cronin A, Davis P, Davies RM, Dowd L, White N, Farrar J, Feltwell T, Hamlin N, Haque A, Hien TT, Holroyd S, Jagels K, Krogh A, Larsen TS, Leather S, Moule S, O’Gaora P, Parry C, Quail M, Rutherford K, Simmonds M, Skelton J, Stevens K, Whitehead S, Barrell BG. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 31.Chiou C-S, Lauderdale T-L, Phung DC, Watanabe H, Kuo J-C, Wang P-J, Liu Y-Y, Liang S-Y, Chen P-C. 2014. Antimicrobial resistance in Salmonella enterica serovar Typhi isolates from Bangladesh, Indonesia, Taiwan, and Vietnam. Antimicrob Agents Chemother 58:6501–6507. doi: 10.1128/AAC.03608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap K-P, Ho WS, Gan HM, Chai LC, Thong KL. 2016. Global MLST of Salmonella Typhi revisited in post-genomic era: genetic conservation, population structure, and comparative genomics of rare sequence types. Front Microbiol 7:270. doi: 10.3389/fmicb.2016.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enterobase. 2018. University of Warwick, Coventry, United Kingdom: http://enterobase.warwick.ac.uk/species/senterica/search_strains?query_st_search. Accessed 2 August 2018. [Google Scholar]
- 34.Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, Lukwesa-Musyani C, Tambatamba B, Mwaba J, Kalonda A, Nakazwe R, Kwenda G, Jensen JD, Svendsen CA, Dittmann KK, Kaas RS, Cavaco LM, Aarestrup FM, Hasman H, Mwansa JCL. 2015. Genomic signature of multidrug-resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol 53:262–272. doi: 10.1128/JCM.02026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidgell C, Reichard U, Wain J, Linz B, Torpdahl M, Dougan G, Achtman M. 2002. Salmonella Typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect Genet Evol 2:39–45. doi: 10.1016/S1567-1348(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 36.Xia X, Li W-H. 1998. What amino acid properties affect protein evolution? J Mol Evol 47:557–564. doi: 10.1007/PL00006412. [DOI] [PubMed] [Google Scholar]
- 37.Moon CP, Fleming KG. 2011. Side-chain hydrophobicity scale derived from transmembrane protein folding into lipid bilayers. Proc Natl Acad Sci U S A 108:10174–10177. doi: 10.1073/pnas.1103979108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holt KE, Baker S, Dongol S, Basnyat B, Adhikari N, Thorson S, Pulickal AS, Song Y, Parkhill J, Farrar JJ, Murdoch DR, Kelly DF, Pollard AJ, Dougan G. 2010. High-throughput bacterial SNP typing identifies distinct clusters of Salmonella Typhi causing typhoid in Nepalese children. BMC Infect Dis 10:144. doi: 10.1186/1471-2334-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyson ZA, Thanh DP, Bodhidatta L, Mason CJ, Srijan A, Rabaa MA, Vinh PV, Thanh TH, Thwaites GE, Baker S, Holt KE. 2017. Whole genome sequence analysis of Salmonella Typhi isolated in Thailand before and after the introduction of a national immunization program. PLoS Negl Trop Dis 11:e0005274. doi: 10.1371/journal.pntd.0005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma V, Dahiya S, Jangra P, Kumar R, Sood S, Kapil A. 2013. Study of the role of efflux pump in ciprofloxacin resistance in Salmonella enterica serotype Typhi. Indian J Med Microbiol 31:374–378. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Cui S, McDermott PF, Zhao S, White DG, Paulsen I, Meng J. 2007. Contribution of target gene mutations and efflux to decreased susceptibility of Salmonella enterica serovar Typhimurium to fluoroquinolones and other antimicrobial. Antimicrob Agents Chemother 51:535–542. doi: 10.1128/AAC.00600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey AM, Ivens A, Kingsley R, Cottell JL, Wain J, Piddock LJ. 2010. RamA, a member of the AraC/XylS family, influences both virulence and efflux in Salmonella enterica serovar Typhimurium. J Bacteriol 192:1607–1616. doi: 10.1128/JB.01517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagès J-M, Amaral L, Fanning S. 2011. An original deal for new molecule: reversal of efflux pump activity, a rational strategy to combat gram-negative resistant bacteria. Curr Med Chem 18:2969–2980. doi: 10.2174/092986711796150469. [DOI] [PubMed] [Google Scholar]
- 44.Nikaido H, Basina M, Nguyen V, Rosenberg EY. 1998. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J Bacteriol 180:4686–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okusu H, Ma D, Nikaido H. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol 178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padilla E, Llobet E, Doménech-Sánchez A, Martínez-Martínez L, Bengoechea JA, Albertí S. 2010. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother 54:177–183. doi: 10.1128/AAC.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaffe A, Chabbert YA, Semonin O. 1982. Role of porin proteins OmpF and OmpC in the permeation of beta-lactams. Antimicrob Agents Chemother 22:942–948. doi: 10.1128/AAC.22.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siu L, Ho P, Yuen K, Wong S, Chau P. 1997. Transferable hyperproduction of TEM-1 β-lactamase in Shigella flexneri due to a point mutation in the pribnow box. Antimicrob Agents Chemother 41:468–470. doi: 10.1128/AAC.41.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed D, Hoque A, Mazumder R, Nahar K, Islam N, Gazi SA, Hossain MA. 2012. Salmonella enterica serovar Typhi strain producing extended-spectrum β-lactamases in Dhaka, Bangladesh. J Med Microbiol 61:1032–1033. doi: 10.1099/jmm.0.044065-0. [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer Y, Matten J, Rabsch W. 2009. Salmonella enterica serovar Typhi with CTX-M β-lactamase, Germany. Emerg Infect Dis 15:1533. doi: 10.3201/eid1509.090567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang W-C, Chan O-W, Wu T-L, Chen C-L, Su L-H, Chiu C-H. 2016. Development of ceftriaxone resistance in Salmonella enterica serotype Oranienburg during therapy for bacteremia. J Microbiol Immunol Infect 49:41–45. doi: 10.1016/j.jmii.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Li W-C, Huang F-Y, Liu C-P, Weng L-C, Wang N-Y, Chiu N-C, Chiang C-S. 2005. Ceftriaxone resistance of nontyphoidal Salmonella enterica isolates in Northern Taiwan attributable to production of CTX-M-14 and CMY-2 β-lactamases. J Clin Microbiol 43:3237–3243. doi: 10.1128/JCM.43.7.3237-3243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker S, Duy PT, Nga TVT, Dung TTN, Phat VV, Chau TT, Turner AK, Farrar J, Boni MF. 2013. Fitness benefits in fluoroquinolone-resistant Salmonella Typhi in the absence of antimicrobial pressure. Elife 2:e01229. doi: 10.7554/eLife.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saha SK. 2018. Commentary: the new typhoid conjugate vaccine marks the dawn of a unique beginning. https://www.jhsph.edu/ivac/2018/03/27/the-new-typhoid-conjugate-vaccine-marks-the-dawn-of-a-unique-beginning/.
- 55.Levine MM, Simon R. 2018. The gathering storm: is untreatable typhoid fever on the way? mBio 9:e00482-18. doi: 10.1128/mBio.00482-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saha SK, Baqui AH, Hanif M, Darmstadt GL, Ruhulamin M, Nagatake T, Santosham M, Black RE. 2001. Typhoid fever in Bangladesh: implications for vaccination policy. Pediatr Infect Dis J 20:521–524. doi: 10.1097/00006454-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 57.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters v8.0:5-9. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf.
- 58.Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 59.Ewels P, Magnusson M, Lundin S, Käller M. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larsen MV, Cosentino S, Lukjancenko O, Saputra D, Rasmussen S, Hasman H, Sicheritz-Pontén T, Aarestrup FM, Ussery DW, Lund O. 2014. Benchmarking of methods for genomic taxonomy. J Clin Microbiol 52:1529–1539. doi: 10.1128/JCM.02981-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasman H, Saputra D, Sicheritz-Ponten T, Lund O, Svendsen CA, Frimodt-Møller N, Aarestrup FM. 2014. Rapid whole genome sequencing for the detection and characterization of microorganisms directly from clinical samples. J Clin Microbiol 52:139. doi: 10.1128/JCM.02452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang S, Yin Y, Jones MB, Zhang Z, Kaiser BLD, Dinsmore BA, Fitzgerald C, Fields PI, Deng X. 2015. Salmonella serotype determination utilizing high-throughput genome sequencing data. J Clin Microbiol 53:1685. doi: 10.1128/JCM.00323-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.R Core Team. 2014. R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- 65.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of genotype 4.3.1 (H58) isolates from Bangladesh, Pakistan, and Nepal in a wgSNP-derived (with singleton) MLT. (a) MLT with all singletons. (b) Divergence among all XDR isolates from Pakistan. All singletons were considered in the consensus SNP data. The tree is colored by country. Different data points, such as those corresponding to lineages, sublineages (details), the presence of different gyrA-83 mutations, MDR, cip resistance, presence of qnr genes, and cro resistance phenotypes, are shown (by colors) in different circles around the tree. Download FIG S1, TIF file, 3.4 MB (3.4MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List and characteristics of detected genes with efflux pump and membrane permeability activity. Download Table S1, DOCX file, 0.01 MB (13.4KB, docx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Annual distribution of genotypes in a wgSNP-derived MLT, showing the mutation profiles, qnr genes, MIC, and CIP resistance phenotypes of our isolates. No singleton was considered in the consensus SNP data. The tree is colored by year, and additional information is displayed in the different circles. Download FIG S2, TIF file, 4.2 MB (4.2MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Annual distribution of dominant H58 sublineages in Bangladesh. Only sublineages Ia, Bdq, and Bd (except Bdq) are considered here. Download FIG S3, TIF file, 3.2 MB (3.2MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of the (a) ciprofloxacin MIC and (b) ceftriaxone MIC for different H58 lineages of isolates from Bangladesh. Download FIG S4, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Association of specific mutations with different genotypes in a wgSNP-derived MLT. No singleton was considered in the consensus SNP data. Mutations such as gyrA S538N, gyrA N529S, and parE A364V are shown in comparison with genotypes 4.3.1, 2.0, and 3.3, respectively. All isolates from Bangladesh (our study), Pakistan, and Nepal are included. Download FIG S5, TIF file, 3.9 MB (3.9MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Summary of our samples sequenced in this study. Data points include the following: sample identifier (id), accession numbers, year of isolation, age, sex, antimicrobial susceptibility patterns (ampicillin, AMP; co-trimoxazole, SXT; chloramphenicol, CHL; ciprofloxacin, CIP; ceftriaxone, CRO), MDR (or non-MDR) status, phenotype, MLST type, genotype, H58 lineages and sublineages, quinolone resistance-determining region (QRDR) mutations (in gyrA/B and parC/E genes), and presence of resistance genes (blaTEM-1B, catA1, dfrA7, sul1, sul2, qnrS1, strA, strB, tetA, and tetB). Download Data Set S1, XLSX file, 0.1 MB (91.3KB, xlsx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of all isolates from Bangladesh in a cgMLST-derived UPGMA tree. The tree is colored according to genotype. Data points such as those corresponding to MLST type, lineage, and sublineage are indicated (by colors) in different circles around the tree. Download FIG S6, TIF file, 4.2 MB (4.3MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Workflow of our study from the biobank of >3,000 S. Typhi isolates to the WGS data analysis of 539 isolates. Download FIG S7, TIF file, 2 MB (2.2MB, tif) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental methods. Download Text S1, DOCX file, 0.01 MB (18.5KB, docx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Summary of detail quality parameters of whole-genome sequences in this study. Different quality parameters at the posttrimming and de novo assembly stages were added separately. Download Data Set S2, XLSX file, 0.1 MB (69.6KB, xlsx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
All sequence data determined in work have been submitted to the European Nucleotide Archive (ENA) (study identifier [ID]: ERP109468).
Summary of detail quality parameters of whole-genome sequences in this study. Different quality parameters at the posttrimming and de novo assembly stages were added separately. Download Data Set S2, XLSX file, 0.1 MB (69.6KB, xlsx) .
Copyright © 2018 Tanmoy et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.