Abstract
The Late Triassic and Early Toarcian extinction events are both associated with greenhouse warming events triggered by massive volcanism. These Mesozoic hyperthermals were responsible for the mass extinction of marine organisms and resulted in significant ecological upheaval. It has, however, been suggested that these events merely involved intensification of background extinction rates rather than significant shifts in the macroevolutionary regime and extinction selectivity. Here, we apply a multivariate modelling approach to a vast global database of marine organisms to test whether extinction selectivity varied through the Late Triassic and Early Jurassic. We show that these hyperthermals do represent shifts in the macroevolutionary regime and record different extinction selectivity compared to background intervals of the Late Triassic and Early Jurassic. The Late Triassic mass extinction represents a more profound change in selectivity than the Early Toarcian extinction but both events show a common pattern of selecting against pelagic predators and benthic photosymbiotic and suspension-feeding organisms, suggesting that these groups of organisms may be particularly vulnerable during episodes of global warming. In particular, the Late Triassic extinction represents a macroevolutionary regime change that is characterized by (i) the change in extinction selectivity between Triassic background intervals and the extinction event itself; and (ii) the differences in extinction selectivity between the Late Triassic and Early Jurassic as a whole.
Keywords: mass extinction, palaeoecology, modelling, Mesozoic, hyperthermal
1. Introduction
The Late Triassic to Early Jurassic interval contains two major extinction events: the Late Triassic mass extinction (LTE; ca 201 Ma) [1] and the Early Toarcian extinction (EToE; ca 187 Ma) [2]. The LTE is recognized as the second largest marine biodiversity loss [3] and third biggest ecological crisis of the Phanerozoic [4], resulting in a global reef crisis [5], the most severe extinction of scleractinian corals [6,7], significant extinctions among ammonoids [8], bivalves [9], and marine vertebrates [10], and the final demise of the conodonts [11]. By comparison, the EToE was smaller in magnitude, but records a similar pattern of selective losses, with an associated reef crisis [5], high levels of extinction among bivalves [12] and ammonoids [13], and the collapse of both benthic and pelagic marine ecosystems [7,8]. Both of these events are associated with, and likely caused by, elevated atmospheric CO2 levels and global warming [14–21]; i.e. they are hyperthermals. In each case, eruptions of Large Igneous Provinces (LIPs) probably caused the rise in CO2 [18,20–25], with proposed extinction drivers including rapid warming [14–17,20,26], ocean anoxia [16,17,26], and ocean acidification [27–29] as a direct result of the volcanic greenhouse gas emissions.
Several biological and ecological traits appear to have been selected against during the LTE, such as possessing a heavily calcified skeleton [27], inhabiting reef and/or inshore environments [30,31], and residing at tropical latitudes [31,32]. The greatest reduction in both taxonomic and functional richness occurred among sessile suspension-feeding guilds, particularly those dwelling in tropical reefs in the Panthalassa Ocean [31]. Despite this evidence for apparent selectivity during the LTE hyperthermal, it has been claimed that there was little change in ‘macroevolutionary regime’ [30] compared to the rest of the Late Triassic and Early Jurassic, and that the LTE simply reflects intensification of the high rates of background extinction already experienced through the Late Triassic [30,33,34]. There has been less research on selectivity during the EToE hyperthermal, although there is some evidence for loss of reef taxa [5], selection against endemic taxa [12], the motile benthos [35], infaunal organisms [12,17,35,36], as well as higher levels of extinction in the restricted basins of north-west Tethys, northeast Panthalassa [35], and the Boreal Ocean [2,36] as well as in the Southern Hemisphere [37]. Whether this represents a macroevolutionary regime shift compared to Jurassic background extinction is unknown.
Here, we provide the first multivariate analysis of ecological selectivity during the Late Triassic and Early Jurassic, in order to determine whether there are any substantial differences between the LTE and EToE hyperthermal events and the periods of normal background extinction, and hence whether a shift in macroevolutionary regime occurred. A macroevolutionary regime shift is recognized when the suite of traits that promote extinction or survivorship are different, and particularly when the direction of selectivity changes so that traits that conferred survivorship during background times become an extinction risk [38]. We apply a generalized linear modelling (GLM) methodology to the largest and most comprehensive global database yet analysed in order to assess the relative importance of a number of intrinsic and extrinsic ecological variables as determinants of extinction in marine ecosystems. We aim to test the following hypotheses: (i) do certain ecological variables (e.g. latitudinal distribution, habitat preference, feeding mode, and calcification) correlate with higher extinction risk during the LTE and EToE hyperthermal events, (ii) are similar trends recorded in both past hyperthermals despite differences in starting conditions and magnitude, and (iii) are similar trends recorded during background times, or do the LTE and EToE hyperthermals represent significantly different extinction selectivity?
2. Methods
We used a database of fossil occurrences of Middle Triassic to Middle Jurassic (Ladinian-Aalenian) marine animal genera collated from the Paleobiology Database (PaleoDB) [39,40]. The total dataset comprises 55 428 occurrences of 2 621 genera, which is more than double the number that was available for previous analyses, e.g. [30,32]. Each genus was then classified according to a number of extrinsic (i.e. abiotic) and intrinsic (i.e. biotic) ecological variables: (table 1; see [31] for detailed download, vetting, and classification information). Proportional generic extinction rates were calculated and plotted at the stage level for guilds of fossil organisms defined by each ecological variable (table 1). In order to account for biases brought about by uneven sampling across space and through time, we applied a subsampling protocol to standardize proportional extinction on the basis of the number of fossil occurrences. All variables were subsampled to n = 250 per stage, for 1 000 iterations apart from feeding, which was subsampled to n = 75, due to the increased number of variable arguments and thus reduced sample sizes after splitting occurrences via feeding mode. Lightly calcified taxa, polar latitude, Boreal Ocean, and reef taxa all fall short of the subsampling requirement for at least one of the time bins and are, therefore, not plotted in the univariate time series. However, when amalgamated with the other variables for the multivariate analyses, they provide sample sizes that are sufficient for the GLM analyses.
Table 1.
Summary of intrinsic and extrinsic ecological determinants of extinction.
| determinant | levels | ref. | |
|---|---|---|---|
| intrinsic | motility | motile non-motile |
[31,41] |
| tiering | pelagic epifaunal infaunal |
[31,41] | |
| feeding | suspension deposit/mining grazing predatory photosymbiotic |
[31,41] | |
| calcification | heavy moderate light |
[31,42] | |
| extrinsic | latitude | polar (>60°) mid-latitude (30–60°) tropical (<30°) |
[31,39] |
| palaeo-ocean basin | Panthalassa Tethys Boreal |
[31,39] | |
| environment | onshore offshore reef |
[31,39] |
Multiple ecological variables are not independent of one another in terms of proportional extinction through time, therefore it is essential to test their effects on extinction within a multivariate framework. For example, pelagic taxa within the database are predominantly predatory and fast-moving as the majority of pelagic taxa are vertebrates or cephalopods. Therefore, it is impossible to determine which, if any, of these three variables is influencing extinction rates in a univariate analysis. We applied GLMs with a binomial distribution and a logit link function (i.e. multiple logistic regression models) to test the effects of multiple ecological variables on proportional generic extinction through the study interval [43]. The major extinction episodes of the LTE (Rhaetian/Hettangian) and EToE (Pliensbachian/Toarcian) were analysed separately and compared to the other stage boundaries which, together, are treated as representing the background intervals of the Triassic and Jurassic. However, because the binomial models were strongly underdispersed, we then used quasi-binomial models and estimated the dispersion parameter from the data [44]. Underdispersal, where the variance is less than the nominal mean [45], can lead to over-conservatism and thus can result in type II errors. We applied the GLMs to two datasets: (i) including all the ecological variables, and (ii) a separate dataset compiled without the depositional setting variable, because reliable depositional setting data only exist for around 50% of the fossil occurrences in the entire dataset. A number of model runs were carried out with different variable combinations for each of the four broad time intervals: Triassic background (Ladinian-Carnian, Carnian-Norian, Norian-Rhaetian); LTE (Rhaetian–Hettangian); Jurassic background (Hettangian-Sinemurian, Sinemurian-Pliensbachian, Toarcian-Aalenian); and EToE (Pliensbachian-Toarcian). Model selection was carried out by using the drop1() command in R, which drops one explanatory variable in turn and each time applies an analysis of deviance test (F-test) [43]. The data for the GLMs were not subsampled, but any ecological guilds with consistently low sample sizes (less than 10 occurrences per bin) were omitted from the analyses. All analyses were carried in R v. 3.4.3 [46].
3. Results
(a). Univariate time series
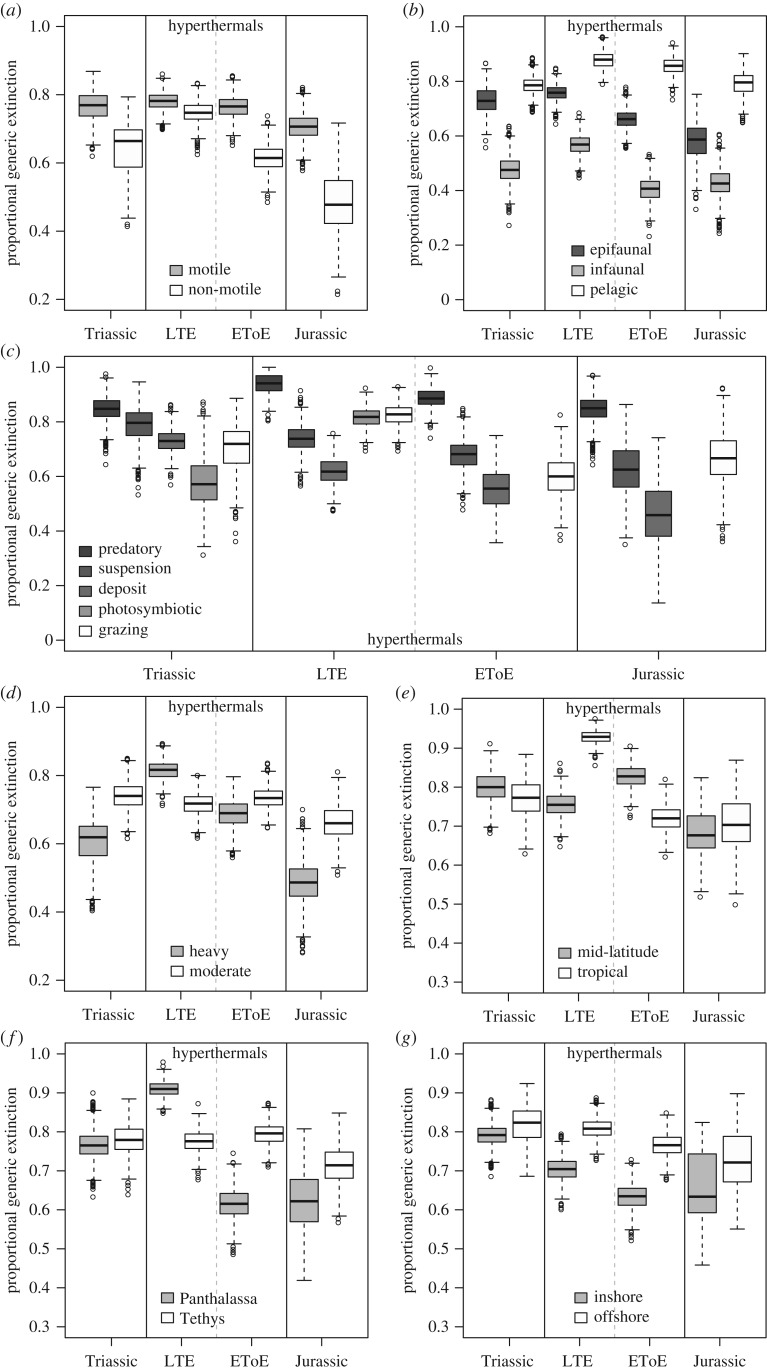
The data show clear differences in extinction magnitude and selectivity between the hyperthermals and background intervals (figure 1). For many, but not all variables (e.g. infaunal taxa at EToE, moderate calcifiers at LTE, mid-latitude taxa at LTE), extinction magnitude is greater during the hyperthermals than during their respective background times, as expected, and the pattern of relative selectivity remains the same (e.g. figure 1a,b). During the LTE, however, feeding, calcification, latitude, and ocean basin all record different patterns of selectivity compared to the Triassic background (figure 1c–f). Photosymbiotic taxa suffered a substantially greater extinction during the LTE than during Triassic background periods, overtaking suspension- and deposit-feeders in relative extinction risk (figure 1c). Likewise, heavily calcified taxa and those that live in the tropics and Panthalassa record the greatest extinction magnitudes during the LTE hyperthermal, which represents a marked shift in selectivity compared to the Triassic background (figure 1d–f). By contrast, the only similar shift in selectivity during the EToE, compared to the Jurassic background, occurs with latitude, with mid-latitude taxa showing greater extinction risk than tropical taxa during the hyperthermal (figure 1e). With a few exceptions, remaining variables all increase during the EToE event, suggesting the event mainly represents an intensification of Jurassic background extinction rates.
Figure 1.
Boxplots across all subsamples of proportional generic extinction per ecological variable through Late Triassic background periods (Triassic), the Late Triassic mass extinction (LTE), the Early Toarcian extinction (EToE), and Early Jurassic background periods (Jurassic) by (a) motility; (b) tiering; (c) feeding; (d) calcification; (e) latitude; (f) ocean basin; (g) depositional setting. Proportional generic extinction is calculated from a subsample of n = 250 across 1 000 iterations except for feeding which is calculated from a subsample of n = 75 across 1 000 iterations. The solid black lines inside the boxes represent the medians, the top and bottom edges of the boxes correspond to the first and third quartiles, and whiskers represent the lowest and highest subsampled values within 1.5 times the interquartile range. Points outside the whiskers are outliers.
Unexpectedly, there also appear to be clear differences in background extinction magnitude and selectivity between the Triassic and Jurassic. Background extinction appears much higher during the Triassic than the Jurassic, with extinction magnitude in some guilds being higher during the Triassic background intervals than during the EToE (figure 1).
(b). Generalized linear modelling
Multivariate analyses demonstrate clear differences between background and mass extinction intervals (table 2 for GLM results). In general, the suite of ecological variables (table 1) analysed in this study explain far less of the recorded extinction during background times compared to the two hyperthermal events. During the Triassic background interval, depositional setting is the only significant predictor of extinction, and only in the single model that considers just the set of extrinsic factors. This is due to reef taxa having significantly lower extinction risk than taxa that live in other settings. During the Jurassic background interval, when all ecological variables are considered, motility, palaeo-ocean basin, and depositional setting are all significant predictors of extinction, with pelagic taxa and taxa residing in the Tethys Ocean having higher extinction and reef dwellers having lower extinction. The best-fitting model identifies only palaeo-ocean (i.e. Tethys) and depositional setting (reefs) as being significant predictors of extinction. The only other model run that identifies a significant predictor of extinction is the one that considers just the extrinsic ecological variables. In that case, palaeo-oceanic basin is again identified as having a significant bearing on extinction, due to the higher extinction in Tethys. In contrast to the background times, during the LTE and EToE events, many more model runs identify significant ecological predictors of extinction. Furthermore, those variables that are identified as being significant are _different to the ones identified during the background times.
Table 2.
Summary of full and best fitting GLMs for predicting extinction through hyperthermal mass extinctions and periods of background extinction. ‘Full model’ includes all variables (see electronic supplementary material for model definitions). ‘Best model’ is the best fitting model following model selection procedure described in methods section. ‘Significant variables' identifies variables and arguments identified as significantly determining extinction and shows which arguments of a particular variables show significantly higher (+) or significantly lower (−) extinction than other arguments of that variables. Numbers in brackets are explained deviance of each model = (null deviance-residual deviance)/null deviance; provides estimate of goodness-of-fit of model to extinction variable. Where no variables are listed, no variables significantly determine extinction.
| full model |
best model |
||||
|---|---|---|---|---|---|
| model | significant variables | explained deviance | significant variables | explained deviance | |
| LTE | all | latitude: tropical (+) | 0.78 | latitude: tropical (+) | 0.32 |
| all no env | feeding: predatory (+) photosymbiotic (+) suspension (+) |
0.60 | feeding: predatory (+) photosymbiotic (+) suspension (+) |
0.43 | |
| extrinsic | latitude: tropical (+) | 0.47 | latitude: tropical (+) | 0.32 | |
| extrinsic no env | — | 0.18 | — | — | |
| intrinsic | — | 0.40 | — | — | |
| intrinsic no env | feeding: predatory (+) photosymbiotic (+) |
0.48 | feeding: predatory (+) photosymbiotic (+) suspension (+) |
0.43 | |
| EToE | all | — | 0.68 | — | — |
| all no env | motility: non-motile (+) feeding: photosymbiotic (+) latitude: polar (−) basin: Boreal (+) calcification: light (−) |
0.71 | feeding: photosymbiotic (+) basin: Boreal (+) |
0.5 | |
| extrinsic | — | 0.23 | — | — | |
| extrinsic no env | basin: Boreal (+) | 0.17 | basin: Panthalassa (−) | 0.14 | |
| intrinsic | — | 0.44 | — | — | |
| intrinsic no env | feeding: photosymbiotic (+) | 0.48 | feeding: photosymbiotic (+) | 0.33 | |
| Triassic background | all | — | 0.34 | — | — |
| all no env | — | 0.27 | — | — | |
| extrinsic | environment: reef (−) | 0.19 | environment: reef (−) | 0.19 | |
| extrinsic no env | — | 0.03 | — | — | |
| intrinsic | — | 0.31 | — | — | |
| intrinsic no env | — | 0.23 | — | — | |
| Jurassic background | all | motility: pelagic (+) basin: Tethys (+) environment: reef (−) |
0.35 | basin: Tethys (+) environment: reef (−) |
0.16 |
| all no env | — | 0.28 | — | — | |
| extrinsic | basin: Tethys (+) | 0.16 | basin: Tethys (+) | 0.07 | |
| extrinsic no env | — | 0.03 | — | — | |
| intrinsic | — | 0.18 | — | — | |
| intrinsic no env | — | 0.25 | — | — | |
During the LTE, feeding or latitude are the only variables identified as being significant predictors of extinction. Latitude alone significantly predicts extinction when (a) all ecological variables are considered and (b) when only extrinsic factors are considered, using the smaller dataset that includes depositional setting. In each case, taxa residing at tropical latitudes show significantly higher extinction than those inhabiting higher latitudes. By contrast, feeding is identified as a significant predictor of extinction in two other model runs, but only using the expanded dataset that excludes depositional setting. In both cases, where (a) all variables or (b) just the intrinsic ones are considered, excluding depositional setting, the best-fitting models identify predatory, photosymbiotic, and suspension-feeding habits as being significant positive predictors of extinction.
For the EToE event, significant predictors of extinction are only identified in model runs that use the expanded dataset that excludes depositional setting. When all variables, apart from depositional setting, are considered, five factors (motility, feeding, latitude, palaeo-ocean basin, and calcification) all appear to significantly predict extinction. Model selection reveals that the best-fitting model identifies just feeding and palaeo-ocean basin, with photosymbiotic taxa and taxa residing in the Boreal Ocean predicting significantly higher extinction than other categories within those variables. When considering just the extrinsic ecological variables, no variable predicts extinction. However, when we use the expanded dataset with no depositional environment data, palaeo-ocean basin significantly predicts extinction with Boreal taxa having higher extinction and, after model selection, Panthalassa taxa show lower extinction than both Boreal and Tethys taxa. When considering only the intrinsic ecological variables, no variable predicts extinction until we use the expanded dataset with no depositional environment variable, after which, feeding significantly predicts extinction, with photosymbiotic taxa showing higher extinction than other feeding guilds.
4. Discussion
There are marked changes in extinction selectivity between periods of normal background and the two hyperthermals (figure 1 and table 2). Extinction magnitude is higher in most ecological guilds during the LTE and, although the EToE generally displays higher levels of extinction than Jurassic background intervals, for some guilds Triassic background extinction is higher than it is during the EToE (figure 1). Not only do the LTE and EToE events represent an increase in extinction intensity above respective Triassic and Jurassic background rates but, more importantly, and contrary to previous claims [30], there are differences in extinction selectivity between times of both background and mass extinction, and between Triassic and Jurassic intervals in general. It is also evident that background extinction was higher in the Late Triassic, prior to the LTE hyperthermal, than it was during the Early Jurassic, in the aftermath of the mass extinction [30,33] (figure 1).
A tropical extinction peak characterizes the LTE, whereas mid-latitude taxa display higher extinction during the EToE and during background times. Although the LTE data are consistent with expectations that an episode of global warming should result in extinction being concentrated in the tropics [32,47], the EToE data suggest that high tropical extinction may not be ubiquitous to all hyperthermal events. Additionally, higher tropical extinction across the LTE is only significant in the models where the data are also partitioned by depositional setting, suggesting that some of the tropical extinction signal is rooted in a particular depositional setting, most probably tropical reefs. Although extinction is higher in the mid-latitudes through the EToE [31], latitude does not significantly predict extinction, suggesting that the higher rates of extinction in the mid-latitudes during the EToE may be governed by other factors such as ocean basin or that the warming was not as intense as during the LTE [2].
The peak in extinction in Panthalassa during the LTE is not replicated in the Tethys Ocean, despite the Tethys showing higher background extinction rates. However, modelling does not show palaeo-ocean as a significant predictor of extinction across the LTE, suggesting that this peak is a result of other factors, such as the high proportion of tropical data in Rhaetian–Hettangian Panthalassa. By contrast, Panthalassa displays significantly lower extinction than the Tethys and Boreal oceans during the EToE. The EToE appears to be characterized by raised extinction rates in the Tethyan and Boreal oceans. This might be expected given the higher prevalence of restricted basins, particularly in north-western Tethys, when considering the repeated dysoxic conditions in the Early Jurassic, of which the EToE is the most severe [2,26]. However, this pattern persists in light of evidence for prolonged anoxia and extinction in some Panthalassa basins [35]. It is also likely that the mid to high palaeolatitude of the Boreal and north-western Tethys basins of Europe are driving the mid-latitudinal peak in extinction intensity through the EToE.
Although difficult to show because of very small sample sizes, the reef crises at the LTE and EToE are evident in the data by the crashes in reef taxa abundances [31] and diversity [7,31]. The reef crises are also highlighted by the high levels of extinction witnessed among photosymbiotic taxa and suspension feeders across the LTE, and photosymbiotic taxa across the EToE. In contrast to the extinction events, background extinction for reef taxa and photosymbiotic feeders was lower than those taxa residing in other depositional settings and feeding via different strategies. This highlights a major change in extinction selectivity during both the LTE and EToE and permits the rejection of the idea that the LTE is merely an intensification of background extinction seen during the Late Triassic [30].
Tiering does not appear to have an influence on extinction selectivity across the LTE, despite there being an increase in extinction magnitude across all guilds. However, the impact of the mass extinction on level-bottom communities was particularly short-lived with full recovery occurring by the upper Hettangian [48–50]. Therefore, the temporary disappearance of the deep infaunal and erect benthic tiers in the earliest Hettangian recorded by previous studies [48,50], would not be detected here because of the coarser nature of the stage-level time bins. There is some evidence of increased extinction risk to pelagic taxa during intervals of background and mass extinction, possibly related to high turnover of ammonoids and vertebrates, which also drives the consistently high levels of extinction in predatory taxa [13,51]. We see a similar pattern in terms of motility, with no apparent selectivity across the LTE or during periods of background extinction. There is some weak evidence for selectivity against non-motile taxa across the EToE, although this is not significant in the best-fitting model. The lack of any selectivity against non-motile and epifaunal taxa across the LTE suggests that the mass extinction did not result in an indirect intensification of the Mesozoic Marine Revolution (MMR) as previously suggested [31] and these previously detected high levels of extinction among non-motile epifauna are a result of elevated extinction among photosymbiotic/suspension-feeding guilds in reef environments, which are predominantly non-motile and epifaunal. Our analyses do detect higher levels of background extinction among non-motile and epifaunal taxa during the Late Triassic compared to the Early Jurassic. Crucially, however, motility and tiering do not predict extinction in the Late Triassic background interval. Therefore, we cannot find solid evidence of selectivity against non-motile epifauna during a time period (Carnian-Norian) that has been identified as key to the MMR [52]. The cause of higher Triassic background extinction versus Jurassic background extinction is likely a result of the high faunal turnover associated with the Carnian Pluvial Event [53], rather than the MMR.
There is a peak in extinction among heavily calcified taxa during the LTE whereas during background periods and the EToE heavy calcifiers display lower extinction than moderate calcifiers. Although this may support the hypotheses that hypercapnia [42] and/or ocean acidification may have played a role in extinction during the LTE [5,27], our modelling results show no evidence that calcification was a significant predictor of extinction during either the LTE or the EToE hyperthermal. The multivariate analyses show no evidence of selectivity against heavy calcifiers during the LTE and only very weak evidence at the EToE, although this result is non-significant in the best-fitting model. Our analyses support previous studies that found no strong link between calcification grade and extinction selectivity [30]. Therefore, it seems unlikely that hypercapnia or ocean acidification were the main or sole drivers of extinction during the LTE and EToE hyperthermals.
The LTE and, albeit to a lesser extent, the EToE are both characterized by shifts in extinction selectivity away from the macroevolutionary regimes of the Late Triassic and Early Jurassic background intervals. Background extinction rates in the Late Triassic were higher than those of the Early Jurassic [30,33], but the LTE was not merely an intensification of those background rates as has been previously suggested [30]. Extinction selectivity changed dramatically across the LTE with the initiation of strong selection against tropical taxa with photosymbiotic, suspension, or predatory feeding strategies. This pattern is consistent with a warming-driven tropical reef crisis. We find little evidence to support previous ideas that palaeo-ocean basin [31] or calcification [27] were important determinants of extinction at the LTE. Despite differences in starting conditions, species involved, and magnitudes of global warming and environmental change, the LTE and EToE show some common patterns of selectivity. Both events record strong extinction selectivity against pelagic predatory guilds and against benthic photosymbiotic and suspension-feeding organisms, suggesting that these groups of marine organisms may be particularly vulnerable during episodes of global warming. The effects of the LTE were most severe in the tropics while the EToE was felt more severely at higher latitudes, which may reflect differences in the magnitude of environmental change or starting conditions, such as palaeogeography. However, the EToE shares some common selectivity patterns with periods of Jurassic background extinction, i.e. high extinction in the Tethys Ocean, suggesting that the EToE may have represented an intensification of Jurassic background extinction, albeit with a switch to selecting against reef-inhabiting photosymbiotic taxa. The LTE shows a clear change in extinction selectivity and thus macroevolutionary regime which is characterized not only by a shift in extinction selectivity from Triassic background intervals across the LTE but also by the difference in extinction selectivity between the Late Triassic and Early Jurassic as a whole.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank Xiaoya Ma, Matthew Clapham and four anonymous reviewers for helpful editorial and analytical reviews that have greatly improved this manuscript. The authors also thank the numerous authors of the original studies that provide the source data on which this study is based, and the many data enterers of the Paleobiology Database for the provision of fossil occurrence data, particularly: Matthew Clapham, Wolfgang Kiessling, Franz Fürsich, Martin Aberhan, Andy Rees, József Pálfy, Matthew Carrano, David Bottjer, Alistair McGowan, Arnold Miller, Luc Villier, Roger Benson, John Alroy, and Richard Butler. This is Paleobiology Database publication 324.
Data accessibility
Additional data is available as part of the electronic supplementary material.
Authors' contributions
A.M.D., W.J.F., and R.J.T. conceived the study. A.M.D. and W.J.F. collected the data. S.A and J.S. wrote analytical code and advised on analytical methods. A.M.D. analysed the data. A.M.D. led the writing of the manuscript and W.J.F., S.A., and R.J.T. contributed to the writing and editing of the manuscript and preparation of figures.
Competing interests
We declare we have no competing interests.
Funding
Funding for this work was provided by a Leverhulme Early Career Fellowship (ECF-2015-044) and Natural Environmental Research Council research grant no. (NE/P013724/1) to A.M.D.
References
- 1.Lindström S, van de Schootbrugge B, Hansen KH, Pedersen GK, Alsen P, Thibault N, Dybkjær K, Bjerrum CJ, Nielsen LH. 2017. A new correlation of Triassic–Jurassic boundary successions in NW Europe, Nevada and Peru, and the Central Atlantic Magmatic Province: a time-line for the end-Triassic mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 478, 80–102. ( 10.1016/j.palaeo.2016.12.025) [DOI] [Google Scholar]
- 2.Little CT, Benton MJ. 1995. Early Jurassic mass extinction: a global long-term event. Geology 23, 495–498. ( 10.1130/0091-7613(1995)023%3C0495:EJMEAG%3E2.3.CO;2) [DOI] [Google Scholar]
- 3.Alroy J. 2010. The shifting balance of diversity among major marine animal groups. Science 329, 1191–1194. ( 10.1126/science.1189910) [DOI] [PubMed] [Google Scholar]
- 4.McGhee GR, Sheehan PM, Bottjer DJ, Droser ML. 2004. Ecological ranking of Phanerozoic biodiversity crises: ecological and taxonomic severities are decoupled. Publication 211, 289–297. ( 10.1016/j.palaeo.2004.05.010) [DOI] [Google Scholar]
- 5.Kiessling W, Simpson C. 2011. On the potential for ocean acidification to be a general cause of ancient reef crises. Glob. Change Biol. 17, 56–67. ( 10.1111/j.1365-2486.2010.02204.x) [DOI] [Google Scholar]
- 6.Flügel E. 2002. Triassic reef patterns. SEPM Special Publication 72, 391–463. [Google Scholar]
- 7.Martindale RC, Berelson WM, Corsetti FA, Bottjer DJ, West AJ. 2012. Constraining carbonate chemistry at a potential ocean acidification event (the Triassic-Jurassic boundary) using the presence of corals and coral reefs in the fossil record. Publication 350, 114–123. ( 10.1016/j.palaeo.2012.06.020) [DOI] [Google Scholar]
- 8.House MR. 1989. Ammonoid extinction events. Phil. Trans. R. Soc. Lond. B 325, 307–326. ( 10.1098/rstb.1989.0091) [DOI] [Google Scholar]
- 9.Ros S, Echevarría J. 2012. Ecological signature of the end-Triassic biotic crisis: what do bivalves have to say? Hist. Biol. 24, 489–503. ( 10.1080/08912963.2011.625568) [DOI] [Google Scholar]
- 10.Benson RBJ, Butler RJ, Lindgren J, Smith AS. 2010. Mesozoic marine tetrapod diversity: mass extinctions and temporal heterogeneity in geological megabiases affecting vertebrates. Proc. R. Soc. B 277, 829–834. ( 10.1098/rspb.2009.1845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bond DPG, Grasby SE. 2017. On the causes of mass extinctions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 478, 3–29. ( 10.1016/j.palaeo.2016.11.005) [DOI] [Google Scholar]
- 12.Aberhan M, Baumiller TK. 2003. Selective extinction among Early Jurassic bivalves: a consequence of anoxia. Geology 31, 1077–1080. ( 10.1130/G19938.1) [DOI] [Google Scholar]
- 13.Dera G, Neige P, Dommergues J-L, Fara E, Laffont R, Pellenard P. 2010. High-resolution dynamics of Early Jurassic marine extinctions: the case of Pliensbachian–Toarcian ammonites (Cephalopoda). J. Geol. Soc. 167, 21–33. ( 10.1144/0016-76492009-068) [DOI] [Google Scholar]
- 14.McElwain JC, Beerling DJ, Woodward FI. 1999. Fossil plants and global warming at the Triassic-Jurassic boundary. Science 285, 1386–1390. ( 10.1126/science.285.5432.1386) [DOI] [PubMed] [Google Scholar]
- 15.McElwain JC, Wade-Murphy J, Hesselbo SP. 2005. Changes in carbon dioxide during an oceanic anoxic event linked to intrusion into Gondwana coals. Nature 435, 479–482. ( 10.1038/nature03618) [DOI] [PubMed] [Google Scholar]
- 16.Danise S, Twitchett RJ, Little CTS. 2015. Environmental controls on Jurassic marine ecosystems during global warming. Geology 43, 263–266. ( 10.1130/g36390.1) [DOI] [Google Scholar]
- 17.Danise S, Twitchett RJ, Little CTS, Clémence M-E. 2013. The impact of global warming and Anoxia on Marine Benthic Community dynamics: an example from the Toarcian (Early Jurassic). PLoS ONE 8, e56255 ( 10.1371/journal.pone.0056255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaller MF, Wright JD, Kent DV. 2011. Atmospheric PCO2 perturbations associated with the Central Atlantic Magmatic Province. Science 331, 1404–1409. ( 10.1126/science.1199011) [DOI] [PubMed] [Google Scholar]
- 19.Blackburn TJ, Olsen PE, Bowring SA, McLean NM, Kent DV, Puffer J, McHone G, Rasbury ET, Et-Touhami M. 2013. Zircon U-Pb geochronology links the end-Triassic extinction with the Central Atlantic Magmatic Province. Science 340, 941–945. ( 10.1126/science.1234204) [DOI] [PubMed] [Google Scholar]
- 20.Whiteside J, Olsen PE, Eglinton T, Brookfield ME, Sambrotto RN. 2010. Compound-specific carbon isotopes from Earth's largest flood basalt eruptions directly linked to the end-Triassic mass extinction. Proc. Natl Acad. Sci. USA 107, 6721–6725. ( 10.1073/pnas.1001706107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhl M, Bonis NR, Reichart G-J, Damsté JSS, Kürschner WM. 2011. Atmospheric carbon injection linked to end-Triassic mass extinction. Science 333, 430–434. ( 10.1126/science.1204255) [DOI] [PubMed] [Google Scholar]
- 22.Burgess SD, Bowring SA, Fleming TH, Elliot DH. 2015. High-precision geochronology links the Ferrar large igneous province with early-Jurassic ocean anoxia and biotic crisis. Earth Planet. Sci. Lett. 415, 90–99. ( 10.1016/j.epsl.2015.01.037) [DOI] [Google Scholar]
- 23.Davies JHFL, Marzoli A, Bertrand H, Youbi N, Ernesto M, Schaltegger U. 2017. End-Triassic mass extinction started by intrusive CAMP activity. Nat. Commun. 8, 15596 ( 10.1038/ncomms15596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corso JD, et al. 2014. The dawn of CAMP volcanism and its bearing on the end-Triassic carbon cycle disruption. J. Geol. Soc. 171, 153–164. ( 10.1144/jgs2013-063) [DOI] [Google Scholar]
- 25.Heimdal TH, Svensen HH, Ramezani J, Iyer K, Pereira E, Rodrigues R, Jones MT, Callegaro S. 2018. Large-scale sill emplacement in Brazil as a trigger for the end-Triassic crisis. Sci. Rep. 8, 141 ( 10.1038/s41598-017-18629-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caruthers AH, Smith PL, Gröcke DR. 2014. The Pliensbachian–Toarcian (Early Jurassic) extinction: a North American perspective. Geol. Soc. Am. Spec. Papers 505, 225–243. ( 10.1130/2014.2505(11)) [DOI] [Google Scholar]
- 27.Hautmann M, Benton MJ, Tomasovych A. 2008. Catastrophic ocean acidification at the Triassic-Jurassic boundary. N. Jb. Geol. Palaont. Abh. 249, 119–127. ( 10.1127/0077-7749/2008/0249-0119) [DOI] [Google Scholar]
- 28.Trecalli A, Spangenberg J, Adatte T, Föllmi KB, Parente M. 2012. Carbonate platform evidence of ocean acidification at the onset of the early Toarcian oceanic anoxic event. Earth Planet. Sci. Lett. 357, 214–225. ( 10.1016/j.epsl.2012.09.043) [DOI] [Google Scholar]
- 29.Hautmann M. 2004. Effect of end-Triassic CO2 maximum on carbonate sedimentation and marine mass extinction. Facies 50, 257–261. ( 10.1007/s10347-004-0020-y) [DOI] [Google Scholar]
- 30.Kiessling W, Aberhan M, Brenneis B, Wagner PJ. 2007. Extinction trajectories of benthic organisms across the Triassic–Jurassic boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 244, 201–222. ( 10.1016/j.palaeo.2006.06.029) [DOI] [Google Scholar]
- 31.Dunhill AM, Foster WJ, Sciberras J, Twitchett RJ. 2018. Impact of the Late Triassic mass extinction on functional diversity and composition of marine ecosystems. Palaeontology 61, 133–148. ( 10.1111/pala.12332) [DOI] [Google Scholar]
- 32.Kiessling W, Aberhan M. 2007. Environmental determinants of marine benthic biodiversity dynamics through Triassic-Jurassic time. Paleobiology 33, 414–434. [Google Scholar]
- 33.Bambach RK. 2006. Phanerozoic biodiversity and mass extinctions. Ann. Rev. Earth Planet. Sci. 34, 127–155. ( 10.1146/annurev.earth.33.092203.122654) [DOI] [Google Scholar]
- 34.Bambach RK, Knoll AH, Wang SC. 2004. Origination, extinction, and mass depletions of marine diversity. Paleobiology 30, 522–542. ( 10.1666/0094-8373(2004)030%3C0522:OEAMDO%3E2.0.CO;2) [DOI] [Google Scholar]
- 35.Martindale RC, Aberhan M. 2017. Response of macrobenthic communities to the Toarcian Oceanic Anoxic Event in northeastern Panthalassa (Ya Ha Tinda, Alberta, Canada). Palaeogeogr. Palaeoclimatol. Palaeoecol. 478, 103–120. ( 10.1016/j.palaeo.2017.01.009) [DOI] [Google Scholar]
- 36.Harries PJ, Little CTS. 1999. The early Toarcian (Early Jurassic) and the Cenomanian–Turonian (Late Cretaceous) mass extinctions: similarities and contrasts. Palaeogeogr. Palaeoclimatol. Palaeoecol. 154, 39–66. ( 10.1016/S0031-0182(99)00086-3) [DOI] [Google Scholar]
- 37.Al-Suwaidi AH, Angelozzi GN, Baudin F, Damborenea SE, Hesselbo SP, Jenkyns HC, Manceñido MO, Riccardi AC. 2010. First record of the early Toarcian Oceanic Anoxic event from the Southern Hemisphere, Neuquén Basin, Argentina. J. Geol. Soc. 167, 633–636. ( 10.1144/0016-76492010-025) [DOI] [Google Scholar]
- 38.Jablonski D. 1986. Background and mass extinctions: the alternation of macroevolutionary regimes. Science 231, 129–133. ( 10.1126/science.231.4734.129) [DOI] [PubMed] [Google Scholar]
- 39.Clapham ME, et al. 2016. Taxonomic occurrences of Triassic to Jurassic marine animals. Palaeobiology Database, accessed 13 April 2016. See http://paleobiodb.org .
- 40.Dunhill AM, Sciberras J, Foster WJ, Twitchett RJ. 2018. Data from: Functional diversity of marine ecosystems across the Late Triassic mass extinction Dryad Digital Repositary. ( 10.5061/dryad.bg30k) [DOI]
- 41.Bambach RK, Bush AM, Erwin DH. 2007. Autecology and the filling of ecospace: key metazoan radiations. Palaeontology 50, 1–22. ( 10.1111/j.1475-4983.2006.00611.x) [DOI] [Google Scholar]
- 42.Knoll AH, Bambach RK, Payne JL, Pruss S, Fischer WW. 2007. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313. ( 10.1016/j.epsl.2007.02.018) [DOI] [Google Scholar]
- 43.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 44.McCullagh P, Nelder J. 1989. Generalized linear models, p. 511 New York, NY: Chapman and Hall. [Google Scholar]
- 45.Hilbe JM. 2011. Negative binomial regression. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 46.R Development Core Team. 2017. R: A language and environment for statistical computing. v 3.4.3. Vienna, Austria: The R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 47.Van de Schootbrugge B, Wignall PB. 2016. A tale of two extinctions: converging end-Permian and end-Triassic scenarios. Geol. Mag. 153, 332–354. ( 10.1017/s0016756815000643) [DOI] [Google Scholar]
- 48.Barras CG, Twitchett RJ. 2007. Response of the marine infauna to Triassic-Jurassic environmental change: ichnological data from southern England. Palaeogeogr. Palaeoclimatol. Palaeoecol. 244, 223–241. ( 10.1016/j.palaeo.2006.06.040) [DOI] [Google Scholar]
- 49.Hautmann M, Stiller F, Huawei C, Jingeng S. 2008. Extinction-recovery pattern of level-bottom faunas across the Triassic-Jurassic boundary in Tibet: implications for potential killing mechanisms. PALAIOS 23, 711–718. ( 10.2110/palo.2008.p08-005r). [DOI] [Google Scholar]
- 50.Mander L, Twitchett RJ, Benton MJ. 2008. Palaeoecology of the Late Triassic extinction event in the SW UK. J. Geol. Soc. Lond. 165, 319–332. ( 10.1144/0016-76492007-029) [DOI] [Google Scholar]
- 51.Thorne PM, Ruta M, Benton MJ. 2011. Resetting the evolution of marine reptiles at the Triassic-Jurassic boundary. Proc. Natl Acad. Sci. USA 108, 8339–8344. ( 10.1073/pnas.1018959108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tackett LS, Bottjer DJ. 2016. Paleoecological succession of Norian (Late Triassic) benthic fauna in eastern Panthalassa (Luning and Gabbs formations, west-central Nevada). PALAIOS 31, 190–202. ( 10.2110/palo.2015.070) [DOI] [Google Scholar]
- 53.Dal Corso J, et al. 2015. Carbon isotope records reveal synchronicity between carbon cycle perturbation and the ‘Carnian Pluvial Event’ in the Tethys realm (Late Triassic). Global Planet. Change 127, 79–90. ( 10.1016/j.gloplacha.2015.01.013) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dunhill AM, Sciberras J, Foster WJ, Twitchett RJ. 2018. Data from: Functional diversity of marine ecosystems across the Late Triassic mass extinction Dryad Digital Repositary. ( 10.5061/dryad.bg30k) [DOI]
Supplementary Materials
Data Availability Statement
Additional data is available as part of the electronic supplementary material.