Abstract
The human birth canal shows a tight fit with the size of the neonate, which can lead to obstetric complications. This is not the case in other apes, and has been explained as the outcome of conflicting evolutionary pressures for bipedal locomotion and parturition of a highly encephalized fetus. Despite the suggested evolutionary constraints on the female pelvis, we show that women are, in fact, extremely variable in the shape of the bony birth canal, with human populations having differently shaped pelvic canals. Neutral evolution through genetic drift and differential migration are largely responsible for the observed pattern of morphological diversity, which correlates well with neutral genetic diversity. Climatic adaptation might have played a role, albeit a minor one, with populations from colder regions showing a more transversally oval shape of the canal inlet. The significant extent of canal shape variation among women from different regions of the world has important implications for modern obstetric practice in multi-ethnic societies, as modern medical understanding has been largely developed on studies of European women.
Keywords: human, pelvis, birth canal, obstetrical constraints, neutral variation, climate
1. Introduction
The shape of the human pelvis is often interpreted as an evolutionary compromise between conflicting selective pressures: a short and compact structure is favoured for bipedal locomotion, but a spacious pelvic canal is essential for parturition of a highly encephalized neonate [1–4] (but see [5–7] for recent challenges to this hypothesis). These contradictory requirements are often referred to as the ‘obstetrical dilemma’ [8], whereby locomotory efficiency poses a limit to the size of the birth canal in our species, sometimes leading to childbirth complications due to cephalo-pelvic disproportion (a mismatch between the size of the fetus' skull and the mother's birth canal). Owing to the tight fit between neonatal size and canal size, the fetus needs to perform a series of rotations to successfully navigate the mother's birth canal. During childbirth, the fetus' head and shoulders align with the wider, transversal diameter of the canal entrance (inlet). Progressing through the pelvis, however, the fetus needs to turn to adapt to the changing shape of the canal, which tends to be sagittally deeper at its midpoint (midplane), and it is often transversally larger again at the outlet [9]. This set of rotations has been explained as a further consequence of the obstetrical dilemma, and in particular of the need to maintain an obstetrically sufficient pelvic canal despite the changes in pelvic shape that accompanied full bipedal adaptation [4].
Because of the effects of the contrasting selective pressures for locomotion and childbirth, it can be expected that human variation in pelvic canal size and shape would be limited by functional constraints. The obstetrical dilemma has been often discussed in terms of constraints on pelvic breadth, especially bi-acetabular breadth, for efficient locomotion [5,10]; however, bipedal adaptations in the pelvis involved wider changes in bone shape and relative position, which affected and potentially constrained all measurements of the birth canal [11]. In contrast to these expectations, there is some evidence that women vary substantially in the shape of the birth canal. The presence of individual variation became obvious with the introduction of X-ray pelvimetry as a standard prenatal examination, during the first half of the twentieth century. Even though the measurements were mostly taken from women of European descent, and therefore had limited geographical diversity, the variation in the shape of the canal was revealed to be substantial. The data were used to define different pelvic types based on the geometry of the pelvic inlet [12,13]. Caldwell and Moloy's typology, in particular, became widely used in the obstetrical literature: depending on the shape of the inlet, the female pelvis was defined as platypelloid (transversally oval inlet), android (heart-shaped inlet), gynecoid (round inlet), or anthropoid (sagittally oval inlet). The different types of pelvis, defined by the shape of the birth canal, have been associated in some studies to different likelihood of childbirth complications, which tends to be higher for the platypelloid and android pelves [12,14–16].
Recent studies have confirmed individual variation in pelvic canal dimensions among women, higher than reported for dimensions of the limbs [17,18]. Moreover, canal shape and overall pelvic shape show significant variation among different ethnic groups [17,19–21]. One possible explanation for population differences in pelvic canal shape is adaptation to local environments. Modern human populations living in high-latitude regions have been shown to have relatively wider pelves than tropical populations [22–25]. This difference has been interpreted as evidence of long-term thermoregulatory adaptation, whereby a wider trunk might help reduce heat loss in cold environments by decreasing the surface-to-volume ratio of the body [24]. If climatic adaptation affected the breadth of the pelvic girdle, and indirectly the breadth of the canal, it could have contributed to generating differences in canal shape between populations.
Obstetrically related selection might have also played a role. Childbirth complications are expected to have a considerable direct effect on the fitness of a woman, which would be decreased in primis by her death or injury, but also by the death or parturition-induced disabilities of the newborn. A recent study suggested that shorter women, who on average have a higher incidence of cephalo-pelvic disproportion [26], tend to have a rounder, obstetrically more effective birth canal [27]. The birth canal of small-bodied populations, such as the KhoeSan, has also been shown to be larger than expected in some dimensions [28].
Neutral processes such as genetic drift and migration can also be expected to generate geographical variation, and have been shown to be important factors in explaining human variation in both cranial and pelvic shape [29–34]. Indeed, these neutral processes likely played a bigger role than climatic selection in shaping population differences in pelvic shape [35].
Understanding the extent to which women vary in the shape and size of the birth canal, and the processes involved in shaping such variation, is important in many respects. Despite some indication of substantial geographical diversity, modern obstetric textbooks are still relying on a typological description of shape variation largely based on women of European descent. An update seems necessary, especially in an increasingly multi-ethnic society. A better understanding of the range of modern birth canal variation is also crucial to interpret the changes in pelvic shape that accompanied hominin evolution and their relationship with obstetrical adaptations.
In this study, we evaluate global variation in the size and shape of the female birth canal, and we test the relative importance of selective and neutral processes in shaping their geographical diversity.
2. Material and methods
Measurements of the main diameters of the birth canal of rearticulated pelves were collected following Kurki [28] (figure 1a) from 348 female individuals from 24 populations (figure 1b and table 1) covering multiple continents. The samples come from different periods, ranging from the second millennium Before Common Era (BCE) to the modern era (electronic supplementary material, table S1).
Figure 1.
Pelvic canal measurements (a) and population samples (b) used in this study. A = anteroposterior (AP) diameter of the inlet, from the sacral promontory to the dorsomedial superior pubis; B = AP diameter of the midplane, from the junction of the fourth and fifth sacral vertebrae to the dorsomedial inferior pubis; C = AP diameter of the outlet, from the apex of the fifth sacral vertebra to the dorsomedial inferior pubis; D = mediolateral (ML) diameter of the inlet, as maximum distance between the linea terminalis; E = ML diameter of the midplane, as distance between the ischial spines (often approximated due to damage to the spines); F = ML diameter of the outlet between the inner margins of the transverse ridge of the ischial tuberosities. (Online version in colour.)
Table 1.
Skeletal samples included in the study. Nbody size is the number of individuals for whom it was possible to estimate body size based on the size of the acetabulum.
| region | Ntot | Nbody size |
|---|---|---|
| Africa | ||
| Botswana, Tswana | 18 | 18 |
| Kenya, Kykuyu | 21 | 21 |
| Lesotho, Sotho | 21 | 21 |
| Nubia | 17 | 16 |
| South Africa, KhoeSan | 12 | 12 |
| Swaziland | 16 | 16 |
| Europe/North Africa | ||
| Austria | 13 | 13 |
| Egypt dynastic | 9 | 9 |
| France | 15 | 15 |
| Italy | 6 | 6 |
| Portugal | 25 | 25 |
| Asia | ||
| Ainu, Japan | 13 | 3 |
| India | 11 | 6 |
| Iran (Iron Age) | 11 | 11 |
| Japan | 21 | 21 |
| Philippines, ‘negritos’ | 8 | 8 |
| Thailand | 21 | 20 |
| America | ||
| Alaska, Ipiutak and Tigara | 21 | 21 |
| Argentina, Patagonia | 7 | 7 |
| Canada, Sadlermiut | 9 | 9 |
| Chile, Fuegians | 9 | 9 |
| Native Californians | 15 | 15 |
| Peru | 13 | 13 |
| South Dakota, Arikara | 16 | 15 |
Sex was determined using standard non-metric methods [36,37], which have been shown to have high accuracy (88–96%) [36,38,39]. The pelvis was rearticulated by fitting as closely as possible the sacrum and the two ossa coxarum at the auricular surface. The gap at the level of the pubic symphysis was filled with plasticine without altering the relative position of the bones, and the rearticulated structure was kept firmly together using elastic bands. An internal caliper was used to measure the anteroposterior and mediolateral diameters of the three planes of the canal. From the raw data, inlet, midplane, and outlet indices were estimated by dividing the respective anteroposterior diameter by the mediolateral diameter, and the indices were used as canal shape data in all the analyses. Canal size was estimated as the sum of the geometric mean of the diameters in each plane. The geometric mean was preferred to the arithmetic mean because it uses a product of the canal diameters, therefore better resembling the area of the canal. The supero-inferior diameter of the acetabulum (from the point on the acetabulum margin where the ilium and ilio-pubic ramus meet to the point furthest away) was measured in most of the individuals, excluding the individuals whose pelvis had been mounted and could not be disassembled (table 1, Nbody size), as a proxy for body size. Following Ruff [40], the femoral head diameter was estimated from the acetabular diameter with the following formula:
The estimated femoral head diameter was then used to calculate likely body mass using the mean result of three different equations, as suggested by Auerbach & Ruff [41]: McHenry's [42] formula for small-bodied samples, Grine et al. [43] formula for large-bodied samples, and Ruff et al. [44] formula for females of European and African ancestry derived from a modern US sample. The combination of the three equations reduces the likelihood of a bias in body mass estimation when using human populations of different body sizes and proportions.
The location of each population was recorded as the coordinates of the centroid of the region that the population likely inhabited (electronic supplementary material, table S2). Geographical distance from central sub-Saharan Africa (8° S 25° E), as the hypothesized region of origin of our species, was estimated as the shortest distance on land avoiding long sea crossings and mountain ranges over 2 000 m in altitude [45]. The average minimum temperature of the coldest month and the average maximum temperature of the warmest month for the population centroids (electronic supplementary material, table S2) were obtained from WorldClim (www.worldclim.org; [46]) as interpolated GIS layers at 30 arc-second definition (ca 1 km).
In the absence of available genetic and morphological data from the same individuals, we assembled a set of populations with genetic information that matched our samples based on geographical location and ancestry. We selected populations from the Human Origin dataset [47], which have been typed for 594 924 single nucleotide polymorphisms (SNPs). As it was impossible to find a suitable match for all skeletal populations, the analyses which involved genetic data were run on a subset of the data (electronic supplementary material, table S3).
(a). Variation in shape and size
To evaluate the geographical pattern of variation in the shape of the birth canal, we compared the variation in pelvic indices among continents using ANOVA and a post hoc Tukey test. North African (here represented by Egypt) and European populations were analysed together, as movement of people across and around the Mediterranean appears to have been higher than across the Sahara Desert [48,49].
The overall worldwide magnitude of shape variation in the birth canal was evaluated by comparing the coefficient of variation (CV) of the three canal indices with other indices of body proportions known to vary among human populations. Brachial (the maximum length of the radius divided by the maximum length of the humerus) and crural (the maximum length of the tibia divided by the maximum length of the femur) indices, and an index of general body shape (bi-iliac breadth divided by maximum femoral length), were calculated for all female individuals in the Goldman dataset (http://web.utk.edu/~auerbach/GOLD.htm; [50]). The dataset is freely available and includes 48 populations from five continents; it is expected to include a wider range of body shape and size variation than the dataset compiled for this study. Worldwide (species) variation in canal size was evaluated by comparing the CV of the six canal measurements from this study with the CV of the length of the limb bones (radius, humerus, tibia, and femur) and pelvic width (bi-iliac breadth) for all female individuals in the Goldman dataset. We calculated an empirical 95% confidence interval by employing a jackknife approach, excluding a population at a time; the confidence interval was estimated by multiplying the standard error by the α-level critical value of a Student's t distribution with number of populations −1 d.f.
(b). Tests of neutral evolution
Neutral processes such as drift and migration are expected to shape genetic and phenotypic variation within a species both in the presence and in the absence of selection, and reflect the vagaries of past population history. Indeed, the geographical pattern of modern human genetic variation has been shown to be largely the outcome of neutral evolution, and has been used to reconstruct the demographic history of our species from its origin in Africa throughout its expansion into other continents [51–53]. Directional selection can also play a role in shaping genetic and phenotypic variation, and many phenotypes have a further layer of variability added by developmental plasticity in response to the environment. Despite these caveats, in the absence of strong selection or high plasticity (i.e. in traits with relatively high heritability) phenotypic variation will reflect past population history [29,54].
For the reasons mentioned above, when evaluating the geographical pattern of human birth canal variation, we start from the null hypothesis that geographical patterns reflect past population history; significant deviations above and beyond this simple expectation can be taken as potentially representing the effects of natural selection. If the shape of the birth canal evolved mainly neutrally in our species, in the absence of strong selection or high environmental plasticity, we would expect a pattern of variation similar, albeit noisier, to the one shown by neutral genetic variation.
The presence of a signature of past population history was evaluated in two different ways:
1. phenotypic diversity should decline with increasing distance from sub-Saharan Africa, due to the iterative founder effect that accompanied the colonization of the other continents [52];
2. there should be a significant correlation between genetic and phenotypic distance between populations.
To test for the signature of the Out-of-Africa expansion, individual within-population variances were calculated as the average of the trace of the variance–covariance phenotypic matrix of canal indices, and regressed on population distances from central sub-Saharan Africa [31].
Relethford & Blangero's [55] method was used to calculate a matrix of between-population phenotypic distances (Q), while correcting for small population sample sizes [56]. This approach treats the canal indices as quantitative traits, whereby different genetic loci have an equal and additive effect [55]. In the absence of an estimate of the additive genetic covariance matrix, the phenotypic covariance matrix was used as an approximation under the assumption of proportionality [57]. Given the lack of heritability estimates for the canal measurements, total heritability was assumed in all analyses. Since phenotypic distances as estimated in the Q matrix are effectively ratios, the distance matrix was normalized using an arcsine square root transformation [33]. For the genetic data, after thinning SNP in linkage disequilibrium using PLINK (v.1.07) [58] with parameters—indep -pairwise 50 10 0.1, we computed pairwise between-population Fst using Arlequin [59]. The correlation between pairwise between-population phenotypic and genetic distances was tested using a Mantel test with 10 000 randomizations.
(c). The effects of climate
Climatic-related directional selection was evaluated by testing for a correlation between pairwise between-population phenotypic distances and temperature differences. In order to take into account the underlying pattern of genetic similarity due to past demographic events, the correlation between phenotypic and temperature differences was also calculated while correcting for genetic distance (in the reduced dataset) using a partial Mantel test. The analyses were repeated for each pelvic index.
(d). Obstetrically related selection
In the absence of a measure of stature, small body mass was used as an indication of women at higher risk of cephalo-pelvic disproportion. It has been suggested that obstetric selective pressures led to a relatively more spacious birth canal in small women [28]. We tested this hypothesis of a nonlinear relationship between canal size and body mass by means of linear, quadratic, and cubic regression analyses. Variation in canal shape with body size was explored by plotting the three indices against body mass, to test whether small women tend to have a rounder and obstetrically more efficient birth canal [27]. All analyses were performed using the software R v.3.4 [60].
3. Results
Human variation in the shape of the birth canal appears to be geographically structured. Figure 2 shows the variation in canal indices in different continents, highlighting the geographical pattern of variation. An analysis of variance (ANOVA) and post hoc Tukey tests showed that most of these differences are highly significant (electronic supplementary material, table S4). Sub-Saharan African populations are overall characterized by a deeper birth canal in the anterior–posterior direction, throughout the three planes (inlet, midplane, and outlet), while Native American populations fall at the other extreme of variation with a more transversally wide canal. Asian and European/North African populations show an intermediate morphology (figure 2). The differences are particularly obvious for the inlet, which tends to be more markedly oval in Americans and Europeans/North Africans (figure 2a), and for the outlet, which tends to be sagittally oval in sub-Saharan Africans and Asians, while it is generally transversally oval in Americans and Europeans/North Africans (figure 2c). It is worth noting, however, that canal variation is continuous and without abrupt differences between regions, when analysed at the level of single populations (figure 3a; electronic supplementary material, figure S1).
Figure 2.

Variation in the inlet (a), midplane (b), and outlet (c) indices within four major geographical regions. The boxes represent the interquartile distance, the whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range, and outliers are highlighted as open circles. The ovals on the left represent the extremes of shape observed in this study for each of the canal planes. The endpoints of the colour bars link pairs of regions with significantly different canal shapes (see results of post hoc Tukey tests in electronic supplementary material, table S4).
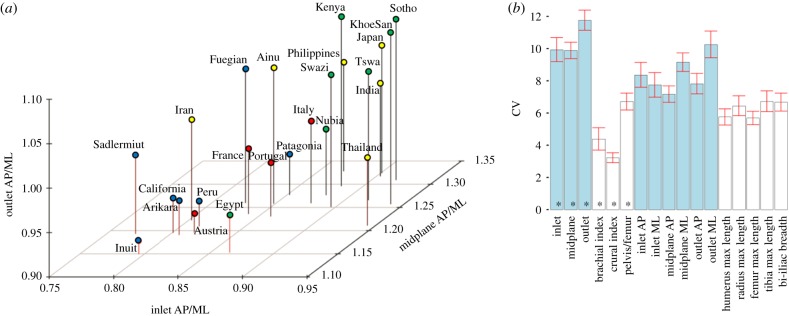
Figure 3.
Three-dimensional plot of population averages for the inlet, midplane, and outlet indices (a) and coefficient of variation (CV) for various postcranial indices and measurements (b). Populations from four geographical regions are highlighted in different colours in (a) (Africa, green; Asia, yellow; Europe and North Africa, red; Americas, blue). (b) The CV for the birth canal (in blue; this study) and other postcranial indices and measurements (in white; Goldman dataset). Indices are highlighted by an asterisk; pelvis/femur refers to pelvic breadth divided by femoral length. The 95% confidence interval obtained after a jackknife procedure is shown in red.
The magnitude of shape variation, CV, is similar among the three planes, and consistently higher than the other body proportion indices and measurements (figure 3b and electronic supplementary material, table S5). The comparative indices are known to vary significantly between human populations, and have been suggested to have been under climatically driven divergent selection [22,23]. The fact that the birth canal indices show a higher CV is, therefore, remarkable and unexpected.
(a). Tests of neutral evolution
Strong evidence of neutral evolution of the shape of the canal comes from the fact that within-population phenotypic diversity tends to decrease with increasing geographical distance from sub-Saharan Africa (R2 = 0.435, F1,22 = 16.95, p < 0.001, figure 4a), mirroring the pattern shown by neutral genetic markers [52] and related to the serial founder events that accompanied the Out-of-Africa expansion. Phenotypic distance between populations is significantly correlated with genetic distance (R2 = 0.242, Mantel p < 0.001; figure 4b), providing additional evidence that neutral evolutionary processes played an important role in shaping canal variation on a global scale.
Figure 4.
Graphical representation of the key results. (a) Plot of within-population phenotypic distance and geographical distance from central sub-Saharan Africa; (b) plot of between-population phenotypic distance and genetic distance; (c) plot of the population average inlet index against minimum temperature; (d) plot of the residuals of the regression of canal size on body mass, against body mass. (Online version in colour.)
(b). The effects of climate
The analyses revealed no significant correlation between overall canal shape differences and temperature differences between populations, before or after correcting for genetic distance (electronic supplementary material, table S6). When the three planes were analysed separately, however, a significant correlation was found between inlet shape differences and temperature differences (electronic supplementary material, table S7). The correlation was still significant after correcting for pairwise genetic distances. The inlet tends to be more transversally oval in colder climates (R2 = 0.394, F1,22 = 14.29, p = 0.001; figure 4c).
(c). Obstetrically related selection
There is a linear increase in birth canal size with body mass, as estimated from the acetabular diameter (R2 = 0.215, F1,325 = 88.74, p < 0.001). The residuals of the linear regression do not show any remaining effect of body mass on canal size (figure 4d); moreover, adding a quadratic (F1,325 = 0.004; p = 949) and cubic term (F1,324 = 0.045; p = 0.835) to the regression model does not improve the fit. The results, therefore, fail to support the hypothesis that smaller women have a larger than expected birth canal. Furthermore, we could not identify any obvious difference in canal shape between women of different body masses (electronic supplementary material, figure S2).
4. Discussion
Women are remarkably variable in the shape of the birth canal. The three planes that define the geometry of the passage vary widely between individuals, in contrast with the expectations of a highly constrained structure under strong selective pressures. The classic narrative of the ‘obstetrical dilemma’ sees the birth canal as a tight compromise between a narrow, locomotory efficient pelvis and a wide, obstetrically sufficient pelvic canal, implying that functional constraints should limit female variation in the shape of the canal. This is clearly incorrect.
Several authors have recently criticized the notion of an ‘obstetrical dilemma’, first of all by showing that the larger female pelvis is not less efficient than the narrower male pelvis during walking or running [5–7] (but see [61]). Grabowski and colleagues [62,63] showed that the human pelvis is a less constrained structure than in other apes, with lower covariance between pelvic traits, allowing it to evolve in more independent directions. The findings presented here provide additional evidence that the classic hypothesis of human pelvic evolution as a strict functional compromise might need to be rethought in a less simplistic way.
On the other hand, injuries or death of the mother and the newborn during parturition are not rare, especially in areas of the world where modern medical assistance is not readily available [64]. Wells and colleagues [18] suggest that obstetrical difficulties could have been exacerbated in recent human evolution by the adoption of agriculture. The shift in diet associated with food production could have increased neonatal growth and level of adiposity, resulting in a tighter fit with the birth canal. Changes in diet and lifestyle in the last century, leading to the modern obesity epidemic, might have further increased relative neonatal size, as evidenced by the positive correlation between maternal obesity and fetal macrosomia (i.e. fetal weight exceeding the 90th percentile of birth weight in a given population) [65]. In this sense, the occurrence of cephalo-pelvic disproportion and related childbirth complications would not be (solely) the consequence of an evolutionary compromise, but evidence of recent shifts in subsistence.
When analysed at a global scale, variation in the shape of the birth canal is geographically structured and women in different regions of the world tend to have, on average, a differently shaped canal.
Climate could be expected to have played a role in increasing canal diversity, as differences in minimum temperature, in particular, have been shown to explain some human variation in body proportions, cranial morphology, and crucially pelvic breadth and the shape of the os coxae [24,25,30,35,66–69]. Unexpectedly, however, differences in overall canal shape between populations are not correlated to temperature differences, and a significant correlation was found only for the shape of the inlet. Instead, a large part of the variation in canal shape is explained by neutral genetic differences between populations, suggesting that most of the observed canal differences might have stemmed from the stochastic effects of genetic drift that accompanied the geographical expansion of our species.
Homo sapiens originated in Africa and dispersed rapidly into new continents in the last 60–100 thousand years, a relatively recent time (see [70] for a recent review of the evidence). The combination of a small founding population size [71] and a quick dispersal meant that a series of genetic bottlenecks accompanied the colonization of new areas of the world. Each founding event, in fact, was achieved by a subpopulation carrying only a portion of the ancestral population's genetic diversity. The signature of these serial founding events is evident in modern populations' genetic variation, whereby genetic diversity decreases with increasing distance from Africa (and number of founding events in the population's history) [52]. Within-population variance in birth canal shape also declines with distance for sub-Saharan Africa, reflecting the effects of the same demographic processes. Not only is this clear evidence that canal variation has been shaped by past population history, but the signal appears to be particularly strong. Distance from Africa explains a remarkable 43.5% of canal diversity within human populations, a proportion higher than that reported for the cranium (R2 = 0.19–0.28; [29,72]) and consistent with previous results for the os coxae (R2 = 0.31–0.47; [31]).
Differences in the shape of the canal between populations are significantly correlated with neutral genetic distances, confirming the important role of neutral evolutionary processes and past demographic events in generating the observed geographical pattern in canal morphology. Shape differences between main geographical regions have likely arisen from a stochastic drift towards different average shapes along the various routes of expansion out of Africa and into new continents.
Recent studies have suggested that selection might have acted in several ways to reduce the risk of cephalo-pelvic disproportion during childbirth. For example, women with a smaller head tend to give birth to small-headed babies [73], while women with a larger head, who are more likely to give birth to babies with a larger head, tend to have a rounder and obstetrically more capable birth canal [27]. Fischer & Mitteroeker [27] also reported a rounder birth canal in shorter women, which are at higher risk of childbirth complications [26], but such a pattern was not observed in this study using body size as a proxy for stature. The use of estimated body mass instead of stature might account for the difference in results, despite stature and femoral head diameter being moderately correlated (r = 0.26–0.71 in European- and African-Americans [74]). We also did not observe a relatively more spacious canal in smaller women, as suggested by Kurki's [28] study comparing small-bodied KhoeSan women and larger-bodied European women.
The remarkable diversity of the human birth canal highlighted by this study, both within and between human populations, has important implications for the interpretation of pelvic morphology in extinct hominins. Palaeoanthropologists have studied the size and shape of the birth canal in several extinct hominin species, to identify at what point in human evolution obstetrical constraints became an important factor. The comparison between hominin and modern human pelves, however, is often performed without explicitly accounting for the range of variation in modern humans [75–77]. This is particularly problematic because of the bias in the human populations used for comparative studies, with populations of European ancestry being over-represented or sometimes used as the sole representative group [78,79].
The canal indices of extinct hominins often fall within the range of modern human variation, especially for the most recent species (electronic supplementary material, table S8). Human variation between geographically diverse modern populations is substantial, and can help put the differences between some of the fossils into perspective. A similar degree of variation in canal morphology between fossil individuals is very likely to be due to the effects of genetic drift; it would not necessarily imply differential adaptation by natural selection and a significant change in obstetrical constraints and birth mechanism in hominin species, as has been suggested before [10,25,80].
The magnitude of canal shape variation in human populations revealed by this study sits in stark contrast with the simplified description of the typical human canal morphology in many anatomy books. The description is often based on the most common shape in European individuals, and does not take into account the wide range of variation showed by our species. The rotation movements required by the fetus to negotiate the twisting passage are also generally reported based on an average European experience. Substantial differences in the shape of the canal in modern populations, especially in the outlet, might translate into differences in fetal movements and presentation. Indeed, X-ray studies of labouring women from the first half of the twentieth century provide some evidence of differences in fetal presentation during labour depending on the shape of the mother's pelvic inlet [16]. The head of the fetus tends to align to the wider diameter of the inlet at engagement. A different rotation of the fetus from the norm might, therefore, occur in women with a differently shaped canal, and should not necessarily be interpreted as a problem. Given the geographical differences in canal shape among modern populations showed by this study, a wider range of variation in childbirth might be expected in modern multi-ethnic societies, and should be taken into account in obstetric training and practice.
Supplementary Material
Supplementary Material
Acknowledgements
Special thanks to Ben Auerbach for making the Goldman dataset available to the scientific community, and to Charles Roseman for useful advice in the early stages of this study. We are grateful to Ben Auerbach, Brendon Billings, Michael Black, Jerome Cybulski, Gisselle Garcia-Pack, Lyman M. Jellema, Natasha Johnson, Maureen Klemp, Osamu Kondo, Robert Kruszynski, Pasuk Mahakkanukrauh, Giorgio Manzi, Philippe Mennecier, Marta Mirazon Lahr, Janet Monge, David Morris, Masaharu Motokawa, Ogeto Mwebi, Tori Randall, Ana Luisa Santos, Maria Teschler-Nicola, Tim White, and Monica Zavattaro for allowing access to the skeletal remains and for general assistance during data collection.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1gk3014 [81].
Authors' contributions
L.B. contributed to the design of the study, collected the pelvic measurements, analysed the data, and wrote the manuscript. A.M. contributed to the design of the study, analysed the genetic data, and contributed to the writing and editing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Wenner-Gren Foundation for Anthropological Research; the Ian and Christine Bolt Scholarship (University of Kent); Sigma Xi Grant; European Union Synthesys Grants; American Museum of Natural History Collection Study Grant; University of Kent PhD Research Scholarship.
References
- 1.Schultz AH. 1949. Sex differences in the pelves of primates. Am. J. Phys. Anthropol. 7, 401–424. ( 10.1002/ajpa.1330070307) [DOI] [PubMed] [Google Scholar]
- 2.Leutenegger W. 1974. Functional aspects of pelvic morphology in simian Primates. J. Hum. Evol. 3, 207–222. ( 10.1016/0047-2484(74)90179-1) [DOI] [Google Scholar]
- 3.Rosenberg K, Trevathan W. 1995. Bipedalism and human birth: the obstetrical dilemma revisited. Evol. Anthropol. 4, 161–168. ( 10.1002/evan.1360040506) [DOI] [Google Scholar]
- 4.Trevathan W, Rosenberg K. 2000. The shoulders follow the head: postcranial constraints on human childbirth. J. Hum. Evol. 39, 583–586. ( 10.1006/jhev.2000.0434) [DOI] [PubMed] [Google Scholar]
- 5.Dunsworth HM, Warrener AG, Deacon T, Ellison PT, Pontzer H. 2012. Metabolic hypothesis for human altriciality. Proc. Natl Acad. Sci. USA 109, 15 212–15 216. ( 10.1073/pnas.1205282109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wall-Scheffler CM. 2012. Energetics, locomotion, and female reproduction: implications for human evolution. Annu. Rev. Anthropol. 41, 71–85. ( 10.1146/annurev-anthro-092611-145739) [DOI] [Google Scholar]
- 7.Warrener AG, Lewton KL, Pontzer H, Lieberman DE. 2015. A wider pelvis does not increase locomotor cost in humans, with implications for the evolution of childbirth. PLoS ONE 10, e0118903 ( 10.1371/journal.pone.0118903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washburn SL. 1960. Tools and human evolution. Sci. Am. 203, 3–15. ( 10.1038/scientificamerican0960-62) [DOI] [PubMed] [Google Scholar]
- 9.Abitbol MM. 1991. Ontogeny and evolution of pelvic diameters in anthropoid primates and in Australopithecus afarensis (AL 288-1). Am. J. Phys. Anthropol. 85, 135–148. ( 10.1002/ajpa.1330850203) [DOI] [PubMed] [Google Scholar]
- 10.Ruff C. 1995. Biomechanics of the hip and birth in early Homo. Am. J. Phys. Anthropol. 98, 527–574. ( 10.1002/ajpa.1330980412) [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg KR. 1992. The evolution of modern human childbirth. Am. J. Phys. Anthropol. 35, 89–124. ( 10.1002/ajpa.1330350605) [DOI] [Google Scholar]
- 12.Caldwell WE, Moloy HC. 1933. Anatomical variations in the female pelvis and their effect in labor with a suggested classification. Am. J. Obstet. Gynecol. 26, 479–505. ( 10.1016/S0002-9378(33)90194-5) [DOI] [PubMed] [Google Scholar]
- 13.Greulich WW, Thoms H. 1938. The dimensions of the pelvic inlet of 789 white females. Anat. Rec. 72, 45–51. ( 10.1002/ar.1090720105) [DOI] [Google Scholar]
- 14.Jagani N, Schulman H, Chandra P, Gonzalez R, Fleischer A. 1981. The predictability of labor outcome from a comparison of birth weight and X-ray pelvimetry. Am. J. Obstet. Gynecol. 139, 507–511. ( 10.1016/0002-9378(81)90508-1) [DOI] [PubMed] [Google Scholar]
- 15.Stålberg K, Bodestedt Å, Lyrenås S, Axelsson O. 2006. A narrow pelvic outlet increases the risk for emergency cesarean section. Acta Obstet. Gynecol. Scand. 85, 821–824. ( 10.1080/00016340600593521) [DOI] [PubMed] [Google Scholar]
- 16.Steer CM. 1975. Moloy's evaluation of the pelvis in obstetrics, 3rd edn New York, NY: Plenum Publishing Corporation. [Google Scholar]
- 17.Kurki HK. 2013. Skeletal variability in the pelvis and limb skeleton of humans: does stabilizing selection limit female pelvic variation? Am. J. Hum. Biol. 25, 795–802. ( 10.1002/ajhb.22455) [DOI] [PubMed] [Google Scholar]
- 18.Wells JCK, DeSilva JM, Stock JT. 2012. The obstetric dilemma: an ancient game of Russian roulette, or a variable dilemma sensitive to ecology? Am. J. Phys. Anthropol. 149, 40–71. ( 10.1002/ajpa.22160) [DOI] [PubMed] [Google Scholar]
- 19.Handa VL, Lockhart ME, Fielding JR, Bradley CS, Brubaker L, Cundiff GW, Ye W, Richter HE. 2008. Racial differences in pelvic anatomy by magnetic resonance imaging. Obstet. Gynecol. 111, 914–920. ( 10.1097/AOG.0b013e318169ce03) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurki HK. 2013. Bony pelvic canal size and shape in relation to body proportionality in humans. Am. J. Phys. Anthropol. 151, 88–101. ( 10.1002/ajpa.22243) [DOI] [PubMed] [Google Scholar]
- 21.Patriquin ML, Steyn M, Loth SR. 2002. Metric assessment of race from the pelvis in South Africans. Forensic Sci. Int. 127, 104–113. ( 10.1016/S0379-0738(02)00113-5) [DOI] [PubMed] [Google Scholar]
- 22.Cowgill LW, Eleazer CD, Auerbach BM, Temple DH, Okazaki K. 2012. Developmental variation in ecogeographic body proportions. Am. J. Phys. Anthropol. 148, 557–570. ( 10.1002/ajpa.22072) [DOI] [PubMed] [Google Scholar]
- 23.Holliday TW, Hilton CE. 2010. Body proportions of circumpolar peoples as evidenced from skeletal data: Ipiutak and Tigara (Point Hope) versus Kodiak Island Inuit. Am. J. Phys. Anthropol. 142, 287–302. ( 10.1002/ajpa.21226) [DOI] [PubMed] [Google Scholar]
- 24.Ruff C. 1994. Morphological adaptation to climate in modern and fossil hominids. Am. J. Phys. Anthropol. 37, 65–107. ( 10.1002/ajpa.1330370605) [DOI] [Google Scholar]
- 25.Weaver TD, Hublin JJ. 2009. Neandertal birth canal shape and the evolution of human childbirth. Proc. Natl Acad. Sci. USA 106, 8151–8156. ( 10.1073/pnas.0812554106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad M, Al-Taher H. 2002. Maternal height and labour outcome. J. Obstet. Gynaecol. 22, 513–515. ( 10.1080/0144361021000003654) [DOI] [PubMed] [Google Scholar]
- 27.Fischer B, Mitteroecker P. 2015. Covariation between human pelvis shape, stature, and head size alleviates the obstetric dilemma. Proc. Natl Acad. Sci. USA 112, 5655–5660. ( 10.1073/pnas.1420325112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurki HK. 2007. Protection of obstetric dimensions in a small-bodied human sample. Am. J. Phys. Anthropol. 133, 1152–1165. ( 10.1002/ajpa.20636) [DOI] [PubMed] [Google Scholar]
- 29.Betti L, Balloux F, Hanihara T, Manica A. 2009. Ancient demography, not climate, explains within-population phenotypic diversity in humans. Proc. R. Soc. B 276, 809–814. ( 10.1098/rspb.2008.1563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betti L, Balloux F, Hanihara T, Manica A. 2010. The relative role of drift and selection in shaping the human skull. Am. J. Phys. Anthropol. 141, 76–82. [DOI] [PubMed] [Google Scholar]
- 31.Betti L, von Cramon-Taubadel N, Manica A, Lycett SJ. 2013. Global geometric morphometric analyses of the human pelvis reveal substantial neutral population history effects, even across sexes. PLoS ONE 8, e55909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvati K, Weaver TD. 2006. Human cranial anatomy and the differential preservation of population history and climate signatures. Anat. Rec. A 288A, 1225–1233. ( 10.1002/ar.a.20395) [DOI] [PubMed] [Google Scholar]
- 33.Roseman CC. 2004. Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proc. Natl Acad. Sci. USA 101, 12 824–12 829. ( 10.1073/pnas.0402637101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betti L. 2017. Human variation in pelvic shape and the effects of climate and past population history. Anat. Rec. 300, 687–697. ( 10.1002/ar.23542) [DOI] [PubMed] [Google Scholar]
- 35.Betti L, von Cramon-Taubadel N, Manica A, Lycett SJ. 2014. The interaction of neutral evolutionary processes with climatically-driven adaptive changes in the 3D shape of the human os coxae. J. Hum. Evol. 73, 64–74. ( 10.1016/j.jhevol.2014.02.021) [DOI] [PubMed] [Google Scholar]
- 36.Phenice TW. 1969. A newly developed visual method of sexing the os pubis. Am. J. Phys. Anthropol. 30, 297–301. ( 10.1002/ajpa.1330300214) [DOI] [PubMed] [Google Scholar]
- 37.Sutherland LD, Suchey JM. 1991. Use of the ventral arc in pubic sex determination. J. Forensic Sci. 36, 501–511. ( 10.1520/JFS13051J) [DOI] [PubMed] [Google Scholar]
- 38.McFadden C, Oxenham MF. 2016. Revisiting the Phenice technique sex classification results reported by MacLaughlin and Bruce (1990). Am. J. Phys. Anthropol. 159, 182–183. ( 10.1002/ajpa.22839) [DOI] [PubMed] [Google Scholar]
- 39.Ubelaker D, Volk C. 2002. A test of the Phenice method for the estimation of sex. J. Forensic Sci. 47, 19–24. [PubMed] [Google Scholar]
- 40.Ruff C. 2010. Body size and body shape in early hominins - implications of the Gona pelvis. J. Hum. Evol. 58, 166–178. ( 10.1016/j.jhevol.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 41.Auerbach BM, Ruff CB. 2004. Human body mass estimation: a comparison of ‘morphometric’ and ‘mechanical’ methods. Am. J. Phys. Anthropol. 125, 331–342. ( 10.1002/ajpa.20032) [DOI] [PubMed] [Google Scholar]
- 42.McHenry HM. 1992. Body size and proportions in early hominids. Am. J. Phys. Anthropol. 87, 407–431. ( 10.1002/ajpa.1330870404) [DOI] [PubMed] [Google Scholar]
- 43.Grine FE, Jungers WL, Tobias PV, Pearson OM. 1995. Fossil Homo femur from Berg Aukas, northern Namibia. Am. J. Phys. Anthropol. 97, 151–185. ( 10.1002/ajpa.1330970207) [DOI] [PubMed] [Google Scholar]
- 44.Ruff CB, Scott WW, Liu AYC. 1991. Articular and diaphyseal remodeling of the proximal femur with changes in body mass in adults. Am. J. Phys. Anthropol. 86, 397–413. ( 10.1002/ajpa.1330860306) [DOI] [PubMed] [Google Scholar]
- 45.Manica A, Prugnolle F, Balloux F. 2005. Geography is a better determinant of human genetic differentiation than ethnicity. Hum. Genet. 118, 366–371. ( 10.1007/s00439-005-0039-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 47.Lazaridis I, et al. 2014. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413. ( 10.1038/nature13673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Font-Porterias N, Solé-Morata N, Serra-Vidal G, Bekada A, Fadhlaoui-Zid K, Zalloua P, Calafell F, Comas D. 2018. The genetic landscape of Mediterranean North African populations through complete mtDNA sequences. Ann. Hum. Biol. 45, 98–104. ( 10.1080/03014460.2017.1413133) [DOI] [PubMed] [Google Scholar]
- 49.Henn BM, et al. 2012. Genomic ancestry of North Africans supports back-to-Africa migrations. PLoS Genet. 8, e1002397 ( 10.1371/journal.pgen.1002397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auerbach BM, Ruff CB. 2006. Limb bone bilateral asymmetry: variability and commonality among modern humans. J. Hum. Evol. 50, 203–218. ( 10.1016/j.jhevol.2005.09.004) [DOI] [PubMed] [Google Scholar]
- 51.Pagani L, et al. 2016. Genomic analyses inform on migration events during the peopling of Eurasia. Nature 538, 238–242. ( 10.1038/nature19792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prugnolle F, Manica A, Balloux F. 2005. Geography predicts neutral genetic diversity of human populations. Curr. Biol. 15, R159–R160. ( 10.1016/j.cub.2005.02.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H, Durbin R. 2011. Inference of human population history from individual whole-genome sequences. Nature 475, 493–496. ( 10.1038/nature10231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manica A, Amos W, Balloux F, Hanihara T. 2007. The effect of ancient population bottlenecks on human phenotypic variation. Nature 448, 346–348. ( 10.1038/nature05951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Relethford JH, Blangero J. 1990. Detection of differential gene flow from patterns of quantitative variation. Hum. Biol. 62, 5–25. [PubMed] [Google Scholar]
- 56.Relethford JH, Crawford MH, Blangero J. 1997. Genetic drift and gene flow in post-famine Ireland. Hum. Biol. 69, 443–465. [PubMed] [Google Scholar]
- 57.Cheverud JM. 1988. A comparison of genetic and phenotypic correlations. Evolution 42, 958–968. ( 10.1111/j.1558-5646.1988.tb02514.x) [DOI] [PubMed] [Google Scholar]
- 58.Purcell S, et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. ( 10.1086/519795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. ( 10.1111/j.1755-0998.2010.02847.x) [DOI] [PubMed] [Google Scholar]
- 60.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 61.Ruff C. 2017. Mechanical constraints on the hominin pelvis and the ‘obstetrical dilemma’. Anat. Rec. 300, 946–955. ( 10.1002/ar.23539) [DOI] [PubMed] [Google Scholar]
- 62.Grabowski MW. 2013. Hominin obstetrics and the evolution of constraints. Evol. Biol. 40, 57–75. ( 10.1007/s11692-012-9174-7) [DOI] [Google Scholar]
- 63.Grabowski MW, Polk JD, Roseman CC. 2011. Divergent patterns of integration and reduced constraint in the human hip and the origins of bipedalism. Evolution 65, 1336–1356. ( 10.1111/j.1558-5646.2011.01226.x) [DOI] [PubMed] [Google Scholar]
- 64.Abou Zahr C, Wardlaw T. 2004. Maternal mortality in 2000: estimates developed by WHO, UNICEF and UNFPA. Geneva: World Health Organization. [Google Scholar]
- 65.Wells JCK. 2017. The new ‘obstetrical dilemma’: stunting, obesity and the risk of obstructed labour. Anat. Rec. 300, 716–731. ( 10.1002/ar.23540) [DOI] [PubMed] [Google Scholar]
- 66.Noback ML, Harvati K, Spoor F. 2011. Climate-related variation of the human nasal cavity. Am. J. Phys. Anthropol. 145, 599–614. ( 10.1002/ajpa.21523) [DOI] [PubMed] [Google Scholar]
- 67.Tilkens MJ, Wall-Scheffler C, Weaver TD, Steudel-Numbers K. 2007. The effects of body proportions on thermoregulation: an experimental assessment of Allen's rule. J. Hum. Evol. 53, 286–291. ( 10.1016/j.jhevol.2007.04.005) [DOI] [PubMed] [Google Scholar]
- 68.Holliday TW. 1997. Body proportions in Late Pleistocene Europe and modern human origins. J. Hum. Evol. 32, 423–447. ( 10.1006/jhev.1996.0111) [DOI] [PubMed] [Google Scholar]
- 69.Katzmarzyk PT, Leonard WR. 1998. Climatic influences on human body size and proportions: ecological adaptations and secular trends. Am. J. Phys. Anthropol. 106, 483–503. ( 10.1002/(sici)1096-8644(199808)106:4%3C483::aid-ajpa4%3E3.0.co;2-k) [DOI] [PubMed] [Google Scholar]
- 70.Nielsen R, Akey JM, Jakobsson M, Pritchard JK, Tishkoff S, Willerslev E. 2017. Tracing the peopling of the world through genomics. Nature 541, 302–310. ( 10.1038/nature21347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schiffels S, Durbin R. 2014. Inferring human population size and separation history from multiple genome sequences. Nat. Genet. 46, 919–925. ( 10.1038/ng.3015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.von Cramon-Taubadel N, Lycett SJ. 2008. Brief communication: human cranial variation fits iterative founder effect model with African origin. Am. J. Phys. Anthropol. 136, 108–113. ( 10.1002/ajpa.20775) [DOI] [PubMed] [Google Scholar]
- 73.Leary S, et al. 2006. Geographical variation in relationships between parental body size and offspring phenotype at birth. Acta Obstet. Gynecol. Scand. 85, 1066–1079. ( 10.1080/00016340600697306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giroux CL, Wescott DJ. 2008. Stature estimation based on dimensions of the bony pelvis and proximal femur. J. Forensic Sci. 53, 65–68. ( 10.1111/j.1556-4029.2007.00598.x) [DOI] [PubMed] [Google Scholar]
- 75.Berge C, Goularas D. 2010. A new reconstruction of Sts 14 pelvis (Australopithecus africanus) from computed tomography and three-dimensional modeling techniques. J. Hum. Evol. 58, 262–272. ( 10.1016/j.jhevol.2009.11.006) [DOI] [PubMed] [Google Scholar]
- 76.Kibii JM, Churchill SE, Schmid P, Carlson KJ, Reed ND, de Ruiter DJ, Berger LR. 2011. A partial pelvis of Australopithecus sediba. Science 333, 1407–1411. ( 10.1126/science.1202521) [DOI] [PubMed] [Google Scholar]
- 77.Marchal F. 2000. A new morphometric analysis of the hominid pelvic bone. J. Hum. Evol. 38, 347–365. ( 10.1006/jhev.1999.0360) [DOI] [PubMed] [Google Scholar]
- 78.Arsuaga J-L, Lorenzo C, Carretero J-M, Gracia A, Martinez I, Garcia N, Bermudez de Castro J-M, Carbonell E. 1999. A complete human pelvis from the Middle Pleistocene of Spain. Nature 399, 255–258. ( 10.1038/20430) [DOI] [PubMed] [Google Scholar]
- 79.Bonmatí A, Gómez-Olivencia A, Arsuaga J-L, Carretero JM, Gracia A, Martínez I, Lorenzo C, Bérmudez de Castro JM, Carbonell E. 2010. Middle Pleistocene lower back and pelvis from an aged human individual from the Sima de los Huesos site, Spain. Proc. Natl Acad. Sci. USA 107, 18 386–18 391. ( 10.1073/pnas.1012131107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tague RG, Lovejoy CO. 1986. The obstetric pelvis of A.L. 288-1 (Lucy). J. Hum. Evol. 15, 237–255. ( 10.1016/S0047-2484(86)80052-5) [DOI] [Google Scholar]
- 81.Betti L, Manica A. 2018. Data from: Human variation in the shape of the birth canal is significant and geographically structured Dryad Digital Repository. ( 10.5061/dryad.1gk3014) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Betti L, Manica A. 2018. Data from: Human variation in the shape of the birth canal is significant and geographically structured Dryad Digital Repository. ( 10.5061/dryad.1gk3014) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1gk3014 [81].