Abstract
Over the past 1000 years, rats (Rattus spp.) have become one of the most successful and prolific pests in human society. Despite their cosmopolitan distribution across six continents and ubiquity throughout the world's cities, rat urban ecology remains poorly understood. We investigate the role of human foods in brown rat (Rattus norvegicus) diets in urban and rural areas over a 100 year period (ca AD 1790–1890) in Toronto, Canada using stable carbon (δ13C) and nitrogen (δ15N) isotope analyses of archaeological remains. We found that rat diets from urban sites were of higher quality and were more homogeneous and stable over time. By contrast, in rural areas, they show a wide range of dietary niche specializations that directly overlap, and probably competed, with native omnivorous and herbivorous species. These results demonstrate a link between rodent diets and human population density, providing, to our knowledge, the first long-term dietary perspective on the relative value of different types of human settlements as rodent habitat. This study highlights the potential of using the historical and archaeological record to provide a retrospective on the urban ecology of commensal and synanthropic animals that could be useful for improving animal management and conservation strategies in urban areas.
Keywords: urban ecology, archaeology, commensalism, Rattus norvegicus, stable isotopes, historical ecology
1. Introduction
Rats (Rattus spp.) have played an important role in many dimensions of human life. Considerable attention has been paid to how the global dissemination of rats, especially black (Rattus rattus), brown (Rattus norvegicus), and Pacific (Rattus exulans) rats, have been implicated in broad scale environmental destruction [1], the spread of deadly zoonotic diseases that pose significant global health risks [2], and the billions of dollars spent annually on pest control [3,4]. Beyond a general understanding of how rats have followed humans owing to the valuable habitat (i.e. food and shelter) that human-structured ecosystems provide, relatively little is known about how and why rats have been so successful at exploiting their relationship with humans at different temporal and spatial scales [5]. In this context, the history of rat dissemination remains sketchy and basic questions persist unanswered, such as, when, where, and which rat populations were involved in early migrations and specifically what aspects of human settlement were most facilitative to their spread [6,7]. Moreover, despite centuries of cohabitation with rats in urban areas, modern ecological studies have yet to establish a unified understanding of urban rat ecology [6].
Very little information is available on the ecology of past rat populations because much of the early spread of rats in human settlements occurred prior to the development of scientific observation. However, because rats were often one of the first invasive mammalian species to be introduced by humans to many regions of the globe, a better understanding of their early ecology may hold significant potential for addressing larger questions about their impact on novel ecosystems. For instance, what impacts did rat introductions, as opposed to direct human exploitation or habitat destruction, have on the extirpation or extinction of local wild taxa? What behavioural modifications have indigenous taxa undergone to adapt to new competition with incoming rat species? Moreover, rats, as initial colonizers of new ecosystems, could have the potential to serve as a comparator and/or a model system for a range of other commensal species such as house mice or fruit flies. For this reason, a better understanding of the mechanisms that facilitated the successful co-global colonization of rat species could be used as an analogy for other, less accessible (archaeologically or otherwise) commensal species by providing improved models for assessing which dimensions the human-commensal relationship are most salient for the spread of anthrodependent species.
In the context of historical ecology and conservation biology, researchers in adjacent fields are increasingly relying on chemical and genetic analyses of historical museum-archived specimens to address questions of species behaviour and long-term environmental variation, especially in the context of understanding how biological communities have adapted to human-altered environments [8,9]. This historical approach could provide a valuable retrospective on how rats have engaged with and adapted to different kinds of anthropogenic systems. Unfortunately, urban rat populations have rarely been the focus of historical or long-term ecological research and historical specimens are therefore rarely present in large quantities in natural history collections. Fortunately, archaeological rat remains could represent a valuable archive of specimens dating to the earliest timeframes of rat–human cohabitation and can provide an invaluable resource for biomolecular research (e.g. isotopic, aDNA). Rat remains are, for instance, commonly found in historical archaeological deposits dating back to some of the earliest European occupations in the New World [10], and on shipwrecks [11] associated with the first arrivals of European settlers to the Americas.
Because food is at the heart of rat–human commensalism, isotopic analyses of rat remains has outstanding potential to illuminate a variety of aspects of early rat ecology and human behaviour. Isotopic analyses of archaeological rat bones can be used to reconstruct patterns in what foods were available to rats in human settlements through time [12] and across space [13]. In addition to providing a baseline for the kinds of food that rats scavenged [14], these data also provide details on how broader cultural (socio-economic shifts related to food production) [12,15] and environmental (impacts on landscapes and native taxa) [13] processes unfold. Moreover, isotopic analyses of archaeological rat remains may be used to reveal patterns in where and how rats have been most successful at exploiting human settlements. In turn, this information could provide a long-term retrospective in which the dynamics of rat infestations in the modern world can be contextualized and better understood.
We approach the question of how early rat–human relationships in urban environments unfolded from the perspective of rat diets. Using collagen peptide mass fingerprinting, also known as zooarchaeology by mass spectrometry (ZooMS) [16], combined with isotopic analyses of bone collagen from 86 archaeological brown rats (R. norvegicus; hereafter referred to as ‘rats’), we assess differences in diet between rat populations from urban and rural habitats as a proxy for relative dependency on human food systems in nineteenth-century Upper Canada. Our working hypothesis is that rat diets will incorporate more high quality foods (including animal protein) in denser human settlements, whereas rats foraging in less human-structured habitats in rural and peripheral settings will have less frequent access to higher quality foods. By comparing stable carbon (δ13C) and nitrogen (δ15N) isotopic compositions of ZooMS-confirmed rat bone collagen from sites associated with different kinds and intensities of human activity, this research aims to investigate the relationship between rat behaviour and human population density in two key areas: (i) the ways in which rat diets differ with proximity to human settlement density; and (ii) whether rats living among denser urban populations enjoy higher quality diets (i.e. diets incorporating more animal fats and protein) more consistently than their rural counterparts.
2. Context, materials, methods
(a). Methodological context
The isotopic composition of collagen from archaeological rat bones can provide information about the kinds of foods these animals consumed (for a review of stable isotopes in archaeology see [17]). Because bone collagen remodels slowly over the life of an individual, the isotopic composition of a rat bone will reflect a long-term average of dietary intake over the lifespan of the individual [18]. For nineteenth-century Upper Canada rural and urban settlements, we expect that rat δ15N values should primarily reflect the trophic level at which an individual fed [19], with lower values suggesting a more herbivorous diet and higher values suggesting increased omnivory (i.e. consumption of more meat and other animal products). Because R. norvegicus is known to be more omnivorous and to preferentially select energy rich foods (particularly meat) when available [20,21], their δ15N values can serve as a proxy for diet quality. Rat δ13C values provide an indicator for the extent to which they had access to foods derived from C3 or C4 plants [22]. In the context of nineteenth-century Upper Canada, most foods were derived from C3 plants, however, it is possible that maize (a major C4 crop) or meat from maize-fed animals could have been available through trade [23].
(b). Sample description
All samples included in this study come from archaeological deposits at 13 sites (figure 1) in the immediate vicinity of the present day city of Toronto, Canada (i.e. within 75 km) and date to the same historical period (AD 1790–1890) of intensification of European settlement in the Lower Great Lake region (see the electronic supplementary material, table S1). To avoid duplicating results from the same individual, samples were selected based on minimum number of individual counts per archaeological context. The urban assemblage of rat bone samples (n = 42) comes from five houses and a hospital in the city of York (now Toronto), in Upper Canada (now southern Ontario, Canada) whereas the rural assemblage of rat bone samples (n = 44) comes from seven sites located up to 75 km from York (figure 1). A range of environmental and cultural processes can contribute to variation in δ15N and δ13C values at the base of the food web (e.g. [24,25]), which can result in spatio-temporal variability in the isotopic composition of consumer tissues [9,26]. For this reason it is critical that rat isotopic compositions are interpreted relative to an isotopic baseline from other species that are from the same time period and local to the study area [27]. Therefore, we also analysed coeval late eighteenth- to late nineteenth-century specimens from local native omnivores (racoons, Procyon lotor; n = 6) and herbivores (groundhogs, Marmota monax; n = 8) from the same region sites to establish a baseline for local wild fauna. To provide a baseline for locally husbanded domestic animals we used previously published data from archaeological livestock (n = 286) from the same region (including some of the same sites) and time period [23].
Figure 1.

Map showing locations of nineteenth-century (AD 1790–1890) archaeological sites from York (now Toronto), Upper Canada (now Ontario, Canada). (Online version in colour.)
(c). Sample preparation
Bones were cleaned of surface contamination and cut into small pieces (approx. 3 mm3). Samples were demineralized over several days in 0.5 M hydrochloric acid (HCl). Demineralized samples were then neutralized in type I water. To remove base-soluble contaminants resulting from diagenetic processes in the archaeological burial environment, samples were subjected to a series of 0.1 M sodium hydroxide (NaOH) treatments in an ultrasonic bath (NaOH solution refreshed every 15 min) until solution remained clear [28]. Collagen was then solubilized in a pH3 solution (pH adjusted with HCl) for 48 h at 65°C. Samples were then centrifuged and the solubilized collagen fraction was transferred to a new tube, frozen, and lyophilized in a freeze dryer.
(d). Isotopic analyses
Isotopic compositions were measured on 0.5 mg collagen subsamples in tin capsules using an Elementar Vario MICRO cube elemental analyser coupled via continuous flow to an Isoprime isotope ratio mass spectrometer in the Archaeology Chemistry Laboratory at the University of British Columbia, Canada, with duplicate analyses being performed on 13% of samples. Calibration and analytical uncertainty is described in the electronic supplementary material. Measured δ13C and δ15N values were calibrated relative to Vienna Pee Dee Belemnite (VPDB) and ambient inhalable reservoir (AIR) respectively using a two point calibration curve anchored to United States Geological Survey (USGS) 40, USGS 41 and USGS 41a [29] (electronic supplementary material, table S2). The accuracy of isotopic measurements was assessed using internal check standards (electronic supplementary material, table S3). For check standards the average absolute difference between observed and known δ-values (i.e. reproducibility or accuracy) was 0.03‰ for δ13C and 0.02‰ for δ15N. For all standards (1σ of check and calibration), measurement precision was ±0.05‰ and ±0.10‰ for δ13C and δ15N respectively. The average difference between duplicate sample pairs was 0.01‰ and 0.05‰ for δ13C and δ15N, respectively. Analytical uncertainty was ±0.10‰ and ±0.12‰ for δ13C and for δ15N, respectively [30] (see the electronic supplementary material). Stable isotope compositions of bone collagen are considered acceptable when accompanied by the following data on collagen integrity criteria [31]: (i) per cent C and N values above 18% and 6%, respectively; and, (ii) atomic C : N values falling between 2.9 and 3.6. In order to ensure appropriate statistical methods for comparing data were applied, we assessed the distribution of our data using Shapiro–Wilk's, Mann–Whitney–Wilcoxon's and, Levene's tests. Statistical analyses for determining difference in means and variance between rat populations were conducted using R (version 3.4.3; Shapiro–Wilk test and Mann–Whitney–Wilcoxon test) as well as the R package car (Levene's test) [32]. For random comparisons between groups the dplyr package was used. To compare niche size between rat populations we calculated their convex hull areas (TA), an area based metric used to quantify niche widths, using the R package, Stable Isotope Bayesian Ellipses in R (SIBER) [33]. A convex hull is the smallest convex polygon in bivariate isotopic space that contains all data within a group, and provides a basis for quantifying and comparing the relative extent of dietary diversity between two or more populations with similar sample sizes [34].
(e). Zooarchaeology by mass spectrometry analyses
Rat dietary behaviour can be influenced by interspecific competition between Rattus species [35] and, for this reason, understanding the taxonomic composition of our rat sample is important for interpretations of urban and rural rat ecology [12]. While it is thought that the early dominance of the brown rat (R. norvegicus) prevented the black rat (R. rattus) from becoming established in the region [36], there remained a possibility that our rat sample could potentially include multiple species of Rattus. Therefore, we used ZooMS to provide species-level taxonomic determination [37,38] for the majority of samples. In brief, several milligrams of collagen per sample, resuspended in 50 mM ammonium bicarbonate, was digested with 0.4 µg sequencing grade trypsin for 18 h at 37°C before being acidified to 0.1% trifluoroacetic acid (TFA). Peptide digests were then applied to an equilibrated C18 solid phase extraction cartridge, washed twice with 0.1% TFA, and fractionated into 10% and 50% (acetonitrile (ACN) in 0.1% TFA) fractions following [16]. These fractions were then evaporated and resuspended with 10 µl 0.1% TFA and 1 µl co-crystalized with an equal volume of 10 mg ml−1 alpha-cyano hydroxycinnamic acid in 50% ACN/0.1% TFA and allowed to air dry. Collagen peptide mass fingerprint spectra were then acquired using a Bruker Ultraflex II Matrix Assisted Laser Desorption Ionization Time of Flight (MALDI-ToF) mass spectrometer with up to 2000 laser acquisitions over the m/z range 700–3700. Spectra were then compared with those of rats published previously [38] as well as those of other rodents (e.g. [37,39]). Of particular significance to this work was distinguishing between the two expected Rattus species through observation of biomarkers at either m/z 2957.4 or m/z 2987.4, when supported with other Rattus markers such as those at m/z 1451.7 and m/z 2143.1 (electronic supplementary material, figure S1).
3. Results
The majority (85%) of rat samples provided collagen peptide mass fingerprint spectra of sufficient quality for species-level determinations (electronic supplementary material, table S1), all but one of which were R. norvegicus, and this provides strong support for the idea that R. rattus was not historically present in the region. The non-Rattus specimen was removed from analyses. The isotopic values of rat bone collagen (figure 2; electronic supplementary material, table S2) were interpreted in the context of a large isotopic baseline for contemporaneous wild omnivores (racoons, n = 6; δ13C = −20.2 ± 1.2‰, δ15N = +7.6 ± 1.1‰) and herbivores (groundhogs, n = 8; δ13C = −22.0 ± 2.1‰, δ15N = +4.4 ± 1.6‰) as well as livestock from the same region; these included cattle (n = 152) and pigs (n = 114), which serve as a baseline for locally produced meat products (n = 256; δ13C = −21.5 ± 0.9‰, δ15N = +6.6 ± 1.1‰) [23]. Analyses show that rats from urban archaeological sites (n = 41) in the historic settlement of York in Upper Canada consistently had the highest δ15N (+10.3 ± 0.9‰; range = 4.6‰) and lowest δ13C (−19.6 ± 0.9‰; range = 3.8‰) values with relatively little variation. These data show that rats living in urban habitats had regular access to higher quality (animal) protein sources. Meanwhile rats from seven rural sites (n = 45) show highly variable and widely ranging δ15N (+8.2 ± 1.9‰; range = 6.5‰) and δ13C (−19.0 ± 2.7‰; range = 9.8‰) values, consistent with a habitat and diet where broader foraging strategies were needed and less reliable food subsidies from human food systems were available. A Shapiro–Wilk test was used to determine that for both urban and rural rat populations, δ13C values were normally distributed (for urban rats, n= 41, W = 0.76647, p ≤ 0.000; for rural rats, n = 44, W = 0.975, p = 0.492) whereas δ15N values were not (for urban rats, n= 41, W = 0.950, p = 0.053; for rural rats, n = 44, W = 0.986, p = 0.878). Levene's and Bartlett's tests were therefore used to determine homogeneity of variance for δ13C and δ15N values respectively, and showed that group variances were unequal (Levene's test, F84 = 8.8192, p= 0.003; Bartlett K-squared = 23.347, d.f. = 1, p ≤ 0.000). Because variances were not equal, a Mann–Whitney–Wilcoxon test was used to compare the difference in means between rat groups. While urban and rural rat populations have significantly different δ15N values (W = 348, p ≤ 0.000), their δ13C values do not differ significantly (W = 774, p = 0.198). Convex hull areas calculated in SIBER for groups of rats show large differences in isotopic niche width, confirming that the rural rat (TA = 47.6) population had much more (over than four times more) dietary niche diversity than their urban (TA = 10.1) counterparts. A similarly large range of intra-site rat isotopic variation can be found at multiple rural sites, suggesting that differing patterns in isotopic variation between rats at urban and rural sites are not related to spatial variables associated with differing local geographies. We also assessed this possibility statistically by bootstrapping 20 samples from urban and rural groups of rats 10 times and comparing their δ15N values with Mann–Whitney–Wilcoxon's tests. Results confirm (for 9 of 10 tests, p ≤ 0.05; see the electronic supplementary material, table S5) that the urban and rural rat groups are significantly different regardless of sites included.
Figure 2.
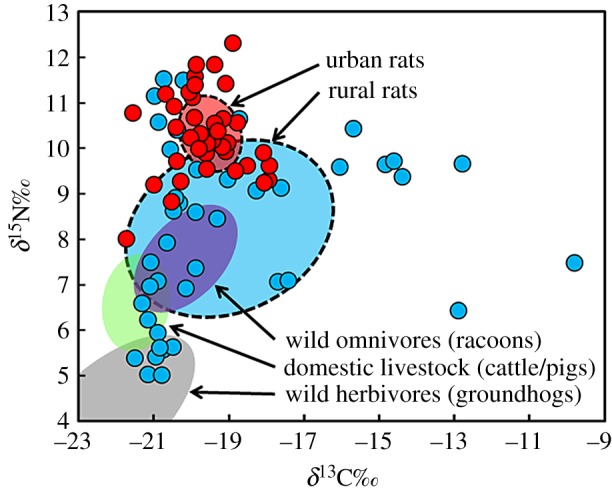
Plot comparing all data included in this study. Urban (n = 42) and rural (n = 44) rat δ13C and δ15N values are shown as red and blue circles respectively. Standard ellipses for urban (red ellipse) and rural (blue ellipse) rats and other animal groups are also shown for comparison: purple ellipse is wild omnivores (six racoons), green ellipse is livestock (256 cattle and pigs) [23] and grey ellipse is wild herbivores (eight groundhogs). A plot comparing all δ13C and δ15N values for individual animals from all taxa is provided in the electronic supplementary material, figure S2. (Online version in colour.)
All rats analysed in this study come from the same general time period (i.e. AD 1790–1890); however, narrower date ranges, based on archaeological context, are available for specimens from some sites. Unfortunately, these date ranges are relatively coarse (spanning between three and nine decades) and therefore only permit assignment of samples to broad and often overlapping temporal categories (i.e. earlier, middle or later nineteenth century). Although the date ranges offered by archaeological context information do not provide the temporal resolution that would be necessary for statistical comparison between time periods, they can, nonetheless, allow for some general observations. While the majority of rats come from archaeological contexts with date ranges spanning the entire timeframe of this study, both urban and rural datasets include a smaller subset of rat samples from archaeological contexts dating from the early-mid and mid-late nineteenth century. For urban rats, these earlier and later nineteenth century subgroups show a similar degree of isotopic variability suggesting that the relative amount of dietary variation for urban rats was consistent through time. Unfortunately, for rural rats, there are too few specimens from narrowly dated archaeological contexts to meaningfully compare time periods. However, given that higher intra-site variation occurs in rat isotope values at multiple sites, and that most rural sites span the entire nineteenth century, we expect that dietary heterogeneity was relatively constant for rural rat populations through time.
4. Discussion and conclusion
This study is, to our knowledge, the first to use isotopic analyses of archaeological fauna to compare urban and rural rodent ecology though time. The isotopic composition of nineteenth-century rat remains from Upper Canada indicates that brown rat populations living within denser human settlement areas probably fared better, from a dietary perspective (i.e. had diets richer in animal-based foods), than those living further away. In the context of modern ecological research on rats, suggesting that diet quality and association with anthropogenic environments can result in greater fecundity and fitness [40,41], these findings serve not only to highlight a close relationship between rat ecology and urbanism, but also can provide a framework for testing archaeological questions about commensality and urbanization.
The long-term relationship (generally spanning AD 1790–1890) observed here between settlement type and rat diet offers a new perspective on the past ecology of urban rodents and could provide a potentially valuable indicator for urbanization in archaeological contexts. Because rat populations inhabiting denser urban environments have access to a larger range of food sources (e.g. [6,42,43]), more individuals should have opportunities to obtain preferred higher quality food items that, particularly for R. norvegicus, can emphasize animal protein/fat sources [20,21]. Our analyses show that this scenario can result in rat diets that, at the population level, are more homogeneous isotopically and consistently include greater proportions of higher trophic level food inputs. By contrast, in less dense settlements human-derived food may be scarcer, leading to rat diets that include lower trophic level foods with greater inter-individual dietary specialization. The association between settlement density and diet quality/variation could therefore provide a marker for degree urbanization (i.e. higher diet quality and lower dietary variation equals greater urbanization). Such a proxy measure could be useful as an independent perspective for tracking settlement density at archaeological sites where rat remains are available from a range of time periods. Further investigation of this possibility, through additional comparisons of rat isotopic compositions at other urban and rural locations, would help to establish the extent to which this dietary relationship is characteristic of rat diets across sites with different human settlement densities.
It is also noteworthy that for rural environments our data show that in some cases, at the individual level, rats still specialized in taking advantage of human food systems. In particular, a small number of rural rat δ13C values suggest their activity in the pilfering of maize, a C4 plant that was a significant crop for European settlers and migrants in many parts of eastern North America during the eighteenth and nineteen centuries, but not in Upper Canada [44]. This is interesting because it demonstrates a pattern that is not shown by the isotopic composition of livestock from the same sites [23]. In the context of nineteenth-century Upper Canada, analyses of herbivores that are probably locally sourced confirm that C4 plants such as maize were not commonly used as a fodder source. Elevated δ13C values from select rats at multiple rural sites therefore suggest that some C4 materials, probably maize based on historical context, could have been present at rural farm sites. Because δ15N baseline values can vary over space and time [9,24], the wide variability in corresponding δ15N values for these C4-feeding rats is difficult to interpret and rat consumption of imported meat products derived from maize-fed animals cannot be ruled out. In the context of historical and archaeological data showing that animal products consumed at the same sites derived mainly from C3-fed animals and that C4 plants were probably not cultivated for animal feed, the most parsimonious interpretation of these C4 rat data is that rats had access to stores of imported maize that was kept on hand for human consumption or perhaps for tasks such as feeding domestic fowl.1 It is also possible that C4-feeding rats could represent intrusive individuals from more recent twentieth-century rat populations, although this explanation is less parsimonious given the fact that these C4-feeding individuals are observed at multiple sites and in a range of well dated archaeological contexts.
Archaeological data from urban rats can also provide insight into the question of why rats have been so successful at exploiting human environments. Because archaeological rat specimens come from multiple sites in different areas of the city and represent animals that lived at different times throughout the nineteenth century, the relatively low degree of isotopic variation suggests that the dietary ecology of urban rats was stable for a long period of time. This suggests that not only has the urban habitat of York provided rats with higher quality foods, but that it had done so consistently over a century. In considering the cost to commensals of inhabiting anthropogenic environments, Hulme-Beanam and colleagues have suggested that, while urban environments today can provide a more steady supply of food to commensal animals, past urban commensal populations would probably have experienced greater variation in food supply [7] and that this unpredictability would have had evolutionary consequences for species dependent on human food systems. Our results suggest that, in at least some areas of the world, food supply in urban settlements was sufficiently abundant and stable to allow for dietary consistency since at least the nineteenth or even the late eighteenth century. From a pest management perspective, the apparent dietary consistency of urban rats suggests that one way of reducing the quality of urban habitat for rats could be disruption, at least periodically, of higher trophic level food supplies. While it is possible that our urban sample is somehow systematically biased to include more rat specimens from particular time periods in which better quality foods were available, our sampling strategy was random and aimed to incorporate rat remains from as many spatiotemporally varied contexts as possible. Therefore the complete lack of individuals with lower trophic level diets in the urban assemblages suggests that this pattern is not greatly influenced by archaeological preservation biases. In this context, we interpret higher quality and more homogeneous rat diets as evidence for a close adaptation between rat ecology and urban environments, in turn, providing support for the idea that the success of rats, and perhaps other urban commensals, in human settlements stems at least partly from the long-term reliability of human food systems.
The retrospect offered by archaeological rat remains may also be able to shed light on the dietary dimensions and evolutionary nature of rat commensality. Banks & Smith [45] have recently argued that some species of rats, R. rattus in particular, are native to urban areas and are in fact only alien once they colonize peri-urban and rural/peripheral environments. This assertion is based not only on the firm association of certain rat species with human settlements, but also on the knowledge that these species are no longer known to exist in a wild state [46]. This idea is supported by our findings, suggesting that rats in urban locations had better and more consistent access to higher quality foods than their rural counterparts, which underwent a range of dietary niche specializations.
From an ecological perspective, the high degree of isotopic variation observed in rats from rural sites can help to better contextualize ecological consequences of rat introductions. Archaeological rats from rural environments adopted a diversity of specialized feeding behaviours that would have put them in competition for niches contemporaneously occupied by native fauna. In this study, for instance, we observe that whereas rats from urban contexts show no overlap with wild taxa (figure 2), rural rats show considerable overlap with both wild herbivores and omnivores, suggesting that racoons, groundhogs, and other rodents may have experienced increased competition for food sources from rat introductions. Given the early dates from some of these specimens (i.e. as early as the later eighteenth century), this archaeological evidence adds considerable temporal depth to the observation that commensal rodent introductions can directly influence native fauna through competition for habitat and food resources.
This study has sought to highlight the potential value of archaeological rat remains as a resource for developing long-term perspectives on urban rat ecology. In the past, researchers have argued that current trends in human population increase [47], urbanization [48], and climate change [49] will serve to significantly expand availability of favourable human-structured habitat for rats. These studies point out that effective mitigation of the risks associated with a growing rat population will depend, in part, on improving ecologically based management strategies [50] that take into account how rats use urban spaces [5,6,51]. Despite large populations and a cosmopolitan distribution in cities across the globe, prominent knowledge gaps exist for developing ecologically based management strategies to control urban rat populations. These gaps can often be attributed to difficulty accessing urban rodent populations [5], which can be challenging to study owing to a complex mixture of social and logistical obstacles. Rat remains are commonly recovered during archaeological excavations and these remains are increasingly being preserved as part of the long-term curation of archaeological faunal assemblages, yet they remain underused. As this study demonstrates, the archaeological record can be used to study historical trends in the dynamics of rat dietary behaviour at a variety of scales and in spatio-temporal contexts that directly foreground many of the issues in rodent ecology being faced in today's modern cities.
Supplementary Material
Acknowledgements
For permission to sample and logistical help: Ron Williamson, Alexis Dunlop, and Caitlin Coleman at Archaeological Services Inc., Dena Doroszenko at the Ontario Heritage Trust, Janet Batchelor and Eliza Brandy at the Toronto Region Conservation Authority, Martin Scott and Peter Popkin at Golder Associates, and Heather Henderson at Historic Horizon Inc. Zooarchaeological and other assistance was provided by Suzanne Needs-Howarth (Perca Zooarchaeology), Eric Tourigny (Newcastle University), and Christina Cheung (Simon Fraser University).
Endnote
Unpublished δ13C values from nineteenth-century chickens (Gallus gallus) from sites in Upper Canada show that C4 plants, probably maize, were fed to some domestic fowl and suggest that at least some maize was kept on hand for this purpose.
Ethics
This paper meets the requirements set out in the Royal Society publishing ethics and policies criteria.
Data accessibility
All data presented in this study can be found in the electronic supplementary material.
Authors' contributions
E.G. designed research and undertook isotopic analyses. M.B. undertook ZooMS analyses. E.G. and M.B. interpreted the data and wrote the paper.
Competing interests
The authors declare that they have no competing interests.
Funding
SSHRC Postdoctoral and Banting Postdoctoral programs and a SSHRC Insight Development Grant (E.G.) and a Royal Society University Research Fellowship (M.B.) (UF120473).
References
- 1.Shiels AB, Pitt WC, Sugihara RT, Witmer GW. 2014. Biology and impacts of Pacific island invasive species. 11. Rattus rattus, the black rat (Rodentia: Muridae). Pac. Sci. 68, 145–184. ( 10.2984/68.2.1) [DOI] [Google Scholar]
- 2.Himsworth CG, Parsons KL, Jardine C, Patrick DM. 2013. Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis. 13, 349–359. ( 10.1089/vbz.2012.1195) [DOI] [PubMed] [Google Scholar]
- 3.Stenseth NC, et al. 2003. Mice, rats, and people: the bio-economics of agricultural rodent pests. Front. Ecol. Environ. 1, 367–375. ( 10.1890/1540-9295(2003)001%5B0367:MRAPTB%5D2.0.CO;2) [DOI] [Google Scholar]
- 4.Pimentel D, Zuniga R, Morrison D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288. ( 10.1016/j.ecolecon.2004.10.002) [DOI] [Google Scholar]
- 5.Parsons MH, Banks PB, Deutsch MA, Corrigan RF, Munshi-South J. 2017. Trends in urban rat ecology: a framework to define the prevailing knowledge gaps and incentives for academia, pest management professionals (PMPs) and public health agencies to participate. J. Urban Ecol. 3, jux005 ( 10.1093/jue/jux005) [DOI] [Google Scholar]
- 6.Feng AY, Himsworth CG. 2014. The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst. 17, 149–162. ( 10.1007/s11252-013-0305-4) [DOI] [Google Scholar]
- 7.Hulme-Beaman A, Dobney K, Cucchi T, Searle JB. 2016. An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends Ecol. Evol. 31, 633–645. ( 10.1016/j.tree.2016.05.001) [DOI] [PubMed] [Google Scholar]
- 8.English PA, Green DJ, Nocera JJ. 2018. Stable isotopes from museum specimens may provide evidence of long-term change in the trophic ecology of a migratory aerial insectivore. Front. Ecol. Evol. 6, 14 ( 10.3389/fevo.2018.00014) [DOI] [Google Scholar]
- 9.Guiry E, Beglane F, Szpak P, Schulting R, McCormick F, Richards MP. 2018. Anthropogenic changes to the Holocene nitrogen cycle in Ireland. Sci. Adv. 4, eaas9383 ( 10.1126/sciadv.aas9383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelso WM. 2006. Jamestown, theburied truth. Charlottesville, VA: University of Virginia Press. [Google Scholar]
- 11.deFrance SD. 2017. Faunal remains. In La Belle: the archaeology of a seventeenth century ship of New World colonization (ed. Bruseth JE.), pp. 749–762. College Station, TX: A&M Press. [Google Scholar]
- 12.Guiry EJ, Gaulton BC. 2016. Inferring human behaviors from isotopic analyses of rat diet: a critical review and historical application. J. Archaeol. Method Theory 23, 399–426. ( 10.1007/s10816-015-9248-9) [DOI] [Google Scholar]
- 13.Swift JA, Miller MJ, Kirch PV. 2017. Stable isotope analysis of Pacific rat (Rattus exulans) from archaeological sites in Mangareva (French Polynesia): the use of commensal species for understanding human activity and ecosystem change. Environ. Archaeol. 22, 283–297. ( 10.1080/14614103.2016.1216933) [DOI] [Google Scholar]
- 14.Guiry EJ, Harpley B, Jones Z, Smith C. 2014. Integrating stable isotope and zooarchaeological analyses in historical archaeology: a case study from the urban nineteenth-century Commonwealth Block site, Melbourne, Australia. Int. J. Hist. Archaeol. 18, 415–440. ( 10.1007/s10761-014-0264-3) [DOI] [Google Scholar]
- 15.Guiry EJ, Noël S, Tourigny E, Grimes V. 2012. A stable isotope method for identifying transatlantic origin of pig (Sus scrofa) remains at French and English fishing stations in Newfoundland. J. Archaeol. Sci. 39, 2012–2022. ( 10.1016/j.jas.2012.03.004) [DOI] [Google Scholar]
- 16.Buckley M, Collins M, Thomas-Oates J, Wilson JC. 2009. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 23, 3843–3854. ( 10.1002/rcm.4316) [DOI] [PubMed] [Google Scholar]
- 17.Lee-Thorp JA. 2008. On isotopes and old bones. Archaeometry 50, 925–950. ( 10.1111/j.1475-4754.2008.00441.x) [DOI] [Google Scholar]
- 18.Hedges REM, Clement JG, Thomas CLD, O'Connell TC. 2007. Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 133, 808–816. ( 10.1002/ajpa.20598) [DOI] [PubMed] [Google Scholar]
- 19.DeNiro MJ, Epstein S. 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351. ( 10.1016/0016-7037(81)90244-1) [DOI] [Google Scholar]
- 20.Schein MW, Orgain H. 1953. A preliminary analysis of garbage as food for the Norway rat. Am. J. Trop. Med. Hyg. 2, 1117–1130. ( 10.4269/ajtmh.1953.2.1117) [DOI] [PubMed] [Google Scholar]
- 21.Yabe T. 1979. The relation of food habits to the ecological distributions of the Norway rat (Rattus norvegicus) and the roof rat (R. rattus). Jpn J. Ecol. 29, 235–244. [Google Scholar]
- 22.DeNiro MJ, Epstein S. 1978. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506. ( 10.1016/0016-7037(78)90199-0) [DOI] [Google Scholar]
- 23.Guiry E, Szpak P, Richards MP. 2017. Isotopic analyses reveal geographical and socioeconomic patterns in historical animal trade between predominantly wheat- and maize-growing agricultural regions in eastern North America. Am. Antiq. 82, 341–352. ( 10.1017/aaq.2016.34) [DOI] [Google Scholar]
- 24.Szpak P. 2014. Complexities of nitrogen isotope biogeochemistry in plant-soil systems: implications for the study of ancient agricultural and animal management practices. Front. Plant Sci. 5, 288 ( 10.3389/fpls.2014.00288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tieszen LL. 1991. Natural variations in the carbon isotope values of plants: implications for archaeology, ecology, and paleoecology. J. Archaeol. Sci. 18, 227–248. ( 10.1016/0305-4403(91)90063-U) [DOI] [Google Scholar]
- 26.Guiry EJ, Hepburn JC, Richards MP. 2016. High-resolution serial sampling for nitrogen stable isotope analysis of archaeological mammal teeth. J. Archaeol. Sci. 69, 21–28. ( 10.1016/j.jas.2016.03.005) [DOI] [Google Scholar]
- 27.Katzenberg MA. 1989. Stable isotope analysis of archaeological faunal remains from southern Ontario. J. Archaeol. Sci. 16, 319–329. ( 10.1016/0305-4403(89)90008-3) [DOI] [Google Scholar]
- 28.Szpak P, Krippner K, Richards M. 2017. Effects of sodium hydroxide treatment and ultrafiltration on the removal of humic contaminants from archaeological bone. Int. J. Osteoarchaeol. 27, 1070–1077. ( 10.1002/oa.2630) [DOI] [Google Scholar]
- 29.Qi H, Coplen TB, Geilmann H, Brand WA, Böhlke J. 2003. Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Commun. Mass Spectrom. 17, 2483–2487. ( 10.1002/rcm.1219) [DOI] [PubMed] [Google Scholar]
- 30.Szpak P, Metcalfe JZ, Macdonald RA. 2017. Best practices for calibrating and reporting stable isotope measurements in archaeology. J. Archaeol. Sci. Rep. 13, 609–616. ( 10.1016/j.jasrep.2017.05.007) [DOI] [Google Scholar]
- 31.DeNiro MJ. 1985. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317, 806–809. ( 10.1038/317806a0) [DOI] [Google Scholar]
- 32.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 33.Jackson AL, Inger R, Parnell AC, Bearhop S. 2011. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. ( 10.1111/j.1365-2656.2011.01806.x) [DOI] [PubMed] [Google Scholar]
- 34.Layman CA, Arrington DA, Montaña CG, Post DM. 2007. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88, 42–48. ( 10.1890/0012-9658(2007)8842:CSIRPF%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 35.Shiels AB, Flores CA, Khamsing A, Krushelnycky PD, Mosher SM, Drake DR. 2013. Dietary niche differentiation among three species of invasive rodents (Rattus rattus R. exulans, Mus musculus). Biol. Invasions 15, 1037–1048. ( 10.1007/s10530-012-0348-0) [DOI] [Google Scholar]
- 36.The City of Toronto. 2012. Mammals of Toronto. In City of Toronto Biodiversity Series (City of Toronto).
- 37.Buckley M. 2018. Zooarchaeology by mass spectrometry (ZooMS) collagen fingerprinting for the species identification of archaeological bone fragments. In Zooarchaeology in practice: case studies in methodology and interpretation in archaeofaunal analysis (eds Giovas CM, LeFebvre MJ), pp. 227–247. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 38.Buckley M, Gu M, Shameer S, Patel S, Chamberlain AT. 2016. High-throughput collagen fingerprinting of intact microfaunal remains; a low-cost method for distinguishing between murine rodent bones. Rapid Commun. Mass Spectrom. 30, 805–812. ( 10.1002/rcm.7483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prendergast ME, et al. 2017. Reconstructing Asian faunal introductions to eastern Africa from multi-proxy biomolecular and archaeological datasets. PLoS ONE 12, e0182565 ( 10.1371/journal.pone.0182565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villafañe IE. G., Cavia R, Vadell MV, Suárez OV, Busch M. 2013. Differences in population parameters of Rattus norvegicus in urban and rural habitats of central Argentina. Mammalia 77, 187–193. [Google Scholar]
- 41.Vadell MV, Villafañe IG, Cavia R. 2014. Are life-history strategies of Norway rats (Rattus norvegicus) and house mice (Mus musculus) dependent on environmental characteristics? Wildl. Res. 41, 172–184. ( 10.1071/WR14005) [DOI] [Google Scholar]
- 42.Traweger D, Travnitzky R, Moser C, Walzer C, Bernatzky G. 2006. Habitat preferences and distribution of the brown rat (Rattus norvegicus Berk.) in the city of Salzburg (Austria): implications for an urban rat management. J. Pest Sci. 79, 113–125. ( 10.1007/s10340-006-0123-z) [DOI] [Google Scholar]
- 43.Tamayo-Uria I, Mateu J, Escobar F, Mughini-Gras L. 2014. Risk factors and spatial distribution of urban rat infestations. J. Pest Sci. 87, 107–115. ( 10.1007/s10340-013-0530-x) [DOI] [Google Scholar]
- 44.McCalla D. 1978. The wheat staple and Upper Canadian development. Hist. Papers 13, 34–46. [Google Scholar]
- 45.Banks PB, Smith HM. 2015. The ecological impacts of commensal species: black rats, Rattus rattus, at the urban–bushland interface. Wildl. Res. 42, 86–97. ( 10.1071/WR15048) [DOI] [Google Scholar]
- 46.Aplin KP, Chesser T, Have JT. 2003. Evolutionary biology of the genus Rattus: profile of an archetypal rodent pest. In Rats, mice and people: rodent biology and management (eds Singleton GR, Hinds LA, Krebs CJ, Spratt DM), pp. 487–498. Canberra, Australia: Australian Centre for International Agricultural Research. [Google Scholar]
- 47.Cohen JE. 2003. Human population: the next half century. Science 302, 1172–1175. ( 10.1126/science.1088665) [DOI] [PubMed] [Google Scholar]
- 48.United_Nations. 2015. World urbanization prospects: the 2014 revision. New York, NY: United Nations Population Division. [Google Scholar]
- 49.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162. ( 10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 50.Singleton GR, Leirs H, Hinds LA, Zhang Z.. 1999. Ecologically-based management of rodent pests–re-evaluating our approach to an old problem. Ecologically-based Management of Rodent Pests. Australian Centre for International Agricultural Research (ACIAR), Canberra, Australia, pp. 17–29.
- 51.Himsworth CG, Jardine CM, Parsons KL, Feng AY, Patrick DM. 2014. The characteristics of wild rat (Rattus spp.) populations from an inner-city neighborhood with a focus on factors critical to the understanding of rat-associated zoonoses. PLoS ONE 9, e91654 ( 10.1371/journal.pone.0091654) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study can be found in the electronic supplementary material.


