Summary
Defective endometrial stromal fibroblasts (EMSFs) contribute to uterine factor infertility, endometriosis, and endometrial cancer. Induced pluripotent stem cells (iPSCs) derived from skin or bone marrow biopsies provide a patient-specific source that can be differentiated to various cells types. Replacement of abnormal EMSFs is a potential novel therapeutic approach for endometrial disease; however, the methodology or mechanism for differentiating iPSCs to EMSFs is unknown. The uterus differentiates from the intermediate mesoderm (IM) to form coelomic epithelium (CE) followed by the Müllerian duct (MD). Here, we successfully directed the differentiation of human iPSCs (hiPSCs) through IM, CE, and MD to EMSFs under molecularly defined embryoid body culture conditions using specific hormonal treatments. Activation of CTNNB1 was essential for expression of progesterone receptor that mediated the final differentiation step of EMSFs before implantation. These hiPSC-derived tissues illustrate the potential for iPSC-based endometrial regeneration for future cell-based treatments.
Keywords: induced pluripotent stem cell, endometrial stromal fibroblast, progesterone signaling, decidualization, WNT/CTNNB1 pathway
Highlights
-
•
We developed a molecularly defined system for differentiating hiPSCs to EMSFs
-
•
hiPSC-derived EMSFs undergo decidualization in response to hormonal stimulation
-
•
D14 embryoid bodies recapitulate the molecular signature of primary EMSFs
-
•
The WNT/CTNNB1 pathway is required for induction of EMSF from hiPSCs
iPSCs provide a patient-specific source that can be differentiated to various cells types. Here, Serdar Bulun and colleagues successfully directed the differentiation of human iPSCs to endometrial fibroblasts (EMSFs) under molecularly defined culture conditions. Activation of CTNNB1 was essential for the induction of EMSFs. These hiPSC-derived tissues illustrate the potential for iPSC-based endometrial regeneration for future cell-based treatments.
Introduction
The endometrium comprises a multilayered mucosa within the uterus. In preparation for the implantation of an embryo, it proliferates, differentiates, degenerates, and regenerates in a cyclical manner in response to ovarian steroid hormones (Maruyama and Yoshimura, 2008, Miyazaki et al., 2012). Estrogen and progesterone bind to their respective receptors ER and PGR and directly regulate the transcription of various genes involved in endometrial physiology. Defective endometrial stromal fibroblast (EMSF) function, particularly abnormal responses to progesterone, play key roles in the development of various types of endometrial disorders, including endometriosis and endometrial cancer. Endometriosis is a painful and persistent gynecological disease that affects approximately 10% of women of reproductive age. Extrauterine growth of endometrium-like tissue results in severe pelvic pain, infertility, and development of adhesions (Bulun, 2009, Dyson et al., 2014, Giudice, 2010, Mahmood and Templeton, 1991). Studies have demonstrated an insufficient response to progesterone in EMSFs from women with endometriosis, which directly affects the stromal-epithelial interactions necessary for normal cycling and function of the endometrium (Kim et al., 2013). Unresponsiveness of EMSFs to progesterone also underlies the pathogenesis of steroid hormone-dependent endometrial cancer (type 1), the most prevalent gynecologic malignancy in the western world, with a rising incidence in the United States (Siegel et al., 2013).
Cell replacement therapies have garnered substantial public and scientific attention as a viable option to replace cells lost or damaged in various disease processes (Daley, 2012, Mutlu et al., 2015). EMSF replacement therapy to restore progesterone responsiveness may similarly provide a novel therapeutic approach to endometrial disease such as endometriosis. The most tractable source of normal EMSFs applicable for clinical use are EMSFs differentiated from patient-derived induced pluripotent stem cells (iPSCs). Human iPSCs (hiPSCs) have been developed using non-integrating episomal factors or synthetic mRNA, producing safer iPSCs with the same efficacy and pluripotency as those derived through viral means (Warren et al., 2010, Yu et al., 2007).
Currently, there is no published method available for differentiating EMSFs from iPSCs. Moreover, the underlying mechanisms for the differentiation of tissue endometrial stem/progenitor cells to EMSFs are not known (Maruyama et al., 2010). We hypothesize that differentiation of iPSCs to EMSFs would mimic the in vivo stages of uterine development during embryogenesis. It is also likely that later stages of this process may simulate the steroid-dependent differentiation of tissue progenitor cells to mature endometrial stromal cells. The uterus is a mesodermal organ that originates from the intermediate mesoderm (IM). During embryogenesis, IM emerges from the posterior primitive streak (PS) and gives rise to the coelomic epithelium (CE). Invagination of CE during fetal development forms the Müllerian duct (MD) (Guioli et al., 2007, Hashimoto, 2003), which then gives rise to the human female reproductive tract, including the oviduct, uterus, and upper vaginal canal (Hashimoto, 2003). Published findings strongly suggest a critical role of the WNT/CTNNB1 pathway in the differentiation of Müllerian tissues (Deutscher and Hung-Chang Yao, 2007, Stewart et al., 2013). Recently, hiPSCs have been differentiated into IM-derived cells that express renal cell lineage markers (Araoka et al., 2014, Morizane et al., 2015), providing a critical starting point for differentiating hiPSCs to EMSFs.
We developed a molecularly defined system for differentiating hiPSCs to EMSFs, whereby embryoid bodies (EBs) of hiPSCs reproducibly recapitulate the hierarchical differentiation stages of PS, IM, CE, and MD. The hiPSC-derived EMSFs expressed the critical endometrial markers HOXA10, HOXA11, and PGR within 14 days of initiation of differentiation (Du and Taylor, 2015, Mote et al., 1999). Prolonged treatment of the hiPSC-derived EMSFs with a time-honored cocktail containing estrogen and progestin, strikingly induced the decidualization (endometrial stromal differentiation) markers FOXO1, HAND2, IGFBP1, and PRL (Buzzio et al., 2006). We predict that histocompatible EMSFs derived from a patients' own cells will permit the development of tailored cell therapies for the endometrial disease. This work represents the first step in developing a cell-based therapeutic approach for women who suffer from uterine factor infertility or endometriosis. The ability to generate functional endometrial tissue from hiPSCs may also create new models for studying endometrial development and pathophysiology, as well as for drug screening. Furthermore, we demonstrate that the WNT/CTNNB1 pathway is a key regulator of PGR expression during differentiation of hiPSCs. This finding may be a game changer for novel molecular therapy to improve progesterone resistance seen in a variety of endometrial diseases.
Results
Differentiation of hiPSCs to Intermediate Mesoderm via the Primitive Streak
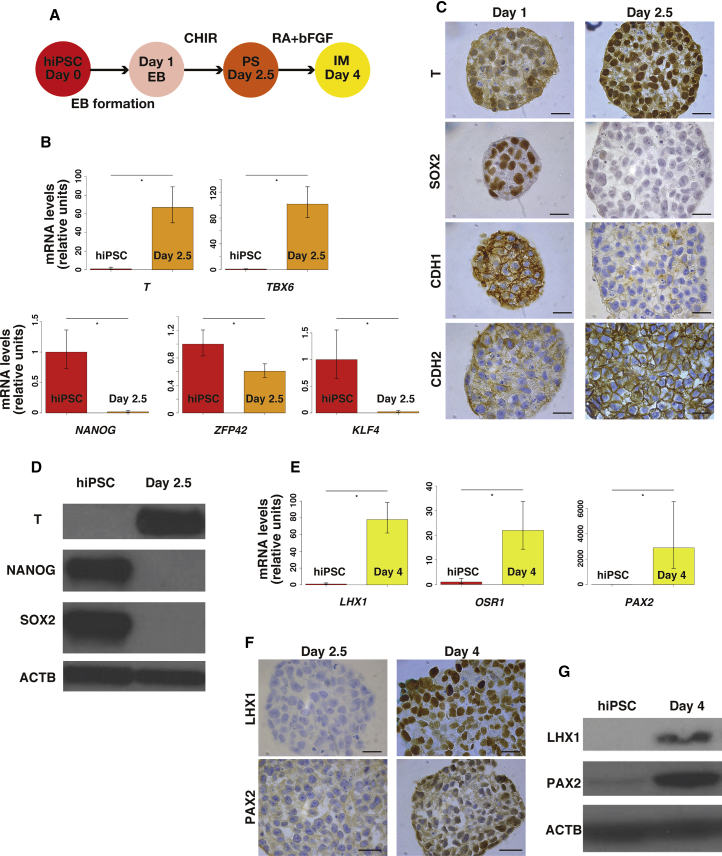
We differentiated hiPSCs to IM via the posterior PS using a previously established protocol (Figure 1A) (Lam et al., 2014). We first cultured hiPSCs for 1 day in plates with microwells designed to facilitate aggregation of pluripotent stem cells into EBs. Day 1 (D1) EBs were treated for 36 hr with 5 mM CHIR99021 (CHIR), a potent GSK3B inhibitor/CTNNB1 pathway agonist, to generate D2.5 EBs. Transcript levels of T and TBX6, genes that are predominantly expressed in PS, were significantly higher in D2.5 EBs versus hiPSCs (Figure 1B) (D'Amour et al., 2005, Gadue et al., 2006). Upregulation of T protein was confirmed by immunostaining (Figure 1C) and immunoblot (Figure 1D). Quantitative analysis of the immunohistochemistry revealed that the percentage of T+ cells was significantly higher in D2.5 compared with D1 (94.5% ± 1.0% versus 9.3% ± 2.3%, p < 0.05) (Figure S1A). Since it is difficult to perform antigen retrieval followed by immunostaining for plated cells in culture, we used D1 EB for immunostaining in place of hiPSC.
Figure 1.
Differentiation of hiPSC into Intermediate Mesoderm via the Primitive Streak
(A) Diagram of differentiation of hiPSCs into IM via PS.
(B) Quantitative RT-PCR of pluripotent stem cell- and PS-specific genes in hiPSCs and day 2.5 EBs. Pluripotent stem cell-specific genes are KLF4, NANOG, and ZFP42, and PS-specific genes are T and TBX6. Error bars represent RQMin and RQMax (N = 9 independent experiments, ∗p < 0.05, Student's t test).
(C) Representative images of immunohistochemistry to detect T, SOX2, CDH1, and CDH2 in D1 and D2.5 EBs. Scale bars represent 20 μm.
(D) Representative immunoblot (N = 3 independent experiments) of T, NANOG, and SOX2 in hiPSCs and D2.5 EBs.
(E) Quantitative RT-PCR of IM-specific genes LHX1, OSR1, and PAX2 in hiPSCs and day 4 EBs. Error bars represent RQMin and RQMax (N = 9 independent experiments, ∗p < 0.05, Student's t test).
(F) Representative images of immunohistochemistry to detect LHX1 and PAX2 in D2.5 EBs and D4 EBs. Scale bars represent 20 μm.
(G) Representative immunoblot (N = 3 independent experiments) of LHX1 and PAX2 in hiPSCs and D4 EBs.
Differentiation of pluripotent stem cells is accompanied by a loss of pluripotency (Lam et al., 2014). Since hiPSCs share many properties with epiblast stem cells (EpiSCs) (Han et al., 2011), we examined the expression of the EpiSC genes NANOG, ZFP42, and SOX2, as well as KLF4, a gene used to generate hiPSCs (Takahashi et al., 2007), to verify the pluripotency of the EBs. Decreases in mRNA levels of NANOG, ZFP42, and KLF4 were observed in D2.5 EBs compared with hiPSCs (Figure 1B). Downregulation of SOX2 was confirmed by immunostaining (Figure 1C) as the quantitative analysis of immunohistochemistry revealed that the percentage of SOX2+ cells was significantly lower in D2.5 compared with D1 (92.9% ± 1.9% versus 1.4% ± 0.3%, p < 0.05) (Figure S1A). Downregulation of NANOG and SOX2 was also confirmed by immunoblot (Figure 1D).
EpiSCs can also differentiate ectoderm cells, early-gastrula organizer, and primordial germ cell precursors as well as PS (Arango and Donahoe, 2008, Davidson and Tam, 2000, Zimmerlin et al., 2017). We evaluated D2.5 EBs for the expression of markers for those derivatives to assess the purity of PS differentiation: PAX6 for ectoderm (Lam et al., 2014), LHX1 for early-gastrula organizer (Davidson and Tam, 2000), and PRDM14 for primordial germ cell precursors (Arango and Donahoe, 2008). We found that mRNA expression was unchanged or downregulated in D2.5 EBs compared with hiPSCs (Figure S1B).
A switch in cadherin protein expression from CDH1 to CDH2 indicates epithelial-mesenchymal transition (EMT) during differentiation of hiPSCs. D2.5 EBs showed decreased expression of CDH1 and increased expression of CDH2 by immunohistochemistry compared with D1 EBs, consistent with cell migration through the PS, as seen during development (Figure 1C) (Ramkumar and Anderson, 2011). Specifically, the quantitative data showed the following: CDH1+, 3.9% ± 0.1% versus 100%, p < 0.05; CDH2+, 100% versus 8.3% ± 0.7%, p < 0.05 (Figure S1A). Together, these findings demonstrated the potency of CHIR to induce differentiation of hiPSCs into PS-like cells via a program that mimics normal development in vivo.
In the next step, D2.5 EBs (PS stage) were treated with 1 mM retinoic acid (RA) and fibroblast growth factor 2 (FGF2) for 36 hr (Figure 1A). We examined whether D4 EBs are committed to the IM lineage by measuring the expression of LHX1, OSR1, and PAX2, which are expressed in the developing IM (Lam et al., 2014, Mugford et al., 2008). A marked increase in LHX1, OSR1, and PAX2 mRNA expression was observed in D4 EBs compared with hiPSCs (Figure 1E). We confirmed protein expression of LHX1 and PAX2 in D4 EBs by immunohistochemistry (Figure 1F) and immunoblot (Figure 1G). Quantitative analysis of the immunohistochemistry revealed that the percentages of LHX1+ cells and PAX2+ cells were significantly higher in D4 compared with D2.5 (96.5% ± 0.5% versus 0%, p < 0.05; 96.0% ± 0.6% versus 1.1% ± 1.1%, p < 0.05, respectively) (Figure S1C).
Because PS can also give rise to lateral plate mesoderm and hemangioblast (Palpant et al., 2017), we also evaluated the D4 EBs for mRNA expression of markers GATA4 (Gao et al., 2016) and CD34 (Young et al., 1995). We found that expression of these markers was downregulated or unchanged compared with D2.5 EBs (Figure S1D). SOX17, which is a marker for endoderm originating from the anterior PS (Familari, 2006), was also unchanged in the D4 EBs (Figure S1D). We therefore concluded that D4 EBs were representative of IM.
Differentiation of Intermediate Mesoderm to Coelomic Epithelium
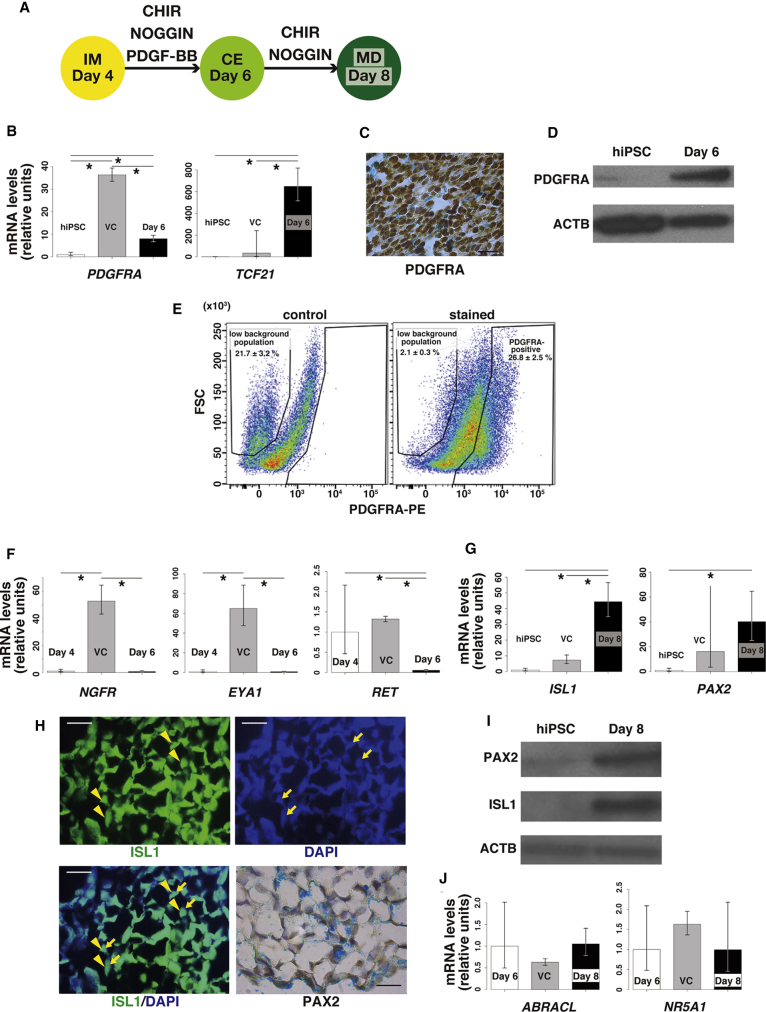
All MD components derive from IM through CE (Hashimoto, 2003), and the WNT/CTNNB1 pathway mediates the development of MD (Deutscher and Hung-Chang Yao, 2007, Stewart et al., 2013). We hypothesized that activation of the WNT/CTNNB1 pathway, in addition to several other growth factors, could reproduce MD development from IM (D4 EBs) in vitro. We added the WNT/CTNNB1 pathway activator CHIR, along with NOGGIN and platelet-derived growth factor BB (PDGF-BB), to D4 EB cultures for 2 days (Figure 2A). Because BMP7 is expressed in the Wolffian duct and induces nephrogenesis from IM (Little et al., 2010), we added the BMP antagonist NOGGIN to suppress nephrogenesis. PDGF-BB was added based on its ability to differentiate human embryonic stem cells into Müllerian lineage cells in a previously published protocol (Yu et al., 2015).
Figure 2.
Differentiation of Intermediate Mesoderm into Müllerian Duct
(A) Diagram of differentiation of IM into MD.
(B) Quantitative RT-PCR of coelomic epithelium (CE)-specific genes PDGFRA and TCF21 in hiPSCs and day 6 EBs. Day 6, D6 EBs treated with CHIR, NOGGIN, and PDGF-BB; VC, D6 EBs treated with vehicle control for 2 days, between D4 and D6. Error bars represent RQMin and RQMax. (N = 9 independent experiments except for VC [N = 3 independent experiments], ∗p < 0.05, Student's t test).
(C) Representative image of immunohistochemistry to detect PDGFRA in D6 EBs. Scale bar represents 20 μm.
(D) Representative immunoblot (N = 3 independent experiments) of PDGFRA in hiPSC and D6 EBs.
(E) The representative dot plots of PDGFRA-positive population in cells dispersed from D6 EBs (N = 3 independent experiments).
(F) Quantitative RT-PCR comparing expression of NGFR, EYA1, and RET in D4 EBs and D6 EBs. Day 6, D6 EBs treated with CHIR, NOGGIN, and PDGF-BB; VC, D6 EBs treated with vehicle control, between D4 and D6. Those genes are expressed in non-CE derivatives from IM. Error bars represent RQMin and RQMax (N = 9 independent experiments except for VC [N = 3 independent experiments], ∗p < 0.05, Student's t test).
(G) Quantitative RT-PCR of Müllerian duct (MD)-specific genes ISL1 and PAX2 in hiPSC and day 8 EBs. Day 8, D8 EBs treated with CHIR and NOGGIN; VC, D8 EBs treated with vehicle control for 2 days, between D6 and D8. Error bars represent RQMin and RQMax (N = 9 except for VC [N = 3 independent experiments], ∗p < 0.05, Student's t test).
(H) Representative immunofluorescence of ISL1 and immunohistochemistry to detect PAX2 in D8 EB. Scale bars represent 20 μm. Yellow arrowheads indicate nuclear staining of ISL1. Yellow arrows indicate DAPI-positive cell nuclei.
(I) Representative immunoblot (N = 3 independent experiments) for ISL1 and PAX2 in hiPSCs and D8 EBs.
(J) Quantitative RT-PCR comparing expression of ABRACL and NR5A1 in D6 EBs and D8 EBs. Day 8, D8 EBs treated with CHIR and NOGGIN; VC, D8 EBs treated with vehicle control for 2 days, between D6 and D8. Those genes are expressed in non-MD derivatives from IM. Error bars represent RQMin and RQMax (N = 9 independent experiments except for VC [N = 3 independent experiments], ∗p < 0.05, Student's t test).
To determine whether EB treatment with CHIR, NOGGIN, and PDGF-BB for 2 days induced the development of IM to CE, we examined the expression of CE markers (and lack of IM markers) in treated or untreated (vehicle control) D6 EBs. mRNA levels of PDGFRA and TCF21, genes that are predominantly expressed in CE (Wilhelm et al., 2007) (Edson et al., 2009), were significantly higher in D6 EBs compared with hiPSCs (Figure 2B) although PDGFRA expression was lower in the treated D6 EBs compared with vehicle-control-treated D6 EBs (Figure 2B). Upregulation of PDGFRA protein was confirmed by immunostaining (Figure 2C) and immunoblot (Figure 2D). We used flow cytometry to assess the distribution of PDGFRA-positive cells in D6 EBs. Via plotting forward-scatter (FSC) against fluorescence intensity in the PE channel obtained from the unstained control, we identified two populations: populations with low and high background of PE (Figure 2E). However, in D6 EBs stained with the PDGFRA antibody, 26.8% ± 2.5% of cells were positive for PDGFRA when the positive gate was positioned to exclude the high PE population although the majority of D6 EB cells were PDGFRA positive after immunostaining (Figures 2C and 2E). This may be due to the variable sensitivity of the different antibodies used for each procedure and also the nature of the individual procedures. Addition of CHIR, NOGGIN, and PDGF-BB also significantly suppressed the expression of markers for mesonephros (NGFR) (Wilhelm et al., 2007) and metanephric mesenchyme (EYA1) (Little et al., 2010) in D6 EBs compared with vehicle-control-treated D6 EBs (Figure 2F). mRNA levels of the Wolffian duct marker RET (Little et al., 2010) were not changed among D4 EBs, treated D6 EBs, and vehicle-control-treated D6 EBs (Figure 2F).
Withdrawal of CHIR significantly increased the mRNA levels of PDGFRA and TCF21 (CE markers), but it also enhanced the expression of EYA1 (metanephric mesenchyme) and RET (Wolffian duct) (Figures S1E and S1F), whereas withdrawal of NOGGIN significantly increased the mRNA level of EYA1 but did not affect the expression of PDGFRA or TCF21 (Figures S1E and S1F). Our data suggested that withdrawal of PDGF-BB enhanced the expression of EYA1 although there was no statistically significant difference (p = 0.06) (Figure S1F). We therefore concluded that the combination of CHIR, NOGGIN, and PDGF-BB is the most optimal cocktail for the differentiation of IM to CE.
We further tested the effects of varying durations of treatment with CHIR, NOGGIN, and PDGF-BB by assaying the expression of differentiation markers from days 5 to 7. Although mRNA levels of PDGFRA and TCF21 were significantly higher in D7 EBs compared with D6 EBs, there was an insignificant trend that D7 EBs expressed more EYA1 and RET compared with D6 EBs (p = 0.06) (Figures S1G and S1H). D5 EBs showed significantly higher expression of EYA1 and RET compared with D6 EBs, whereas mRNA levels of PDGFRA and TCF21 were comparable between D5 EBs and D6 EBs (Figures S1G and S1H). We therefore concluded that D6 is the best time point for CE before proceeding to MD.
Differentiation of Coelomic Epithelium into Müllerian Duct
We then cultured D6 EBs with CHIR and NOGGIN for an additional 2 days (i.e., removed PDGF-BB) (Figure 2A), and examined the expression of MD markers and lack of expression of genes typically found in other CE derivatives. mRNA levels of ISL1 and PAX2, genes that are predominantly expressed in MD (Kobayashi and Behringer, 2003), were significantly increased in D8 EBs compared with vehicle control D8 EBs or hiPSCs (Figure 2G). Protein expression of those markers was confirmed by immunostaining (Figure 2H) and immunoblot (Figure 2I). One hundred percent of cells in D6 EBs were ISL1 positive, whereas 90.3% ± 0.8% of cells were PAX2 positive (N = 3) (Figure 2H). Conversely, expression of marker genes for other CE derivatives, such as ovarian mesenchymal stroma and testicular interstitial cells (ABRACL) (Jameson et al., 2012) and gonadal somatic cells (NR5A1) (Edson et al., 2009), was not significantly different among D6 EBs, treated D8 EBs, and vehicle control D8 EBs (Figure 2J). Therefore, we considered the D8 EBs to be a putative MD cell population. Notably, H&E staining revealed a drastic change in cell morphology during the transition from D6 to D8, such that D6 EB cells were primarily “cobblestone” shaped, epithelium-like cells, whereas the majority of cells in D8 EBs were spindle-shaped, fibroblast-like cells (Figure S2).
Differentiation of Müllerian Duct into EMSFs
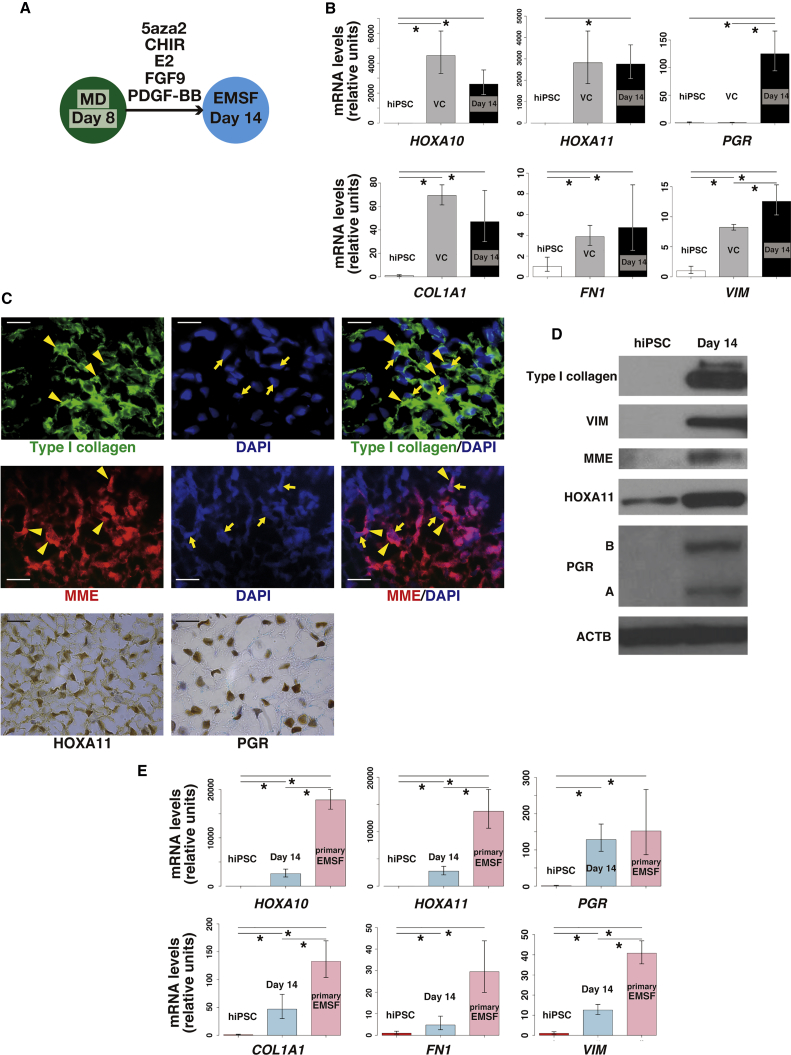
Next, we cultured D8 EBs with 5′-aza-2′-deoxycytidine (5aza2), CHIR, 17β-estradiol (E2), FGF9, and PDGF-BB for 6 days (Figure 3A). 5aza2 is a DNA methyltransferase inhibitor that induces pluripotent stem cell differentiation into several cell types (Banerjee and Bacanamwo, 2010, Horrillo et al., 2013). E2 at a moderate concentration (10−8 M) was used to reproduce the levels present in the fetus (Peterson et al., 1975), consistent with a previous protocol used to differentiate human embryonic stem cell into cells of the Müllerian lineage (Yu et al., 2015). FGF9 was added as a known autocrine endometrial stromal growth factor (Tsai et al., 2002), and PDGF-BB was used because it supports clonogenicity of endometrial stromal cells (Chan et al., 2004).
Figure 3.
Differentiation of Müllerian Duct into EMSFs
(A) Diagram of differentiation of MD into EMSFs.
(B) Quantitative RT-PCR of EMSF-specific genes HOXA10, HOXA11, PGR, COL1A1, FN1, and VIM in hiPSCs and day 14 EBs. Day 14, D14 EBs treated with 5aza2, CHIR, E2, FGF9, and PDGF-BB; VC, D14 EBs treated with vehicle control for 6 days, between D8 and D14. Error bars represent RQMin and RQMax (N = 9 independent experiments except for VC [N = 3 independent experiments], ∗p < 0.05, Student's t test).
(C) Representative immunofluorescence of type I collagen and MME, and immunohistochemistry to detect HOXA11 and PGR in D14 EB. Scale bars represent 20 μm. Yellow arrowheads indicate intracellular staining of type I collagen or MME staining on cell membranes. Yellow arrows indicate DAPI-positive cell nuclei.
(D) Representative immunoblot (N = 3 independent experiments) for COL1A1, VIM, MME, HOXA11, and PGR in hiPSCs and D14 EBs.
(E) Quantitative RT-PCR comparing expression of HOXA10, HOXA11, PGR, COL1A1, FN1, and VIM in hiPSCs, D14 EBs, and primary EMSFs. Error bars represent RQMin and RQMax (N = 9 independent experiments except for primary EMSFs [N = 3 independent experiments], ∗p < 0.05, Student's t test).
After 6 days of treatment or (vehicle control treatment), we examined whether the D14 EBs had committed to the EMSF lineage based on positive expression of EMSF markers. HOXA10 and HOXA11 are known to play critical roles in developmental patterning of the reproductive tract (Benson et al., 1996, Gendron et al., 1997) and are also necessary for endometrial receptivity (Du and Taylor, 2015). HOXA10 is expressed in the developing uterus (Du and Taylor, 2015) but minimally expressed in fallopian tube tissue (Salih and Taylor, 2004). In fact, the homeotic transformation of the anterior part of the uterus into an oviduct-like structure takes place in response to HOXA10 deficiency (Du and Taylor, 2015). HOXA11 is found in the primordial lower uterus and cervix (Du and Taylor, 2015). MME is a type II transmembrane glycoprotein predominantly expressed in EMSFs (Imai et al., 1992), as is PGR (Mote et al., 1999), which mediates progesterone effects in the endometrium; PGR expression is low in the vagina (Di Carlo et al., 1985). Expression levels of general fibroblast markers, including COL1A1, FN1, and VIMs were also examined (Akamatsu et al., 2013, Busch et al., 2017, Cheng et al., 2016).
mRNA levels of HOXA10, HOXA11, PGR, COL1A1, FN1, and VIM significantly increased in D14 EBs compared with hiPSCs (Figure 3B), although expression of HOXA10, HOXA11, COL1A1, and FN1 was comparable between treated and vehicle control D14 EBs (Figure 3B). Protein expression of MME, HOXA11, PGR, type I collagen, and VIM was confirmed by immunostaining (Figure 3C) and immunoblot (Figure 3D). One hundred percent of cells in D14 EB were positive for MME and type I collagen, whereas 98.3% ± 1.0% of cells were HOXA11 positive. PGR was expressed in 94.3% ± 1.3% of cells (N = 3) (Figure 3C). Withdrawal of 5aza2 or E2 between D8 and D14 significantly decreased the expression of EMX2 (Figure S1I), a gene highly expressed in proliferative phase endometrium (Daftary and Taylor, 2004).
Of note, mRNA expression of PGR in D14 EBs was comparable with that in cultured primary EMSFs, although expression of HOXA10, HOXA11, COL1A1, and VIM in primary EMSFs was significantly higher than that in D14 EBs (Figure 3E). Our data suggested that primary EMSFs also express more FN1 compared with D14 EBs although there was no statistically significant difference (p = 0.06). The cells in D14 EBs were fibroblast-like cells, with no epithelial glandular formation as shown by H&E staining (Figure S2). Taken together, we considered these cells as an EMSF population.
The transcript levels of SUSD2, which is a marker of endometrial mesenchymal stem cells (eMSCs), increases from D4 to D8 (up to 10-fold), and decreases significantly from D8 to D14 (Masuda et al., 2012). This supports the notion that, during the differentiation protocol, these cells acquire some of the eMSC properties at D8, followed by their differentiation to EMSFs, as evident by a decline in SUSD2 expression (Figure S1J).
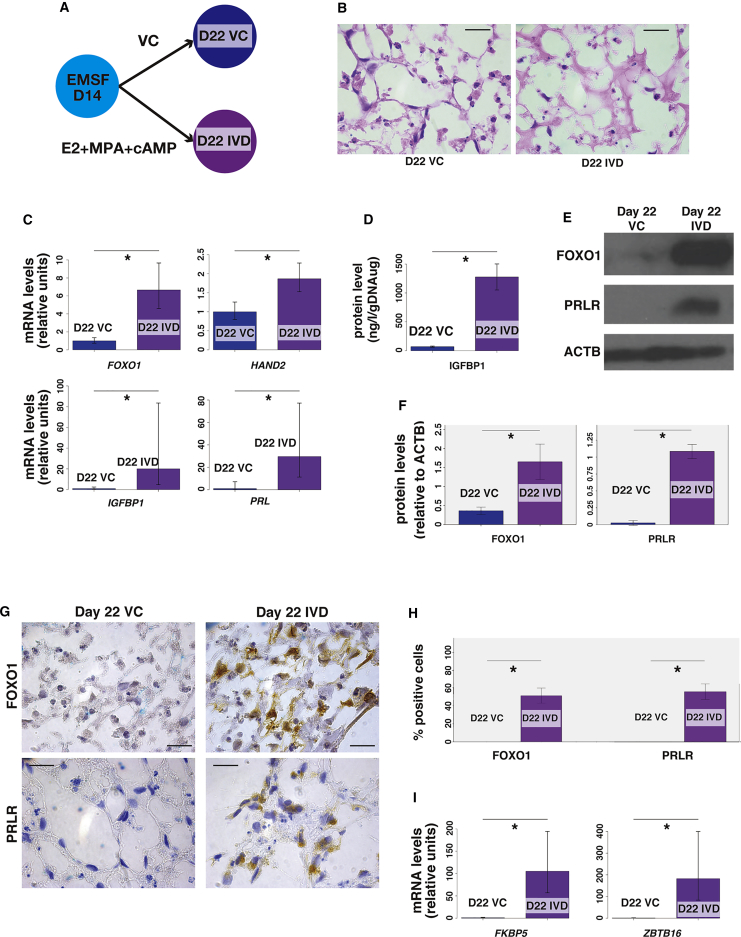
D14 EBs Undergo Decidualization in Response to Hormonal Stimulation
During the secretory phase of the human menstrual cycle, EMSFs undergo decidualization in response to hormonal stimulation in preparation for implantation of an embryo (Buzzio et al., 2006). The high levels of expression of PGR in D14 EBs prompted us to further examine the hormone responsiveness of D14 EBs as putative EMSFs. We mimicked the secretory phase hormonal environment in culture to stimulate in vitro decidualization (IVD) by treating D14 EBs with E2, progestin (MPA), and 8-bromoadenosine 3′,5′-cyclic monophosphate (cAMP) (Figure 4A). After 8 days of treatment, there was a significant increase in the cytoplasmic volumes of treated cells, as demonstrated by H&E staining at higher magnification (Figure 4B). Quantification of cytoplasmic area demonstrated the significant increase in the cytoplasmic volume in D22 EBs treated with hormones (D22 IVD) compared with D22 EBs treated with vehicle control (D22 VC) (266.2 ± 29.1 m2 versus 76.4 ± 4.2 m2, p < 0.05, respectively) (Figure S3A). Furthermore, D22 IVD expressed significantly higher mRNA levels of decidualization markers such as FOXO1, HAND2, IGFBP1, and PRL (Buzzio et al., 2006) compared with D22 VC (Figure 4C). A similar profile was observed in two independent clones although the upregulation of two of four decidualization markers (IGFBP1 and PRL) observed in primary EMSFs were more prominent compared with D22 EBs (Figures S3B and S3C). ELISA was used to confirm the protein levels of secreted IGFBP1 (Figure 4D). Immunoblot and immunohistochemistry were used to confirm the protein expression of FOXO1 (Figures 4E and 4G). The levels of PRLR, another marker of decidualization (Graubner et al., 2017), was also increased in the D22 IVD versus D22 VC by immunoblot (Figure 4E) and immunohistochemistry (Figure 4G). Quantitative analysis of immunoblot densitometry showed that the protein levels of FOXO1 and PRLR were significantly higher in D22 IVD compared with D22 VC (Figure 4F). The quantitative analysis of immunohistochemistry revealed that the percentages of FOXO1+ cells and PRLR+ cells were significantly higher in D22 IVD compared with D22 VC (51.1% ± 4.2% versus 0%, p < 0.05; 54.2% ± 4.2% versus 0%, p < 0.05, respectively) (Figure 4H). Furthermore, the mRNA expression of FKBP5 (Su et al., 2012) and ZBTB16 (Kommagani et al., 2016), which are direct PGR-target genes, was significantly higher in D22 IVD compared with D22 VC (Figure 4I), showing the progesterone responsiveness of the differentiated cells.
Figure 4.
In Vitro Decidualization of D14 EBs
(A) Diagram of in vitro decidualization (IVD) protocol in which D14 EBs are treated with E2, MPA, and cAMP (D22 IVD), or with vehicle control (D22 VC) for 8 days, between D14 and D22.
(B) Representative images of H&E staining of D22 VC and D22 IVD. Scale bars represent 20 μm.
(C) Quantitative RT-PCR of decidualization-specific genes FOXO1, HAND2, IGFBP1, and PRL in D22 VC and D22 IVD differentiated from clone 1 hiPSCs. Error bars represent RQMin and RQMax (N = 9 independent experiments, ∗p < 0.05, Student's t test).
(D) ELISA to detect IGFBP1 in D22 VC and D22 IVD. Values are normalized with genomic DNA amount. Error bars represent mean ± SEM (N = 9 independent experiments, ∗p < 0.05, Student's t test).
(E) Representative immunoblot (N = 3 independent experiments) of FOXO1 and PRLR in D22 VC and D22 IVD.
(F) Immunoblot densities quantified with ImageJ software showing protein levels of FOXO1 and PRLR in day 22 VC and IVD. Data represent means ± SEM (N = 3 independent experiments, ∗p < 0.05, Student's t test).
(G) Representative images of immunohistochemistry to detect FOXO1 and PRLR in D22 VC and D22 IVD. Scale bars represent 20 μm.
(H) Quantification of cells with positive staining for FOXO1 and PRLR in day 22 VC and IVD. Data represent means ± SEM (N = 3 independent experiments, ∗p < 0.05, Welch test for FOXO1, Student's t test for PRLR).
(I) Quantitative RT-PCR of PGR-target genes FKBP5 and ZBTB16 in D22 VC and D22 IVD. Error bars represent RQMin and RQMax (N = 9 independent experiments; ∗p < 0.05; Student's t test).
To define a genetic signature of decidualization in the differentiated D22 EBs, we performed RNA sequencing (RNA-seq) to identify differentially expressed genes (DEGs) in D22 IVD relative to D22 VC. Pathway enrichment analysis of the DEGs was performed using MetaCore. The differentially regulated pathways for the DEGs included the cAMP signaling pathway (false discovery rate [FDR] <0.01) (Figure S4A), which is important for the decidual transformation of EMSFs (Brar et al., 1997). See Table S1 for the list of pathways with FDR values obtained in the pathway enrichment analysis. We then compared the D22 DEGs with those from our previously published expression microarray analysis of primary human EMSFs that underwent IVD treatment (Dyson et al., 2014). A total of 1,404 DEGs were upregulated or downregulated similarly in IVD-treated primary EMSFs and hiPSC-derived D22 EMSFs (treated with IVD) compared with respective vehicle controls (adjusted p < 0.05) (Figure S3D). Using real-time PCR, we verified major decidualization markers such as FOXO1, HAND2, IGFBP1, which were present among commonly upregulated DEGs in both cell types, supporting our hypothesis that iPSC-derived EMSFs are molecularly quite similar to primary EMSFs (Buzzio et al., 2006). Taken together, we confirmed the decidualization capacity of D14 EBs, and characterized molecular similarities between decidualization of primary EMSFs and iPSC-derived EMSFs, also designated as D14 EBs.
D14 EBs Recapitulate the Molecular Signature of Primary EMSFs
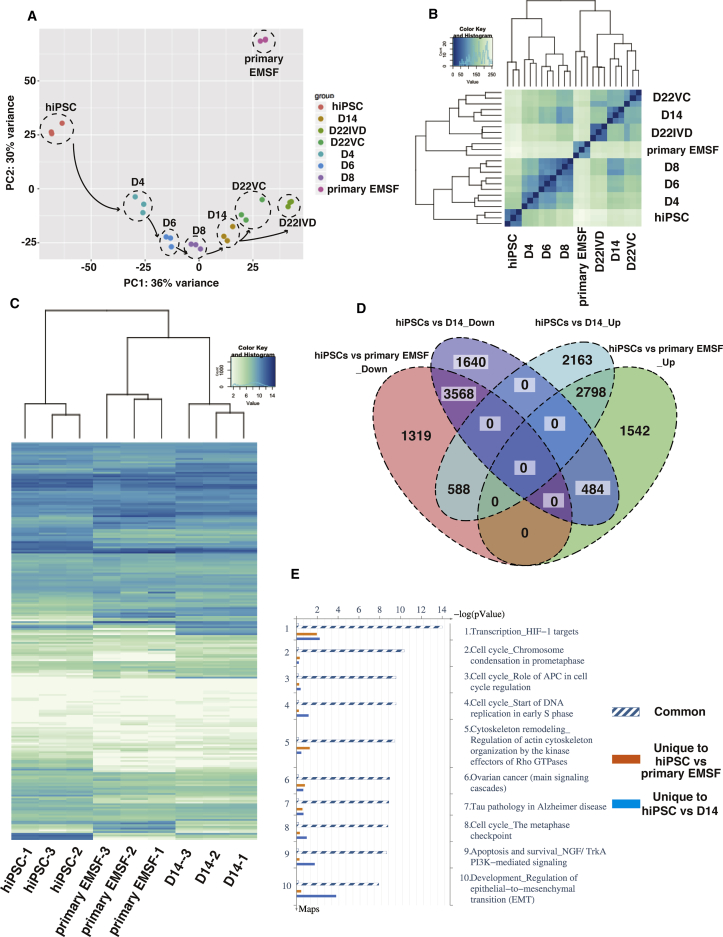
To assess the changes in molecular signatures that occur during the differentiation of hiPSCs in our protocol and how they compare with that in primary EMSFs, we analyzed global gene expression profiles of EBs at each stage (hiPSC, D2.5 EB, D4 EB, D6 EB, D8 EB, D14 EB, D22 VC, and D22 IVD) and of primary EMSFs using RNA-seq. The expression levels of 25,369 genes were measured simultaneously. Unsupervised principal component analysis (PCA) revealed a seamless transition in the transcriptional profiles during differentiation (Figure 5A). Unsupervised hierarchical clustering analysis showed strong correlations between technical replicates in each group and that the D14 EB and D22 EB signatures cluster more closely to primary EMSFs, confirming their resemblance to EMSFs (Figure 5B). Clustered heatmap analysis of 1,434 transcription factor genes revealed greater similarity between the transcriptomes of primary EMSFs and D14 EBs compared with hiPSCs (Figure 5C). See Table S2 for the list of genes used in Figure 5C.
Figure 5.
Transcriptome Changes during Differentiation of hiPSCs to EMSFs
(A) Principal component analysis (PCA) of RNA-seq obtained from EBs at each stage of EMSF differentiation from hiPSCs (N = 3 independent experiments).
(B) Hierarchical clustering analysis based on RNA-seq data obtained from EBs at each stage of EMSF differentiation from hiPSCs (N = 3 independent experiments). A total of 25,369 genes analyzed on RNA-seq were used in (A) and (B).
(C) Clustered heatmap analysis comparing the expression of 1,434 transcription factor genes in hiPSCs, D14 EBs, and primary EMSFs (N = 3 independent experiments).
(D) Based on RNA-seq, similar DEGs were identified between D14 EBs and primary EMSFs, relative to hiPSCs (N = 3 independent experiments, FDR adjusted p value <0.05). The Venn diagram shows the total numbers of up- and downregulated genes identified in each comparison. A total of 11,241 and 10,299 DEGs were identified in D14 EBs and primary EMSFs, respectively, compared with hiPSCs (N = 3 independent experiments, FDR adjusted p value <0.05). Since D14 EBs and primary EMSFs share 7,438 DEGs regardless of the direction of the expression, this Venn diagram includes 14,102 (= 11,241 + 10,299 − 7,438) different genes.
(E) Top ten common pathways identified by pathway enrichment analysis performed on the same gene list used in (D) (N = 3 independent experiments, FDR adjusted p value <0.05).
To identify the genes that drive the induction of EMSFs from hiPSCs, we sought common DEGs in primary EMSFs and D14 EBs relative to hiPSCs. A total of 11,241 and 10,299 DEGs were identified in D14 EBs and primary EMSFs, respectively, compared with hiPSCs (adjusted p < 0.05). Since D14 EBs and primary EMSFs share 7,438 DEGs regardless of the direction of expression, 14,102 (= 11,241 + 10,299 – 7,438) different sets of genes were used to perform downstream analyses. A total of 6,366 DEGs were similarly upregulated or downregulated in primary EMSFs and D14 EBs compared with hiPSCs (adjusted p < 0.05), as demonstrated by the Venn diagram in Figure 5D. Thirty genes (15 upregulated and 15 downregulated genes in D14 and primary EMSFs compared with hiPSCs) with the highest fold changes are listed in the clustered heatmap (Figure S5A). The top 15 downregulated DEGs include pluripotency markers such as SOX2 and NANOG, whereas the top 15 upregulated genes include genes that play crucial roles in endometrial function, such as WNT2, GBP1, and POSTN (Burmenskaya et al., 2017, Kumar et al., 2001, Li et al., 2017). Endometrium-specific transcription factors (HOXA9, HOXA10, HOXA11, and PGR) and EMSF markers (MME, COL1A1, FN1, and VIM) were present among other common DEGs. Pathway enrichment analysis by MetaCore comparing a total of 14,102 genes included in DEGs in primary EMSFs and D14 EBs relative to hiPSCs showed common enrichment for genes in the cell-cycle regulation pathway (FDR <0.01) and EMT pathway involving transforming growth factor (TGF) and WNT/CTNNB1 pathways (FDR <0.01), indicating that similar pathways are active in primary EMSFs and D14 EBs (Figures 5E and S4B). See Table S3 for the list of pathways with FDR values obtained in the pathway enrichment analysis. Gene ontology (GO) analysis using the same gene list showed common enrichment for “cellular component organization” and “system development” (Figure S5B). See Table S4 for the list of pathways with FDR values obtained in the pathway enrichment analysis. Heatmap analysis based on 133 genes involved in cell-cycle regulation revealed significant similarities between primary EMSFs and D14 EBs compared with hiPSCs (Figure S5C), confirming the result of the pathway enrichment analysis. By contrast, the similarity between primary EMSFs and D14 EBs was relatively weak with regard to the cytoskeleton remodeling pathway or EMT pathway as shown by heatmap analysis of 232 genes involved in these two pathways (Figure S5D). See Table S2 for the list of genes used in Figures S5C and S5D.
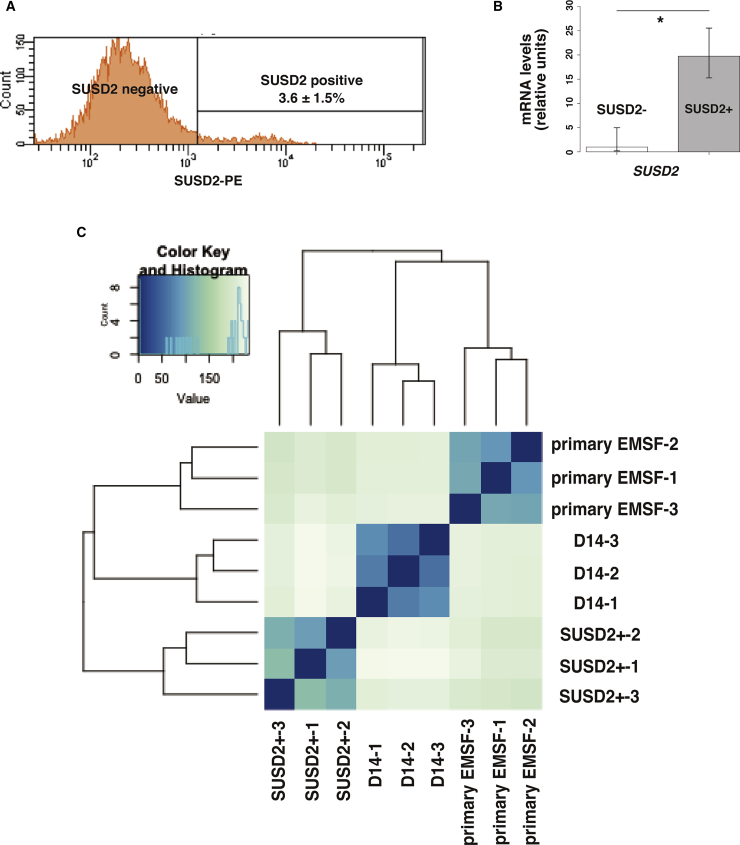
To examine the similarity between D14 EBs and eMSCs, we sorted SUSD2-positive eMSCs from freshly isolated primary EMSFs using flow cytometry, and performed RNA-seq. The percentage of SUSD2+ eMSCs among primary EMSFs was 3.6% ± 1.5% (N = 3) (Figure 6A). The mRNA expression of SUSD2 was significantly higher in the SUSD2-positive population compared with the SUSD2-negative population (Figure 6B), showing the efficient isolation of SUSD2-positive eMSCs. We then performed RNA-seq on the SUSD2-positive population, and compared its global gene expression profile with D14 and primary EMSFs. Unsupervised hierarchical clustering analysis revealed greater similarity between the transcriptomes of primary EMSFs and D14 EBs compared with eMSCs (Figure 6C). These results collectively suggest the proximity of D14 EBs to primary EMSFs, but not eMSCs.
Figure 6.
Isolation of SUSD2-Positive Population and Comparison of Its Transcriptome with hiPSC-Derived EMSFs
(A) Representative histogram (N = 3 independent experiments) of the SUSD2-positive population in freshly isolated primary EMSFs.
(B) Quantitative RT-PCR comparing expression of SUSD2 in the SUSD2-positive and SUSD2-negative population. Error bars represent RQMin and RQMax (N = 3 independent experiments, ∗p < 0.05, Student's t test).
(C) Hierarchical clustering analysis based on RNA-seq data obtained from D14 EBs, primary EMSFs, and SUSD2+ cells (N = 3 independent experiments). A total of 25,369 genes analyzed on RNA-seq were used in (C).
The WNT/CTNNB1 Pathway Is Required for Induction of EMSFs from hiPSCs
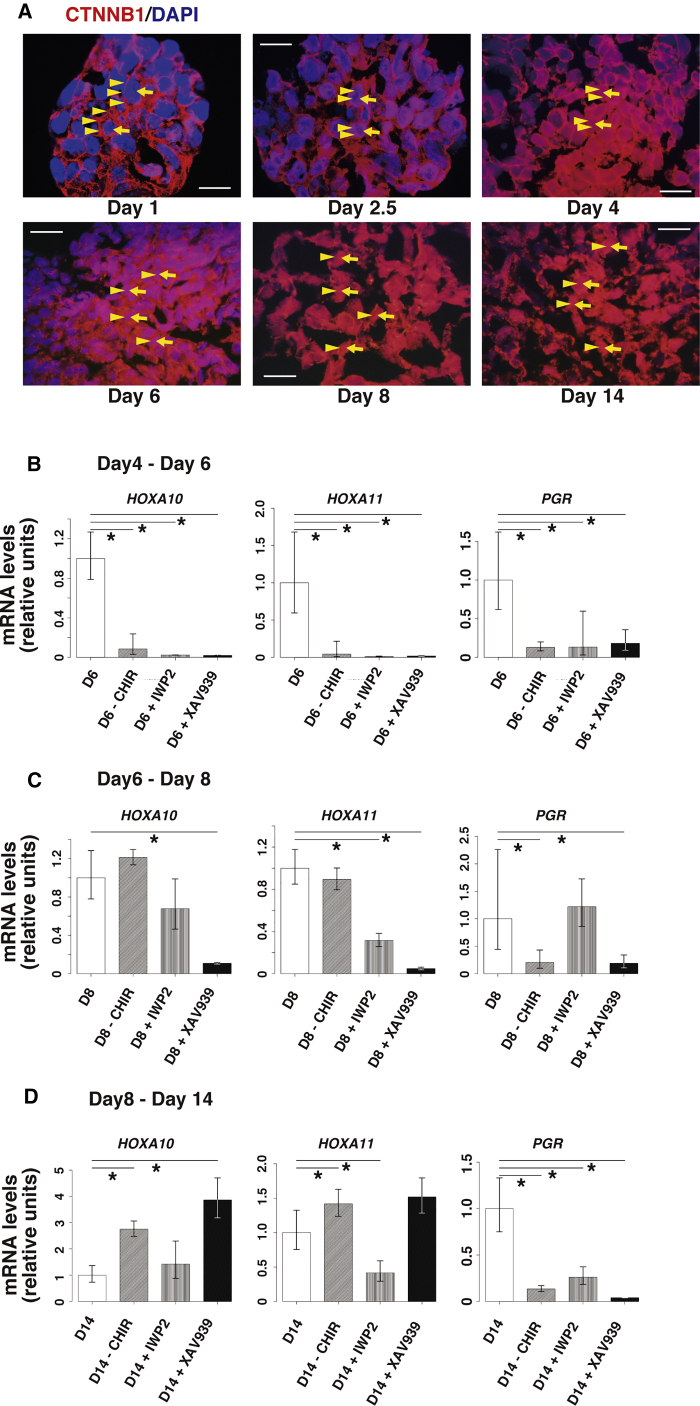
Based on the critical effect of CHIR, a CTNNB1 pathway agonist for EMSF induction from hiPSCs in our previous experiments, we hypothesized that the canonical CTNNB1 signaling pathway may play a key role in the differentiation of hiPSCs to EMSFs. Indeed, pathway enrichment analysis showed greater similarity of gene expression in the WNT signaling pathway between primary EMSFs and D14 EBs relative to hiPSCs (Figures 5E and S3B). Since activation of the canonical WNT/CTNNB1 signaling pathway results in the accumulation of CTNNB1 in the nucleus (Nusse, 2005), we performed immunofluorescence of CTNNB1 to determine its cellular localization in EBs. Progressive translocation of CTNNB1 from the plasma membrane to the nucleus was observed at each EB differentiation stage, confirming the activation of the canonical CTNNB1 pathway (Figures 7A and S6).
Figure 7.
Inhibition of the Canonical WNT/CTNNB1 Pathway
(A) Representative immunofluorescence image of CTNNB1 in hiPSC, D2.5, D4, D6, D8, and D14 EB. Scale bars represent 20 μm. Yellow arrowheads indicate CTNNB1 staining. Yellow arrows indicate DAPI-positive cell nuclei. These images are re-used in Figure S6.
(B–D) Quantitative RT-PCR of EMSF-specific genes HOXA10, HOXA11, and PGR in D6 EBs, D8 EBs, and D14 EBs.
(B) D6 EBs were treated with CHIR, NOGGIN, and PDGF-BB; with NOGGIN and PDGF-BB only; with CHIR, NOGGIN, PDGF-BB, and IWP2; or with CHIR, NOGGIN, PDGF-BB, and XAV939 for 2 days, between D4 and D6. Error bars represent RQMin and RQMax (N = 3 independent experiments except for D6 [N = 9 independent experiments], ∗p < 0.05, Student's t test).
(C) D8 EB were treated with CHIR and NOGGIN; with NOGGIN only; with CHIR, NOGGIN, and IWP2; or with CHIR, NOGGIN, and XAV939 for 2 days, between D6 and D8. Error bars represent RQMin and RQMax (N = 3 independent experiments except for D8 [N = 9 independent experiments], ∗p < 0.05, Student's t test).
(D) D14 EBs were treated with 5aza2, CHIR, E2, FGF9, and PDGF-BB; with 5aza2, E2, FGF9, and PDGF-BB only; with 5aza2, CHIR, E2, FGF9, PDGF-BB, and IWP2; or with 5aza2, CHIR, E2, FGF9, PDGF-BB, and XAV939 for 6 days, between D8 and D14. Error bars represent RQMin and RQMax (N = 3 independent experiments except for D14 [N = 9 independent experiments], ∗p < 0.05, Student's t test).
To determine whether the WNT/CTNNB1 pathway is required for differentiation of hiPSCs to EMSFs, we maintained cultures of D4 EBs (IM-like stage), D6 EBs (CE-like stage), and D8 EBs (MD-like stage) in the absence of CHIR or in the presence of CHIR plus or minus CTNNB1 inhibitors until D6 (CE-like stage), D8 (MD-like stage), and D14 (EMSF-like stage), respectively. Two types of CTNNB1 inhibitors were used. IWP2, an inhibitor of Porcn, was used to block the autocrine secretion of WNT proteins. XAV939 is a Tankyrase inhibitor that stimulates CTNNB1 degradation. We then examined the effects of treatment on expression of PGR, HOXA10, and HOXA 11. Interestingly, withholding CHIR or the addition of CTNNB1 pathway inhibitors (to the regular CHIR-containing cocktail) significantly decreased the expression of PGR at every EB stage (Figures 7B–7D), indicating the importance of WNT/CTNNB1 signaling for PGR expression at every stage of differentiation of hiPSCs. In contrast, while withholding CHIR or the addition of CTNNB1 pathway inhibitors between D4 and D6 significantly decreased the expression of HOXA10 and HOXA11 (Figure 7B), CHIR withdrawal between D6 and D8 had no effect on HOXA gene expression levels (Figure 7C). Furthermore, withholding CHIR or CTNNB1 inhibition between D8 and D14 increased the expression of HOXA10 and HOXA11 (Figure 7D). These data suggest that, while the WNT/CTNNB1 pathway is necessary during all stages of endometrial differentiation, it serves diverse functions and seems to be essential for progesterone responsiveness.
Discussion
We defined a protocol to induce the differentiation of EMSFs from hiPSCs under molecularly delineated EB culture conditions. Although the differentiation of human embryonic stem cells and/or hiPSCs to cardiac, hepatic, pancreatic, kidney, or neuronal cell lineages has been widely reported (Batchelder et al., 2009, Chambers et al., 2009, Mae et al., 2013, Song et al., 2009, Zhang et al., 2009a, Zhang et al., 2009b), our study represents a demonstration of the successful production of endometrial lineage cells from hiPSCs.
Only a few previous studies have attempted to derive cells of endometrial cell lineage from human embryonic stem cells (Song et al., 2015, Ye et al., 2011, Yu et al., 2015). Ye and colleagues induced mesoderm from a human embryonic stem cell line, co-cultured the mesoderm with murine neonatal endometrial cells, and then transplanted the differentiated cells under the kidney capsule of immunodeficient mice. In the graft, they found epithelium with TUBB (tubulin beta)-positive cilia, which expressed female reproductive tract epithelium markers CK18, CA125, ESR1, and HOXA10 (Ye et al., 2011). Yu and colleagues also demonstrated induction of an endometrium-like cell population, which expressed significantly higher PRL after treating a human embryonic stem cell line with a combination of PDGF-BB and WNT5a (Yu et al., 2015). Song et al. (2015) reported differentiation of a human embryonic stem cell line into endometrium-like cells expressing several endometrial markers, using conditioned media from human EMSFs. Of these three previous papers, only Ye et al. (2011) reported the expression of Müllerian duct cell markers during differentiation from mesoderm, whereas changes in gene profiles during the differentiation of the human embryonic stem cells in the studies by Yu et al. (2015) and Song et al. (2015) are not clear. While Yu et al. (2015) did demonstrate possible hormone receptivity of the differentiated endometrium-like cells, since human dermal fibroblast cells also express PRL after IVD (Richards and Hartman, 1996), they were unable to distinguish “endometrium-like cells” from dermal fibroblast cells.
The published protocols to produce Müllerian duct cells from human embryonic stem cells have several limitations. First, these protocols used poorly defined components, such as conditioned medium obtained from endometrial cell culture (Song et al., 2015) and co-culture with endometrium obtained from newborn mice (Ye et al., 2011), which would not be suitable for clinical applications. Second, since human embryonic stem cells are established from the inner cell mass of an embryo, the tissues derived from these cells cannot be transplanted to patients without an immune rejection.
Although hiPSCs are more difficult to differentiate than human embryonic stem cells (Kuzmenkin et al., 2009), hiPSCs present fewer ethical problems than human embryonic stem cells and are able to produce histocompatible tissues for autologous transplantation (Kondo et al., 2017). Thus, hiPSC research is expected to lead to regenerative therapies for various disorders, possibly including uterine factor infertility and endometriosis.
Here, we attempted to reproduce each stage of EMSF development, confirming the lineage of the differentiated cells at each step based on the presence and absence of cell-specific marker genes. We used an established protocol for the first step, IM induction, using a previously established protocol from Lam et al. (2014). We detected robust upregulation of LHX1 and PAX2 via PS differentiation using sequential treatment with CHIR, FGF2, and RA, consistent with the findings of Lam et al. (2014). We then used a WNT/CTNNB1 pathway agonist along with other chemical cocktails following the published findings that suggest a critical role of the WNT/CTNNB1 pathway in the differentiation of Müllerian tissues (Deutscher and Hung-Chang Yao, 2007, Stewart et al., 2013). With each step, our protocol produced EBs with gene expression profiles similar to CE, MD, and endometrial cells.
Importantly, we also demonstrated that the transcriptomes of the hiPSC-derived EMSFs and primary EMSFs were similar in the hierarchical analysis and heatmap analysis. Temporal changes in transcription factor genes are critical for development, as they turn on or off the appropriate genes in response to various growth factors to regulate changes in cell morphology and function that determine cell fate and differentiation (Lobe, 1992). For example, the HOX transcription factor family is important for proper body pattern formation (Moens and Selleri, 2006). A characteristic spatial distribution of HOXA9-13 is seen throughout the MD of vertebrates (Goodman, 2002). HOXA9 is expressed in the oviduct, HOXA10 is expressed in the uterus, HOXA11 is found in the lower uterus and cervix, and HOXA13 is seen in the ectocervix and upper vagina (Du and Taylor, 2015). Interestingly, HOXA9, 10, and 11, but not 13, were similarly regulated in D14 EBs and EMSFs, but distinct from hiPSCs.
The PCA partly supported the similarity of hiPSC-derived EMSFs and primary EMSFs: on the PC1 axis, the transcriptome distance between primary EMSFs and EBs became progressively closer during the differentiation process. To assess the gene signature that drives the induction of EMSFs, we identified 30 common DEGs with the highest fold changes in D14 and primary EMSFs relative to hiPSCs. Although the PCA was generated from the whole transcriptome, it is inferable that these DEGs significantly contributed to the transition observed in PCA.
Pathway enrichment analysis further revealed that EMSFs and D14 EBs show common enrichment for genes in the cell-cycle regulation pathway, EMT pathway, and cytoskeleton remodeling pathway that involves TGF and WNT, relative to hiPSCs. However, while a heatmap of cell-cycle regulation gene expression clearly demonstrated the similarity between D14 EBs and primary EMSFs, a heatmap of EMT pathway and cytoskeleton pathway genes showed only weak similarity between D14 EBs and primary EMSFs. This discrepancy between pathway enrichment analysis and heatmap gene expression may be explained by the difference in fold change between primary EMSFs and D14 EBs relative to hiPSCs, as pathway enrichment analysis only relies on the direction of gene regulation (i.e., upregulation or downregulation).
Hormone responsiveness is a defining characteristic of the endometrial stroma. In the secretory phase of the human menstrual cycle, progesterone exerts its action on EMSFs by binding to PGR, a member of the steroid hormone receptor superfamily of ligand-activated transcription factors, promoting differentiation of the cells in a process termed decidualization (Gellersen and Brosens, 2003). When hiPSC-derived EBs were treated with estrogen, progestin, and a cAMP analog, they underwent decidualization, and the resulting D22 EBs had a similar transcriptome expression pattern as primary EMSFs, with approximately 20%–30% of all DEGs similarly up- or downregulated in response to IVD treatment. The upregulation of direct PGR-target genes in response to the hormone treatment further supported the active PGR signaling pathway in the differentiated cells.
Our method of using CHIR to induce differentiation into Müllerian lineage is consistent with the important role of WNT/CTNNB1 signaling during embryonic MD development (Deutscher and Hung-Chang Yao, 2007, Stewart et al., 2013). Indeed, the addition of CTNNB1 inhibitors led to downregulation of PGR, suggesting a critical relationship between the canonical WNT/CTNNB1 signaling pathway and PGR expression, at least in the developing MD. Further study is needed to clarify the interaction between these signaling cascades.
hiPSC-derived EMSFs cultured as EBs displayed many specific characteristics of primary EMSFs such as expression of the critical genes including PGR, HOXA11, PRL, and IGFBP1. There were, however, some quantitative differences between the two cell types. For example, progesterone fold induction of PRL or IGFBP1 was lower in hiPSC-derived EMSFs. Moreover, the expression levels of general fibroblast markers such as VIM were lower in hiPSC-derived EMSFs compared with primary EMSFs. We anticipate that further optimization of the current protocol and experimental model will improve these aspects in the future.
Further study is also needed to generate endometrial epithelial cells from hiPSCs. Whereas EMSFs are relatively easy to grow on a 2D culture dish, endometrial epithelial cells are more difficult to maintain. Recently, Turco et al. (2017) reported a method for long-term, hormone-responsive organoid culture of human endometrium in a chemically defined medium. Their approach may help elucidate the distinct physiology of endometrial epithelial cells and lead to a new protocol to generate endometrial epithelial cells from hiPSCs in the future.
With regard to the potential clinical applications of our work, we envision that the protocol to differentiate EMSFs from hiPSCs will one day be amenable to cell replacement therapy for endometrial diseases such as endometriosis or early-stage low-grade endometrial cancer, in which abnormal EMSFs contribute to pathogenesis (Janzen et al., 2013, Kim et al., 2013). Cell replacement therapy may also be effective for the treatment of uterine factor infertility, such as Asherman syndrome (intrauterine adhesions). Future studies will explore the use of natural or synthetic scaffolds of differentiated iPSC-derived EMSFs for transplantation. In fact, endometrium-like tissues were successfully regenerated from decellularized rat uterine matrix reseeded with rat uterine cells (Miyazaki and Maruyama, 2014). This technology may become a component of a novel therapeutic strategy for treating uterine agenesis. Also, further work is needed to elucidate the precise molecular pathways within the WNT/CTNNB1 signaling cascade for the regulation of PGR during differentiation of stem cells, which can lead to a novel molecular therapy for a variety of endometrial diseases since abnormal responses to progesterone play key roles in the pathogenesis of such disorders.
In conclusion, defined sequential incubation with a CTNNB1 activator and a number of other specific hormones induces differentiation of hiPSCs into EBs with similar gene expression patterns as EMSFs; these cells are capable of decidualization in response to a time-honored hormonal stimulation. We also demonstrated that the canonical CTNNB1 signaling pathway is essential for the expression of PGR, the key steroid hormone receptor and master regulator of the most important function of the endometrium, i.e., implantation of an embryo (Kim et al., 2013). The establishment of a protocol for differentiating hiPSCs into cells of the endometrial stromal cell lineage will have implications for cell-based therapies and bioengineering of endometrial tissue for the treatment of various endometrial diseases such as endometriosis, early-stage endometrial cancer, and uterine factor infertility.
Experimental Procedures
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Serdar E. Bulun (s-bulun@northwestern.edu).
Experimental Model and Subject Details
Ethics Statement
The acquisition of human tissue for this study was approved by the Northwestern Institutional Review Board for Human Research (1375–005). Written informed consent from each subject was obtained before surgery.
Method Details
hiPSC Culture
Two hiPSC lines (clone 1, ACS-1028; clone 2, ACS-1030) were purchased from American Type Culture Collection (Manassas, VA). Clones 1 and 2 were derived from bone marrow CD34+ cells obtained from a healthy African American female donor and a white female donor, respectively, with Sendai viral expression of OCT4, SOX2, KLF4, and MYC genes. The cells were routinely cultured on feeder layers of mitomycin C-treated mouse embryonic fibroblast feeder cells (Applied StemCells, Milpitas, CA), seeded on gelatin-coated dishes (MilliporeSigma, Burlington, MA), in hiPSC maintenance medium (DMEM/F12 + 20% KnockOut Serum Replacement + 1 mM nonessential amino acids + 2 mM GlutaMAX + 0.55 mM 2-mercaptoethanol [all from Thermo Fisher Scientific, Carlsbad, CA]) supplemented with 10 ng/mL recombinant human FGF2 (PeproTech, Rocky Hill, NJ) (passages 9–13). Cell plastics were from TPP (St. Louis, MO). Cultures were passaged using calcium-/magnesium-free PBS (Thermo Fisher Scientific) supplemented with 0.5 mM EDTA (VWR, Radnor, PA) and 30.8 mM NaCl (MilliporeSigma) at a 1:4 split ratio every 3–5 days.
See Supplemental Experimental Procedures for method details.
Author Contributions
K.M., T.M., and S.E.B. conceived and designed the experiments. K.M., M.T.D., J.S.C., Y.F., B.D.Y., and S.E.B. performed the experiments. K.M., M.T.D., and S.E.B. analyzed the data. K.M., M.T.D., J.S.C., and S.E.B. contributed reagents/materials/analysis tools. K.M. and S.E.B. wrote the manuscript. K.M., M.T.D., T.M., and S.E.B. reviewed the manuscript.
Acknowledgments
The authors thank Dr. Masashi Toyoda for his valuable advice on hiPSC culture. The authors thank Dr. Matthew Schipma for his technical support for RNA-seq data analysis. The authors thank Dr. Demirkan Gursel for his technical support for immunostaining. This study was supported by funding from NIH grant R37-HD36891 (S.E.B.) and R03-HD082558 (M.T.D.), Grant-in-Aid for Scientific Research (Young Scientists B) (K.M.), the Uehara Memorial Foundation (K.M.), and the Kanzawa Medical Research Foundation (K.M.).
Published: November 1, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and seven tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.10.002.
Accession Numbers
RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number ArrayExpress: E-MTAB-7292.
Supplemental Information
References
- Akamatsu T., Arai Y., Kosugi I., Kawasaki H., Meguro S., Sakao M., Shibata K., Suda T., Chida K., Iwashita T. Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair. 2013;6:15. doi: 10.1186/1755-1536-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango N.A., Donahoe P.K. StemBook. Harvard Stem Cell Institute; 2008. Sex differentiation in mouse and man and subsequent development of the female reproductive organs.https://www.ncbi.nlm.nih.gov/books/NBK47454/ [PubMed] [Google Scholar]
- Araoka T., Mae S., Kurose Y., Uesugi M., Ohta A., Yamanaka S., Osafune K. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS One. 2014;9:e84881. doi: 10.1371/journal.pone.0084881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Bacanamwo M. DNA methyltransferase inhibition induces mouse embryonic stem cell differentiation into endothelial cells. Exp. Cell Res. 2010;316:172–180. doi: 10.1016/j.yexcr.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder C.A., Lee C.C., Matsell D.G., Yoder M.C., Tarantal A.F. Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation. 2009;78:45–56. doi: 10.1016/j.diff.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson G.V., Lim H., Paria B.C., Satokata I., Dey S.K., Maas R.L. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Brar A.K., Frank G.R., Kessler C.A., Cedars M.I., Handwerger S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6:301–307. doi: 10.1007/BF02820507. [DOI] [PubMed] [Google Scholar]
- Bulun S.E. Endometriosis. N. Engl. J. Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Burmenskaya O.V., Bozhenko V.K., Smolnikova V.Y., Kalinina E.A., Korneeva I.E., Donnikov A.E., Beyk capital Ie C., Naumov V.A., Aleksandrova N.V., Borovikov P.I. Transcription profile analysis of the endometrium revealed molecular markers of the personalized 'window of implantation' during in vitro fertilization. Gynecol. Endocrinol. 2017;33:22–27. doi: 10.1080/09513590.2017.1404236. [DOI] [PubMed] [Google Scholar]
- Busch S., Andersson D., Bom E., Walsh C., Stahlberg A., Landberg G. Cellular organization and molecular differentiation model of breast cancer-associated fibroblasts. Mol. Cancer. 2017;16:73. doi: 10.1186/s12943-017-0642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzio O.L., Lu Z., Miller C.D., Unterman T.G., Kim J.J. FOXO1A differentially regulates genes of decidualization. Endocrinology. 2006;147:3870–3876. doi: 10.1210/en.2006-0167. [DOI] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.W., Schwab K.E., Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- Cheng F., Shen Y., Mohanasundaram P., Lindstrom M., Ivaska J., Ny T., Eriksson J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc. Natl. Acad. Sci. U S A. 2016;113:E4320–E4327. doi: 10.1073/pnas.1519197113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour K.A., Agulnick A.D., Eliazer S., Kelly O.G., Kroon E., Baetge E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Daftary G.S., Taylor H.S. EMX2 gene expression in the female reproductive tract and aberrant expression in the endometrium of patients with endometriosis. J. Clin. Endocrinol. Metab. 2004;89:2390–2396. doi: 10.1210/jc.2003-031389. [DOI] [PubMed] [Google Scholar]
- Daley G.Q. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10:740–749. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B.P., Tam P.P. The node of the mouse embryo. Curr. Biol. 2000;10:R617–R619. doi: 10.1016/s0960-9822(00)00675-8. [DOI] [PubMed] [Google Scholar]
- Deutscher E., Hung-Chang Yao H. Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev. Biol. 2007;307:227–236. doi: 10.1016/j.ydbio.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo F., Racca S., Gallo E., Conti G., Russo A., Mondo F., Francalanci S. Estrogen and progesterone receptors in the human vagina. J. Endocrinol. Invest. 1985;8:131–134. doi: 10.1007/BF03350667. [DOI] [PubMed] [Google Scholar]
- Du H., Taylor H.S. The role of hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb. Perspect. Med. 2015;6:a023002. doi: 10.1101/cshperspect.a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson M.T., Roqueiro D., Monsivais D., Ercan C.M., Pavone M.E., Brooks D.C., Kakinuma T., Ono M., Jafari N., Dai Y. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10:e1004158. doi: 10.1371/journal.pgen.1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson M.A., Nagaraja A.K., Matzuk M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familari M. Characteristics of the endoderm: embryonic and extraembryonic in mouse. ScientificWorldJournal. 2006;6:1815–1827. doi: 10.1100/tsw.2006.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P., Huber T.L., Paddison P.J., Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.R., Li S., Zhang J., Liang C., Chen E.N., Zhang S.Y., Chuai M., Bao Y.P., Wang G., Yang X. Excess imidacloprid exposure causes the heart tube malformation of chick embryos. J. Agric. Food Chem. 2016;64:9078–9088. doi: 10.1021/acs.jafc.6b03381. [DOI] [PubMed] [Google Scholar]
- Gellersen B., Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J. Endocrinol. 2003;178:357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- Gendron R.L., Paradis H., Hsieh-Li H.M., Lee D.W., Potter S.S., Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol. Reprod. 1997;56:1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- Giudice L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman F.R. Limb malformations and the human HOX genes. Am. J. Med. Genet. 2002;112:256–265. doi: 10.1002/ajmg.10776. [DOI] [PubMed] [Google Scholar]
- Graubner F.R., Reichler I.M., Rahman N.A., Payan-Carreira R., Boos A., Kowalewski M.P. Decidualization of the canine uterus: from early until late gestational in vivo morphological observations, and functional characterization of immortalized canine uterine stromal cell lines. Reprod. Domest. Anim. 2017;52(Suppl 2):137–147. doi: 10.1111/rda.12849. [DOI] [PubMed] [Google Scholar]
- Guioli S., Sekido R., Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev. Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Han D.W., Greber B., Wu G., Tapia N., Arauzo-Bravo M.J., Ko K., Bernemann C., Stehling M., Scholer H.R. Direct reprogramming of fibroblasts into epiblast stem cells. Nat. Cell Biol. 2011;13:66–71. doi: 10.1038/ncb2136. [DOI] [PubMed] [Google Scholar]
- Hashimoto R. Development of the human Mullerian duct in the sexually undifferentiated stage. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;272:514–519. doi: 10.1002/ar.a.10061. [DOI] [PubMed] [Google Scholar]
- Horrillo A., Pezzolla D., Fraga M.F., Aguilera Y., Salguero-Aranda C., Tejedo J.R., Martin F., Bedoya F.J., Soria B., Hmadcha A. Zebularine regulates early stages of mESC differentiation: effect on cardiac commitment. Cell Death Dis. 2013;4:e570. doi: 10.1038/cddis.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Maeda M., Fujiwara H., Okamoto N., Kariya M., Emi N., Takakura K., Kanzaki H., Mori T. Human endometrial stromal cells and decidual cells express cluster of differentiation (CD) 13 antigen/aminopeptidase N and CD10 antigen/neutral endopeptidase. Biol. Reprod. 1992;46:328–334. doi: 10.1095/biolreprod46.3.328. [DOI] [PubMed] [Google Scholar]
- Jameson S.A., Natarajan A., Cool J., DeFalco T., Maatouk D.M., Mork L., Munger S.C., Capel B. Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet. 2012;8:e1002575. doi: 10.1371/journal.pgen.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen D.M., Rosales M.A., Paik D.Y., Lee D.S., Smith D.A., Witte O.N., Iruela-Arispe M.L., Memarzadeh S. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 2013;73:4697–4710. doi: 10.1158/0008-5472.CAN-13-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Kurita T., Bulun S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Behringer R.R. Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Kommagani R., Szwarc M.M., Vasquez Y.M., Peavey M.C., Mazur E.C., Gibbons W.E., Lanz R.B., DeMayo F.J., Lydon J.P. The promyelocytic leukemia zinc finger transcription factor is critical for human endometrial stromal cell decidualization. PLoS Genet. 2016;12:e1005937. doi: 10.1371/journal.pgen.1005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Toyoda T., Inagaki N., Osafune K. iPSC technology-based regenerative therapy for diabetes. J. Diabetes Invest. 2017;9:234–243. doi: 10.1111/jdi.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Li Q., Dua A., Ying Y.K., Bagchi M.K., Bagchi I.C. Messenger ribonucleic acid encoding interferon-inducible guanylate binding protein 1 is induced in human endometrium within the putative window of implantation. J. Clin. Endocrinol. Metab. 2001;86:2420–2427. doi: 10.1210/jcem.86.6.7534. [DOI] [PubMed] [Google Scholar]
- Kuzmenkin A., Liang H., Xu G., Pfannkuche K., Eichhorn H., Fatima A., Luo H., Saric T., Wernig M., Jaenisch R. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009;23:4168–4180. doi: 10.1096/fj.08-128546. [DOI] [PubMed] [Google Scholar]
- Lam A.Q., Freedman B.S., Morizane R., Lerou P.H., Valerius M.T., Bonventre J.V. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J. Am. Soc. Nephrol. 2014;25:1211–1225. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Li S., Wang Y., Wang J., Wang K., Liu X., Li Y., Liu J. Decreased expression of WNT2 in villi of unexplained recurrent spontaneous abortion patients may cause trophoblast cell dysfunction via downregulated Wnt/beta-catenin signaling pathway. Cell Biol. Int. 2017;41:898–907. doi: 10.1002/cbin.10807. [DOI] [PubMed] [Google Scholar]
- Little M., Georgas K., Pennisi D., Wilkinson L. Kidney development: two tales of tubulogenesis. Curr. Top. Dev. Biol. 2010;90:193–229. doi: 10.1016/S0070-2153(10)90005-7. [DOI] [PubMed] [Google Scholar]
- Lobe C.G. Transcription factors and mammalian development. Curr. Top. Dev. Biol. 1992;27:351–383. doi: 10.1016/s0070-2153(08)60539-6. [DOI] [PubMed] [Google Scholar]
- Mae S., Shono A., Shiota F., Yasuno T., Kajiwara M., Gotoda-Nishimura N., Arai S., Sato-Otubo A., Toyoda T., Takahashi K. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat. Commun. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood T.A., Templeton A. Prevalence and genesis of endometriosis. Hum. Reprod. 1991;6:544–549. doi: 10.1093/oxfordjournals.humrep.a137377. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr. J. 2008;55:795–810. doi: 10.1507/endocrj.k08e-067. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Masuda H., Ono M., Kajitani T., Yoshimura Y. Human uterine stem/progenitor cells: their possible role in uterine physiology and pathology. Reproduction. 2010;140:11–22. doi: 10.1530/REP-09-0438. [DOI] [PubMed] [Google Scholar]
- Masuda H., Anwar S.S., Buhring H.J., Rao J.R., Gargett C.E. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012;21:2201–2214. doi: 10.3727/096368911X637362. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35:8791–8800. doi: 10.1016/j.biomaterials.2014.06.052. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Maruyama T., Masuda H., Yamasaki A., Uchida S., Oda H., Uchida H., Yoshimura Y. Stem cell-like differentiation potentials of endometrial side population cells as revealed by a newly developed in vivo endometrial stem cell assay. PLoS One. 2012;7:e50749. doi: 10.1371/journal.pone.0050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens C.B., Selleri L. Hox cofactors in vertebrate development. Dev. Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Morizane R., Lam A.Q., Freedman B.S., Kishi S., Valerius M.T., Bonventre J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015;33:1193–1200. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote P.A., Balleine R.L., McGowan E.M., Clarke C.L. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 1999;84:2963–2971. doi: 10.1210/jcem.84.8.5928. [DOI] [PubMed] [Google Scholar]
- Mugford J.W., Sipila P., McMahon J.A., McMahon A.P. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev. Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu L., Hufnagel D., Taylor H.S. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol. Reprod. 2015;92:138. doi: 10.1095/biolreprod.114.126771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Palpant N.J., Pabon L., Friedman C.E., Roberts M., Hadland B., Zaunbrecher R.J., Bernstein I., Zheng Y., Murry C.E. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat. Protoc. 2017;12:15–31. doi: 10.1038/nprot.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A.J., Hunter J.T., Welch R.A., Fairclough R.J. Oestrogens in bovine fetal and maternal plasma near term. J. Reprod. Fertil. 1975;43:179–181. doi: 10.1530/jrf.0.0430179. [DOI] [PubMed] [Google Scholar]
- Ramkumar N., Anderson K.V. SnapShot: mouse primitive streak. Cell. 2011;146:488–488.e2. doi: 10.1016/j.cell.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Richards R.G., Hartman S.M. Human dermal fibroblast cells express prolactin in vitro. J. Invest. Dermatol. 1996;106:1250–1255. doi: 10.1111/1523-1747.ep12348944. [DOI] [PubMed] [Google Scholar]
- Salih S.M., Taylor H.S. HOXA10 gene expression in human fallopian tube and ectopic pregnancy. Am. J. Obstet. Gynecol. 2004;190:1404–1406. doi: 10.1016/j.ajog.2004.01.066. [DOI] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Song Z., Cai J., Liu Y., Zhao D., Yong J., Duo S., Song X., Guo Y., Zhao Y., Qin H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- Song T., Zhao X., Sun H., Li X., Lin N., Ding L., Dai J., Hu Y. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng. Part A. 2015;21:353–361. doi: 10.1089/ten.tea.2014.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.A., Wang Y., Bonilla-Claudio M., Martin J.F., Gonzalez G., Taketo M.M., Behringer R.R. CTNNB1 in mesenchyme regulates epithelial cell differentiation during Mullerian duct and postnatal uterine development. Mol. Endocrinol. 2013;27:1442–1454. doi: 10.1210/me.2012-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Blackwelder A.J., Grossman G., Minges J.T., Yuan L., Young S.L., Wilson E.M. Primate-specific melanoma antigen-A11 regulates isoform-specific human progesterone receptor-B transactivation. J. Biol. Chem. 2012;287:34809–34824. doi: 10.1074/jbc.M112.372797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tsai S.J., Wu M.H., Chen H.M., Chuang P.C., Wing L.Y. Fibroblast growth factor-9 is an endometrial stromal growth factor. Endocrinology. 2002;143:2715–2721. doi: 10.1210/endo.143.7.8900. [DOI] [PubMed] [Google Scholar]
- Turco M.Y., Gardner L., Hughes J., Cindrova-Davies T., Gomez M.J., Farrell L., Hollinshead M., Marsh S.G.E., Brosens J.J., Critchley H.O. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017;19:568–577. doi: 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D., Palmer S., Koopman P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Ye L., Mayberry R., Lo C.Y., Britt K.L., Stanley E.G., Elefanty A.G., Gargett C.E. Generation of human female reproductive tract epithelium from human embryonic stem cells. PLoS One. 2011;6:e21136. doi: 10.1371/journal.pone.0021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P.E., Baumhueter S., Lasky L.A. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995;85:96–105. [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu W.Z., Chen X.M., Niu W.B., Wang F., Sun B., Sun Y.P. Role of Wnt5a in the differentiation of human embryonic stem cells into endometrium-like cells. Int. J. Clin. Exp. Pathol. 2015;8:5478–5484. [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Jiang W., Liu M., Sui X., Yin X., Chen S., Shi Y., Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wilson G.F., Soerens A.G., Koonce C.H., Yu J., Palecek S.P., Thomson J.A., Kamp T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin L., Park T.S., Zambidis E.T. Capturing human naive pluripotency in the embryo and in the dish. Stem Cells Dev. 2017;26:1141–1161. doi: 10.1089/scd.2017.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.