Abstract
T lymphocytes are critical mediators of the adaptive immune system and they can be harnessed as therapeutic agents against pathogens and in cancer immunotherapy. T cells can be isolated and expanded from patients and potentially generated in vitro using clinically relevant systems. An ultimate goal for T-cell immunotherapy is to establish a safe, universal effector cell type capable of transcending allogeneic and histocompatibility barriers. To this end, human pluripotent stem cells offer an advantage in generating a boundless supply of T cells that can be readily genetically engineered. Here, we review emerging T-cell therapeutics, including tumor-infiltrating lymphocytes, chimeric antigen receptors and progenitor T cells (proT cells) as well as feeder cell-free in vitro systems for their generation. Furthermore, we explore their potential for adoption in the clinic and highlight the challenges that must be addressed to increase the therapeutic success of a universal immunotherapy.
Keywords: CAR T cells, immunotherapy, T-cell development, T-cell progenitors
Producing proT cells for immunotherapy
Introduction
T cells are important mediators of adaptive immune defense against pathogens and cancer. Their absence, as seen in the case of hereditary or acquired immunodeficiencies, can result in deregulated immune function, a broad spectrum of diseases and increased cancer burden. On the other hand, T cells are also potent mediators of tissue destruction, graft rejection and graft-versus-host disease (GVHD). For example, some autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosus, can occur, at least in part, because of altered immune function (1). A discrepancy in regulatory T cells (Treg cells) can lead to a lack of regulation of autoreactive T cells and result in organ-specific autoreactivity, leading to type 1 diabetes (2, 3). Therefore, administration of beneficial versus deleterious T-cell subtypes and directing T-cell maturation are important for regulating the activation versus inhibitory balance during normal health and for controlling the composition of therapeutic T-cell products (4, 5). In this review, we will highlight current T-cell-based therapies for cancer immunotherapy, followed by a deeper examination of the advances and limitations in establishing clinically relevant methods for T-cell generation. Designing effector T cells with custom-designed, high-affinity, high-specificity receptors should be a priority in order to develop effective therapeutic tools specific to eliminating cancer.
Human T-cell development
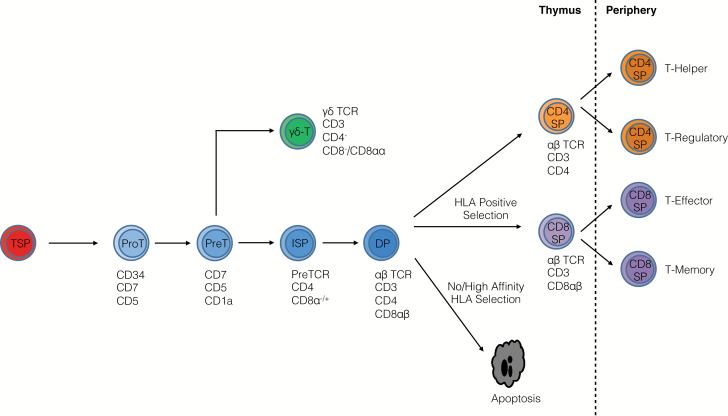
T cells develop from bone marrow (BM)-derived precursors within the thymus and are distinguished from other immune cell types on the basis of cell surface marker expression and their rearrangement of T-cell receptor (TCR) variable (V), diversity (D) and joining (J) gene segments in the thymus. TCR-bearing T cells undergo positive and negative selection, with the resulting T cells being self-tolerant to tissue-restricted antigens. Most T cells exiting the thymus are naive until encounter with antigen in the periphery, where they undergo expansion and acquire effector or memory functions. Selection events within the thymus as well as antigen-priming events are reliant on TCR engagement with human leukocyte antigen (HLA)–peptide complexes on antigen-presenting cells (APCs), in combination with co-stimulatory receptor engagement by ligands expressed on APCs (Fig. 1) (6, 7). T cells and the thymic microenvironments required for their development have been reviewed elsewhere in more detail (6–12).
Fig. 1.
Human T-cell development. Thymus-seeding progenitors (TSPs) that are derived from HSCs enter the thymus and differentiate into proT cells. The outcome of TCR rearrangements determines their fate as αβ T cells or γδ T cells. Successful rearrangement of the TCRβ leads to β-selection and subsequent emergence of CD4+ immature single-positive (ISP) cells. The CD4+ ISP then transitions into a CD4+CD8+ double-positive (DP) cell that has begun to rearrange its TCRα locus. DPs undergo apoptosis unless they receive a TCR-mediated survival signal established by interaction with self-HLA during positive selection. Following this process, DPs adopt either a CD4+CD8– or CD4–CD8+ fate and are termed single-positive (CD4 SP or CD8 SP) cells. Negative selection ensues within the thymus to delete any self-reactive cells. T cells migrate to the periphery and differentiate into effector cells after contact with APCs.
T-cell-based adoptive immunotherapy
Adoptive immunotherapy with expanded patient-derived T cells is an effective therapeutic strategy for the recognition and eradication of cancer and infectious diseases. In the context of cancer immunotherapies, these strategies employ either autologous or allogeneic cells, which encompass their own advantages and disadvantages. Allogeneic T cells are an attractive cell source for transplants as they are derived from healthy donors (13, 14). However, the recipient has a higher risk of GVHD. In contrast, autologous T cells, which are harvested from the transplant recipient (15), are well tolerated but are often functionally impaired and unable to confer therapeutic graft-versus-leukemia (GVL) or anti-cancer potential.
The first therapeutic use of T cells was during allogeneic hematopoietic stem cell transplantation (HSCT), where healthy whole BM grafts were infused into patients for replacement of leukemic BM. The grafts contained donor T cells, and thus enabled beneficial GVL responses against allogeneic antigens present on leukemic cells. However, these effects were concomitant with detrimental GVHD, given the robust allogeneic response against the cells in the recipient (16). The identification of human HLA and therefore better HLA matching, followed by the development of immunosuppressive drugs and the addition of lymphodepletion for the prevention of GVHD (17–19), paved the way for the successful use of HSCT as a curative modality for hematologic malignancies. Nevertheless, the precise balance between GVL and GVHD, and graft rejection and opportunistic infections due to prolonged immunodeficiency in the host, remain major complications that limit the versatility of HSCT.
Thus, numerous T-cell therapies have since been developed including transfer of autologous cytokine-activated killer cells and tumor-infiltrating lymphocytes (TILs) for patients with melanoma and, to a lesser extent, other solid tumors (20–22). Furthermore, genetic engineering of autologous peripheral T cells to express chimeric antigen receptors (CARs) has been gaining momentum for targeting leukemias through the rapid generation of T cells with increased potency and re-targeted specificity against tumor antigens (23). Introduction of Treg cells, which provide immunosuppressive balance, into CAR-based therapies could protect patients from GVHD or the cytokine storm that follows GVL syndromes (24, 25). Finally, progenitor T cells (proT cells) are developmentally immature cells that can undergo positive and negative selection in the recipient thymus, preventing GVHD while reconstituting the T-cell compartment during periods of immunodeficiency after HSCT (26).
Tumor-infiltrating lymphocytes
In some cancers, different immune populations, including leukocytes, macrophages, dendritic cells, mast cells and T cells infiltrate the tumor microenvironment to mediate anti-tumor immunity. TILs are defined as a selected population of T cells with high immunological reactivity against the tumor that they are invading. They can be surgically removed and cultured from different tumor types, such as melanomas (21, 22, 27, 28), with the goal of in vitro clonal expansion and autologous administration to the patient. Unfortunately, the clinical use of TILs has been limited in its success (29–31). For instance, in an earlier clinical trial, 93 melanoma patients were treated with autologous TILs, with only 22% achieving complete remission (29). Providing insight into these findings, Saito et al. (20) later revealed that TILs are typically composed of terminally differentiated effector cells that possess an exhausted phenotype and therefore are functionally impaired. Furthermore, not all tumor types contain a rich source of TILs, and combination therapy with high-dose IL-2 for expansion of the TIL population resulted in significant toxicity (32, 33). Nevertheless, TILs have a potential prognostic role in various cancers including melanoma, lung, breast and ovarian cancers, where immunohistochemistry-based analysis of tumor tissues can help to assess the predictive value of TILs in several tumor types. In both melanoma and ovarian cancer, for example, the accumulation of TILs leads to high production of the inflammatory cytokine IFN-γ. This ultimately leads to high expression of the immune checkpoint inhibitor, PD-L1 in the tumor, thus resulting to a more potent immunotherapy response (34). Similarly, exploiting immune mechanisms could also be achieved by genetically modifying immune cells with specific function towards an intended target, as in the case of CAR T cells.
CAR T cells
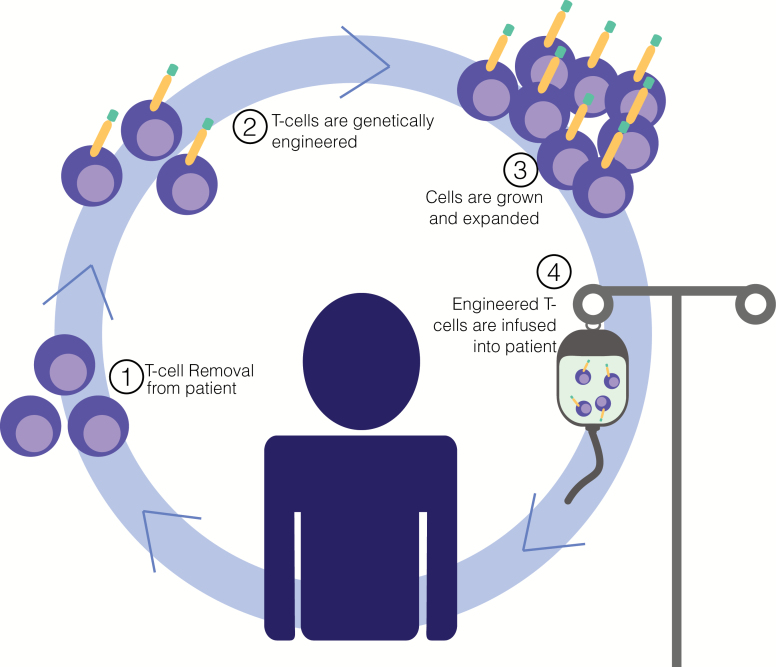
CAR T cells are T lymphocytes that are isolated from patients and genetically engineered to express a tumor-specific receptor (Fig. 2). Rapidly generated tumor-targeted cells overcome the obstacles that impede the induction of effective anti-tumor responses in an individual. The receptor design, which has evolved since its conception, is a chimera of the TCR/CD3 signaling motif from CD3ζ combined with an antigen recognition domain of an immunoglobulin-derived single-chain variable fragment (scFV) (35). As such, CAR T cells can recognize tumor antigens in an HLA-independent fashion.
Fig. 2.
Genetically engineered T cells for immunotherapy. T-cell immunotherapy involves isolating a patient’s own cells and engineering them to recognize a tumor-specific protein, enabling them to seek out and destroy the cancer upon re-infusion. CAR T cells represent an important T-cell immunotherapy approach.
One of the benefits of CARs is that recognition of non-protein targets, such as lipids and carbohydrates, can also be achieved. Furthermore, next-generation CARs mediate both antigen recognition and initiate co-stimulation to enhance the potency of T-cell responses and prolong their persistence. CD28 and 4-1BB co-stimulatory domains are among those used to demonstrate drastically improved responses in clinical trials (36). CD28-CARs build up the potency of T cells, resulting in their rapid activation and proliferation, but they have limited persistence. In contrast, 4-1BB-CARs allow for increased T-cell persistence, despite lower effector responses. Therefore, CARs can differentially activate T cells depending on their composition (35).
The first target for CAR-based therapies was CD19 on B-cell malignancies (37). Of note, CD19 is an antigen that is expressed both on cancerous cells and on healthy cells. Initially, targeting of CD19 was expected to induce B-cell aplasia, but there was strong rationale supporting its use, including the fact that B-cell elimination is clinically manageable by infusion of immunoglobulins, plus the removal of B cells would prevent the generation of anti-CAR antibodies (38). Numerous clinical trials have been completed using CD28-based or 4-1BB-based CARs in patients with multiple B-cell malignancies (39–46). The results of these trials were extremely encouraging in both adult and pediatric patients with acute lymphoblastic leukemia (37, 39, 40, 47) and CD19+ lymphomas (48).
A prominent challenge, as highlighted by these studies, is that cytotoxic conditioning (radiation/chemotherapy given before therapy) is both required for and potentially detrimental to the outcome of CAR-based therapies. CD19-targeting CAR T cells are typically administered following conditioning regimens that deplete the patient’s endogenous lymphocytes. Over-extensive conditioning can induce neurotoxicities and systemic toxicities including cytokine-release syndrome (37, 38, 49). Thus far, these toxicities have been managed by administration of corticosteroids and antibodies that block the IL-6 receptor, and adjusting the conditioning intensity and cell-dosing regimens. Another major obstacle in the translation of CAR therapies is that even when tumor antigens are available, the immunosuppressive tumor microenvironment limits the potency of effector cells capable of anti-tumor responses (35). Enhancements in CAR technology could be made to regulate specificity and off-target effects and modulate the cancer microenvironment. To this end, combinatorial antigen recognition (50) and synthetic receptors capable of driving custom outputs (51) can be employed, allowing for tumor-localized delivery of therapeutic payloads and induction of effector responses to overcome tumor-associated immunosuppression. Alternately, other groups have established strategies using drug-loaded lipid nanoparticles that are conjugated to the surface of pathogen-specific CD8+ cytotoxic T lymphocytes to enable drug release on target antigen recognition (52). It is conceivable that current CARs combined with the potential for T-cell engineering will likely advance T-cell-based therapies in the near future.
ProT-cell therapy
The use of mature T cells can lead to GVHD, underscoring the importance of utilizing a safe and easily accessible cell type for transplant. Human proT cells may become attractive candidates for adoptive T-cell therapies as they robustly home to, and settle and mature within, the thymus of immunodeficient recipient mice (53–55). ProT cells are immature T-cell precursors, which undergo positive and negative selection in the host thymus. Thus, they become restricted to the recipient’s major histocompatibility complex (MHC) yielding host tolerant T lymphocytes that bypass the clinical challenges associated with GVHD.
Several groups have identified the ability of proT cells to overcome the paucity of T-cell reconstitution that is seen after HSCT. Lethally irradiated immunodeficient mice injected with hematopoietic stem cells (HSCs) alone showed high levels of engraftment within the BM, but lacked thymus engraftment for up to 8 weeks post-transplant (54). When proT cells were co-injected with HSCs into irradiated hosts, mice receiving both HSCs and proT cells had a proT-cell-derived population within the thymus, but also a phenotypically distinct HSC-derived population as early as 3 weeks post-transplant. Therefore, proT cells facilitated HSC-derived thymic engraftment.
To further investigate the mechanism underlying these effects, Awong et al. (54) demonstrated that proT-cell entry into the thymus induced changes in the thymic architecture, including cortical and medullary epithelial restructuring, and enhanced expression of chemokine genes such as Ccl21, Ccl19 and Ccl25, which are critical for the recruitment of BM-derived thymus-seeding progenitors and for directing migration of developing progenitors throughout the thymus. Furthermore, cell surface expression of RANKL was identified on proT cells, which, in the context of mouse thymus organogenesis, is a known critical player in thymic epithelial cell differentiation (56). Therefore, the interactions between RANKL expressed on proT cells and RANK present on the thymic epithelial cells likely contributed to the recovery of the host thymus, thereby facilitating the recruitment of thymus-seeding progenitors from the BM.
ProT cells provide many possibilities for clinical application, including the potential to generate tumor-targeting T cells. Zakrzewski et al. (57) demonstrated that mouse proT cells could be modified to express transgenic CARs. CARs were only expressed after proT cells matured within the thymus and egressed into the periphery of mice. Importantly, the mice remained GVHD-free and CAR-bearing T cells were able to reduce the tumor load in an HLA-independent fashion within an allogeneic mouse model. Thus, CAR-proT cells provide an opportunity to immediately restore T-cell immunity in patients afflicted with cancer, while simultaneously providing tumor antigen-specific effector responses without a potential for GVHD.
Arguably, the greatest advantage of using proT cells is that histocompatibility needs are flexible between donors and recipients. As a proof of principle, this therapy was successful in mice with fully MHC-mismatched cells at an equal efficiency to autologous cells (57). Bearing in mind that clinically relevant strategies are being developed for proT-cell production, it would be encouraging to test the translation of proT-cell-based therapies in the clinic in the near future.
In vitro feeder cell-free systems for T-cell production
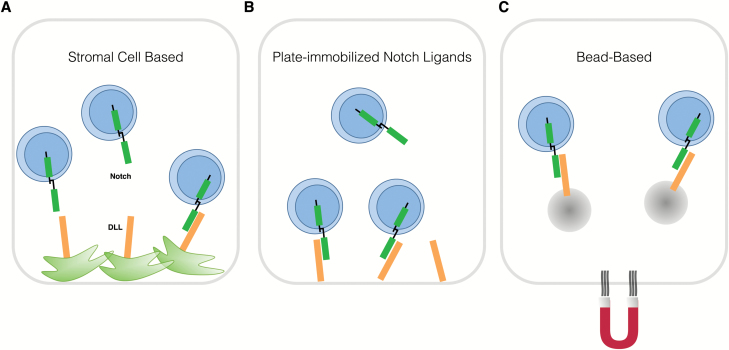
The generation of large numbers of mature T cells from HSCs to restore the immune system in lymphopenic and cancer-afflicted patients has been difficult to achieve both in vitro and in vivo (7). Conventional in vitro approaches make use of a mouse-derived OP9 stromal cell line that ectopically expresses the Notch ligand Delta-like-1 (Dll1; OP9-DL1) or Delta-like-4 (Dll4; OP9-DL4) (Fig. 3A) (58, 59). The accessibility and flexibility of this system has provided a revolutionary tool for studying human T-cell developmental processes and producing large numbers of cells, which is often a limiting factor in most systems. However, the generation of mature T cells in this system is not efficient, and serum-containing medium and xenogeneic stromal cells limit its use for translation to the clinic. Based on the understanding of the instrumental role of Notch signaling for inducing T-cell differentiation from HSCs (60), the development of feeder-free and serum-free culture systems has created an exciting prospect for immediate translational impact.
Fig. 3.
In vitro approaches for T-cell generation. Signaling through the receptor Notch, which engages delta-like ligands (DLLs), is pivotal for proT-cell generation. DLLs can be expressed on stromal cells (A), as with the conventional OP9-DL co-culture system. Alternatively, in serum-free and stromal cell-free approaches, which are amenable for clinical translation, Notch ligands can be immobilized to polystyrene plates (B) or presented on magnetic microbeads (C) for engaging Notch on developing thymocytes. Microbeads can be extracted from the system using magnetic forces.
T-cell differentiation using plate-immobilized Notch ligands
Co-cultures of hematopoietic precursor cells with engineered fusion proteins containing Notch ligands demonstrate an increase in proT cells (61, 62). The success of this approach was reliant on the immobilization of Notch ligands on the plate surface, as presentation of soluble Notch ligands using nanoparticles failed to activate Notch and could inhibit the effects of immobilized ligands (63). Varnum-Finney et al. (63) immobilized the Notch ligand Delta1ext–IgG, which is a fusion between the extracellular domain of DL1 and the Fc domain of human immunoglobulin G1, and showed an increase in the number of mouse proT cells that could be obtained. Interestingly, these proT cells were capable of both short- and long-term reconstitution in immunodeficient mice.
In a similar approach, Ikawa et al. (64) used DL4–Fc immobilized on plastic plates, successfully differentiating mouse HSCs to double-positive cells. Furthermore, this study revealed the importance of exogenous factors including cytokine administration for targeting transcriptional control points at different stages of T-cell development. Gehre et al. (65) demonstrated that in vitro-derived mouse proT cells from DL4–Fc-coated plates could engraft mice upon adoptive transfer. In a mouse model of allogeneic HSCT, these cells had the capacity to accelerate T-cell reconstitution after transplantation (66).
The field has also made significant progress towards understanding human T-cell development using immobilized Notch ligands (Fig. 3B). Cord blood-derived CD34+ HSCs that were cultured in the presence of immobilized DL1 and cytokines resulted in the development of a CD34+CD7+ proT-cell population, which was capable of maturation into CD3+ T cells in the thymus of immunodeficient mice (67). Furthermore, Reimann et al. developed an ex vivo culture approach that utilizes immobilized human DL4–Fc to produce proT cells from umbilical cord blood (UCB) and mobilized peripheral blood-derived CD34+ cells, which were capable of accelerating T-cell reconstitution in immunodeficient mice, compared to injection of HSCs alone (54, 55, 68). Their studies showed production of early thymic progenitors including proT1 cells (CD34+CD7+CD5–) and proT2 cells (CD34+CD7+CD5+) [defined by Awong et al. (53)], as well as CD1a+ T-lineage-committed cells. Cells emerging from their system also possessed the phenotypic and molecular signatures of immature thymic precursors.
Although this approach was stromal cell-free, fetal bovine serum supplementation still limits its versatility. To address this, Shukla et al. (69) recently established a ‘minimal thymic-like niche’, combining DL4–Fc and vascular adhesion molecule-1 with cytokine supplementation. Their system allowed for directing mouse and human HSCs to the proT-cell stage. The human CD7+ proT cells isolated from this system showed thymus-seeding potential and the ability to reconstitute a functional peripheral T-cell compartment in immunodeficient mouse recipients. However, it remains to be tested whether this will also be the case in adult patients, which are the patient population that is majorly afflicted by immunodeficiency post-HSCT.
The systems described thus far demonstrate the potential for in vitro generation of T-lineage progenitor cells, with an opportunity to introduce CAR transgenes or genetic modifications in clinical-grade proT cells for therapeutic use. However, numerous disadvantages exist including the high expense and low stability of the plate-bound ligands for long-term cultures. Furthermore, the ability to attain mature human T-cell phenotypes in long-term cultures remains unclear, as none of these studies have demonstrated the generation of T-lineage cells beyond the proT-cell stage; nor do they incorporate signals that would allow positive selection of CD4+CD8– or CD4–CD8+ single-positive αβ T cells. Overcoming this developmental roadblock through investigation of factors and signals required for maturation beyond the CD1a+ preT-cell stage will inform future protocols. For example, one group found that optimized media conditions and inclusion of ascorbic acid in immobilized DL4–Fc cultures made it possible to develop CD4+CD8+ double-positive, and TCRαβ+CD3+ single-positive (CD4+ or CD8+) T cells in their system (70).
Lastly, the utility of these approaches to generate proT cells for therapy may be limited because of the potential need for scale-up processing for clinical manufacture, as current systems are not as robust as the conventional OP9-DL system. New methods involving the use of small molecules such as StemRegenin (71) and UM171 (72), which are capable of HSC expansion, may be used in combination with these T-cell generation systems for potentially limitless expansion of T-cell progenitors. The use of bioreactors to enhance the production of T-lineage cells would also aid in achieving this goal.
T-cell differentiation using soluble Notch ligands
The presentation of Notch ligand on microbeads is a unique strategy that could allow large-scale bioreactor-based suspension cultures that overcome the scalability drawbacks associated with plate-immobilization approaches (Fig. 3C). In one study, superparamagnetic polystyrene beads were functionalized with DL4 (73). The prospect was exciting given that the magnetic nature of the bead facilitates easy retrieval of T cells, eliminating the difficulties involved with obtaining a pure population. Some mouse proT cells were successfully generated from BM HSCs. Furthermore, comparison to co-culture of BM HSCs with OP9-DL cells directly or indirectly using Transwell inserts, which allow soluble factors through but can prevent direct cell–cell contact, revealed that although the presence of stromal cells was required, contact between stem cells and stromal cells was not required. Therefore, it is likely that soluble growth factors or exosomes secreted by stromal cells were needed to support differentiation (73), and these factors may complement current protocols using functionalized DL4.
It is important to note that cultures with DL4 functionalized beads gave rise to CD19+ B cells in this study. As such, it is possible that these B cells emerged from the inaccessibility of some HSCs to the Notch signals because of an uneven distribution of the beads, or that this finding is consistent with the fact that soluble Notch ligands are inhibitory towards T-lineage fate (63). These types of studies have yet to be performed using human CD34+ cells. Nevertheless, the results demonstrate the prospect of DL4 bead-based systems for the generation of T cells from progenitor cell populations. The ability to scale up these approaches in bioreactor suspension cultures and applying them to human HSCs will greatly increase the likelihood of developing a true ‘off-the-shelf’ T-cell therapy.
Looking forward: pluripotent stem cells for the generation of T-cell therapies
Given the relative scarcity of antigen-specific T cells that can be isolated from a host, and the inherent variability between patients, the development of cellular therapeutics from functionally validated, banked, histocompatible cell types would allow for universal applicability and reduced cost of adoptive T-cell therapies. Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced PSCs (iPSCs), can serve as a renewable supply of therapeutic antigen-specific T lymphocytes with several functionalities including cytotoxic, helper and regulatory function, and can be either TCRαβ+ or TCRγδ+. Furthermore, the PSC platform enables the use of additional engineering strategies intended to enhance the therapeutic potential of induced T cells.
Many groups have demonstrated that T lymphocytes can be generated from human ESCs and iPSCs in vitro (74–78). However, this process is inefficient, producing low T-cell yields. Efforts to study the niche signals and regulatory mechanisms that direct human PSCs towards an HSC fate will greatly facilitate the advance of this technique for clinical applications (7).
Mouse PSC protocols have been optimized for the timed addition of Flt3L and IL-7, which are critical factors for inducing a T-lineage fate, and these methods enabled the generation of functional CD8+ cells in vitro (79). In vitro differentiation of human PSCs to mature T cells has been more difficult. A combination of initial culture on OP9 cells, followed by in vivo differentiation within human thymus tissue grafted in immunodeficient mice, allowed ESCs to fully differentiate into mature T cells in one study (80). Insights from this and other studies have revealed that PSC-derived HSC-like cells have a strong natural killer (NK) cell bias at the expense of T-cell development, as compared with CD34+ cells from UCB (81). Further studies using human ESC cultures on OP9 cells revealed that the CD34+CD43lo fraction formed hematopoietic zones and, when transferred to OP9-DL cultures, generated functionally mature TCRαβ and TCRγδ T cells (77).
Kennedy et al. (74) defined a differentiation strategy in which human iPSCs could be directed towards a definitive HSC-like cell capable of T-cell differentiation on OP9-DL4 cells. In this study, inhibition of the Activin/Nodal pathway using SB431542 and stimulation of the Wnt pathway during mesoderm specification produced CD34+CD43– cells with an enhanced capacity to generate definitive proT2 cells (74). Additionally, iPSC-derived T cells displayed a broad TCR repertoire (82).
The differentiation of PSCs to T cells provides an opportunity to exploit current methodologies to achieve three critical goals of immunotherapy: antigen specificity, effector function and broad histocompatibility.
Antigen specificity
Antigen specificity is mediated by the TCR. One of the difficulties with direct translation of ESCs and iPSCs is that their TCRα and β loci exist in germline configuration, therefore allowing for random VDJ rearrangements for the generation of a polyclonal T-cell repertoire with undefined specificity and HLA restriction. One approach to circumvent this issue involves rendering T cells with known specificity into the PSC fate, for later re-differentiation back into T cells (75, 78, 83). This approach would be difficult to implement, as it is reliant on the availability of suitable antigen-specific and HLA-restricted T cells for prospective recipients.
Therefore, another strategy is to genetically engineer cells to express a receptor with a known specificity. Themeli et al. (76) demonstrated that T cells could be programmed to iPSCs and forced to express a CD19-targeted CAR (CAR-TiPSC), which was a useful way to harness both the unlimited proliferative potential of iPSCs and the antigen specificity of iPSC-derived T cells. Adoptive transfer of these CAR-TiPSCs into immunodeficient mice transplanted with a CD19+ lymphoma graft revealed remarkable anti-tumor activity when compared with mice receiving no treatment. The use of a CAR, rather than a TCR, allowed for enhanced potency in their approach.
Effector function
The effector function of T-lineage cells is determined during their development and differentiation (Fig. 1). The critical developmental decision that is made by T cells as they develop within the thymus is whether to rearrange an αβ or a γδ TCR. PSC-derived progenitors largely seem to follow these steps during in vitro differentiation towards the T lineage, as αβ T cells reprogrammed into TiPSCs (T-cell-derived iPSCs) give rise to αβ-expressing T cells upon re-differentiation. However, these cells possessed unique differences compared with conventional αβ T cells (83). On the basis of microarray gene analyses, in vitro-generated CAR-TiPSC-T cells behaved like innate γδ T cells, despite possessing an endogenous αβ-TCR (76). When tested in a tumorigenic context, these cells generated functional responses that were comparable to γδ T cells emerging from the same donor and transduced with the same CAR.
Similarly, Saito et al. reprogrammed TILs into iPSCs and then subsequently used them as an unlimited source to generate autologous tumor-specific polyclonal T cells for cancer immunotherapy. This study provided a proof-of-concept approach for overcoming many of the limitations of current TIL approaches including poor T-cell survival following infusion into patients, and terminal differentiation of exhausted effector cells with functional impairments (84). Taken together, these studies indicate that some critical differences exist between conventional T cells and T cells that are generated from T-reprogrammed iPSCs.
Interestingly, in another study performed by Maeda et al. (83), cells that were CD4+CD8α+TCR+ cells exclusively generated CD8αα+ innate-like γδ T cells after antigen stimulation. CD8αα+ T cells have less antigen-specific cytotoxic activity than CD4αβ+ or CD8αβ+ T cells. Other studies consistently showed minimal generation of early double-positive CD4+CD8α+ cells, and detection of late double-positive cells expressing CD8αβ has remained difficult. However, a recent study provided a protocol using TCR/CD3 stimulation of isolated CD4+CD8α+ cells that allowed for the generation of CD8αβ+ cells (83). Therefore, a better understanding of the requirements for producing CD4+CD8αβ+ cells will pave the way for generating true CD8+ and CD4+ T cells that have effector and regulatory function.
With respect to functionality, in vivo persistence and sustained functionality of PSC-derived T cells will be critically important to assess. There is little data on the functionality of ESC-derived or iPSC-derived T cells in vitro or upon adoptive transfer in vivo. Given that TCR rearrangements are random, the feasibility of studying antigen-specific expansion and function of PSC-T cells has been limited. Only in vitro assays showing IFN-γ production and target cell lysis have been reported, although these experiments were done using non-specific stimulations (75, 77). These studies provide limited proof of principle showing that human PSC-derived T lymphocytes generated in vitro possess functional capacity through cytokine secretion and anti-tumor function. Further studies will better define the in vivo capabilities of PSC-derived T cells.
Histocompatibility
Immune rejection of non-histocompatible T cells may limit their efficacy, highlighting the requirement for sufficient persistence and long-term engraftment for a T-cell immunotherapy product. Groups have tried to circumvent this issue by banking cells with common HLA haplotypes, including T cells with specific reactivities (85, 86). Nevertheless, the widespread implementation of this approach is limited by donor availability. Consequently, iPSC or ESC banks provide a valuable step towards broader applicability (87). The challenge with establishing PSC lines is again constrained by the need to generate lines from multiple donors. For applicability towards specific disease contexts, there is a further need to identify or target T cells towards particular specificities and HLA restrictions. With a lot of genetic manipulation to be performed, perhaps most importantly, the need to perform extensive safety studies for PSC-derived sources precludes their implementation in the clinic.
An alternative approach to banking lines with common HLA haplotypes could be to genetically target HLA genes towards generation of histocompatible cell products (88). While targeting multiple HLA loci may be feasible, the high technical manipulation of cell products will raise the bar for biosafety considerations and regulations. Furthermore, HLA engineering may be easier to perform in PSCs rather than primary T cells (88), which is particularly problematic given the low efficiency of generating T cells from PSC lines. An important biological consideration for targeting HLA loci would be that NK cells have increased recognition, and therefore rejection, of cells lacking HLA class I on their cell surface. To this end, additionally engineering cells to overexpress various classes of type 1 HLA alleles has been proposed as a mechanism to confer NK cell resistance (88, 89).
Conclusions and Perspectives
T-cell immunotherapy is becoming a promising approach to combat a variety of conditions including infectious diseases and cancer. TILs were among the first T cells to demonstrate therapeutic potential. Newly emerging synthetic engineering approaches have enabled the generation of CAR T cells, which have increased specificity for target antigen and enhanced immune potency compared with previous strategies. Histocompatibility barriers and GVHD are two major hurdles that must be overcome for generation of a broadly applicable, universal T-cell immunotherapy. The development of clinically relevant systems for the generation of large numbers of proT cells will be critical for overcoming these challenges. Finally, while there is excitement surrounding the use of PSC-derived immune cells for therapies, the generation of T cells from PSCs remains difficult. Rigorous testing will be imperative for demonstrating the safety and benefit of PSC-derived T cells prior to clinical implementation.
Acknowledgement
The authors thank Edward L.Y. Chen for help with figures.
Conflicts of interest statement: the authors declared no conflicts of interest.
Funding
This work was supported by grants to J.C.Z.-P. from Canadian Institutes of Health Research (CIHR FND154332), the Ontario Institute for Regenerative Medicine, Medicine by Design: A Canada First Research Excellence Fund Program at the University of Toronto, and The Krembil Foundation.
References
- 1. Yang Z., Matteson E. L., Goronzy J. J. and Weyand C. M. 2015. T-cell metabolism in autoimmune disease. Arthritis Res. Ther. 17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bluestone J. A., Trotta E. and Xu D. 2015. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert Opin. Ther. Targets 19:1091. [DOI] [PubMed] [Google Scholar]
- 3. Yang J., Chow I. T., Sosinowski T. et al. . 2014. Autoreactive T cells specific for insulin B:11–23 recognize a low-affinity peptide register in human subjects with autoimmune diabetes. Proc. Natl Acad. Sci. USA 111:14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saraceni F., Shem-Tov N., Olivieri A. and Nagler A. 2015. Mobilized peripheral blood grafts include more than hematopoietic stem cells: the immunological perspective. Bone Marrow Transplant. 50:886. [DOI] [PubMed] [Google Scholar]
- 5. Huenecke S., Bremm M., Cappel C. et al. . 2016. Optimization of individualized graft composition: CD3/CD19 depletion combined with CD34 selection for haploidentical transplantation. Transfusion 56:2336. [DOI] [PubMed] [Google Scholar]
- 6. Chen L. and Flies D. B. 2013. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brauer P. M., Singh J., Xhiku S. and Zúñiga-Pflücker J. C. 2016. T cell genesis: in vitro veritas est?Trends Immunol. 37:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spits H. 2002. Development of alphabeta T cells in the human thymus. Nat. Rev. Immunol. 2:760. [DOI] [PubMed] [Google Scholar]
- 9. Taghon T., Waegemans E. and Van de Walle I. 2012. Notch signaling during human T cell development. Curr. Top. Microbiol. Immunol. 360:75. [DOI] [PubMed] [Google Scholar]
- 10. Vicente R., Swainson L., Marty-Grès S. et al. . 2010. Molecular and cellular basis of T cell lineage commitment. Semin. Immunol. 22:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petrie H. T. and Zúñiga-Pflücker J. C. 2007. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol. 25:649. [DOI] [PubMed] [Google Scholar]
- 12. Takahama Y. 2006. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 6:127. [DOI] [PubMed] [Google Scholar]
- 13. Gratwohl A., Baldomero H., Aljurf M. et al. ; Worldwide Network of Blood and Marrow Transplantation. 2010. Hematopoietic stem cell transplantation: a global perspective. JAMA 303:1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poirot L., Philip B., Schiffer-Mannioui C. et al. . 2015. Multiplex genome-edited T-cell manufacturing platform for “Off-the-Shelf” adoptive T-cell immunotherapies. Cancer Res. 75:3853. [DOI] [PubMed] [Google Scholar]
- 15. Rosenberg S. A., Lotze M. T., Muul L. M. et al. . 1986. A new approach to the therapy of cancer based on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2. Surgery 100:262. [PubMed] [Google Scholar]
- 16. Ferrara J. L. and Deeg H. J. 1991. Graft-versus-host disease. N. Engl. J. Med. 324:667. [DOI] [PubMed] [Google Scholar]
- 17. Champlin R. E., Passweg J. R., Zhang M. J. et al. . 2000. T-cell depletion of bone marrow transplants for leukemia from donors other than HLA-identical siblings: advantage of T-cell antibodies with narrow specificities. Blood 95:3996. [PubMed] [Google Scholar]
- 18. Ho V. T. and Soiffer R. J. 2001. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 98:3192. [DOI] [PubMed] [Google Scholar]
- 19. Jakubowski A. A., Small T. N., Kernan N. A. et al. . 2011. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol. Blood Marrow Transplant. 17:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito H., Okita K., Fusaki N., Sabel M. S., Chang A. E. and Ito F. 2016. Reprogramming of melanoma tumor-infiltrating lymphocytes to induced pluripotent stem cells. Stem Cells Int. 2016:8394960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meng Q., Liu Z., Rangelova E. et al. . 2016. Expansion of tumor-reactive T cells from patients with pancreatic cancer. J. Immunother. 39:81. [DOI] [PubMed] [Google Scholar]
- 22. Tran E., Ahmadzadeh M., Lu Y. C. et al. . 2015. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sadelain M., Rivière I. and Brentjens R. 2003. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 3:35. [DOI] [PubMed] [Google Scholar]
- 24. Dawson N. A. J. and Levings M. K. 2017. Antigen-specific regulatory T cells: are police CARs the answer?Transl. Res. 187:53. [DOI] [PubMed] [Google Scholar]
- 25. Dawson N. A. J., Vent-Schmidt J. and Levings M. K. 2017. Engineered tolerance: tailoring development, function, and antigen-specificity of regulatory T cells. Front. Immunol. 8:1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Awong G., LaMotte-Mohs R. and Zúñiga-Pflücker J. C. 2010. Key players for T-cell regeneration. Curr. Opin. Hematol. 17:327. [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg S. A., Restifo N. P., Yang J. C., Morgan R. A. and Dudley M. E. 2008. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu R., Forget M. A., Chacon J. et al. . 2012. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 18:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg S. A., Yang J. C., Sherry R. M. et al. . 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17:4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Besser M. J., Shapira-Frommer R., Treves A. J. et al. . 2010. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 16:2646. [DOI] [PubMed] [Google Scholar]
- 31. Dudley M. E., Gross C. A., Langhan M. M. et al. . 2010. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin. Cancer Res. 16:6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hinrichs C. S. and Rosenberg S. A. 2014. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 257:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenberg S. A., Yannelli J. R., Yang J. C. et al. . 1994. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl Cancer Inst. 86:1159. [DOI] [PubMed] [Google Scholar]
- 34. Abiko K., Matsumura N., Hamanishi J. et al. . 2015. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sadelain M., Rivière I. and Riddell S. 2017. Therapeutic T cell engineering. Nature 545:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hombach A. A. and Abken H. 2011. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int. J. Cancer 129:2935. [DOI] [PubMed] [Google Scholar]
- 37. Brentjens R. J., Latouche J. B., Santos E. et al. . 2003. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9:279. [DOI] [PubMed] [Google Scholar]
- 38. Kochenderfer J. N., Dudley M. E., Feldman S. A. et al. . 2012. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119:2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davila M. L., Riviere I., Wang X. et al. . 2014. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maude S. L., Shpall E. J. and Grupp S. A. 2014. Chimeric antigen receptor T-cell therapy for ALL. Hematology. Am. Soc. Hematol. Educ. Program 2014:559. [DOI] [PubMed] [Google Scholar]
- 41. Lee D. W., Kochenderfer J. N., Stetler-Stevenson M. et al. . 2015. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turtle C. J., Hanafi L. A., Berger C. et al. . 2016. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 8:355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qasim W., Zhan H., Samarasinghe S. et al. . 2017. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9:eaaj2013. [DOI] [PubMed] [Google Scholar]
- 44. Kochenderfer J. N., Dudley M. E., Kassim S. H. et al. . 2015. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kochenderfer J. N., Dudley M. E., Carpenter R. O. et al. . 2013. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122:4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turtle C. J., Hanafi L. A., Berger C. et al. . 2016. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126:2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barrett D. M., Liu X., Jiang S., June C. H., Grupp S. A. and Zhao Y. 2013. Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Hum. Gene Ther. 24:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramos C. A., Savoldo B. and Dotti G. 2014. CD19-CAR trials. Cancer J. 20:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maude S. L., Barrett D., Teachey D. T. and Grupp S. A. 2014. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 20:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zah E., Lin M. Y., Silva-Benedict A., Jensen M. C. and Chen Y. Y. 2016. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 4:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roybal K. T., Williams J. Z., Morsut L. et al. . 2016. Engineering T cells with customized therapeutic response programs using synthetic Notch receptors. Cell 167:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jones R. B., Mueller S., Kumari S. et al. . 2017. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials 117:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Awong G., Herer E., Surh C. D., Dick J. E., La Motte-Mohs R. N. and Zúñiga-Pflücker J. C. 2009. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood 114:972. [DOI] [PubMed] [Google Scholar]
- 54. Awong G., Singh J., Mohtashami M. et al. . 2013. Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood 122:4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reimann C., Six E., Dal-Cortivo L. et al. . 2012. Human T-lymphoid progenitors generated in a feeder-cell-free Delta-like-4 culture system promote T-cell reconstitution in NOD/SCID/γc(-/-) mice. Stem Cells 30:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takahama Y., Ohigashi I., Baik S. and Anderson G. 2017. Generation of diversity in thymic epithelial cells. Nat. Rev. Immunol. 17:295. [DOI] [PubMed] [Google Scholar]
- 57. Zakrzewski J. L., Suh D., Markley J. C. et al. . 2008. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat. Biotechnol. 26:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schmitt T. M. and Zúñiga-Pflücker J. C. 2002. Induction of T cell development from hematopoietic progenitor cells by Delta-like-1 in vitro. Immunity 17:749. [DOI] [PubMed] [Google Scholar]
- 59. La Motte-Mohs R. N., Herer E. and Zúñiga-Pflücker J. C. 2005. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood 105:1431. [DOI] [PubMed] [Google Scholar]
- 60. Radtke F., Wilson A., Stark G. et al. . 1999. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10:547. [DOI] [PubMed] [Google Scholar]
- 61. Varnum-Finney B., Brashem-Stein C. and Bernstein I. D. 2003. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood 101:1784. [DOI] [PubMed] [Google Scholar]
- 62. Dallas M. H., Varnum-Finney B., Martin P. J. and Bernstein I. D. 2007. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood 109:3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Varnum-Finney B., Wu L., Yu M. et al. . 2000. Immobilization of Notch ligand, Delta-1, is required for induction of Notch signaling. J. Cell Sci. 113:4313. [DOI] [PubMed] [Google Scholar]
- 64. Ikawa T., Hirose S., Masuda K. et al. . 2010. An essential developmental checkpoint for production of the T cell lineage. Science 329:93. [DOI] [PubMed] [Google Scholar]
- 65. Gehre N., Nusser A., von Muenchow L. et al. . 2015. A stromal cell free culture system generates mouse pro-T cells that can reconstitute T-cell compartments in vivo. Eur. J. Immunol. 45:932. [DOI] [PubMed] [Google Scholar]
- 66. Zakrzewski J. L., Kochman A. A., Lu S. X. et al. . 2006. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat. Med. 12:1039. [DOI] [PubMed] [Google Scholar]
- 67. Ohishi K., Varnum-Finney B. and Bernstein I. D. 2002. The Notch pathway: modulation of cell fate decisions in hematopoiesis. Int. J. Hematol. 75:449. [DOI] [PubMed] [Google Scholar]
- 68. Simons L., Ma K., de Chappedelaine C. et al. . 2018. Generation of adult human T-cell progenitors for immunotherapeutic applications. J. Allergy Clin. Immunol. 141:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shukla S., Langley M. A., Singh J. et al. . 2017. Progenitor T-cell differentiation from hematopoietic stem cells using Delta-like-4 and VCAM-1. Nat. Methods 14:531. [DOI] [PubMed] [Google Scholar]
- 70. Huijskens M. J., Walczak M., Koller N. et al. . 2014. Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 96:1165. [DOI] [PubMed] [Google Scholar]
- 71. Boitano A. E., Wang J., Romeo R. et al. . 2010. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fares I., Chagraoui J., Gareau Y. et al. . 2014. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Taqvi S., Dixit L. and Roy K. 2006. Biomaterial-based Notch signaling for the differentiation of hematopoietic stem cells into T cells. J. Biomed. Mater. Res. A. 79:689. [DOI] [PubMed] [Google Scholar]
- 74. Kennedy M., Awong G., Sturgeon C. M. et al. . 2012. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2:1722. [DOI] [PubMed] [Google Scholar]
- 75. Nishimura T., Kaneko S., Kawana-Tachikawa A. et al. . 2013. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell 12:114. [DOI] [PubMed] [Google Scholar]
- 76. Themeli M., Kloss C. C., Ciriello G. et al. . 2013. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 31:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Timmermans F., Velghe I., Vanwalleghem L. et al. . 2009. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J. Immunol. 182:6879. [DOI] [PubMed] [Google Scholar]
- 78. Vizcardo R., Masuda K., Yamada D. et al. . 2013. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell 12:31. [DOI] [PubMed] [Google Scholar]
- 79. Schmitt T. M., Ciofani M., Petrie H. T. and Zúñiga-Pflücker J. C. 2004. Maintenance of T cell specification and differentiation requires recurrent Notch receptor-ligand interactions. J. Exp. Med. 200:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Galic Z., Kitchen S. G., Kacena A. et al. . 2006. T lineage differentiation from human embryonic stem cells. Proc. Natl Acad. Sci. USA 103:11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martin C. H., Woll P. S., Ni Z., Zúñiga-Pflücker J. C. and Kaufman D. S. 2008. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood 112:2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chang C. W., Lai Y. S., Lamb L. S. Jr and Townes T. M. 2014. Broad T-cell receptor repertoire in T-lymphocytes derived from human induced pluripotent stem cells. PLoS One 9:e97335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maeda T., Nagano S., Ichise H. et al. . 2016. Regeneration of CD8αβ T cells from T-cell-derived iPSC imparts potent tumor antigen-specific cytotoxicity. Cancer Res. 76:6839. [DOI] [PubMed] [Google Scholar]
- 84. Saito H., Iwabuchi K., Fusaki N. and Ito F. 2016. Generation of induced pluripotent stem cells from human melanoma tumor-infiltrating lymphocytes. J. Vis. Exp 117:54375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gallot G., Vollant S., Saïagh S. et al. . 2014. T-cell therapy using a bank of EBV-specific cytotoxic T cells: lessons from a phase I/II feasibility and safety study. J. Immunother. 37:170. [DOI] [PubMed] [Google Scholar]
- 86. Leen A. M., Bollard C. M., Mendizabal A. M. et al. . 2013. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121:5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Themeli M., Rivière I. and Sadelain M. 2015. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell 16:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Riolobos L., Hirata R. K., Turtle C. J. et al. . 2013. HLA engineering of human pluripotent stem cells. Mol. Ther. 21:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Torikai H., Reik A., Liu P. Q. et al. . 2012. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119:5697. [DOI] [PMC free article] [PubMed] [Google Scholar]