Abstract
On deep time scales, changing climatic trends can have a predictable influence on macroevolution. From evidence of mass extinctions, we know that rapid climatic oscillations can indirectly open niche space and precipitate adaptive radiation, changing the course of ecological diversification. These dramatic shifts in the global climate, however, are rare events relative to extended periods of protracted climate change and biome turnover. It remains unclear whether during gradually changing periods, shifting habitats may instead promote non-adaptive speciation by facilitating allopatry and phenotypic conservatism. Using fossil-calibrated, species-level phylogenies for five Australian radiations comprising more than 800 species, we investigated temporal trends in biogeography and body size evolution. Here, we demonstrate that gradual Miocene cooling and aridification correlates with the restricted phenotypic diversification of multiple ecologically diverse vertebrate groups. This probably occurred as species ranges became fractured and isolated during continental biome restructuring, encouraging a shift towards conservatism in body size evolution. Our results provide further evidence that abiotic changes, not only biotic interactions, may act as selective forces influencing phenotypic macroevolution.
Keywords: macroevolution, marsupials, reptiles, phenotypic evolution, adaptive radiation, comparative methods
1. Introduction
Changes to the global climate can promote macroevolutionary and macroecological turnover by either abiotic or biotic drivers, or both [1]. Climatic changes may proceed over long or short time periods, varying in intensity from mild to extreme, and as a result, changes to macroevolutionary patterns may respond in kind. To date, the overwhelming majority of literature on this topic has been concerned with the effects of rapid climatic change on the pace and process of diversification. As a result of dramatic events, species richness and ecological diversity may first plummet, then swiftly accumulate. This probably occurs due to opening niche space or release from biotic competitive constraint owing to elevated extinction and provides a popular explanation for adaptive radiations that follow periods of climatic flux [2]. However, such extreme events are rare in evolutionary time, and our knowledge of the influence of the much longer intervening periods of gradual climatic change on macroevolution remains limited. Periods of prolonged climatic change are common in palaeoclimatic history and are often defined by more dramatic events which precede and follow them [3,4]. Despite this, identifying signals of the influence of protracted climate change has been difficult. Whereas rapid environmental change may leave obvious fossil and phylogenetic signatures as a result of shifting origination and/or extinction rates, diversification during gradual climate change may outwardly resemble constant-rate processes. This may result in less obvious anagenetic and assemblage changes and provide the appearance of evolutionary stasis [5–7].
The Miocene epoch (23–5.3 Ma) has figured prominently in the diversification of many extant faunal groups. This is largely the result of climatic instability, fluctuating atmospheric CO2 concentrations and floral biome turnover [8–11]. Following the Middle Miocene climatic optimum warm phase (17–15 Ma), the latter half of this epoch (12–5.3 Ma) exhibited a global cooling trend (−0.5°C per million years), exemplified by dropping sea surface temperatures and Antarctic glaciation [12,13]. Global cooling coincided with the birth and expansion of arid biomes and contraction of more mesic ones [13]. In Africa and Asia, ecological replacement of C3 forest and woodland plants with C4 savannah in the Mid-to-Late Miocene forced an ecological transition in herbivorous mammals from browsers to grazers [5]. Turnover in the Miocene ungulate assemblage also resulted in directional morphological trends, including a general increase in body size [14,15]. This suggests that prolonged change to the global climate may have indirect influences on macroevolutionary trajectories of some groups. But to what extent are morphological changes consistent among coexisting radiations, and are these changes detectable from contemporary data?
The rise of arid habitats in the Miocene, and the impact of these biomes on diversification patterns, provides an ideal opportunity to investigate their influence across ecologically divergent organismal groups. The Miocene climate developed the Gobi and Sahara deserts [16,17], and in Australia, aridification created the red centre of the continent (‘outback’ Australia). This resulted in the expansion of sclerophyllous vegetation, and shrinking and fracturing of closed and rainforest biomes [18,19]. The growth of the Australian arid zone during this period has been simultaneously implicated in the rapid speciation of some vertebrate groups [20,21], and range restriction and extinction of other, mesic-adapted groups [22–24]. The implications of habitat turnover in the late Miocene for the morphological evolution of Australian vertebrates, however, have not been investigated. Using fossil-calibrated phylogenies and discrete and continuous characters of five Australian vertebrate groups (>800 species), we investigated the influence of protracted Miocene aridification on phenotypic evolution. These focal radiations (agamid lizards, marsupial mammals, meliphagoid birds, pygopodoid geckos, sphenomorphine skinks) cover a diversity of species richness (100–235 spp.), ecology (fossorial to aerial, dietary specialists and generalists) and age (crown 26–60 Ma), to represent a comprehensive sample of extant Australian terrestrial vertebrate biodiversity. More importantly, this sampling enables us to identify the strength and congruence of signatures from multiple independent clades.
To specifically address macroevolutionary change during this period of flux, we focus on body size and historical biogeography. Body size (as body length or mass) is the most commonly used measurement for studies of ecomorphological diversification owing to its ubiquitous influence on life-history traits and ecology [25–27]. Similarly, species' distributions are representative of both their ecological niche (e.g. habitat/biome types), as well as geographical distribution (e.g. explicit proximity or overlap with other species). With recent advances in phylogenetic comparative methods, we can now model changes in morphology and distribution as temporal trends, providing insight into changes both among and along branches of phylogenetic trees.
Given that periods of intense climatic change may precipitate adaptive radiation, we suggest the opposite may be true for periods of gradual change. Whereas ecomorphological radiation follows mass extinction, instead, non-adaptive processes dictate speciation during periods of protracted biome turnover. To address this concept, we started by investigating signature of Miocene biome rearrangement using likelihood methods to determine temporal trends in the geographical mode of speciation, either allopatric or sympatric. We anticipated that changes to the global and Australian climate during this period facilitated an increase in allopatry by fracturing existing mesic habitats. In this case, we consider a strong link between the geographical speciation process of allopatry and the trait evolutionary process of niche conservatism [28,29]. So, we fitted a series of models to the body size data which follow a narrative of increasing late Miocene phenotypic conservatism. These included mode-shifting processes that increasingly retained ancestral body sizes, via declining evolutionary rates and variances in the late Miocene and Pliocene. Our findings are consistent with our hypothesis that prolonged abiotic environmental changes may indirectly constrain phenotypic evolution. These gradual climatic pressures appear to similarly influence the macroevolutionary trajectories of ecologically diverse contemporaneous groups.
2. Material and methods
(a). Phylogenies, and morphological and biogeographic data
Recently developed analytical methods for modelling and visualizing macroevolutionary trends have facilitated the investigation of diversification dynamics of a number of Australian groups [30,31]. Comparatively few studies, however, have looked into the evolutionary tempo of phenotypic evolution in Australian clades [21,32]. We compiled or generated fossil-calibrated phylogenies of Australian radiations spanning squamate reptiles [21,30], birds (honeyeaters) [33] and mammals [34] (see the electronic supplementary material for tree-building details). The breadth of our focal phylogenies (ecology, age, size) aims to analyse a diverse representation of the most conspicuous and abundant Australian vertebrate groups. Though timing and biogeographic patterns of Australian taxa since the Mid-Miocene onset of aridification has been extensively addressed (see [35] for review), we focus on the influence of environmental turnover and biome rearrangement on the tempo and mode of ecomorphological differentiation.
To model body size macroevolution, we collected body size measurements from the literature, relevant to each phylogenetic group: squamate reptiles—snout-vent length (mm); birds—mass (g), mammals—body length (mm) and log-transformed these to normalize data for all analyses. To address biogeographic changes as a result of changing climate and environments, we treated species distributions in two ways. First, by coding occupancy among biomes. Climatic conditions determine the distribution and suitability of biomes largely by influencing the floral assemblage. In Australia, the primary contemporary biome stressor is precipitation and so we partitioned Australia into five discrete biomes that attempt to best encapsulate the intersection of floral community and precipitation. This biome classification system is modified from the widely used objective Köppen–Geiger system [36] and follows Brennan and Oliver [21]. Second, species distributions were described by spatial occurrence data. We downloaded species occurrence records from the Atlas of Living Australia (ALA; www.ala.org.au) then transformed them into spatial data geometries for further analyses (for specifics of data handling, see the electronic supplementary material). Ultimately, both sets of data were used to reconstruct ancestral occupancy and distribution, to determine pairwise geographical overlap among species.
(b). Analyses of body size evolution
To investigate the tempo and mode of body size evolution, we used maximum-likelihood to fit a series of rate constant, rate variable, mode variable and mode and rate variable models to our continuous data. To account for intraspecific variation and trait measurement error (ME) as a potential source of bias in model selection and parameter estimation [37], we jointly estimated ME as an additional parameter during model fitting. We began with Brownian motion (BM) and Ornstein–Uhlenbeck (OU) models as implemented in Geiger [38]; however, our hypothesis of phenotypic evolution focuses on temporal variation in processes and rates. To address this, we also implemented a series of time–variable evolutionary models. These included early burst (EB), multi-era BMOU [39], environmentally dependent [40] and Lévy jump models [41]. We discuss the assumptions and behaviour of these models more extensively in the electronic supplementary material.
During the late Miocene, aridification resulted in the fragmentation of closed forest habitats [22], potentially leading to elevated allopatric speciation, exaggerating niche conservatism and constraining ecomorphological diversification. Morphological conservatism following the Mid-Miocene climatic optimum (MMCO) may be best modelled by a change in the mode of trait evolution, towards a more constrained process akin to OU. To model this indirect environmental constraint on body size evolution, we implement a mode variable BMOU process. We design two models which are methodologically identical to the BMOU and BMOUi models used in mvMORPH [39] and build on the comparative methods literature of time-stratified evolutionary processes [27,42]. These models allow the trait of interest to evolve under BM from the group's origin until tshift, at which point they transition to an OU process with trait evolution constrained by the α parameter. The first, BMOU, estimates only a single rate (σ2) of trait evolution along the whole tree. The BMOU model fits a narrative where body size evolved unconstrained until a given point in time, after which size evolution became bounded around a stationary peak. The second, BMOUi, is similar to the BMOU; however, the trait evolution rate also changes (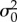 under BM,
under BM, 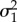 under OU), allowing BM and OU processes to independently explain the accumulated variance of trait evolution in different eras (temporal regimes) of the tree. Because joint estimation of ME is not currently incorporated into mvMORPH, we have built this parameter into our BMOU models found in the ‘fitContinuous_paleo’ script provided in the supplemental material of Slater [27]. This material is available at the GitHub repository for this publication. To determine our ability to recover mode-shifting models and distinguish them from existing models, we performed a series of simulations outlined in the electronic supplementary material.
under OU), allowing BM and OU processes to independently explain the accumulated variance of trait evolution in different eras (temporal regimes) of the tree. Because joint estimation of ME is not currently incorporated into mvMORPH, we have built this parameter into our BMOU models found in the ‘fitContinuous_paleo’ script provided in the supplemental material of Slater [27]. This material is available at the GitHub repository for this publication. To determine our ability to recover mode-shifting models and distinguish them from existing models, we performed a series of simulations outlined in the electronic supplementary material.
Advances in macroevolutionary modelling have provided ever-more complex methods which may better describe the idiosyncracies of evolution. We tested the performance of the process and pattern-driven models alongside several recently developed methods. These model trait evolution as jumps across Simpsonian landscapes (models: Jump-Normal, Normal Inverse Gaussian), with varied waiting times between jumps in trait values [41], or trait variance may instead accumulate in response to additional time-sampled variables like global palaeotemperature (model: ENV) or dispersal rates inferred from an external source (model: BGB—dispersal through time as estimated from BioGeoBears), fitted using RPANDA (function ‘fit_t_env’) [43]. All models in this study were iteratively applied to 100 trees randomly sampled from the post burn-in posterior distribution from dating analyses of each vertebrate clade, as well as the maximum clade credibility tree (MCC) as summarized by TreeAnnotator v. 2.4.2. To compare models against one another, we calculated Akaike information criterion correction (AICc) values from our likelihood scores and the number of parameters in the given model and estimated the AICc weights (AICcWt) as the contribution of the model to the total fit. We combined the AICcWt results across all trees of a given radiation and used this to compute a mean AICcWt and standard error per model, to determine the best fitting models for each radiation. We plotted the results of our iterative model fitting and comparison using ggplot2 [44].
In the process of comparing models for each given tree, we calculated the ΔAICc between each model and the best fitting model (lowest AICc), deeming ΔAICc ≥ 4 as significant evidence of model preference and retained all equally plausible models (ΔAICc < 4) following Burnham and Anderson [45]. We then extracted parameter estimates (all applicable: timing of shift, body size optima θ, constraint α, evolutionary rate σ2, beta) of those preferred (best) models to evaluate the tempo and mode of trait evolution prior to and following the Late Miocene shift at time tshift (electronic supplementary material, figures S3–S6).
(c). Simulating extinction
Macroevolutionary inferences of trait evolution can be improved upon by the inclusion of fossil taxa [46]. This is particularly true on geological time scales, where extinction is considered to be appreciable [47]. Unfortunately, meaningful fossil records are scant for most terrestrial Australian vertebrate groups, save marsupials. Because of this, it is important to take into account that our use of extant-only phylogenies may introduce bias in our inference of trends in biogeographical and body size macroevolution. To directly address the influence of unobserved extinction, we undertook an exercise using our empirical phylogenies to simulate trees and data under a series of plausible extinction scenarios. These assumed extinction throughout the trees to be phylogenetically and temporally (i) stochastic, (ii) elevated in the Pliocene-Pleistocene, or (iii) elevated in the Late Miocene. The specifics of the design and implementation of this extinction exercise are detailed in the electronic supplementary material.
(d). Biogeographic histories
To investigate if the signal of historical biome turnover is detectable and can be modelled from contemporary distributions, we focused on the frequency and timing of cladogenetic dispersal events. We undertook this initially by summarizing species distributions as their occurrence across Australian biomes, then fitting dispersal models using BioGeoBears [48] in R [49] and RStudio [50]. This framework allowed us to account for uncertainty in ancestral distributions using biogeographic stochastic maps, and the ability to simulate data under the generating dispersal model for comparison against empirical results. From this, we summarized the proportion of cladogenetic events deemed allopatric (occurring between biomes) and plotted temporal trends for both the empirical and simulated data (figure 1b). Ultimately, clade-specific dispersal trends (figure 3—‘proportion of divergence events'; electronic supplementary material, figure S1) were then used as a time-sampled variable to explain body size evolution (model BGB) in our comparative model fitting analyses.
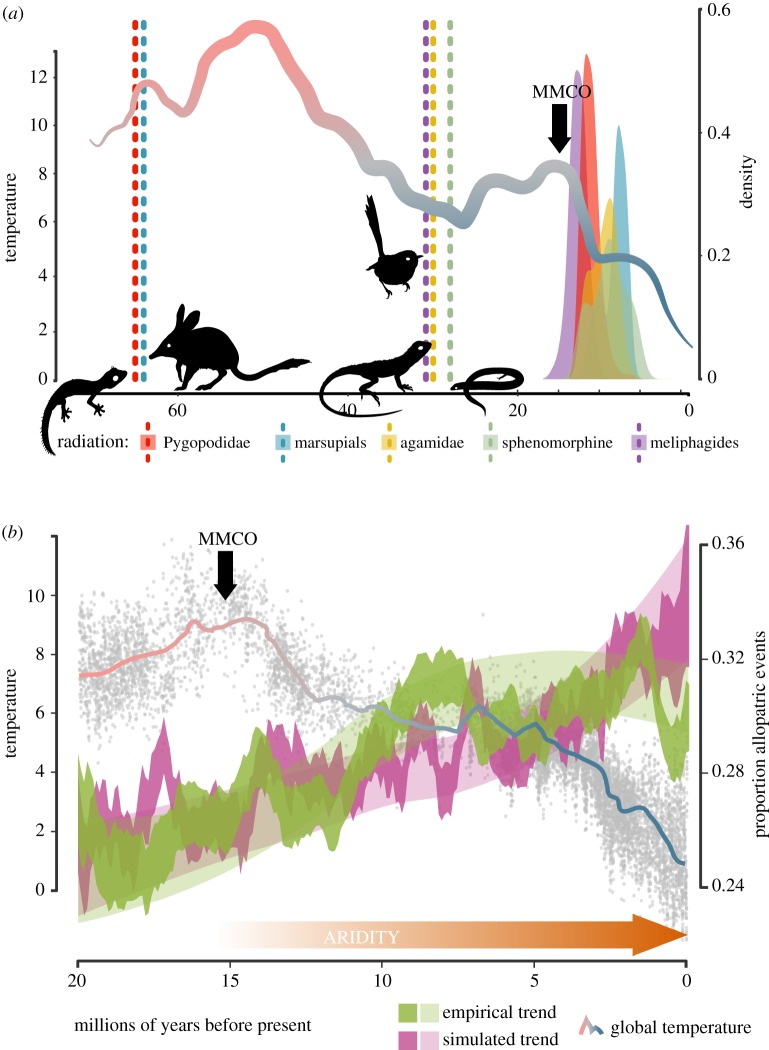
Figure 1.
Shifts in evolutionary mode (BM to OU) of body size evolution are temporally clustered in the Late Miocene and congruent with a shift in dispersal histories. (a) Dotted vertical lines and density distributions are colour-coded by clade and indicate crown divergences and inferred shift timing of each focal radiation. Shifts between rates or modes (or both) of trait evolution are tightly constrained to the Late Miocene (11–5 Ma). Red-to-blue coloured line shows a global trend in palaeotemperature through the Cenozoic, data from Zachos et al. [3]. (b) Trends in the dispersal history of Australian radiations from the Early Miocene to present, as inferred from BioGeoBears analyses of empirical and simulated data (i.e. species distributions are observed as biome occurrences). The observed trend in the proportion of allopatric dispersal events (in green) exceeds the expected simulated proportion (purple), in the Late Miocene, coinciding with constraints on body size evolution of select Australian vertebrate clades. Jagged and Loess-smoothed dispersal curves represent two visualization methods of the same trend. Grey dots show palaeotemperature data and the red-to-blue line shows a best fit trend in palaeotemperature data. Note: scales of temperature and time differ between (a) and (b). MMCO, Mid-Miocene climatic optimum.
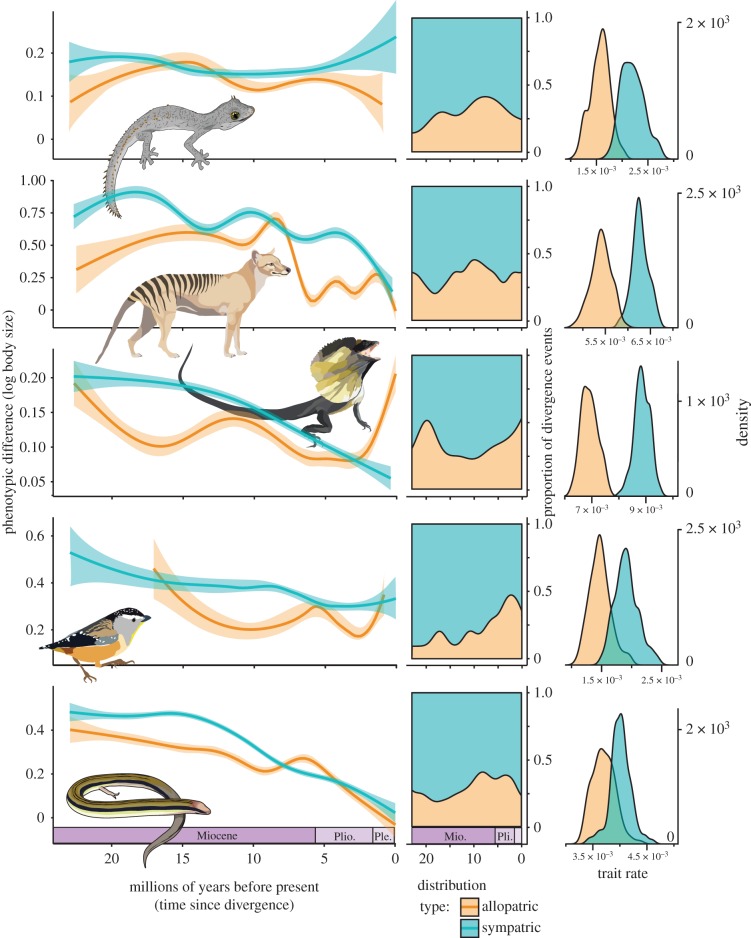
Figure 3.
Sympatric and allopatric species pairs display differing trends in body size evolution. Left and centre columns show trends in allopatry and sympatry as inferred using extant species occurrence data and reconstructed ancestral ranges from rase [51]. Left column: allopatric species pairs (orange lines) exhibit less phenotypic disparity than sympatric relatives (blue lines) and show more pronounced declines in trait disparity through the Miocene. Centre column: the proportion of divergence events which are allopatric (orange fill) increase through the Miocene in most radiations. These estimates differ slightly from those presented in the electronic supplementary material, figure S1 because of the data used (spatial occurrence records versus biome codings), but see the electronic supplementary material, figure S1 for comparison of trends across all clades using both geographical data. Right column: multi-rate Brownian Motion separate model estimates identify greater evolutionary rates (sigma) for sympatric (blue) sister taxa than allopatric (orange).
Alternatively, we used spatial records from the ALA to describe species ranges. Using contemporary point data we modelled ancestral distributions using a BM dispersal method implemented in rase [51]. This allowed us to determine pairwise overlap among taxa within each tree and plot temporal trends in allopatric and sympatric speciation (figure 3; electronic supplementary material, figure S1). For specifics on our biogeographic methods, see the electronic supplementary material.
(e). Intersecting geographical and phenotypic histories
To investigate body size evolution and determine if conservatism is the result of temporal changes in the prevailing geographical mode of speciation (allopatry or sympatry), we combined our phenotypic and spatial occurrence data. We began by creating a pairwise distance matrix between all tips (terminal nodes) and internal nodes of the tree, representing patristic distances between taxa in millions of years. We repeated this process using trait distances (absolute value of sp1–sp2) to determine the amount of phenotypic divergence between species pairs and again using spatial data geometries to ascertain pairwise overlap in distribution (binary: allopatric or sympatric). Unfortunately, shifting species ranges through time, as a result of habitat tracking or evolving niches [52], may erase the signature of the geographical mode of speciation, causing an erroneous signal. To address this, we trimmed these matrices to include only sister pair relationships (terminal node to terminal node, or terminal node to internal node) [53] and plotted the results from 100 trees to visualize temporal trends in phenotypic evolution comparing sympatric to allopatric species pairs (figure 3, left panel). To further explore the relationship between evolutionary rates and phenotypic variance accumulated between allopatric and sympatric taxa, we mapped range overlap as a binary trait onto our trees and estimated independent evolutionary rates using the BMS model in OUwie [54] (figure 3, right panel). To account for intraspecific variation or error, we provided a uniform value of ME per clade, extracted from the empirical model fits.
3. Results
(a). Body size evolution
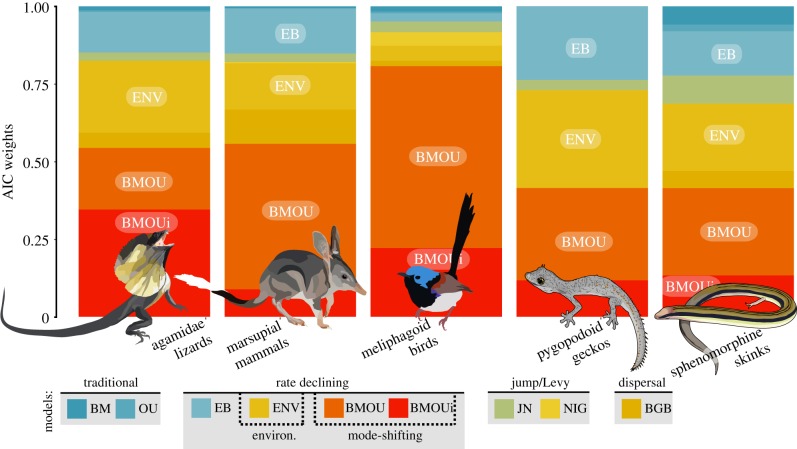
The results of comparative model fitting identified three model classes which account for a combined more than 0.75 AICcWt (and up to 0.97) in all five radiations: mode variable (BMOU, BMOUi), global temperature-dependent (ENV) and exponentially declining (EB) (figure 2; electronic supplementary material, figure S2). All four models describe declining evolutionary rates of phenotypic evolution towards the present, with varied intensities and temporal aspects. In the BMOU and BMOUi models, phenotypic variance slows as the evolutionary process shifts in the Mid-to-Late Miocene, with shifts among radiations temporally clustered (11–5 Ma) but not necessarily concurrent (electronic supplementary material, figures S3 and S4). In all focal groups, estimates of beta for the ENV model suggest a positive relationship between Cenozoic temperature fluctuations and body size evolution. As the global temperature dropped following the MMCO, phenotypic rates followed (electronic supplementary material, figure S5). Finally, evolutionary rates decay exponentially (negative beta values) under the EB model, resulting in a considerable slowdown in the accumulation of phenotypic variance towards the tips of the trees (electronic supplementary material, figure S6).
Figure 2.
Comparative fit of models to body size data of Australian vertebrate clades finds a preference for rate-declining models (BMOU, BMOUi, EB, ENV). Models are categorized below the plot. BMOU, BMOUi and ENV models are not methodologically explicit rate-declining, but instead, empirical parameter estimates (electronic supplementary material, figures S3,S4,S6) inform this trend. The y-axis indicates the relative support for each model as Akaike weights (averaged across 100 posterior trees). The top models which account for a combined more than 0.75 of the AICc weight for each clade are noted on each stacked bar graph.
(b). Effects of extinction
In agreement with studies elsewhere [27,46], we find that in the absence of fossil information, false support for non-generating models does increase (electronic supplementary material, figures S7 and S8; for a description of methods, see the electronic supplementary material). In our simulations, this never results in a shift away from the generating model as preferred. This includes the ‘worst-case’ scenario in which extinction is elevated specifically in the Late Miocene. It is important to note, however, that parameter estimates are dictated by the data provided, and so in the absence of valid fossil information, we rely exclusively on extant taxa for our estimated model values.
(c). Biogeographic histories
Investigating temporal trends in biome dispersal history revealed an increase (5–25% of all events) in the proportion of allopatric events in the Late Miocene. These events, in which sister species (or nodes) do not overlap geographically, increase in relation to sympatric events (figures 1 and 3; electronic supplementary material, figure S1). This result does not appear sensitive to the geographical data used, i.e. if species distributions are coded solely by biome inhabitance (figure 1; electronic supplementary material, figures S1 and S9), or as spatially explicit occurrences (figure 3; electronic supplementary material, figure S1). Elevated trends in allopatric speciation among biomes extend beyond what we would expect from simulations generated under the preferred biogeographic model (always Dispersal Extinction Cladogenesis + jump; see Material and methods). The proportion of allopatric events also increases in a combined analysis across all radiations under both geographical datasets (biome codings and occurrence records).
(d). Geographical mode of speciation and phenotypic evolution
Using spatial records, trends in body size evolution through time differ between allopatric and sympatric species pairs. Irrespective of time, allopatric taxa exhibit less disparity in body size, and through time, exhibit a greater decrease in disparity in the Late Miocene (figure 3). Lower disparity translates into a lower estimated rate of phenotypic evolution in allopatric taxa in all focal radiations (figure 3, right column). In most cases, the frequency of allopatry as the geographical mode of speciation increases and is temporally consistent with an accelerated decline in phenotypic diversity (figure 3, centre column).
4. Discussion
On deep time scales, ebbs and flows in species richness have generally been attributed to abiotic factors, particularly rapid environmental changes [2,55,56]. In comparison, phenotypic macroevolutionary patterns are most often explained by biotic interactions [57–59]. A growing body of work, however, is beginning to draw attention to the influence of abiotic environmental factors on trait evolution, often across ecologically diverse groups [15,40,60–62]. Here, we investigated the impacts of climate change on body size evolution using an extant continental vertebrate fauna. We first sought to determine if the signal of the process of gradual Miocene biome rearrangement remains detectable from current species distributions by modelling biogeographic histories. Second, we investigated how habitat turnover may have influenced phenotypic evolution. We hypothesized that shifting Miocene habitats may have increased allopatric speciation and by association, reduced rates of body size evolution causing an impression of evolutionary stasis. Our results show that biogeographic dispersal histories across all radiations trend towards increasing allopatry through the Miocene. While it may seem obvious that allopatry is a process independent of trait evolution, it is also a primary cause of niche conservatism, which is not [28,29]. Trends towards increasing allopatry are temporally concordant with a shift towards more conservative body size evolution (decreasing rates and variance). We link these two patterns by observing differing temporal dynamics in the evolution of body size between sympatric and allopatric species. Our results imply a climate-driven shift in the evolution of Australian vertebrate body sizes, and that in the face of changing global climates, macroevolutionary responses across diverse clades may be predictable.
(a). Biome turnover and allopatry
Evidence from palaeontological and neontological data suggest that the Miocene was a period of dramatic climatic and environmental flux across Australia [35,63]. The rise of eucalypts, acacias and chenopods ushered in the birth of modern arid biomes and initiated many common geographical barriers to gene-flow [64]. As habitats shifted, species either shifted their own distribution to track preferable habitat (causing local extinctions) or stayed in place and adjusted to habitat changes (local adaptation), else they went extinct [65]. This resulted in well-documented relictual lineages [22,23,66], particularly in low-vagility groups such as reptiles [67–69] and dasyurid mammals [70,71]. Evidence from the fossil record corroborates this and suggests that habitat tracking may reduce phenotypic variance, promoting morphological stasis and allopatry [52,72]. We observe this in extant allospecies which include exceptional examples of cryptic diversity [68,71,73,74]. These taxa exist in similar habitats, with similar ecologies and morphologies, but are fragmented by suitable habitat and isolated by sometimes tens of millions of years, all hallmarks of conservatism. Broadly across our data, these patterns are consistent: slowdowns in phenotypic evolution are associated with allopatric species pairs, which show less body size disparity than sympatric relatives. It is important to note that there is, however, variation in the intensity and tempo of clade-specific trends, which suggests that environmental pressures may act on intrinsic factors such as ecology to dictate the strength and pace of response.
(b). Declining rates of body size evolution
Australia is home to a number of iconic adaptive radiations that are the result of the continent's extended geographical isolation [75]. These radiations include immense ecological variety, from semelparous carnivorous (Antechinus) to gliding herbivorous (Petaurus) marsupial mammals, and from arboreal leaf-tailed (Carphodactylidae) to limbless fossorial (Pygopodidae) geckos. To determine the impact of a changing global climate on phenotypic evolution across such diverse groups, we fitted several models which attempt to account for a transition in evolutionary pace and process during the Miocene. Best supported models all suggest declining evolutionary rates over the course of each group's history. This is explained as a result of early accumulation of variance and subsequent decay (EB—electronic supplementary material, figure S6), a positive relationship to cooling global temperatures (ENV—electronic supplementary material, figure S5), or a single shift in process and rate around the Mid-to-Late Miocene time period (BMOU—electronic supplementary material, figure S3, BMOUi—electronic supplementary material, figure S4). Regardless of the specifics, parameter estimates from these models all distinguish between trait evolution occurring at deep and shallower time scales, suggesting differing periods of temporal phenotypic evolution (electronic supplementary material, figure S3–S6).
Outwardly, declining rates result in reduced accumulation of variance and the appearance of periods of morphological stasis [6,7]. To date, the majority of evidence linking phenotypic slowdowns with environmental drivers has been limited largely to fossil data [15,52]. In the absence of reliable fossil records (particularly for squamate reptiles), however, we tentatively suggest the same using molecular and trait data solely from extant taxa. This appearance of stasis may, however, be the result of alternative processes dictating phenotypic evolution. Unpredictable climates might have favoured incremental or gradual steps in trait change (instead of significant jumps), or filtered extreme phenotypes, selecting for generalists. Long-sustained habitats may have also encouraged convergence towards similar trait values, mimicking evolutionary rate declines. Admittedly, all of these are plausible alternatives that are difficult to distinguish with neontological data alone, but present interesting directions for future study. Given existing data and inferred changes to temporal patterns in the process of geographical speciation, we opt to link observed slowdowns in Australian vertebrate trait evolution with gradually shifting global climates and local Australian biome rearrangement.
(c). A changing landscape and phenotypic conservatism
At present, studies investigating periods of protracted climate change are far outnumbered by those studying the effects of dramatic climate turnover. This is probably owing to the more conspicuous diversification and phenotypic shifts which occur following rapid climate change. However, this fails to recognize that periods of gradual climate change predominate geological time. By looking across several cohabiting clades, we find that signature of biome rearrangement may still be readable from extant species, despite the self-effacing nature of evolution and differing community responses to the expansion of arid habitats [76,77].
Protracted biome rearrangement in Late Miocene Australia has been implicated in allopatric and often cryptic, speciation of mammals [71], reptiles [20], amphibians [78], freshwater fishes [79], spiders [80] and cicadas [23] among others. In some clades (see Gehyra, Diplodactylus, Oedura and Crenadactylus geckos; Ctenotus and Lerista skinks; Planigale dasyurids; Uperoleia frogs) it is only because of the availability of molecular studies that we have begun to grasp the incredible amount of cryptic diversity that exists. Here, we synthesize trends from several iconic clades to show that conservative phenotypic evolution driven by a cooling global climate and allopatry is a common pattern in Australia. We suggest that for extant taxa, this process is more prevalent than has been previously thought and identify a consistent temporal driver, Miocene climate change. Finally, we find it encouraging that this process is visible on a continental scale, where broad-scale Miocene turnover in terrestrial biomes has accounted for the observed pattern of constrained trait evolution across Australia.
While Australia is unique in its forms of diversity, its biogeographic and phenotypic patterns have probably been shaped by the same processes occurring elsewhere. Changes to the global climate dictate the evolution and succession of biomes, including the expansion of Miocene deserts [16,17]. Though the incredible diversity of body forms of Australian vertebrates appear to have developed early in their evolution, perhaps equally intriguing is the more recent climate-mediated shift towards a non-adaptive process in the Miocene. Results from our study offer evidence that similar processes may have dictated patterns in dispersal history and trait evolution among terrestrial organisms occurring on other continents.
Supplementary Material
Acknowledgements
We would like to thank the Keogh laboratory group for discussion and comments throughout the development of this project, Rob Lanfear for an unfiltered critique, Alex Skeels for an introduction to spatial data, and Zoe K. M. Reynolds for critical comments and troubleshooting scripts. Thank you to Liam Revell and Graham Slater for help with simulating trait data under varied evolutionary models. Susanne Fritz, Julien Clavel and Michael Landis provided exceptionally thoughtful and constructive suggestions on a previous version of this manuscript. A considerable thank you to the curators and staff of the many Australian Museums and databases (Australian Museum, Northern Territories Museum, South Australian Museum, Queensland Museum, Western Australian Museum, Atlas of Living Australia) for access to tissues and locality data that made this work possible.
Data accessibility
All data and R code used in this study, as well as supporting materials, results and figures are available on GitHub: https://github.com/IanGBrennan/MioceneAustralia. Details of museum sample numbers and specimen identities can be found in the electronic supplementary material.
Authors' contributions
I.G.B. conceived of the study and collected and analysed the data. I.G.B. and J.S.K. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This work has been funded by an Australian Research Council Discovery grant no. (ARC DP150102403) to J.S.K., and an Australian National University International Postgraduate Research Scholarship to I.G.B.
References
- 1.Ezard THG, Aze T, Pearson PN, Purvis A. 2011. Interplay between changing climate and species' ecology drives macroevolutionary dynamics. Science 332, 349–351. ( 10.1126/science.1203060) [DOI] [PubMed] [Google Scholar]
- 2.Jablonski D. 1989. The biology of mass extinction: a palaeontological view. Proc. Soc. Lond. B 325, 357–368. ( 10.1098/rstb.1989.0093) [DOI] [PubMed] [Google Scholar]
- 3.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 4.Cramer BS, Miller KG, Barrett PJ, Wright JD. 2011. Late Cretaceous–Neogene trends in deep ocean temperature and continental ice volume: reconciling records of benthic foraminiferal geochemistry (δ18O and Mg/Ca) with sea level history. J. Geophys. Res. Oceans 116, 1–23. ( 10.1029/2011JC007255) [DOI] [Google Scholar]
- 5.Badgley C, Barry JC, Morgan ME, Nelson SV, Behrensmeyer AK, Cerling TE, Pilbeam D. 2008. Ecological changes in Miocene mammalian record show impact of prolonged climatic forcing. Proc. Natl Acad. Sci. USA 105, 12145–12149. ( 10.1073/pnas.0805592105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brett CE, Ivany LC, Schopf KM. 1996. Coordinated stasis: an overview. Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 1–20. ( 10.1016/S0031-0182(96)00085-5) [DOI] [Google Scholar]
- 7.Eldredge N, et al. 2005. The dynamics of evolutionary stasis. Paleobiology 31, 133–145. ( 10.1666/0094-8373(2005)031%5B0133:tdoes%5D2.0.co;2) [DOI] [Google Scholar]
- 8.Hoorn C, et al. 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931. ( 10.1126/science.1194585) [DOI] [PubMed] [Google Scholar]
- 9.Winkler IS, Mitter C, Scheffer SJ. 2009. Repeated climate-linked host shifts have promoted diversification in a temperate clade of leaf-mining flies. Proc. Natl Acad. Sci. USA 106, 18 103–18 108. ( 10.1073/pnas.0904852106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagani M, Freeman KH, Arthur MA. 1999. Late Miocene atmospheric CO2 concentrations and the expansion of C4 grasses. Science 285, 876–879. ( 10.1126/science.285.5429.876) [DOI] [PubMed] [Google Scholar]
- 11.Kürschner WM, Kvaček Z, Dilcher DL. 2008. The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proc. Natl Acad. Sci. USA 105, 449–453. ( 10.1073/pnas.0708588105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savin SM, Abel L, Barrera E, Hodell D, Kennett JP, Murphy M, Keller G, Killingley J, Vincent E. 1985. The evolution of Miocene surface and near-surface marine temperatures: oxygen isotopic evidence. Geol. Soc. Am. Mem. 163, 49–82. ( 10.1130/MEM163-p49) [DOI] [Google Scholar]
- 13.Herbert TD, Lawrence KT, Tzanova A, Peterson LC, Caballero-Gill R, Kelly CS. 2016. Late Miocene global cooling and the rise of modern ecosystems. Nat. Geosci. 9, 843–847. ( 10.1038/ngeo2813) [DOI] [Google Scholar]
- 14.Janis C. 2008. An evolutionary history of browsing and grazing ungulates. In The ecology of browsing and grazing (eds Gordon IJ, Prins HHT), pp. 21–45. Berlin, Germany: Springer. [Google Scholar]
- 15.Vrba SE. 2005. Mass turnover and heterochrony events in response to physical change. Paleobiology 31, 157–174. ( 10.1666/0094-8373(2005)031%5B0157:MTAHEI%5D2.0.CO;2) [DOI] [Google Scholar]
- 16.Caves JK, et al. 2016. The Neogene de-greening of Central Asia. Geology 44, 887–890. ( 10.1130/g38267.1) [DOI] [Google Scholar]
- 17.Zhang Z, Ramstein G, Schuster M, Li C, Contoux C, Yan Q. 2014. Aridification of the Sahara desert caused by Tethys Sea shrinkage during the Late Miocene. Nature 513, 401–404. ( 10.1038/nature13705) [DOI] [PubMed] [Google Scholar]
- 18.Martin HA. 2006. Cenozoic climatic change and the development of the arid vegetation in Australia. J. Arid Environ. 66, 533–563. ( 10.1016/j.jaridenv.2006.01.009) [DOI] [Google Scholar]
- 19.Crisp MD, Cook LG. 2013. How was the Australian flora assembled over the last 65 million years? A molecular phylogenetic perspective. Annu. Rev. Ecol. Evol. Syst. 44, 303–324. ( 10.1146/annurev-ecolsys-110512-135910) [DOI] [Google Scholar]
- 20.Rabosky DL, Donnellan SC, Talaba AL, Lovette IJ. 2007. Exceptional among-lineage variation in diversification rates during the radiation of Australia's most diverse vertebrate clade. Proc. R. Soc. B 274, 2915–2923. ( 10.1098/rspb.2007.0924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan IG, Oliver PM. 2017. Mass turnover and recovery dynamics of a diverse Australian continental radiation. Evolution 71, 1351–1365. ( 10.1111/evo.13207) [DOI] [PubMed] [Google Scholar]
- 22.Bryant LM, Krosch MN. 2016. Lines in the land: a review of evidence for eastern Australia's major biogeographical barriers to closed forest taxa. Biol. J. Linn. Soc. 119, 238–264. ( 10.1111/bij.12821) [DOI] [Google Scholar]
- 23.Owen CL, Marshall DC, Hill KBR, Simon C. 2017. How the aridification of Australia structured the biogeography and influenced the diversification of a large lineage of Australian cicadas. Syst. Biol. 66, 569–589. ( 10.5061/dryad.1580p) [DOI] [PubMed] [Google Scholar]
- 24.Cardillo M, Weston PH, Reynolds ZKM, Olde PM, Mast AR, Lemmon EM, Lemmon AR, Bromham L. 2017. The phylogeny and biogeography of Hakea (Proteaceae) reveals the role of biome shifts in a continental plant radiation. Evolution 71, 1928–1943. ( 10.1111/evo.13276) [DOI] [PubMed] [Google Scholar]
- 25.Peters RH. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Wilson DS. 1975. The adequacy of body size as a niche difference. Am. Nat. 109, 769–784. ( 10.1086/283042) [DOI] [Google Scholar]
- 27.Slater GJ. 2013. Phylogenetic evidence for a shift in the mode of mammalian body size evolution at the Cretaceous-Palaeogene boundary. Methods Ecol. Evol. 4, 734–744. ( 10.1111/2041-210X.12084) [DOI] [Google Scholar]
- 28.Wiens JJ. 2004. Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution 58, 193–197. ( 10.1554/03-447) [DOI] [PubMed] [Google Scholar]
- 29.Pyron RA, Costa GC, Patten MA, Burbrink FT. 2015. Phylogenetic niche conservatism and the evolutionary basis of ecological speciation. Biol. Rev. Camb. Philos. Soc. 90, 1248–1262. ( 10.1111/brv.12154) [DOI] [PubMed] [Google Scholar]
- 30.Rabosky DL, Donnellan SC, Grundler M, Lovette IJ. 2014. Analysis and visualization of complex macroevolutionary dynamics: an example from Australian scincid lizards. Syst. Biol. 63, 610–627. ( 10.1093/sysbio/syu025) [DOI] [PubMed] [Google Scholar]
- 31.Smissen PJ, Rowe KC. 2016. Repeated biome transitions in the evolution of Australian rodents. Mol. Phylogenet. Evol. 128, 182–191. ( 10.1016/j.ympev.2018.07.015) [DOI] [PubMed] [Google Scholar]
- 32.Toussaint EFA, Condamine FL, Hawlitschek O, Watts CH, Porch N, Hendrich L, Balke M. 2015. Unveiling the diversification dynamics of Australasian predaceous diving beetles in the Cenozoic. Syst. Biol. 64, 3–24. ( 10.1093/sysbio/syu067) [DOI] [PubMed] [Google Scholar]
- 33.Marki PZ, Jonsson KA, Irestedt M, Nguyen JM, Rahbek C, Fjeldsa J. 2017. Supermatrix phylogeny and biogeography of the Australasian Meliphagides radiation (Aves: Passeriformes). Mol. Phylogenet. Evol. 107, 516–529. ( 10.1016/j.ympev.2016.12.021) [DOI] [PubMed] [Google Scholar]
- 34.Mitchell KJ, et al. 2014. Molecular phylogeny, biogeography, and habitat preference evolution of marsupials. Mol. Biol. Evol. 31, 2322–2330. ( 10.1093/molbev/msu176) [DOI] [PubMed] [Google Scholar]
- 35.Byrne M, et al. 2008. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Mol. Ecol. 17, 4398–4417. ( 10.1111/j.1365-294X.2008.03899.x) [DOI] [PubMed] [Google Scholar]
- 36.Stern H, De Hoedt G, Ernst J. 2000. Objective classification of Australian climates. Aust. Meteorol. Mag. 49, 87–96. [Google Scholar]
- 37.Silvestro D, Kostikova A, Litsios G, Pearman PB, Salamin N, Münkemüller T. 2015. Measurement errors should always be incorporated in phylogenetic comparative analysis. Methods Ecol. Evol. 6, 340–346. ( 10.1111/2041-210x.12337) [DOI] [Google Scholar]
- 38.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 39.Clavel J, Escarguel G, Merceron G. 2015. mvmorph: anrpackage for fitting multivariate evolutionary models to morphometric data. Methods Ecol. Evol. 6, 1311–1319. ( 10.1111/2041-210x.12420) [DOI] [Google Scholar]
- 40.Clavel J, Morlon H. 2017. Accelerated body size evolution during cold climatic periods in the Cenozoic. Proc. Natl Acad. Sci. USA 114, 4183–4188. ( 10.1073/pnas.1606868114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landis MJ, Schraiber JG. 2017. Pulsed evolution shaped modern vertebrate body sizes. Proc. Natl Acad. Sci. USA 114, 13 224–13 229. ( 10.1073/pnas.1710920114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ree RH, Smith SA. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 57, 4–14. ( 10.1080/10635150701883881) [DOI] [PubMed] [Google Scholar]
- 43.Morlon H, Lewitus E, Condamine FL, Manceau M, Clavel J, Drury J. 2016. RPANDA: an R package for macroevolutionary analyses on phylogenetic trees. Methods Ecol. Evol. 7, 589–597. ( 10.1111/2041-210X.12526) [DOI] [Google Scholar]
- 44.Wickham H. 2009. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 45.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference, xxvi+488 p New York, NY: Springer. [Google Scholar]
- 46.Slater GJ, Harmon LJ, Alfaro ME. 2012. Integrating fossils with molecular phylogenies improves inference of trait evolution. Evolution 66, 3931–3944. ( 10.1111/j.1558-5646.2012.01723.x) [DOI] [PubMed] [Google Scholar]
- 47.Jablonski D. 2008. Extinction and the spatial dynamics of biodiversity. Proc. Natl Acad. Sci. USA 105(Supplement 1), 11528–11535. ( 10.1073/pnas.0801919105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matzke NJ. 2013. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 5, 242–248. [Google Scholar]
- 49.R Core Team. 2000. R language definition. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 50.R Studio Team. 2017. RStudio: integrated development environment for R. Boston, MA: RStudio, Inc. [Google Scholar]
- 51.Quintero I, Keil P, Jetz W, Crawford FW. 2015. Historical biogeography using species geographical ranges. Syst. Biol. 64, 1059–1073. ( 10.1093/sysbio/syv057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raia P, Passaro F, Fulgione D, Carotenuto F. 2012. Habitat tracking, stasis and survival in Neogene large mammals. Biol. Lett. 8, 64–66. ( 10.1098/rsbl.2011.0613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drury JP, Grether GF, Garland T Jr, Morlon H. 2018. An assessment of phylogenetic tools for analyzing the interplay between interspecific interactions and phenotypic evolution. Syst. Biol. 67, 413–427. ( 10.1093/sysbio/syx079) [DOI] [PubMed] [Google Scholar]
- 54.Beaulieu J, O'Meara B. 2012. OUwie: analysis of evolutionary rates in an OU framework. R package version 1. See https://cran.r-project.org/web/packages/OUwie/.
- 55.Tennant JP, Mannion PD, Upchurch P, Sutton MD, Price GD. 2016. Biotic and environmental dynamics through the Late Jurassic–Early Cretaceous transition: evidence for protracted faunal and ecological turnover. Biol. Rev. 92, 776–814. ( 10.1111/brv.12255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jezkova T, Wiens JJ. 2017. What explains patterns of diversification and richness among animal phyla? Am. Nat. 189, 201–212. ( 10.1086/690194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber MG, Wagner CE, Best RJ, Harmon LJ, Matthews B. 2017. Evolution in a community context: on integrating ecological interactions and macroevolution. Trends Ecol. Evol. 32, 291–304. ( 10.1016/j.tree.2017.01.003) [DOI] [PubMed] [Google Scholar]
- 58.Clarke M, Thomas GH, Freckleton RP. 2017. Trait evolution in adaptive radiations: modeling and measuring interspecific competition on phylogenies. Am. Nat. 189, 121–137. ( 10.1086/689819) [DOI] [PubMed] [Google Scholar]
- 59.Etienne RS, Haegeman B. 2012. A conceptual and statistical framework for adaptive radiations with a key role for diversity dependence. Am. Nat. 180, E75–E89. ( 10.1086/667574) [DOI] [PubMed] [Google Scholar]
- 60.Olalla-Tárraga MÁ, et al. 2011. Climatic niche conservatism and the evolutionary dynamics in species range boundaries: global congruence across mammals and amphibians. J. Biogeogr. 38, 2237–2247. ( 10.1111/j.1365-2699.2011.02570.x) [DOI] [Google Scholar]
- 61.Condamine FL, Rolland J, Morlon H. 2013. Macroevolutionary perspectives to environmental change. Ecol. Lett. 16, 72–85. ( 10.1111/ele.12062) [DOI] [PubMed] [Google Scholar]
- 62.Vrba ES. 1993. Turnover-pulses, the Red Queen, and related topics. Am. J. Sci. 293, 418–452. ( 10.2475/ajs.293.A.418) [DOI] [Google Scholar]
- 63.Byrne M, et al. 2011. Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. J. Biogeogr. 38, 1635–1656. ( 10.1111/j.1365-2699.2011.02535.x) [DOI] [Google Scholar]
- 64.Edwards RD, Crisp MD, Cook DH, Cook LG. 2017. Congruent biogeographical disjunctions at a continent-wide scale: quantifying and clarifying the role of biogeographic barriers in the Australian tropics. PLoS ONE 12, e0174812 ( 10.1371/journal.pone.0174812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mairal M, Sanmartín I, Pellissier L. 2017. Lineage-specific climatic niche drives the tempo of vicariance in the Rand Flora. J. Biogeogr. 44, 911–923. ( 10.1111/jbi.12930) [DOI] [Google Scholar]
- 66.Schneider CJ, Cunningham M, Moritz C. 1998. Comparative phylogeography and the history of endemic vertebrates in the wet tropics of Australia. Mol. Ecol. 7, 487–498. ( 10.1046/j.1365-294x.1998.00334.x) [DOI] [Google Scholar]
- 67.Smith L, Henry J. 1999. Aprasia picturata (Squamata: Pygopodidae), a new legless lizard from the interior of Western Australia. J. R. Soc. West. Aust. 82, 75–77. [Google Scholar]
- 68.Oliver PM, Adams M, Doughty P. 2010. Molecular evidence for ten species and Oligo-Miocene vicariance within a nominal Australian gecko species (Crenadactylus ocellatus, Diplodactylidae). BMC Evol. Biol. 10, 1–11. ( 10.1186/1471-2148-10-386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujita MK, McGuire JA, Donnellan SC, Moritz C. 2010. Diversification and persistence at the arid-monsoonal interface: Australia-wide biogeography of the Bynoe's gecko (Heteronotia binoei; Gekkonidae). Evolution 64, 2293–2314. ( 10.1111/j.1558-5646.2010.00993.x) [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Navas V, Westerman M. 2018. Niche conservatism and phylogenetic clustering in a tribe of arid-adapted marsupial mice, the Sminthopsini. J. Evol. Biol. 31, 1204–1215. ( 10.1111/jeb.13297) [DOI] [PubMed] [Google Scholar]
- 71.Westerman M, Blacket MJ, Hintz A, Armstrong K, Woolley PA, Krajewski C. 2016. A plethora of planigales: genetic variability and cryptic species in a genus of dasyurid marsupials from northern Australia. Aust. J. Zool. 64, 303 ( 10.1071/zo16052) [DOI] [Google Scholar]
- 72.Fortelius M, Eronen JT, Kaya F, Tang H, Raia P, Puolamäki K. 2014. Evolution of neogene mammals in Eurasia: environmental forcing and biotic interactions. Annu. Rev. Earth Planet Sci. 42, 579–604. ( 10.1146/annurev-earth-050212-124030) [DOI] [Google Scholar]
- 73.Oliver PM, Adams M, Lee MS, Hutchinson MN, Doughty P. 2009. Cryptic diversity in vertebrates: molecular data double estimates of species diversity in a radiation of Australian lizards (Diplodactylus, Gekkota). Proc. R. Soc. B 276, 2001–2007. ( 10.1098/rspb.2008.1881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oliver PM, McDonald PJ. 2016. Young relicts and old relicts: a novel palaeoendemic vertebrate from the Australian Central Uplands. R. Soc. open sci. 3, 160018 ( 10.1098/rsos.160018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–639. ( 10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 76.Lanier HC, Edwards DL, Knowles LL. 2013. Phylogenetic structure of vertebrate communities across the Australian arid zone. J. Biogeogr. 40, 1059–1070. ( 10.1111/jbi.12077) [DOI] [Google Scholar]
- 77.Powney GD, Grenyer R, Orme CDL, Owens IPF, Meiri S. 2010. Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds. Glob. Ecol. Biogeogr. 19, 386–396. ( 10.1111/j.1466-8238.2009.00521.x) [DOI] [Google Scholar]
- 78.Catullo RA, Keogh JS. 2014. Aridification drove repeated episodes of diversification between Australian biomes: evidence from a multi-locus phylogeny of Australian toadlets (Uperoleia: Myobatrachidae). Mol. Phylogenet. Evol. 79, 106–117. ( 10.1016/j.ympev.2014.06.012) [DOI] [PubMed] [Google Scholar]
- 79.Unmack PJ, Bagley JC, Adams M, Hammer MP, Johnson JB. 2012. Molecular phylogeny and phylogeography of the Australian freshwater fish genus Galaxiella, with an emphasis on dwarf galaxias (G. pusilla). PLoS ONE 7, e38433 ( 10.1371/journal.pone.0038433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rix MG, Harvey MS. 2012. Phylogeny and historical biogeography of ancient assassin spiders (Araneae: Archaeidae) in the Australian mesic zone: evidence for Miocene speciation within Tertiary refugia. Mol. Phylogenet. Evol. 62, 375–396. ( 10.1016/j.ympev.2011.10.009) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and R code used in this study, as well as supporting materials, results and figures are available on GitHub: https://github.com/IanGBrennan/MioceneAustralia. Details of museum sample numbers and specimen identities can be found in the electronic supplementary material.