Abstract
Background
Agitation is very common in patients with acute stage schizophrenia, and injection of antipsychotics and clonazepam is widely used. Network meta-analysis of these comparisons among three injection treatments has been seldom reported.
Aim
To compare the efficacy and safety of various injections for agitation symptoms in Chinese patients with schizophrenia.
Methods
Searches were made in PubMed, Embase and Web of Knowledge, Cochrane Library, Wanfang data, CNKI, SinoMed and VIP databases up to 18 February 2018. Standard search strategies were performed by two reviewers according to the Cochrane Review Group. The Consolidated Standards of Reporting Trials statement was used to assess the methodological quality of the studies. STATA was used to perform meta-analysis. The Cochrane Grades of Recommendation, Assessment, Development and Evaluation (GRADE) was used to assess the strength of evidence.
Results
A total of 15 studies were included in the network meta-analysis. There were 11 studies comparing ziprasidone with haloperidol, and four studies comparing haloperidol with clonazepam. The results showed that ziprasidone is more effective than haloperidol and clonazepam (sucra: 77.2, 72.8 and 0) in the treatment of agitation symptoms. There was the effect size (standardised mean difference (SMD)) in the three groups: haloperidol: SMD=2.278, 95% CI 1.836 to 2.719; ziprasidone: SMD=2.536, 95% CI 2.082 to 2.990; and clonazepam: SMD=1.360, 95% CI 0.127 to 2.593. The acceptability was assessed by the incidence of excessive sedation, which showed that ziprasidone and haloperidol were similar with both being superior to clonazepam (sucra: 0.3, 0.7 and 99.0). Ziprasidone had significantly less adverse effects than haloperidol in effects of extrapyramidal system (EPS) (z=5.01, p<0.001). There were no statistically significant differences between haloperidol and ziprasidone in tachycardia and abnormal ECG (z=1.69, p=0.091; z=0.87, p=0.386; respectively). Based on GRADE, the strength of the evidence for primary outcome was ‘medium’.
Conclusion
Our results suggested that ziprasidone was more suitable than haloperidol and clonazepam in the treatment of agitation symptoms in Chinese patients with schizophrenia, according to the efficacy and acceptability of these three intramuscular injection medications.
Keywords: schizophrenia, agitation, ziprasidone, haloperidol, clonazepam, network meta-analysis
Background
Schizophrenia is a common serious mental disorder, and the prevalence of schizophrenia is up to 0.8% in China according to the Phillips(2009).1 Agitation is often seen in the acute stage of schizophrenia,2 and studies have shown that 64.3% of the newly admitted psychiatric patients with agitation are patients with schizophrenia, with 31.0% of them having severe agitation.3 Agitation caused by mental disorders such as schizophrenia has an acute onset, rapid progression and can present with violent aggression, which makes it an emergency and thus needing to be handled in a timely and appropriate manner.4 The first-generation and second-generation of fast-acting injected antipsychotics and benzodiazepines (clonazepam injection) are widely used in clinical practice to control agitation quickly, but there are neither conclusions of current studies nor studies evaluating the safety and tolerance of these injections simultaneously.3 Besides considering the differences in drug metabolism and adverse drug reactions between Chinese and foreign populations5, we conducted a comprehensive systematic review of studies of quick-acting injections for the treatment of agitation symptoms in Chinese patients with schizophrenia. To compare the efficacy and safety of these quick-acting injections, we also conducted a network meta-analysis about the efficacy and acceptability, and we hope to provide evidence for drug selection in clinical practice.
Materials and methods
Data sources and search strategy
We searched PubMed, Web of Knowledge, Embase and Cochrane Library using the English keywords including schizophrenia, agitation, Chinese, China, injection, and intramuscular and searched Wanfang data, CNKI, SinoMed and VIP databases using the Chinese keywords including schizophrenia, agitation, injection, and intramuscular. Search strategy was based on the Cochrane Collaboration Handbook,6 and we used truncation to improve recall ratio. In addition, we hand-searched articles published in the past 6 months in the related psychiatric journals (Chinese Journal of Psychiatry, Chinese Journal of Nervous and Mental Diseases, Shanghai Archives of Psychiatry and so forth) and references of the retrieved articles (to identify studies not captured by our primary search strategy).7 8
Search results
The search was not restricted to initial published time, and the final search was run on 18 February 2018. A total of 227 retrieved results from four English databases and four Chinese databases were obtained, including 15 English articles and 212 Chinese articles (figure 1).
Figure 1.
Flow chart of the study.
Study selection
Inclusion and exclusion criteria were according to the Consolidated Standards of Reporting Trials statement.9 In general, the trials were considered eligible if they met the following criteria: (1) all the subjects were Chinese patients with schizophrenia; (2) randomised controlled trials (RCTs) with a clear randomised method; (3) correct statistical methods; and (4) complete data including efficacy and/or safety data for meta-analysis. The following criteria were used for data exclusion: (1) non-randomised controlled trials (non-RCTs) or self-control study; (2) statistical data for meta-analysis were unavailable; (3) reviews, case reports, animal experiments and so forth and (4) repeated reports, resulting in unreliable results.
We found 65 articles for screening and after excluding non-conforming studies (n=48), repeated reports (n=2), we included 15 trials for meta-analysis (figure 1).
Quality assessment and data extraction
Two independent reviewers (LS and ZL) assessed the quality of the included studies. Disagreements between the two reviewers were regularly resolved by consulting a third reviewer (SS) or the expert (YX). Major assessment items included: (1) description of research design, research sites and research environment; (2) randomisation, blinding methods, (3) statistical data, description of statistical methods and (4) whether the data of efficacy and side effects were complete. Data extraction and entry of the efficacy indicators and side effects of agitation were performed independently by two evaluators, and then they conducted a cross-check. The descriptions of studies included in the meta-analysis are shown in table 1.
Table 1.
Characteristics of the 15 studies included in the network meta-analysis
| ID | Author | Year | Drugs | Average age (year) | Gender composition | Study design | Inclusion criteria | Treatment programmes |
| 1 | Hongyan Zhang16 | 2013 | ZIP versus HAL | 32; 31. | Male: 48%; male: 48%. | Randomised double blinded. | ICD-10 | ZIP group: 10-20 mg/time, maximum dose 40 mg/day; HAL group: 5 mg/time, maximum dose 20 mg/day. |
| 2 | Jicai Wang21 | 2007 | ZIP versus HAL | 29; 26. | Male: 38%; male: 62%. | Randomised double blinded. | CCMD-3 | Randomly assigned into ZIP or HAL group, 3 days in total ZIP group: initial dose 10 mg twice a day, 2-3 days 10–20 mg twice a day; HAL group: initial dose 5 mg twice a day, 2-3 day, 5-10 mg twice a day. |
| 3 | Xiaoqin Jiang22 | 2008 | ZIP versus HAL | 33; 32. | Male: 21; male: 19. | Randomised double blinded | CCMD-3 | ZIP group: the initial dose is 10 mg intramuscularly, and can be reused after 4-6 hours, the maximum dose is 40 mg/day. HAL group: the initial dose is 5-10 mg intramuscular injection, which can be reused after 4-6 hours, the maximum dose is 40 mg/day. The course of treatment is 3 days. After 3 days, the muscles were changed to oral treatment. |
| 4 | Renchang Wang23 | 2005 | ZIP versus HAL | 27.0; 27.4. | Male: 19/female: 11; male: 20/female: 10. | Randomised double blinded. | CCMD-3 | ZIP group: 10-20 mg intramuscular injection, can be reused after 4-6 hours, the maximum dose of 40 mg/day. HAL group: 5-10 mg intramuscular injection, can be reused after 4–6 hours, the maximum dose of 30 mg/day. The course of treatment is 3 days. |
| 5 | MengYe24 | 2009 | ZIP versus HAL | 27.4; 28.3. | Male: 19/female: 11; Male: 20/female: 10. | Randomised double blinded | DSM-IV | ZIP group: 10–20 mg intramuscular injection can be reused after 4–6 hours, the maximum dose of 40 mg/day. HAL group: 5-10 mg intramuscular injection can be reused after 4–6 hours, the maximum dose of 30 mg/day. The course of treatment is 3 days. |
| 6 | Yuehua Li25 | 2006 | ZIP versus HAL | 34; 33. | Male: 75/female: 40; male: 73/female: 43. | Randomised double blinded. | CCMD-3 | ZIP group: 10-20 mg intramuscular injection, can be reused after 4-6 hours, the maximum dose of 40 mg/day. HAL group: 5-10 mg intramuscularinjection, can be reused after 4-6 hours, the maximum dose of 30 mg/day. The course of treatment is 3 days. |
| 7 | Guangtao Hu26 | 2014 | ZIP versus HAL | 28.44; 27.57. | Male: 26/female: 17; male: 20/female: 23. | Randomised double blinded. | ICD-10 | ZIP group: 10-20mg/intramuscular injection, the maximum dose of 40 mg/day; HAL group: 5-10 mg/intramuscular injection, the maximum dose of 30 mg/day. The two groups of drugs can be reused after 4-6 hours according tothe condition, and the course of treatment is observed for 3 days. |
| 8 | Xiaoyang Zhang19 | 2015 | ZIP versus HAL | 28.12; 28.35. | Male: 28/female: 19; male: 30/female: 17. | Randomised double blinded. | ICD-10 | The ZIP group: the initial dose is 10-20 mg intramuscular injection, and can be reused after 4-6 hours, the maximum dose is 40 mg/day, and the daily average dose is (34.35±8.26) mg. HAL group: the initial dose is 5-10 mg intramuscular injection, can bereused after 4-6 hours, the maximum dose is 30 mg/day, the average dose is (15.59±6.04) mg/day. |
| 9 | Fangyu Deng27 | 2011 | ZIP versus HAL | 32.1; 35.2. | Not reported. | Randomised double blinded. | CCMD-3 | ZIP group (n=35): 10-20 mg/day intramuscular injection; HAL group (n=35): 10–30 mg/day intramuscular injection. Four days for one observation period. |
| 10 | Jingy u Chen17 | 2008 | ZIP versus HAL | 32.7; 31.8. | Female: 64; female: 64. | Randomised double blinded. | CCMD-3 | ZIP group: 10 mg/intramuscular injection, 10 mg can be repeated after 6-8 hours, the maximum dose is 30 mg/day. HAL group: 5-10 mg/intramuscular injection, repeated injection after 6-8 hours, the maximum dose of 30 mg/day. The course of treatment is 3 days. |
| 11 | Guangcai Chen28 | 2010 | ZIP versus HAL | 31; 34. | Male: 24/female: 16; male: 26/female: 14. | Randomised double blinded. | CCMD-3 | ZIP group: initial dose 10-20 mg/intramuscular injection, the highest dose 40 mg/day. HAL group: initial dose 5-10 mg/intramuscular injection, the highest dose of 40 mg/day. |
| 12 | Zhaoxiang Zeng29 | 1997 | CLO versus HAL | 33; 34. | Male: 16; male: 17. | Randomised double blinded. | CCMD-2 | CLO group: 1-3 mg/time, two times/day; HAL group: 10-20 mg/time, two times/day; the course of treatment is 1 week. |
| 13 | Changyun Du30 | 2013 | CLO versus HAL | 32; 31. | Male: 20/female: 14; male: 18/female: 16. | Randomised double blinded. | CCMD-3 | CLO group: injection 3 mg/time; HAL group: injection 10 mg/time. The OAS score, BPRS score and adverse reactions of the two groups before injection, 1 hour after injection and 24 hours after injection were observed. |
| 14 | Hongfang Qu31 | 1999 | CLO versus HAL | 31.93; 31.93. | Male: 16/female: 6; male: 16/female: 8. | Randomised double blinded. | CCMD-2-R | Double-blind administration of intramuscular injection of two drugs, the doctor used OAS to assess each time before and after the injection 2 hours and 24 hours; using BPRS to assess the severity, each time before and after the injection 24 hours. |
| 15 | Zhao Li18 | 2007 | CLO versus HAL | 31.4; 34.8. | Male: 6/female: 9; male: 7/female: 8. | Randomised double blinded. | CCMD-3 | The initial dose of HAL in the placebo group was 5 mg intramuscularly, and the maximum dose was 15 mg/day. The initial dose of CLO in the placebo group was 2 mg intramuscularly, with a maximum dose of 6 mg/day. |
ICD-10:The International Classification of Diseases, Tenth Revision
CCMD-3: Chinese Classification of Mental Disorders, Third Edition
OAS: Overt Aggression Scale
BPRS: Brief Psychiatric Rating Scale
DSM-IV:Diagnostic and Statistical Manual of Mental Disorders, 4th. Edition
CLO, clonazepam; HAL, haloperidol; ZIP, ziprasidone.
Data analysis
The network meta-analysis was done with multivariate random-effects meta-analysis and meta-regression (mvMeta),10 and network graphs package11 12 in STATA statistical software. The primary outcome was the efficacy towards agitation symptoms, that is, the reduced scores of the Agitation-Calmness Evaluation Scale (ACES), and the secondary outcome was incidence of adverse side effects like excessive sedation, EPS, tachycardia and ECG Corrected QT Interval (QTc) interperiod extension. The specific statistical methods were listed in the published literature,5 13 14 and it should be made clear that the network diagram was a visualisation of network relationships between interventions, each node of which presented an intervention and the size of node indicated the sample size. Moreover, a solid line connecting two points indicated that two nodes had direct comparative evidence. The more comparisons were made, the thicker the connection was. The two points that were not connected can be used to discuss indirect comparative evidence according to the network relationship.11 The publication bias of the included literature were assessed by funnel plot.10 One of the most important functions of network meta-analysis was to sort the interventions by the effect value. In the end, we chose network rank15 to rank the therapeutic effects and adverse reactions of the interventions and used cluster rank14 for comprehensive ranking. Grades of Recommendation, Assessment, Development and Evaluation (GRADE) was used to evaluate the overall level of the evidence.
Results
Included studies characteristics
A total of 15 studies were included in the meta-analysis, including 1 English paper and 14 Chinese papers. The risk bias of four studies were rated as ‘low risk’ (≥5 low-risk items), while 11 studies were rated as ‘high risk’ (≥2 high-risk items). The level of evidence for primary outcome was medium according to GRADE. There were 11 studies comparing ziprasidone and haloperidol and four comparisons between haloperidol and clonazepam in 15 articles. However, searches about chlorpromazine were excluded from the meta-analysis due to intravenous infusion. All the papers included were RCTs and 14 studies reported the efficacy data (ziprasidone vs haloperidol: n=11; haloperidol vs clonazepam: n=3), while the data of main adverse reactions caused by drugs were as follows: excessive sedation were reported by 12 articles (ziprasidone vs haloperidol: n=8; haloperidol vs clonazepam: n=4). Moreover, 12 articles reported EPS, 11 studies reported tachycardia and 8 studies reported abnormal EKG data; all the studies above were a comparison of ziprasidone and haloperidol. The mean age, gender composition and specific therapies are shown in table 1.
Primary outcome: efficacy for agitation symptoms
We were unable to perform an inconsistency test for circulated comparisons of the three drugs (i.e., direct comparison of ziprasidone and clonazepam, no triangular or quadratic loops) (figure 2). Test for heterogeneity were carried by direct comparisons of the drugs (haloperidol vs ziprasidone: Q=16.15, n=10, p=0.10, I2=38.07%; haloperidol vs clonazepam: Q=3.49, n=2, p=0.17, I2=42.65%) but heterogeneity was not statistically significant. Efficacy indicated the primary outcome, that is, the standardised mean difference (SMD) and SE of the differences in the scores of the ACES before and after treatment (haloperidol: SMD=2.278, 95% CI 1.836 to 2.719; ziprasidone: SMD=2.536, 95% CI 2.082 to 2.990; clonazepam: SMD=1.360, 95% CI 0.127 to 2.593). The results of the network meta-analysis suggested that three intramuscularly administered drugs were all effective for agitation symptoms in Chinese patients with schizophrenia. Furthermore, ziprasidone was better than haloperidol and clonazepam in accordance with effect value (see table 2).
Figure 2.
Network meta-analysis of three injections (left: the network diagram, right: funnel plot). h, ziprasidone; z: haloperidol; c, clonazepam.
Table 2.
The order of efficacy of the three injections for treating agitation symptoms
| Therapy | Sucra | PrBest | MeanRank |
| Clonazepam | 0.0 | 0.0 | 3.0 |
| Haloperidol | 72.8 | 45.6 | 1.5 |
| Ziprasidone | 77.2 | 54.4 | 1.5 |
The maximum score of sucra is 100%, the bigger the score is, the better the interventions work; PrBest is the probability that the treatment will be the best treatment; MeanRank refers to the average order of the treatment, the below is the same.
*Note: the maximum score of sucra is 100%, the bigger the score is, the better the interventions work; PrBest is the probability that the treatment will be the best treatment; MeanRank refers to the average order of the treatment, the below is the same.
Secondary outcome indexes: comparison of excessive sedation
The target of interventions was alleviation of agitation symptoms and reducing the risk of impulsive or self-injurious behaviours. Also these interventions were given to enable patients to cooperate with the necessary assessments and related examinations and give them a smooth transition to subsequent standardised treatment. In this study, we used the acceptability indicators as secondary outcome measures, namely the proportion of excessive sedation in adverse events. The results of the network meta-analysis showed that clonazepam has the strongest sedative effect, while haloperidol was similar to ziprasidone (see table 3). The results of the overall efficacy and acceptability are shown in figure 3.
Table 3.
The order of excessive sedation caused by three injections
| Therapy | Sucra | PrBest | MeanRank |
| Clonazepam | 99.3 | 99.0 | 1.0 |
| Haloperidol | 41.0 | 0.7 | 2.2 |
| Ziprasidone | 9.7 | 0.3 | 2.8 |
Figure 3.

The efficacy and acceptability of three injections for agitation symptoms (the closer to the top right, the better).
Comparison of other adverse reactions such as EPS, tachycardia and abnormal ECG
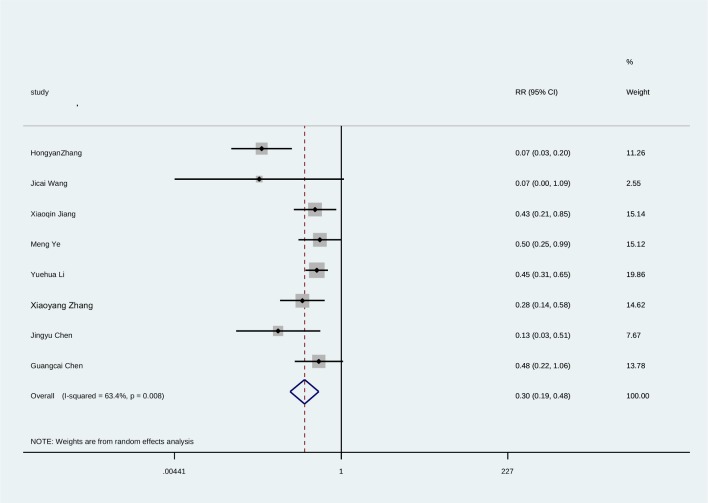
Since the studies of clonazepam did not report adverse reactions data for EPS, tachycardia and abnormal ECG, we performed a traditional meta-analysis of the above adverse reactions by aligning the two injections of ziprasidone and haloperidol. The results suggested that the incidence of EPS was significantly higher in haloperidol than in ziprasidone (z=5.01, p<0.001) (see figure 4). There were no significant differences between the two groups in the adverse reactions of tachycardia and abnormal ECG (the combined statistics were: z=1.69, p=0.091; z=0.87, p=0.386), (see figures 5 and 6).
Figure 4.
Forest plot of meta-analysis of EPS caused by ziprasidone and haloperidol injection.
Figure 5.
Forest plot of meta-analysis of tachycardia caused by ziprasidone and haloperidol injection.
Figure 6.
Forest plot of meta-analysis of abnormal ECG caused by ziprasidone and haloperidol injection.
Discussion
Main findings
Evidence of intramuscular injections for treating agitation symptoms in patients with schizophrenia have not been seen in studies in China and abroad. Our study indicates that ziprasidone and haloperidol work better than clonazepam in the treatment of agitation symptoms in patients with schizophrenia. As for the acceptability, the incidences of excessive sedation in clonazepam are higher than haloperidol and ziprasidone. It is necessary for drug interventions that treat agitation symptoms in schizophrenia to work as quickly as possible and to make patients calm but not excessively sedated. Regarding the efficacy and acceptability of intramuscular administration, ziprasidone is superior to haloperidol and clonazepam, which is consistent with the research findings in China and internationally.16 17 18
Studies have shown that the incidence of aggression in mental disorders is 2 10 times higher than that in healthy individuals, with the highest incidence among those with schizophrenia.2 Agitation caused by schizophrenia has an acute onset, rapid progression and can present with violent aggression.3 Schizophrenia patients often have poor compliance, particularly in the acute phase of agitation symptoms and tend to refuse oral medication. Intramuscular injections work fast so they can alleviate agitation-related symptoms quickly and reduce the risk of violent behaviour or tendencies.19 Intramuscular injections are important in clinical practice to control agitation symptoms.
Ziprasidone and haloperidol stand as new and traditional antipsychotics and the oral dosage forms of which are widely used in the treatment of patients with schizophrenia. As for adverse reactions, haloperidol injections have a low risk of sedation and hypotension, but a high risk of EPS and QTc interval extension especially in patients with high-risk factors (such as heart diseases, electrolyte imbalance, combined with other prolonged QTc interval drugs and so forth).3 The chances for EPS occurrences with ziprasidone are small, but tachycardia and QTc interval extension have a possibility of happening (patients with high-risk factors).19 Benzodiazepines such as clonazepam have strong sedative effects and can also be used for the treatment of agitation symptoms, but it can induce excessive sedation, respiratory depression, hypotension, falls and so forth.2 This study tells us that the risks of EPS induced by haloperidol are higher than ziprasidone, and excessive sedations caused by clonazepam are obvious, which is consistent with the above findings. However, what surprised us is that after the treatment with ziprasidone or haloperidol injections, the occurrence probability of QTc interval extension is not high, and this may be related to the small sample size and the strict requirements of the RCT study for the enrolled samples.
Intravenous administration of benzodiazepines or antipsychotics can quickly work; however, it can also cause respiratory depression, hypotension, pain and thrombophlebitis easily.3 4 If we have to apply it to the patients in this way in clinical practice, we should have complete assurance of cardiopulmonary resuscitation equipment and pay close attention to changes in the patient's vital signs. Due to the high safety of intramuscular injection (I.M)., it is rare to report on intravenous injections, and previous studies did not meet the inclusion criteria of quality, so no study of intravenous injection drugs was included in this study.
Limitations
Limitations are as follows: first, studies included are few, and some of them have small sample sizes, which may lead to statistical bias. Second, due to lack of direct comparison of ziprasidone and clonazepam, the assessment of consistency tests is inadequate; the risk of bias in some studies is ‘high risk’; and the level of GRADE evidence for primary outcome measures is ‘medium’. Therefore, the accuracy and interpretation of the research results need to be handled with caution. Last but not least, we only performed a traditional meta-analysis of some adverse reactions because not all the studies reported the data of efficacy and adverse reactions. The conclusions we got need further study to obtain more comprehensive conclusions, which are more conducive to the clinical practice of agitation symptom therapy in patients with schizophrenia.
Research significance
Compared with traditional meta-analysis, network meta-analysis can simultaneously evaluate multiple interventions. In the absence of direct comparisons, indirect comparisons can also provide valuable information for health policy. When direct comparisons exist, combining results of direct and indirect comparisons can increase the accuracy of findings.20 At the same time, network meta-analysis can sort the interventions by their efficacies and calculate the probability of optimal interventions.14 In the present study, although there were not direct comparisons between clonazepam and ziprasidone, the network meta-analysis still showed that ziprasidone has the advantage in the efficacy and the probability of excessive sedation related to ziprasidone is smaller than that of clonazepam.
Acknowledgments
We would like to thank Professor Kaida Jiang for his guidance and help in the topic selection and conclusion discussion and thank Yi Xu for his help in data entry and document retrieval.
Biography
Liang Su obtained the bachelor degree from Anhui Medical University in Anhui, China in 2000, and a Master and PhD degree from the School of Medicine, Fudan University in Shanghai, China in 2005 and 2008. He have started to work in Shanghai Mental Health Center and Huashan Hospital affiliated to Fudan University since 2005. Now he is working as a deputy chief physician in psychiatry at the department of psychiatry of Shanghai Mental Health Center, Shanghai, China. In addition, he is also a deputy chief physician at the department of psychiatry of Huashan Hospital, Shanghai, China. His research interests include the evidence-based medicine and neuroimaging in psychiatry.

Footnotes
Correction notice: This article has been corrected since it was first published. Figures 4-6 were incorrect at the time of publication. They have been amended.
Contributors: LS: document retrieval, data entry, statistical analysis and thesis writing. ZL: data entry and thesis writing. SS: document retrieval and thesis writing.
YX: literature evaluation, data checking and thesis writing.
Funding: Shanghai Municipal Hospital's Emerging Frontier Technology Joint Research Project (SHDC12012109) Shanghai Open Project of Key Laboratory of Severe Psychiatry (13dz2260500).
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet 2009;373:2041–53. 10.1016/S0140-6736(09)60660-7 [DOI] [PubMed] [Google Scholar]
- 2. Nordstrom K, Allen MH. Managing the acutely agitated and psychotic patient. CNS Spectr 2007;12(10 Suppl 17):5–11. 10.1017/S1092852900026286 [DOI] [PubMed] [Google Scholar]
- 3. Schizophrenia Cooperation Group, Psychiatric Branch of the Chinese Medical Association Expert consensus on agitation in mental disorders [J]. Chinese Journal of Psychiatry 2017;50. [Google Scholar]
- 4. Miao G. Agitation: identification and disposal [J]. Journal of Clinical Psychiatry 2016;26:429–30. [Google Scholar]
- 5. Bai Z, Wang G, Cai S, et al. Efficacy, acceptability and tolerability of 8 atypical antipsychotics in Chinese patients with acute schizophrenia: A network meta-analysis. Schizophr Res 2017;185:73–9. 10.1016/j.schres.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 6. Higgins Jpt GS. Cochrane handbook for systematic reviews of interventions Version 5.1.0[J]. Cochrane database of systematic reviews 2011;2011:S38. [Google Scholar]
- 7. Su L, Cai Y, Xu Y, et al. Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry 2014;14:1–7. 10.1186/s12888-014-0321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang SU, Shen-xun SHI, Zheng LU. Electroconvulsive treatment for geriatric depression: a systematic review of randomized clinical trials. J of Shanghai Jiaotong University(Medical Science) 2018;38:76–80. [Google Scholar]
- 9. Schulz KF, Altman DG, Moher D, et al. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palmer TM, Peters JL, Sutton AJ. Meta-analysis in stata: an updated collection from the stata journal. Stata Press Books 2009.
- 11. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015;15:905–50. [Google Scholar]
- 13. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382:951–62. 10.1016/S0140-6736(13)60733-3 [DOI] [PubMed] [Google Scholar]
- 14. White IR. Network meta-analysis. Stata Journal 2015;15:951–85. [Google Scholar]
- 15. White I. Network: stata module to perform network meta-analysis. Statistical software components 2017.
- 16. Zhang H, Wang G, Zhao J, et al. Intramuscular ziprasidone versus haloperidol for managing agitation in Chinese patients with schizophrenia. J Clin Psychopharmacol 2013;33:178–85. 10.1097/JCP.0b013e3182839612 [DOI] [PubMed] [Google Scholar]
- 17. Jingyu C, Xiangdong Y, Aiping S. Intramuscular ziprasidone versus haloperidol in the treatment of acute agitation in schizophrenia. Shanghai Archives of Psychiatry 2008;20:363–6. [Google Scholar]
- 18. Li Chao ZS, Huifang W, Hua C. Safety and efficacy of clonazepam, haloperidol and haloperidol combined with clonazepam in the treatment of schizophrenia with excitement and agitation. Shanghai Archives of Psychiatry 2007;19:150–2. [Google Scholar]
- 19. Xiao-yang Z, Xiao-jiang Y, Zhe-wei W. Clinical comparative study of intramuscular ziprasidone versus haloperidol in the treatment of acute agitation symptoms of schizophrenia. Sichuan Mental Health 2015;1:42–4. [Google Scholar]
- 20. Miladinovic B, Chaimani A, Hozo I. Indirect treatment comparison. Stata Journal 2014;14:76–86. [Google Scholar]
- 21. Ji-cai W, Xiu-feng XU, Hong O. The second phase clinical trials of intramuscular Ziprasidone for the treatment of acute psychotic agitation of the schizophrenia. Nervous Diseases and Mental Health 2007;7:364–70. [Google Scholar]
- 22. Xiaoqin J, Kairen Y, Bo Z. A clinical study of intramuscular ziprasidone in treating acute agitation in schizophrenia. Shanghai Archives of Psychiatry 2008;20:234–6. [Google Scholar]
- 23. Ren-chang W, Le-hua L, Yi-chao Z. Ziprasidone made in China and Haloperidol in treating acute onset of schizophrenia: a randomized, single blinded, double modelling, parallel control study. Chinese Journal of Clinical Rehabilitation 2005;9:83–5. [Google Scholar]
- 24. Meng YE, Mao-sheng F, Le-hua LI, et al. Domestic ziprasidone mesylate injection in the treatment of acute agitation of schizophrenia. Jof Xinxiang Med College 2009;26:63–5. [Google Scholar]
- 25. Le-hua, ZHAO Jing-ping LI, Xiu-feng XU, Hua-qing M, et al. A comparative study of intramuscular ziprasidone and haloperidol in treating acute agitation in schizophrenia. Chinese Journal of Psychiatry 2006;39:216–9. [Google Scholar]
- 26. Guangtao H, Hang S, Guowei W. Control study on ziprasidone versus haloperidol injection in treating acute agitation of schizophrenia. Chongqing Med 2014;35:4766–8. [Google Scholar]
- 27. Fang-yu D, Qiong WEI. A Control Study of Ziprasidone Injection and Haloperidol Injection for Schizophrenia in the Acute Phase. Occupation and Health 2011;27:2952–3. [Google Scholar]
- 28. Guang-cai C, Fang-yu D, De-sui W, et al. A control study of ziprasidone injection in treating schizophrenia during acute phase. Anhui Med and Pharmaceutical J 2010;14:1072–3. [Google Scholar]
- 29. Zhao-xiang Z, Lian-cheng S, Yong-dong Z. Clonazepam, droperidol and haloperidol in the treatment of psychomotor excitability: a comparative study. Sichuan Mental Health 1997;1:29–30. [Google Scholar]
- 30. Chang-yun D. Comparison of efficacy of clonazepam and haloperidol on psychotic destructive behavior. Modern Diagnosis & Treatment 2013;15:3443–4. [Google Scholar]
- 31. Hong-fang Q, Zhen Z. Comparison of efficacy of clonazepam and haloperidol on psychotic destructive behavior. Heath Psychology Journal 1999;2:134–5. [Google Scholar]